Key Points
Thirty-six percent of patients with thrombocytopenia after ChAdOx1 nCov-19 test positive for antiplatelet antibodies.
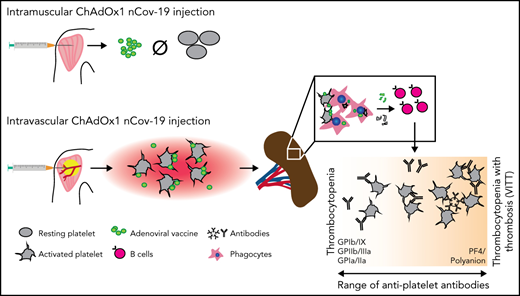
IV, but not IM, injection of ChAdOx1 nCov-19 triggers thrombocytopenia and platelet-directed immunity including antibody formation in mice.
Abstract
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are based on a range of novel platforms, with adenovirus-based approaches (like ChAdOx1 nCov-19) being one of them. Recently, a novel complication of SARS-CoV-2–targeted adenovirus vaccines has emerged: immune thrombocytopenia, either isolated, or accompanied by thrombosis (then termed VITT). This complication is characterized by low platelet counts, and in the case of VITT, also by platelet-activating platelet factor 4 antibodies reminiscent of heparin-induced thrombocytopenia, leading to a prothrombotic state with clot formation at unusual anatomic sites. Here, we detected antiplatelet antibodies targeting platelet glycoprotein receptors in 30% of patients with proven VITT (n = 27) and 42% of patients with isolated thrombocytopenia after ChAdOx1 nCov-19 vaccination (n = 26), indicating broad antiplatelet autoimmunity in these clinical entities. We use in vitro and in vivo models to characterize possible mechanisms of these platelet-targeted autoimmune responses leading to thrombocytopenia. We show that IV but not intramuscular injection of ChAdOx1 nCov-19 triggers platelet-adenovirus aggregate formation and platelet activation in mice. After IV injection, these aggregates are phagocytosed by macrophages in the spleen, and platelet remnants are found in the marginal zone and follicles. This is followed by a pronounced B-cell response with the emergence of circulating antibodies binding to platelets. Our work contributes to the understanding of platelet-associated complications after ChAdOx1 nCov-19 administration and highlights accidental IV injection as a potential mechanism of platelet-targeted autoimmunity. Hence, preventing IV injection when administering adenovirus-based vaccines could be a potential measure against platelet-associated pathologies after vaccination.
Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the ensuing pandemic has led to the development of vaccines eliciting an immune response to the virus spike protein in an unprecedented time frame. Multiple vaccine platforms have been deployed successfully, with mRNA- and adenovirus vector–based vaccines carrying the bulk of global vaccination efforts.1 Recently, a potentially novel adverse effect of vaccination termed vaccine-induced immune thrombotic thrombocytopenia (VITT) has been described in patients receiving adenovirus-based vaccines (ChAdOx1 nCov-19 [AstraZeneca] and Ad26.COV2.S [Johnson&Johnson]) 4 to 30 days before symptoms.2-8 This rare disease resembles heparin-induced thrombocytopenia type II (HIT II) with a positive platelet factor 4 (PF4) antibody assay required for diagnosis.9,10 The recognized epitope of VITT PF4 antibodies differs from HIT antibodies as it targets the PF4 heparin-binding site and does not require heparin for immune complex formation and FcγRIIa-dependent platelet activation.11,12 It remains unclear what triggers the formation of these autoantibodies. Hypotheses include that charge-dependent PF4-adenoviral vector binding occurs, which then renders this complex immunogenic, or that direct platelet–adenoviral vector interaction leads to formation of antibodies.13 In addition, thrombocytopenia without thrombosis has also been described clinically14,15 and is associated with ChAdOx1 nCov-19 administration.16-18 Whether these patients also show antiplatelet immunity is not known. Also, why thrombocytopenia with or without thrombosis develops only in a small fraction of vaccinated individuals in response to ChAdOx1 nCov-19 vaccination remains poorly understood, and potential preventive measures are not established.19,20
Methods
Patient data
All sera of patients with confirmed thrombocytopenia (platelet count < 150 × 109/L) and suspicion for VITT after ChAdOx1 nCov-19 vaccination sent to the department of transfusion medicine of the University Medicine Greifswald in April 2021 were included in the study. Sera were tested for anti-PF4 antibodies by in-house immunoglobulin G (IgG)-specific PF4/polyanion enzyme immunoassay and PF4-induced platelet activation test (PIPA). Those sera that tested positive in both assays were considered definite VITT. Patients with thrombocytopenia after vaccination testing negative in both the anti-PF4 enzyme immunoassayand the PIPA test were considered anti–PF4-IgG seronegative patients.
Furthermore, employees of the University Medicine Greifswald who were vaccinated with ChAdOx1 nCov-19 were used as healthy controls. Blood samples of the cohort were collected 7 to 9 days after vaccination as part of the SeCo study,21 and none of the study participants developed thrombosis or clinically evident thrombocytopenia. The median age of female controls was 42.82 ± 10.42 years and that of male controls was 46.7 ± 10.61 years.
The use of whole blood and platelets from healthy donors and serum from patients was approved by the Ethics Board at University Medicine Greifswald and was conducted in accordance with the Declaration of Helsinki.
Platelet activation assays
PIPA was performed as previously described.22 Briefly, washed platelets from healthy donors and heat-inactivated patient serum were incubated in a microtiter plate in the presence of either buffer, 0.2 U/mL anti–factor X low-molecular-weight heparin (reviparin, Abbott), 100 IU/mL unfractionated heparin or PF4 (10 µg/mL final concentration, Chromatec, Greifswald, Germany). During the 45-minute incubation at room temperature, the transparency of the suspension was assessed every 5 minutes, and the assay was considered positive for platelet activation if clearance of the solution and visible aggregates occurred.
Monoclonal antibody-specific immobilization of platelet antigens assay
All sera sent to the platelet laboratory of the University Medicine Greifswald in April 2021 for thrombocytopenia with and without thrombotic complications were screened for platelet-specific antibodies by the monoclonal antibody-specific immobilization of platelet antigens (MAIPA) assay.23,24
Mouse strains
C57BL/6 mice were purchased from The Jackson Laboratory. AID−/−sIgM−/− and TgH(KL25) mice were bred and maintained at our animal facility. Mice were between 12 and 16 weeks of age for the experiments and were age- and sex-matched for intergroup analyses. All mice live in standardized conditions where temperature, humidity, and hours of light and darkness are maintained at a constant level and provided water and food ad libitum. All animal experiments were performed in compliance with the relevant ethical regulations and were approved by the local administrative authority for the protection of animals (Regierung von Oberbayern, München).
Vaccine acquisition
Only residual content of discarded BNT162b2 (Pfizer-BioNTech) and ChAdOx1 nCov-19 (AstraZeneca) vials, that, according to the supervising physicians’ body (Kassenärztliche Vereinigung Bayerns), could not be used for human application, were used for this study. For ChAdOx1 nCov-19, this meant a storage period greater than 48 hours after first withdrawal of a dose from a multidose vial, and for BNT162b2, it meant a storage period greater than 24 hours after first withdrawal of a dose from a multidose vial. Both vaccines were stored continuously at 4°C. Experiments were conducted as soon as possible after vaccine acquisition.
Human platelet isolation
Human blood for in vitro assays was obtained after informed consent from healthy male and female donors aged 20 to 35 years. Blood was collected with a syringe containing 1/7 of the blood volume acid dextrose citrate (39 mM citric acid, 75 mM sodium citrate, 135 mM dextrose). The blood was further diluted 1:1 with Tyrode’s buffer (137 mM NaCl, 2.8 mM KCl, 12 mM NaHCO3, 5.5 mM glucose, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH = 6.5) and centrifuged at 70g for 35 minutes, and the supernatant platelet-rich plasma (PRP) was taken. PRP was further spun down after adding 0.1 mg/mL PGI2 at 1200g for 10 minutes. The resulting washed platelet pellet was resuspended in pH 7.2 Tyrode’s buffer and used for subsequent experiments.
Mouse platelet isolation
Mouse blood was obtained via retro-orbital blood collection, after anesthesia with medetomidine, midazolam, and fentanyl. Blood was collected via a capillary and mixed with 1/7 of the blood volume citrate. The blood was further diluted 1:1 with Tyrode’s buffer (137 mM NaCl, 2.8 mM KCl, 12 mM NaHCO3, 5.5 mM glucose, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH = 6.5) and centrifuged at 70g for 10 minutes, and the supernatant PRP was taken. PRP was further spun at 1200g for 10 minutes. The resulting washed platelet pellet was resuspended in pH 7.2 Tyrode’s buffer and used for subsequent experiments. For some experiments, platelets were incubated with prostacyclin (0.1 mg/mL) or 100 µM of adenosine diphosphate and 1 mM calcium for 15 minutes before incubation with ChAdOx1 nCov-19.
Platelet incubation with vaccines
Approximately 108 washed platelets were incubated with ∼5 × 107 ChAdOx1 nCov-19 viral particles (100 µL), 100 µL BNT162b2, or phosphate-buffered saline (PBS) for 20 minutes. After this, 1:200 X649 (mouse, emfret Analytics) or CD41 (human, Biolegend) was added, further incubated for 10 minutes, and then analyzed via flow cytometry. For transfusion, after separate vaccine incubation with ChAdOx1 nCov-19 and BNT162b2, platelets were stained with 1:200 X649 or X488 (emfret Analytics), respectively, for 10 minutes. X488 and X649 labeling was switched for half of the mice to ensure no color bias. The platelets were spun down at 1200g for 10 minutes, resuspended in 200 µL pH 7.2 Tyrode’s buffer, and immediately injected separately via tail vein injection.
Mouse vaccine injection and blood collection
Five (low dose) or 50 µL ChAdOx1 nCov-19, BNT162b2, PBS, heat-inactivated ChAdOx1 nCov-19, or ADV-004 (Cell Biolabs, Inc.) was used for injections. Heat inactivation of ChAdOx1 nCov-19 was achieved by heating the vaccine to 99°C for 30 minutes and then spinning the resulting protein precipitate down at 10 000g for 3 minutes and taking the supernatant. Injection was performed either via tail vein or in the medial aspect of the thigh of isoflurane anesthetized mice. At specified time points, vaccine-injected and transfused mice were briefly anesthetized with isoflurane, and blood was obtained via facial vein puncture and collected in EDTA capillaries. Blood counts were measured with the Sysmex XN-V Series XN-1000V cell counter; only blood counts that passed internal quality control were used.
Platelet-bound immunoglobulin detection and anti–spike protein antibody detection
Mouse washed platelets were incubated with 1:4 plasma of mice 6 days after inoculation and control mice for 40 minutes. After adding 1:100 CD41 Pacific blue, anti-IgG Cy3, and anti-IgM fluorescein isothiocyanate for a 20-minute incubation, washed platelets were fixed, diluted, and analyzed via flow cytometry. Anti–spike antibody concentration was measured with an enzyme-linked immunosorbent assay (ELISA) for mouse anti–SARS-CoV-2 Spike Trimer according to manufacturer instructions (RAS-T023, Acro Biosystems). Complement binding of the antiplatelet antibodies was assessed by incubating washed platelets with C1-esterase inhibitor (0.5 mg/mL; Sigma Aldrich) for 15 minutes before proceeding with antibody detection as described above.
Intravital imaging of the spleen
Coinjection of platelets was performed as previously described.25 After injection of X488- or X649-labeled platelets incubated with ChAdOx1 nCov-19 or PBS, mice were also injected with 15 µL each of CD169 PE and F4/80 BV421 (Biolegend), anesthetized with medetomidine, midazolam, and fentanyl, and shaved, and the spleen exposed via a small lateral cut. Subsequently, the spleen was mobilized and imaged with a Zeiss LSM 880 confocal microscope (×20 objective) in airyscan mode. Regular blood flow in the spleen was observed throughout imaging, and z-Stack images (1-µm slice thickness) and videos were taken.
Data analysis and statistics
Analysis of histology and intravital imaging was done using ImageJ v2.1 or Imaris (Bitplane). Intravital imaging cell tracking was done manually on randomly selected cells that could be traced for at least 5 frames. Statistics were computed with Imaris; the meandering index was defined as net displacement/total track length. Unpaired t tests with Welch’s correction were used for all cell tracking analyses. For area quantification, thresholds were taken with ImageJ, and overlap was quantified by percent area overlapping between thresholds. Minimal Gaussian blur (≤0.5 µm) was applied to some histologic images and videos for clarity. For all statistical tests, Prism (GraphPad) was used. Unpaired t tests were used unless otherwise noted. Percentages derived from patient numbers were rounded to the next integer. All data are shown as mean ± standard error of the mean (SEM). When more than 3 t tests were applied on the same data set, multiple testing correction was performed.
Results
Broad antiplatelet immunity as a rare side effect of ChAdOx1 nCov-19 vaccination
Reports on thrombotic and thrombocytopenic complications after adenovirus-based SARS-CoV-2 vaccination include an increased rate of isolated thrombocytopenia and anti-PF4 IgG-seronegative cases of sinus vein thromboses.17,26 We therefore asked whether additional immune-mediated mechanisms beyond anti-PF4 antibodies might be involved in the pathogenesis of thrombocytopenia associated with adenoviral vector anti–SARS-CoV2 vaccines.
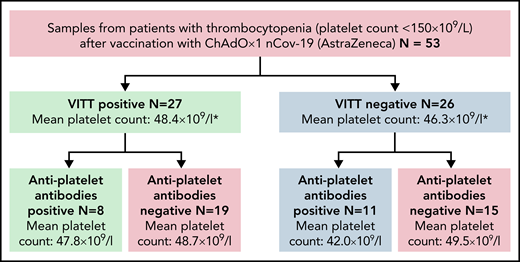
We screened sera from a cohort of patients with confirmed VITT for antiplatelet autoantibodies (n = 27 patients), using the gold standard indirect glycoprotein-specific ELISA (MAIPA).23 All patients tested were positive for anti-PF4 antibodies and fulfilled diagnostic criteria for VITT. We discovered antiplatelet antibodies in 8 of 27 cases (30% of patients; Figure 1; supplemental Table 1). Of these, 2 patients were positive for 1 antibody (either anti-GPIa/IIa or anti-GPIb/IX), 1 patient was positive for both anti-GPIa/IIa and anti-GPIb/IX, and another 5 patients were pan-reactive for all 3 antiplatelet antibodies (Table 1).
Antiplatelet antibody screening of VITT and thrombocytopenia patients after ChAdOx1 nCov-19 administration. A total of n = 53 patients with thrombocytopenia after ChAdOx1 nCov-19 vaccination were screened for antiplatelet antibodies. N = 27 of these patients had positive anti–PF4-heparin antibody tests, fulfilling the criteria for VITT. N = 26 patients had isolated thrombocytopenia. Of these groups, n = 8 and n = 11 patients had antiplatelet antibodies present in the serum, respectively. The mean platelet counts between positive and negative antiplatelet antibody cohorts did not differ significantly (Welch’s t test).
Antiplatelet antibody screening of VITT and thrombocytopenia patients after ChAdOx1 nCov-19 administration. A total of n = 53 patients with thrombocytopenia after ChAdOx1 nCov-19 vaccination were screened for antiplatelet antibodies. N = 27 of these patients had positive anti–PF4-heparin antibody tests, fulfilling the criteria for VITT. N = 26 patients had isolated thrombocytopenia. Of these groups, n = 8 and n = 11 patients had antiplatelet antibodies present in the serum, respectively. The mean platelet counts between positive and negative antiplatelet antibody cohorts did not differ significantly (Welch’s t test).
Clinical parameters and VITT diagnostic results of VITT-positive samples
| Patient . | Platelet nadir (×109/L) . | Clinical symptoms . | Symptoms onset (no. of days after vaccination) . | Anti-PF4-heparin IgG ELISA (OD)* . | PF4-dependent platelet-activation assay (activated cells)† . | IvIgG prior blood withdrawal . | MAIPA‡ . | ||
|---|---|---|---|---|---|---|---|---|---|
| GPIIb/IIIa antibodies . | GPIb/IX antibodies . | GPIa/IIa antibodies . | |||||||
| Female, 40 y | 36 | Initially with severe headache and nausea, 5 d later CSVT | 6 | 2.94 | Positive | No | 0.145 | 0.12 | 1.915 |
| Female, 57 y | 24 | CSVT | 3 | 2.87 | Positive | No | 0.19 | 0.245 | 0.09 |
| Female, 61 y | 50 | Flu-like symptoms with headache and fever | 9 | 3.2 | Positive | Yes | 0.25 | 0.305 | 0.44 |
| Male, 20 y | 99 | Initially with headache, later pulmonary embolism and CSVT | Not known | 3.56 | Positive | Yes | 0.2 | 0.21 | 0.235 |
| Male, 21 y | 94 | Initially with headache, later CSVT | ∼2 | 3.12 | Positive | No | 0.18 | 0.265 | 0.34 |
| Female, 61 y | 46 | Initially with headache, later dissection of the A. vertebralis and infarct of the medulla oblongata. | 11 | 2.63 | Positive | Yes | 0.305 | 0.215 | 0.305 |
| Female, 74 y | 12 | Initially with headache, flu-like symptoms, limb pain, discomfort, petechia | 1 | 1.93 | Positive | Not known | 0.265 | 0.235 | 0.3 |
| Female, 55 y | 21 | Thrombosis of the V. ophthalmica, acute ischemic stroke, later focal seizures | 7 | 2.55 | Positive | No | 0.685 | 0.645 | 2.2 |
| Patient . | Platelet nadir (×109/L) . | Clinical symptoms . | Symptoms onset (no. of days after vaccination) . | Anti-PF4-heparin IgG ELISA (OD)* . | PF4-dependent platelet-activation assay (activated cells)† . | IvIgG prior blood withdrawal . | MAIPA‡ . | ||
|---|---|---|---|---|---|---|---|---|---|
| GPIIb/IIIa antibodies . | GPIb/IX antibodies . | GPIa/IIa antibodies . | |||||||
| Female, 40 y | 36 | Initially with severe headache and nausea, 5 d later CSVT | 6 | 2.94 | Positive | No | 0.145 | 0.12 | 1.915 |
| Female, 57 y | 24 | CSVT | 3 | 2.87 | Positive | No | 0.19 | 0.245 | 0.09 |
| Female, 61 y | 50 | Flu-like symptoms with headache and fever | 9 | 3.2 | Positive | Yes | 0.25 | 0.305 | 0.44 |
| Male, 20 y | 99 | Initially with headache, later pulmonary embolism and CSVT | Not known | 3.56 | Positive | Yes | 0.2 | 0.21 | 0.235 |
| Male, 21 y | 94 | Initially with headache, later CSVT | ∼2 | 3.12 | Positive | No | 0.18 | 0.265 | 0.34 |
| Female, 61 y | 46 | Initially with headache, later dissection of the A. vertebralis and infarct of the medulla oblongata. | 11 | 2.63 | Positive | Yes | 0.305 | 0.215 | 0.305 |
| Female, 74 y | 12 | Initially with headache, flu-like symptoms, limb pain, discomfort, petechia | 1 | 1.93 | Positive | Not known | 0.265 | 0.235 | 0.3 |
| Female, 55 y | 21 | Thrombosis of the V. ophthalmica, acute ischemic stroke, later focal seizures | 7 | 2.55 | Positive | No | 0.685 | 0.645 | 2.2 |
PF4–heparin enzyme-linked immunosorbent assay (ELISA) was done as described by Juhl et al.27 Reactivity on ELISA was defined according to the optical-density units (reference value OD < 0.50).
PF4-dependent platelet-activation assay was performed as recently described by Greinacher et al.49 The cutoff for a negative result is 0.50 optical-density units.
The cutoff for a negative result is OD < 0.2. Italic entries represent antiplatelet antibody detection.
Because ChAdOx1 nCov-19 vaccination is also associated with an increased risk for isolated thrombocytopenia, we asked whether these antiplatelet antibodies can also be found in patients without VITT. We hence screened additional (n = 26) anti–PF4-IgG seronegative patients that developed thrombocytopenia after ChAdOx1 nCov-19 vaccination. Eleven of these 26 patients had antiplatelet antibodies (42% of patients; Figure 1; supplemental Table 1). Here, 2 patients were positive for 1 antibody (either anti-GPIa/IIb or anti-GPIb/IX), 2 patients were positive for both anti-GPIa/IIa and anti-GPIIb/IIIa, and 7 patients were pan-reactive for all 3 antiplatelet antibodies (Table 2). No antiplatelet antibodies were found in the control group (n = 52) of health care workers vaccinated with ChAdOx-1 nCoV who experienced no clinical symptoms of thrombosis or thrombocytopenia (supplemental Table 2).
Clinical parameters and VITT diagnostic results of VITT-negative samples
| Patient . | Platelet nadir (×109/L) . | Clinical symptoms . | Symptoms onset (no. of days after vaccination) . | Anti-PF4-heparin IgG ELISA (OD)* . | PF4-dependent platelet-activation assay (activated cells)† . | IvIgG prior blood withdrawal . | MAIPA‡ . | ||
|---|---|---|---|---|---|---|---|---|---|
| GPIIb/IIIa antibodies . | GPIb/IX antibodies . | GPIa/IIa antibodies . | |||||||
| Male, 30 y | 110 | Petechia, known primary sclerosing cholangitis | 2 | 0.23 | Negative | No | 0.78 | 2.105 | 2.195 |
| Male, 62 y | 111 | Thrombosis (site of thrombosis not indicated) | 14 | 0.15 | Negative | Yes | 0.21 | 0.17 | 0.355 |
| Female, 30 y | 55 | Initially with headache, fever, limb pain and flu-like syndroms; elevated d-dimer level | 1 | 0.14 | Negative | No | 0.08 | 0.515 | 0.105 |
| Female, 73 y | 30 | Not indicated | 17 | 0.35 | Negative | No | 0.52 | 0.9 | 0.895 |
| Male, 59 y | 17 | Gastric mucosal bleeding | 45 | 0.44 | Negative | Yes | 0.23 | 0.29 | 0.36 |
| Male, 78 y | 4 | Petechia, epistaxis | 20 | 0.38 | Negative | Yes | 1.145 | 0.52 | 0.65 |
| Male, 69 y | 5 | Petechia and oral bleeding, discomfort | Not known | 0.1 | Negative | No | 0.555 | 0.865 | 0.385 |
| Male, 71 y | 2 | Not known | 10 | 0.1 | Negative | Not known | 0.665 | 1.28 | 0.75 |
| Female, 21 y | 13 | Petechia, hematoma | ∼21 | 0.27 | Negative | Not known | 0.215 | 0.185 | 0.25 |
| Male, 62 y | 5 | Petechia | 15 | 0.34 | Negative | Yes | 0.21 | 0.245 | 0.325 |
| Female, 70 y | 110 | Not known | Not known | 0.26 | Negative | Not known | 0.09 | 0.145 | 1.535 |
| Patient . | Platelet nadir (×109/L) . | Clinical symptoms . | Symptoms onset (no. of days after vaccination) . | Anti-PF4-heparin IgG ELISA (OD)* . | PF4-dependent platelet-activation assay (activated cells)† . | IvIgG prior blood withdrawal . | MAIPA‡ . | ||
|---|---|---|---|---|---|---|---|---|---|
| GPIIb/IIIa antibodies . | GPIb/IX antibodies . | GPIa/IIa antibodies . | |||||||
| Male, 30 y | 110 | Petechia, known primary sclerosing cholangitis | 2 | 0.23 | Negative | No | 0.78 | 2.105 | 2.195 |
| Male, 62 y | 111 | Thrombosis (site of thrombosis not indicated) | 14 | 0.15 | Negative | Yes | 0.21 | 0.17 | 0.355 |
| Female, 30 y | 55 | Initially with headache, fever, limb pain and flu-like syndroms; elevated d-dimer level | 1 | 0.14 | Negative | No | 0.08 | 0.515 | 0.105 |
| Female, 73 y | 30 | Not indicated | 17 | 0.35 | Negative | No | 0.52 | 0.9 | 0.895 |
| Male, 59 y | 17 | Gastric mucosal bleeding | 45 | 0.44 | Negative | Yes | 0.23 | 0.29 | 0.36 |
| Male, 78 y | 4 | Petechia, epistaxis | 20 | 0.38 | Negative | Yes | 1.145 | 0.52 | 0.65 |
| Male, 69 y | 5 | Petechia and oral bleeding, discomfort | Not known | 0.1 | Negative | No | 0.555 | 0.865 | 0.385 |
| Male, 71 y | 2 | Not known | 10 | 0.1 | Negative | Not known | 0.665 | 1.28 | 0.75 |
| Female, 21 y | 13 | Petechia, hematoma | ∼21 | 0.27 | Negative | Not known | 0.215 | 0.185 | 0.25 |
| Male, 62 y | 5 | Petechia | 15 | 0.34 | Negative | Yes | 0.21 | 0.245 | 0.325 |
| Female, 70 y | 110 | Not known | Not known | 0.26 | Negative | Not known | 0.09 | 0.145 | 1.535 |
PF4–heparin enzyme-linked immunosorbent assay (ELISA) was done as described by Juhl et al.27 Reactivity on ELISA was defined according to the optical-density units (reference value OD < 0.50).
PF4-dependent platelet-activation assay was performed as recently described by Greinacher et al.49 The cutoff for a negative result is 0.50 optical-density units.
The cutoff for a negative result is OD < 0.2. Italic entries represent antiplatelet antibody detection.
Of the total patients (n = 53) screened with thrombocytopenia (with or without VITT) after ChAdOx1 nCov-19 vaccination, 36% (n = 19) presented antiplatelet antibodies. This points towards a broad antiplatelet adaptive immune response beyond anti-PF4 antibodies in a considerable subset of patients with ChAdOx1 nCov-19 induced thrombocytopenia.
IV application of ChAdOx1 nCov-19 induced platelet activation and thrombocytopenia in mice
Platelet autoantigen presentation and subsequent antibody emergence can occur on clearance of activated platelets by antigen presenting cells (APCs), for example, splenic macrophages.28 We asked whether the adenoviral vector vaccine interacts with platelets and if this, in turn, induces phenotypic changes, potentially leading to thrombocytopenia and antiplatelet antibodies.
To test this, we studied direct adenoviral vector–platelet interaction and the consequences on the platelet phenotype in vitro. We incubated healthy human platelets with ChAdOx1 nCov-19, mRNA-based vaccine BNT162b2 (Pfizer-BioNTech), or PBS. Incubation with anti–pan-adenovirus antibody revealed binding of ChAdOx1 nCov-19 to human platelets (Figure 2A-B). This binding resulted in a shift of platelets toward an activated phenotype with increased activated GPIIb/IIIa integrin (Figure 2C; supplemental Figure 1A). Similarly, mouse platelets also strongly bound ChAdOx1 nCov-19 in vitro (supplemental Figure 1B). Activated platelets displayed a trend toward a higher binding, whereas prostacyclin incubation had no effect compared with naïve washed platelets (supplemental Figure 1C).
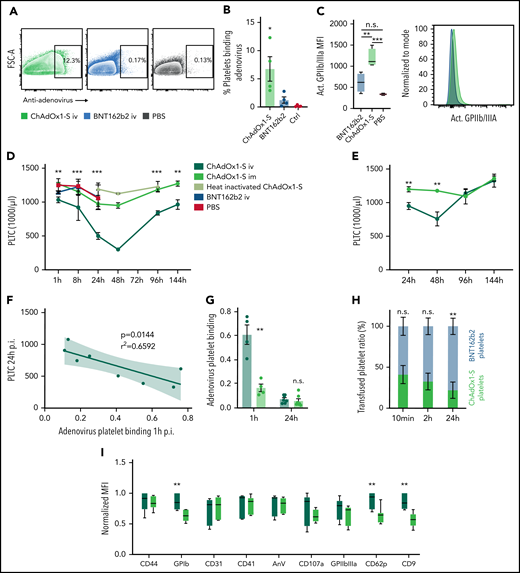
IV but not IM injection of ChAdOx1 nCov-19 triggers ChAdOx1 nCov-19-platelet binding and thrombocytopenia. (A) Exemplary flowcytometric contour plots of human washed platelets with ChAdOx1 nCov-19, BNT162b2, or PBS. Two percent contour with outliers shown gate shows adenovirus positive platelets. (B) Quantification of adenovirus platelet binding according to panel A. One-way analysis of variance with post hoc Tukeýs test. Comparison of ChAdOx1 nCov-19 to both controls. n ≥ 3 human donors per group. (C) Platelet expression of activated GPIIbIIIa mean fluorescent intensity incubated with ChAdOx1 nCov-19, BNT162b2, or PBS control. One-way analysis of variance with post hoc Tukey’s multiple comparison test, n = 4 per group. Exemplary histogram of activated GPIIbIIIa is also shown. (D) Platelet counts of mice over time. Multiple t tests with Holm-Sidak correction of ChAdOx1 nCov-19 IV and IM is shown. n ≥ 3 per time point for of ChAdOx1 nCov-19 groups; n ≥ 2 per time point for other groups. (E) Platelet counts of mice over time with 0.5 µL ChAdOx1 nCov-19 injection. Multiple t tests with Holm-Sidak correction of ChAdOx1 nCov-19 IV and IM is shown. n ≥ 6 per time point and group. (F) Linear regression of platelet count at 24 hours after inoculation and adenovirus binding to platelets 1 hour after inoculation. 95% confidence interval shown; P value denotes line deviation from zero. (G) Adenovirus-platelet binding after IM or IV administration of ChAdOx1 nCov-19 1 and 24 hours after inoculation. Unpaired t tests. n ≥ 4 per group. (H) Ratio of transfused BNT162b2 and ChAdOx1 nCov-19 platelets (total transfused platelets are normalized to 100%) over time. Unpaired t tests. n = 4 per time point. (I) Platelet surface marker expression of mouse platelets 24 hours after administration of ChAdOx1 nCov-19 IV or IM. Normalized mean fluorescent intensity. Multiple t tests with Holm-Sidak correction. n = 7 mice per group. Error bars are mean ± SEM. *P < .05; **P < .01; ***P < .001. n.s., not significant.
IV but not IM injection of ChAdOx1 nCov-19 triggers ChAdOx1 nCov-19-platelet binding and thrombocytopenia. (A) Exemplary flowcytometric contour plots of human washed platelets with ChAdOx1 nCov-19, BNT162b2, or PBS. Two percent contour with outliers shown gate shows adenovirus positive platelets. (B) Quantification of adenovirus platelet binding according to panel A. One-way analysis of variance with post hoc Tukeýs test. Comparison of ChAdOx1 nCov-19 to both controls. n ≥ 3 human donors per group. (C) Platelet expression of activated GPIIbIIIa mean fluorescent intensity incubated with ChAdOx1 nCov-19, BNT162b2, or PBS control. One-way analysis of variance with post hoc Tukey’s multiple comparison test, n = 4 per group. Exemplary histogram of activated GPIIbIIIa is also shown. (D) Platelet counts of mice over time. Multiple t tests with Holm-Sidak correction of ChAdOx1 nCov-19 IV and IM is shown. n ≥ 3 per time point for of ChAdOx1 nCov-19 groups; n ≥ 2 per time point for other groups. (E) Platelet counts of mice over time with 0.5 µL ChAdOx1 nCov-19 injection. Multiple t tests with Holm-Sidak correction of ChAdOx1 nCov-19 IV and IM is shown. n ≥ 6 per time point and group. (F) Linear regression of platelet count at 24 hours after inoculation and adenovirus binding to platelets 1 hour after inoculation. 95% confidence interval shown; P value denotes line deviation from zero. (G) Adenovirus-platelet binding after IM or IV administration of ChAdOx1 nCov-19 1 and 24 hours after inoculation. Unpaired t tests. n ≥ 4 per group. (H) Ratio of transfused BNT162b2 and ChAdOx1 nCov-19 platelets (total transfused platelets are normalized to 100%) over time. Unpaired t tests. n = 4 per time point. (I) Platelet surface marker expression of mouse platelets 24 hours after administration of ChAdOx1 nCov-19 IV or IM. Normalized mean fluorescent intensity. Multiple t tests with Holm-Sidak correction. n = 7 mice per group. Error bars are mean ± SEM. *P < .05; **P < .01; ***P < .001. n.s., not significant.
Vaccines are routinely administered intramuscularly (IM) and trigger immune responses mainly in the draining lymph nodes.29 Based on our finding that (1) adenoviral vaccine binds to and activates blood platelets and (2) broad antiplatelet immunity emerges after vaccine application in some individuals, we hypothesized that accidental IV injection of adenoviral vaccine might lead to platelet-adenovirus aggregate formation with platelet activation and possibly ensuing platelet clearance.
When we modeled accidental IV injection in mice, we observed systemic distribution of injected Evans blue dye on intravascular needle placement and blood aspiration, whereas local application without evidence of blood return did not lead to systemic Evans blue distribution (supplemental Figure 1D). IV but not IM injection of ChAdOx1 nCov-19 triggered transient thrombocytopenia in mice (Figure 2D). IV injection of PBS, BNT162b2, or heat-inactivated ChAdOx1 nCov-19 injection had no effect on platelet counts over an extended time course (Figure 2D; supplemental Figure 1E). Recombinant adenovirus ADV-004 showed platelet binding in vitro but had no effect on platelet counts or parameters over time, pointing toward serotype-dependent effects (supplemental Figure 1F). Thrombocytopenia with a significant drop in platelet counts at 24 and 48 hours after IV injection of ChAdOx1 nCov-19 also occurred when we applied reduced doses to mice that were equivalent to human vaccination doses (0.5 µL; Figure 2E). Thrombocytopenia induced by IV injection of ChAdOx1 nCov-19 was not caused by fluid shift or changes in platelet production, as indicators like platelet distribution width, mean platelet volume, and platelet larger cell ratio did not show variations (supplemental Figure 1G). Furthermore, we did not detect evidence for a difference in platelet production, as megakaryocytes in the bone marrow of IV- and IM-injected mice did not differ in terms of number and size (supplemental Figure 1H). The decline in platelet count correlated directly with adenovirus-positive platelets circulating in the blood 1 hour after IV injection (Figure 2F; supplemental Figure 1I). AID−/−sIgM−/− mice lacking IgM and IgG antibodies displayed the same platelet count kinetics as wild type mice, indicating that development of thrombocytopenia within 48 hours after IV injection of ChAdOx1 nCov-19 does not depend on antibodies (supplemental Figure 1J).30,31 In line with this, previous exposure to IV ChAdOx1 did not affect platelet count kinetics after re-exposure to ChAdOx1 via a second IV injection (supplemental Figure 1K). IV but not IM injection of ChAdOx1 nCov-19 resulted in a strong increase in platelet-adenovirus aggregates in vivo, which confirms binding of adenoviral particles to platelets even in the presence of plasma proteins (Figure 2G). These aggregates had a short dwell time and rapidly disappeared from the circulation (Figure 2G). Disappearance of platelet-adenovirus aggregates preceded recovery of platelet counts (Figure 2D,G). We did not observe changes in the amount of platelet extracellular vesicles in IV- or IM-injected mice, excluding extensive terminal platelet activation in the vasculature (supplemental Figure 1L). We also did not observe changes in platelet leukocyte aggregate formation, which suggests that platelet leukocyte aggregates do not play a major role in the clearance of virus-coated platelets (supplemental Figure 1M). To further investigate if binding of adenovirus is responsible for enhanced platelet clearance, we coinjected labeled platelets incubated with either ChAdOx1 nCov-19 or BNT162b2. We observed that ChAdOx1 nCov-19–incubated platelets were selectively removed from the circulation (Figure 2H; 2.8 ± 1.1-hour half-life for ChAdOx1 nCov-19–incubated platelets vs 10 ± 3.7-hour half-life for BNT162b2-incubated platelets). At 24 hours after IV ChAdOx1 nCov-19 administration, mouse platelets displayed an activated phenotype with increased P-selectin (CD62P), CD9, and GPIb levels compared with IM injection (Figure 2I). Therefore, IV but not IM injection of ChAdOx1 nCov-19 induces thrombocytopenia in mice.
Removal of ChAdOx1 nCov-19 binding platelets by splenic macrophages
Platelets have been shown to bind viruses and bacterial products as part of their role as innate immune cells.32,33 Specifically, platelets are known to bind and shuttle blood-borne pathogens to professional phagocytes in the spleen and liver to mount an adaptive immune response.34,35 In the spleen, platelets are abundant in the red pulp during steady-state conditions but on pathogen binding deposit pathogens in the marginal zone and follicles, which are predominant regions of adaptive immune responses.36 We therefore analyzed platelet localization in the spleen after IV compared with IM vaccine application. To robustly label all platelets, we injected a fluorescent non-activating GPIb antibody at the time of vaccine injection. Analysis of overall GPIb content in the spleen after IV injection in contrast to IM injection revealed prominent accumulation of GP1b+ material in the marginal zone and follicles; these areas are devoid of platelets under steady-state conditions (Figure 3A). In IM-injected animals, the GPIb signal identified round and intact platelets in the splenic red pulp (Figure 3A). In contrast, the GPIb signal in the IV injection group appeared morphologically different, with follicular and marginal zone clusters reminiscent of platelet remnants (Figure 3A; supplemental Figure 2A). Professional phagocytes, particular red pulp macrophages, phagocytose activated platelets in the spleen.37 In line with this, we found increased platelet uptake by splenic F4/80 macrophages after ChAdOx1 nCov-19 IV compared with IM administration (Figure 3B). In summary, these data show phagocytic uptake and processing of platelets and adenovirus in the spleen.
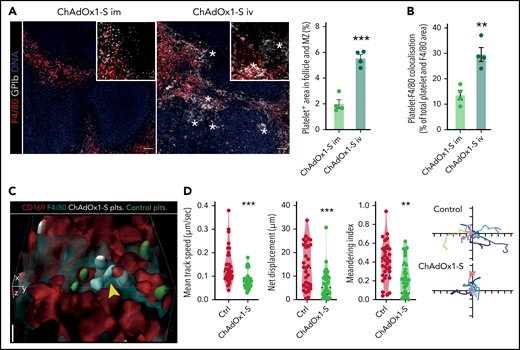
Platelet-adenovirus aggregates are taken up by macrophages in the spleen. (A) Images of X649 endogenous platelet labeling mice 24 hours after IM and IV ChAdOx1 nCov-19 administration. Cut out of red pulp to follicle transition is shown on the upper left. Stars are next to GPIb + agglomerations that are morphologically reminiscent of platelet remnants. Scale bars for overview are 50 µm and for cut outs are 10 µm. Quantification of platelet (×649) area in the marginal and follicle zone as percent of marginal and follicular area. n = 4 per group, unpaired t test. (B) Quantification of platelet-F4/80 co-localization in the splenic red pulp. Colocalization is shown as percent of total platelet-F4/80 area. n = 4 per group, unpaired t test. (C) 3D reconstruction of in vivo microscopy of the spleen with transfused platelets pretreated with either ChAdOx1 nCov-19 (white) or PBS (green). ChAdOx1 nCov-19 pretreated platelets are taken up by F4/80+/CD169+ macrophages, whereas control treated platelets are not. Scale bar is 5 µm. (D) Platelet tracking parameters of intraviral imaging. n = 31 platelets tracked per group of 1 mouse. Ten exemplary tracked paths per group are shown on the left as x and y displacement; ticks signify 5-µm intervals. Unpaired t test with Welch’s correction. Error bars are mean ± SEM. **P < .01; ***P < .001.
Platelet-adenovirus aggregates are taken up by macrophages in the spleen. (A) Images of X649 endogenous platelet labeling mice 24 hours after IM and IV ChAdOx1 nCov-19 administration. Cut out of red pulp to follicle transition is shown on the upper left. Stars are next to GPIb + agglomerations that are morphologically reminiscent of platelet remnants. Scale bars for overview are 50 µm and for cut outs are 10 µm. Quantification of platelet (×649) area in the marginal and follicle zone as percent of marginal and follicular area. n = 4 per group, unpaired t test. (B) Quantification of platelet-F4/80 co-localization in the splenic red pulp. Colocalization is shown as percent of total platelet-F4/80 area. n = 4 per group, unpaired t test. (C) 3D reconstruction of in vivo microscopy of the spleen with transfused platelets pretreated with either ChAdOx1 nCov-19 (white) or PBS (green). ChAdOx1 nCov-19 pretreated platelets are taken up by F4/80+/CD169+ macrophages, whereas control treated platelets are not. Scale bar is 5 µm. (D) Platelet tracking parameters of intraviral imaging. n = 31 platelets tracked per group of 1 mouse. Ten exemplary tracked paths per group are shown on the left as x and y displacement; ticks signify 5-µm intervals. Unpaired t test with Welch’s correction. Error bars are mean ± SEM. **P < .01; ***P < .001.
Even under steady-state conditions, contacts between passing platelets and local macrophages are frequently observed in the red pulp.38 To understand the dynamics of platelet-adenovirus trafficking in more detail, we cotransfused labeled control and ChAdOx1 nCov-19–incubated platelets and performed 4-dimensional intravital microscopy of the spleen (supplemental Figure 2B). We observed increased phagocytosis of ChAdOx1 nCov-19–pretreated platelets by CD169+ and F4/80+ macrophages in vivo (Figure 3C; supplemental Figure 2C-D), again indicating preferential targeting of virus-loaded platelets to professional phagocytes in the spleen. As a result, single-cell tracking in the spleen revealed decreased motility and enhanced adherence of the ChAdOx1 nCov-19–pretreated platelets to macrophages (Figure 3D; supplemental Video 1).
Formation of antiplatelet antibodies in response to IV ChAdOx1 nCov-19 application in mice
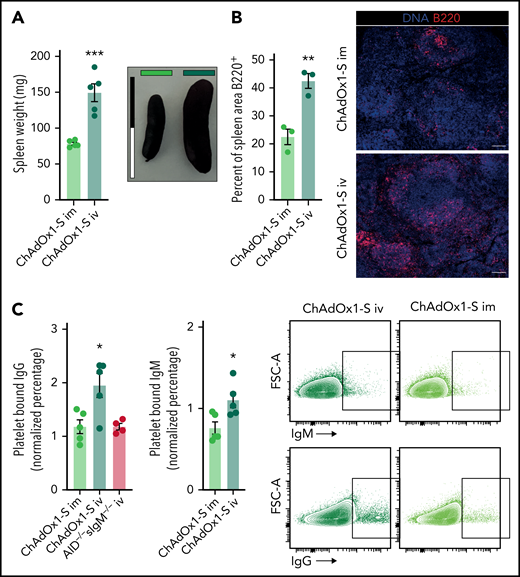
Based on the platelet uptake by splenic macrophages, we hypothesized that presentation of platelet antigens in the spleen might trigger antiplatelet antibody production observed in our cohort of patients with thrombocytopenia after ChAdOx1 nCov-19 vaccination. This could result in a second delayed wave of platelet depletion occurring with a characteristic lag time, which resembles the kinetics of clinically apparent vaccine-associated thrombocytopenia reported to occur typically 4 to 30 days after vaccination. To test this hypothesis, we assessed adaptive immune responses in the spleen on IM/IV ChAdOx1 nCov-19 application. IV ChAdOx1 nCov-19 led to significant splenomegaly with enlarged B-cell follicles in the spleen (Figure 4A-B; supplemental Figure 2E). Next, we determined whether this immune response results in production of antibodies targeting platelets. We incubated control mouse platelets with plasma from IM or IV ChAdOx1 nCov-19–treated animals 6 days after injection and control plasma. Naïve ChAdOx1 nCov-19–unexperienced platelets incubated with plasma from IV-injected mice showed increased IgM and IgG binding, pointing toward circulating antiplatelet antibodies (Figure 4C). In contrast, plasma from IV-injected ChAdOx1 nCov-19 antibody-deficient AID−/−sIgM−/− mice showed a signal comparable to controls (Figure 4C). The platelet-binding antibodies did not lead to platelet activation, most likely because mouse platelets, unlike their human counterparts, lack Fc receptors (supplemental Figure 2F). Binding of the platelet-binding antibodies was independent of C1-dependent activation of the classical complement pathway as well, suggesting a direct binding mechanism via Fab (supplemental Figure 2G). In addition, we did not detect increased IgG binding in a cohort of IV-injected heavy chain transgenic TgH(KL25) mice, which selectively produce antibodies against lymphocytic choriomeningitis virus (supplemental Figure 2H).39 This additional control group indicates that specific antibody binding is necessary to mediate platelet–IgG interaction in our setting. We did not detect any difference in thrombosis and vessel occlusions in necropsies of IV- and IM-injected mice, both macroscopically and microscopically in the liver (supplemental Figure 2I). We also did not detect an increase in D-dimer, a serum marker for thrombosis (supplemental Figure 2J). Anti–spike protein antibody formation did not differ between IM and IV injections, with both groups showing similar anti–spike protein antibody formation compared with AID−/−sIgM−/− mice (supplemental Figure 2K). This suggests that the antiplatelet antibody formation is independent of anti–spike protein antibody formation after vaccination, in line with previous reports in humans for anti-PF4 antibodies.40
Formation of platelet-binding antibodies in response to IV ChAdOx1 nCov-19 application. (A) Spleen weights of animals 6 days after inoculation. Representative images of spleens of both groups are shown; scale bar is 2 cm. Unpaired t test. n = 5 per group. (B) Quantification of B220+ are in the spleen of IV or IM ChAdOx1 nCov-19–injected mice 6 days after inoculation as percentage of total splenic area. Unpaired t test; n = 3 per group. Representative images of a splenic micrograph of both groups are shown; scale bar is 100 µm. (C) Control mouse platelets positive for bound IgM and IgG after incubation with plasma from mice 6 days after inoculation with either IM or IV ChAdOx1 nCov-19 or ChAdOx1 nCov-19 AID−/−sIgM−/− mice for IgG additionally. n = 5 per group for IV or IM; n = 4 for AID−/−sIgM−/−. Unpaired t tests between IV ChAdOx1 nCov-19 and the other groups. Percent of platelets binding IgM or IgG are normalized to the mean of 4 control plasmas. Error bars are mean ± SEM. *P < .05; **P < .01; ***P < .001.
Formation of platelet-binding antibodies in response to IV ChAdOx1 nCov-19 application. (A) Spleen weights of animals 6 days after inoculation. Representative images of spleens of both groups are shown; scale bar is 2 cm. Unpaired t test. n = 5 per group. (B) Quantification of B220+ are in the spleen of IV or IM ChAdOx1 nCov-19–injected mice 6 days after inoculation as percentage of total splenic area. Unpaired t test; n = 3 per group. Representative images of a splenic micrograph of both groups are shown; scale bar is 100 µm. (C) Control mouse platelets positive for bound IgM and IgG after incubation with plasma from mice 6 days after inoculation with either IM or IV ChAdOx1 nCov-19 or ChAdOx1 nCov-19 AID−/−sIgM−/− mice for IgG additionally. n = 5 per group for IV or IM; n = 4 for AID−/−sIgM−/−. Unpaired t tests between IV ChAdOx1 nCov-19 and the other groups. Percent of platelets binding IgM or IgG are normalized to the mean of 4 control plasmas. Error bars are mean ± SEM. *P < .05; **P < .01; ***P < .001.
Taken together, these findings point toward platelet-glycoprotein targeted autoimmunity after vaccination with the adenovirus vector–based vaccine ChAdOx1 nCoV19, independent of the pathognomonic formation of anti-PF4 antibodies in VITT.
Discussion
Increased frequency of immune thrombocytopenic purpura (ITP)-like vaccine-associated thrombocytopenia and the novel clinical entity VITT have been linked to adenoviral-based COVID-19 vaccinations.9,16,18
Our data provide experimental evidence for the potential sequence of events that lead to thrombocytopenia in some patients after ChAdOx1 nCov-19 vaccination: accidental IV vaccine injection leads to platelet-adenovirus aggregation, which in turn triggers platelet activation and subsequent splenic clearance (supplemental Figure 3). This process is antibody independent and most likely clinically inapparent. Phagocytosis and processing of platelet-adenovirus aggregates by professional phagocytes and then creates a cross-reactive immune response, which could culminate in the delayed emergence of a spectrum of antibodies binding to platelets. Depending on epitope and antibody titer, this could lead to platelet clearance and ensuing thrombocytopenia. Moreover, if the host immune system targets PF4, strongly activating antibodies could lead to fulminant platelet activation in vivo and thrombogenicity. Potentially, this could explain the variable clinical presentation: if this immune response leads to the formation of platelet-activating PF4 antibodies, thrombocytopenia is accompanied by thrombosis.22
In line with this concept, we detected a range of platelet glycoprotein-specific autoantibodies in the sera of 30% of VITT cases and in 42% of patients with isolated thrombocytopenia (total, n = 53 patients). This confirms the existence of broad antiplatelet immunity beyond PF4 antibodies, ranging from antibodies classically associated with ITP to strongly activating PF4 antibodies in VITT. This is likely an underestimation of the presence of antiplatelet autoantibodies in VITT, as the indirect assay used here has only a sensitivity of about 10% compared with detecting platelet autoantibodies directly on the patient’s platelets.33-35 Unfortunately, direct assays using autologous platelets of patients requires fresh blood samples, which were not available. In this context, it is important to note that we have not observed platelet anti-autoantibodies in hundreds of healthy subjects serving as negative controls for the used MAIPA assay (data not shown) and did not detect evidence of antiplatelet immunity in asymptomatic subjects (n = 52) after ChAdOx1 nCov-19 vaccination.
Glycoprotein-specific antibodies are of potential major clinical importance in the setting of vaccine-associated thrombocytopenia: The phenotype of affected patients likely varies depending on the individual antibody pattern from clinically inapparent to severe. There is ample mechanistic evidence from translational studies that antiplatelet antibodies against platelet glycoprotein epitopes directly contribute to thrombocytopenia.41-43 In some patients, platelet-binding antibodies might induce a second wave of clinically apparent thrombocytopenia through platelet opsonization, contributing to the increased incidence in ITP-like thrombocytopenia observed after adenoviral vector vaccination.16 This could also contribute to the observed secondary bleeding complications in VITT. Severe major bleeding in a subgroup of patients with VITT might not only be secondary to cerebral venous sinus thrombosis but cotriggered by platelet autoantibodies. In addition, the observation that steroids seem to be beneficial in VITT patients with rather low platelet counts < 30 000/µL might also be partly related to their effect on platelet autoantibody formation or reduced phagocytic potential of macrophages.44-46
Adenovirus as a potential trigger of thrombocytopenia has been described in an earlier study by Othman et al.47 Others have established platelet-adenovirus phagocytosis and shuttling in the spleen48; however, our study is the first to describe this mechanism for an adenovirus vaccine to date. Most important, our study is the first showing that VITT and platelet autoantibodies frequently occur concomitantly.
Intact adenovirus is the most likely culprit, as we could not observe the same effect with heat-inactivated adenovirus, ruling out other heat-stable ingredients of the vaccine, such as glycosaminoglycans, which play a major role in HIT. The vaccine contains intact viral particles.49 Intact adenovirus binding by platelets has been shown for other adenoviridae34,47 and has also been observed for ChAdOx1 nCov-19.50 Although our study includes experiments that show thrombocytopenia with doses of ChAdOx1 nCov-19 in mice approximately equivalent to human vaccine doses, dose-dependent effects of ChAdOx1 nCov-19 cannot be fully excluded. Interestingly, we did not detect thrombocytopenia after IV application of ADV-004, another adenovirus strain. Hence, adenovirus subtype might determine platelet clearance and ensuing complications. Therefore, optimization of adenoviral vector design with negligible effects on platelets could increase safety.
Accidental IV deltoid injection is considered an unlikely event for anatomic reasons.51 Our findings might therefore explain the rare incidence of vaccine-induced thrombocytopenia and potentially also for VITT occurring in about 1:50 000 and 1:100 000 vaccinations.45 Further prospective studies with human subjects are needed to pinpoint frequencies and mechanisms of inadvertent IV deltoid injection. Whether the capillary leakage effect induced by EDTA in the vaccine further contributes to transmission of the virus into the circulation requires further studies.50,52
It is important to note that we used an animal model that inherently cannot fully recapitulate disease pathophysiology observed in humans. Murine platelets do not express Fcy receptors and therefore are not suited as a model of antibody-mediated platelet activation and thrombocytopenia (ie, upon injection of heparin and anti-PF4 antibody).53 Similarly, we did not observe delayed antibody-dependent thrombocytopenia or thrombus formation after IV adenovirus injection in mice and did not detect platelet activation on antibody binding. In addition, because of methodical challenges, we cannot further specify the exact platelet antigen of the identified IgG and IgM antibodies after IV ChAdOx1 inoculation in our mouse model, but our data support the concept that specific antibody binding to platelets is required. Because of these limitations of the murine model used here, the relevance of the identified mechanism for human vaccine-related ITP and VITT remains to be determined.
Further research is necessary to fully characterize the antibody responses in patients with thrombocytopenia or VITT as a vaccination side effect, as well as (large) animal studies to define mechanisms and targets of the mounted adaptive immune response in depth. Crucially, it is important to elucidate whether anti-PF4 antibodies develop independently of the observed antiplatelet immune response or are dictated by the same mechanisms. Furthermore, our identified mechanism neither explains the increased incidence of VITT/thrombocytopenia after the first vaccine dose compared with the second dose nor does it explain the finding that de novo or worsening of preexisting ITP can also occur after vaccination with mRNA-based vaccines.54
In the case of adenovirus-based vaccines, measures to prevent IV injection during vaccination could protect against induction of an antiplatelet immune response as a vaccination side effect. As shown in this work qualitatively in an animal model, aspiration might be an option for preventing IV injection. Currently, the Centers for Disease Control do not recommend aspiration during IM application of vaccine,55 and more studies will be needed to evaluate whether aspiration can prevent IV injection during vaccination. Finally, the authors want to emphasize that the findings in this paper do not represent an evaluation of ChAdOx1 nCov-19 efficacy for preventing or attenuating COVID-19 disease or SARS-CoV-2 infection. Safety reviews by national and international medical authorities have consistently found the benefits of ChAdOx1 nCov-19 to outweigh potential adverse effects.56,57 Hence, the findings in this paper describe a possible mechanism for a rare side effect of ChAdOx1 nCov-19 administration and offer a discussion of potential measures to prevent this side effect.
Acknowledgments
The authors thank all laboratory members for valuable input and help and Wolfgang Schneider for substantial support.
This study was supported by the Deutsche Herzstiftung e.V., Frankfurt a.M. (to L.N.), Deutsche Forschungsgemeinschaft (DFG) Sonderforschungsbereich (SFB) 914 (to S.M., B02 and Z01; to K. Stark, B02), DFG SFB 1123 (to S.M., B06; to K. Stark, A07), DFG FOR 2033 (to S.M.), the German Centre for Cardiovascular Research (DZHK; Clinician Scientist Programme to L.N.; Munich Heart Alliance (MHA) 1.4VD to S.M.; FP7 program project 260309, PRESTIGE to S.M.), and the DFG Clinician Scientist Programme PRIME (413635475 to K.P. and R.K.). The work was also supported by the European Research Council (ERC-2018-ADG “IMMUNOTHROMBOSIS” to S.M. and ERC-“T-MEMORE” to K. Stark).
Authorship
Contribution: L.N. and A.L. conceived and initiated the study; L.N. conceptualized the study; L.N., A.L., K.P., K. Stark, and S.M. planned the experiments; L.N., A.L., K.P., M.E., A.A., E.R., V.E., M.M., M.-L.H., Z.Z., D.K., V.P., L.E., D.R., J.R., and A.T. conducted the experiments; A.L., L.N., K.P., M.E., A.A., V.E., Z.Z., R.E., and R.K. analyzed the results; A.L. visualized the findings; L.N. wrote the initial draft; L.N., A.L., K.P., K. Stark, and S.M. edited the manuscript with input from all authors; T.P., K. Spiekermann, M.I., T.T., and A.G. provided resources; and L.N., K.P., K. Stark, and S.M. provided oversight, resources, and funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leo Nicolai, Medizinische Klinik und Poliklinik I, University Hospital Ludwig-Maximilian-University Munich, Marchioninistr 15, 81377 Munich, Germany; e-mail: leo.nicolai@med.uni-muenchen.de; Alexander Leunig, Medizinische Klinik und Poliklinik I, University Hospital Ludwig-Maximilian-University Munich, Marchioninistr 15, 81377 Munich, Germany; e-mail: alexander.leunig@med.un-muenchen.de; Kami Pekayvaz, Medizinische Klinik und Poliklinik I, University Hospital Ludwig-Maximilian-University Munich, Marchioninistr 15, 81377 Munich, Germany; e-mail: kami.pekayvaz@med.uni-muenchen.de; and Konstantin Stark, Medizinische Klinik und Poliklinik I, University Hospital Ludwig-Maximilian-University Munich, Marchioninistr 15, 81377 Munich, Germany; e-mail: konstantin.stark@med.uni-muenchen.de.
Materials are shared upon reasonable request by e-mail.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
L.N., A.L., and K.P. contributed equally to this study.
K. Stark and S.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal