In this issue of Blood, Atanackovic et al1 and Oh et al2 separately report on T-cell immune responses following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccination in patients with non-Hodgkin lymphoma (NHL) receiving CD19 chimeric antigen receptor (CAR) T-cell therapy. Collectively, their findings suggest a potential seatbelt of immune protection against coronavirus disease 2019 (COVID-19) for patients whose treatment journey requires traveling by CD19 CAR T cells.
COVID-19 is caused by SARS-CoV-2, a single-stranded RNA virus that requires host cells for replication. In this regard, SARS-CoV-2 affixes itself to the host cell using its spike (S) protein comprising S1 and S2 subunits, the former containing the receptor binding protein (RBD) that binds to angiotensin-converting enzyme 2 receptor, enabling the virus to enter the host cell and use its replicative machinery.3 Effective eradication of SARS-CoV-2 involves coordinated innate and adaptive antiviral responses, while dysregulated host immunity leads to systemic hyperinflammation underlying severe COVID-19.4
In December 2020, the Food and Drug Administration (FDA) issued emergency use authorization for SARS-CoV-2 mRNA vaccines, BNT162b2 (age >16 years) and mRNA-1273 (age >18 years), as prevention against severe COVID-19. Since FDA approval of BNT162b2 and mRNA-1273, SARS-CoV-2 mRNA booster vaccination and revaccination have been recommended for patients receiving hematopoietic cell transplantation and CAR T-cell therapy.5 These effective vaccines induce antiviral innate and adaptive immune responses in immunocompetent persons, most notably antibodies against the S and RDB proteins as well as viral-specific CD4+ and CD8+ T cells.6,7 Variants of the ancestral SARS-CoV-2 strain, including Delta (B.1.617.2) and Omicron (B.1.1.529), and the variants themselves (ie, Omicron variant, BA.2) are associated with decreased vaccine efficacy attributed to S mutations that attenuate neutralizing antibody effect.8
Patients with hematologic malignancies are at significant risk for severe COVID-19, resulting from primary disease and therapy-associated, aberrant or absent immune function.9 For example, patients receiving CD19 CAR T-cell therapy for relapsed/refractory NHL experience prolonged and profound B-cell aplasia and hypogammaglobulinemia, placing them at higher risk for severe COVID-19 regardless of their receiving supplemental IV immunoglobulin.10 Although attenuated humoral responses to SARS-CoV-2 mRNA vaccination have been noted in patients receiving CD19 CAR T-cell therapy, vaccine-associated cellular immune responses remain largely undefined.
To this end, Oh and colleagues compare adaptive immune responses following BNT162b2 vaccination between 8 patients with B-cell lymphoma who received CD19 4-1BB-CD3z CAR T-cell therapy and 26 healthy controls. Specifically, the authors measure neutralizing antibody, whole-blood S-peptide-induced interferon-γ (IFN-γ) and interleukin-2, IFN-γ enzyme-linked immune absorbent spot, and activation-induced marker CD4+ and CD8+ T cells before and after vaccination (10, 21, and 90 days following first and second inoculations) as well as vaccine-associated cellular responses against SARS-CoV-2 variants, Delta and Omicron. Notably, second BNT162b vaccination induced levels of functional S-specific T cells in CD19 CAR T-cell recipients, which were greater than those in healthy controls. Furthermore, vaccine-associated T-cell responses were largely preserved against SARS-CoV-2 variants (ie, neutralization of Delta being greater than that of Omicron).
Atanackovic and colleagues compare adaptive immune responses following SARS-CoV-2 mRNA vaccination between 18 patients with NHL receiving mostly CD19 4-1BB-CD3z CAR T-cell therapy (10 received mRNA-1273, 8 received BNT162b2) and 10 healthy controls. Interestingly, despite reduced levels of antibody against SARS-CoV-2 proteins (S1, S2, RBD), even after 3 inoculations and lower levels of peripheral blood B cells and quantitative immunoglobulins, patients receiving CAR T cells had normal levels of immunoglobulin G against recall antigens (influenza A, tetanus toxoid, Epstein-Barr virus, and herpes simplex) and higher numbers of plasma cells (CD19−CD38−) relative to controls. In contrast, levels of vaccine-induced anti-S CD4+ and CD8+-specific T cells in patients receiving CAR T cells were comparable to or even higher than control levels. In addition, vaccine-induced T-cell reactivity against the Omicron variant was present albeit decreased relative to activity against ancestral SARS-CoV-2 in patients receiving CAR T cells.
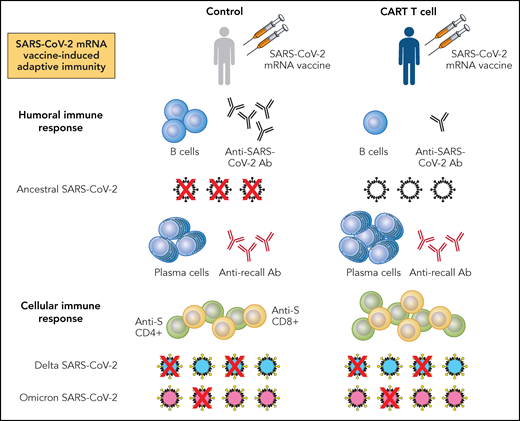
What do these studies tell us? First, relative to healthy controls, patients with NHL receiving CD19 CAR T cells have compromised humoral immune responses following SARS-CoV-2 mRNA vaccination, reflected by decreased vaccine-induced B cells, anti-S and RBD antibody, and neutralizing antibody against ancestral SARS-CoV-2 (see figure). Second, these patients mount vaccine-induced T-cell responses directed against SARS-CoV-2 proteins, which demonstrate neutralizing activity against ancestral virus. Moreover, patients receiving CAR T cells mount higher levels of viral-specific CD4+ and CD8+ cells following SARS-CoV-2 mRNA vaccination. In this regard, it is interesting to speculate a potential compensatory, vaccine-induced immune response reflecting redundancy in host defense against pathogen. Last, these studies show that vaccine-induced, antiviral T-cell activity is present even against SARS-CoV-2 variants, regardless of changes in their S protein epitopes.11
Summary findings from Oh et al and Atanackovic et al for SARS-CoV-2 mRNA vaccine-induced adaptive immune responses in patients with NHL receiving CD19 CAR T-cell therapy. Patients with NHL receiving CD19 CAR T-cell therapy and at least 2 doses of either BNT162b2 or mRNA-1273 mRNA vaccines have reduced numbers of B cells and anti-S or RBD antibody (Ab), associating with decreased neutralization of ancestral SARS-CoV-2 relative to healthy controls receiving similar vaccination. Furthermore, in comparison with healthy controls, patients with NHL receiving CD19 CAR T-cell therapy have greater numbers of plasma cells and similar levels of Ab directed against recall antigens. Last, patients with NHL receiving CD19 CAR T-cell therapy have higher levels of anti-S CD4+ and CD8+ T cells than controls. These antiviral T cells associate with preserved, but variable neutralization of SARS-CoV-2 variants, with neutralization of Delta being greater than that of Omicron. Professional illustration by Patrick Lane, ScEYEnce Studios.
Summary findings from Oh et al and Atanackovic et al for SARS-CoV-2 mRNA vaccine-induced adaptive immune responses in patients with NHL receiving CD19 CAR T-cell therapy. Patients with NHL receiving CD19 CAR T-cell therapy and at least 2 doses of either BNT162b2 or mRNA-1273 mRNA vaccines have reduced numbers of B cells and anti-S or RBD antibody (Ab), associating with decreased neutralization of ancestral SARS-CoV-2 relative to healthy controls receiving similar vaccination. Furthermore, in comparison with healthy controls, patients with NHL receiving CD19 CAR T-cell therapy have greater numbers of plasma cells and similar levels of Ab directed against recall antigens. Last, patients with NHL receiving CD19 CAR T-cell therapy have higher levels of anti-S CD4+ and CD8+ T cells than controls. These antiviral T cells associate with preserved, but variable neutralization of SARS-CoV-2 variants, with neutralization of Delta being greater than that of Omicron. Professional illustration by Patrick Lane, ScEYEnce Studios.
What more do we need to know? Although informative, these studies are preliminary observations that need to be verified in larger groups of patients and include more robust immunologic analyses in defining vaccine-induced T-cell function and virus killing. In addition, these findings are limited to a select number of patients with NHL who received mainly 1 type of CD19 CAR T-cell therapy (CD19 4-1BB-CD3z). Most importantly, no definitive conclusions or correlations can be made regarding clinical protection against breakthrough COVID-19 and immunologic responses measured.
Notwithstanding, these works describe intact T-cell–mediated mechanisms following SARS-CoV-2 mRNA vaccination in patients at very high risk for severe COVID-19 that may contribute to conferring protection against COVID-19. As COVID-19 transitions from a pandemic to an endemic phase,12 patients with hematologic malignancies will continue to be at higher risk for severe COVID-19, requiring antiviral therapies and prevention strategies, including booster vaccination and revaccination following cell therapies.13 Therefore, studies by Oh and Atanackovic and their colleagues may illuminate the road ahead for patients requiring CAR T cells as well as further investigation into defining immune responses following mRNA vaccination. In so doing, primary and booster SARS-CoV-2 mRNA vaccination likely is the protection needed to avert a future catastrophic encounter with COVID-19.
Conflict-of-interest disclosure: The author is an employee of the National Marrow Donor Program. He declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal