Key Points
Axicabtagene ciloleucel induced long-term survival with no new safety signals in patients with refractory LBCL.
Durable responses were associated with expansion of chimeric antigen receptor T cells early after intravenous infusion.
Abstract
In phase 2 of ZUMA-1, a single-arm, multicenter, registrational trial, axicabtagene ciloleucel (axi-cel) autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy demonstrated durable responses at 2 years in patients with refractory large B-cell lymphoma (LBCL). Here, we assessed outcomes in ZUMA-1 after 5 years of follow-up. Eligible adults received lymphodepleting chemotherapy followed by axi-cel (2 × 106 cells per kg). Investigator-assessed response, survival, safety, and pharmacokinetics were assessed in patients who had received treatment. The objective response rate in these 101 patients was 83% (58% complete response rate); with a median follow-up of 63.1 months, responses were ongoing in 31% of patients at data cutoff. Median overall survival (OS) was 25.8 months, and the estimated 5-year OS rate was 42.6%. Disease-specific survival (excluding deaths unrelated to disease progression) estimated at 5 years was 51.0%. No new serious adverse events or deaths related to axi-cel were observed after additional follow-up. Peripheral blood B cells were detectable in all evaluable patients at 3 years with polyclonal B-cell recovery in 91% of patients. Ongoing responses at 60 months were associated with early CAR T-cell expansion. In conclusion, this 5-year follow-up analysis of ZUMA-1 demonstrates sustained overall and disease-specific survival, with no new safety signals in patients with refractory LBCL. Protracted B-cell aplasia was not required for durable responses. These findings support the curative potential of axi-cel in a subset of patients with aggressive B-cell lymphomas. This trial was registered at ClinicalTrials.gov, as #NCT02348216.
Introduction
Chimeric antigen receptor (CAR) T-cell therapies targeting CD19 induce considerable clinical benefit and have advanced the treatment of multiply-relapsed large B-cell lymphoma (LBCL) since initial reports.1-3 Axicabtagene ciloleucel (axi-cel), an autologous CAR T-cell therapy, was initially approved for relapsed or refractory (R/R) LBCL after ≥2 lines of systemic therapy based on the pivotal ZUMA-1 study.4,5 In ZUMA-1, treatment with axi-cel demonstrated an 83% objective response rate (ORR), with a 58% complete response (CR) rate.6 The safety profile of axi-cel was manageable, with serious adverse events (AEs) mostly occurring early after infusion.1,6 After a median of 27.1 months of follow-up, 39% of patients were in ongoing response and the median overall survival (OS) was not yet reached.6 In a propensity analysis comparing the ZUMA-1 study with the SCHOLAR-1 retrospective study of non−CAR T-cell salvage regimens, compared with conventional chemotherapy, axi-cel had a 73% reduction in the risk of death.7
More recently, axi-cel has been approved in the United States for R/R LBCL within 12 months of first-line chemoimmunotherapy.4 As of March 2022, >6000 patients worldwide have received axi-cel.8 Real-world analyses of postapproval axi-cel have demonstrated largely comparable outcomes with ZUMA-1.9-11
Herein, we report long-term efficacy and safety of axi-cel in patients with refractory LBCL from phase 2 of ZUMA-1 after 5 years of follow-up, including exploratory analyses to assess durability of response and long-term survival.
Methods
Patients and study design
ZUMA-1 is a multicenter, single-arm, registrational phase 1/2 study conducted at 22 sites across the United States and Israel, and is registered at ClinicalTrials.gov/NCT02348216. Participating sites were previously reported.1,6 ZUMA-1 was conducted in compliance with the principles of the Declaration of Helsinki. All enrolled patients provided written informed consent to participate in the study. The protocol was approved by the institutional review board at each site. The study was designed in a collaboration between the study sponsor (Kite) and the investigators. All authors had access to the clinical trial data and contributed to the study conduct, data analysis and interpretation, and manuscript development.
Patients eligible for enrollment were aged ≥18 years and had histologically confirmed LBCL (World Health Organization 2008 classification), including diffuse LBCL (DLBCL, phase 2, cohort 1), or primary mediastinal B-cell lymphoma or transformed follicular lymphoma (phase 2, cohort 2). Patients had refractory disease, defined as progressive or stable disease as best response to the most recent prior therapy or relapse within 12 months of autologous stem cell transplantation (SCT). Full eligibility criteria were previously described.1
Procedures and end points
Patients underwent leukapheresis followed by lymphodepleting chemotherapy (fludarabine 30 mg/m2 per day and cyclophosphamide 500 mg/m2 per day) on days −5 through −3. On day 0, patients received a single intravenous infusion of axi-cel at a target dose of 2 × 106 CAR T cells per kilogram of body weight. Bridging therapy was not permitted.
The primary end point of ZUMA-1 was investigator-assessed ORR per the International Working Group Response Criteria for Malignant Lymphoma.12 Secondary end points included duration of response (DOR), progression-free survival (PFS), OS, incidence of select AEs of interest, and blood levels of CAR T cells. Additional efficacy end points included in this long-term analysis were event-free survival (EFS; time from axi-cel infusion until disease progression, initiation of new anticancer therapy, excluding SCT, or any-cause death), time to progression (time from axi-cel infusion to progressive disease), time to next therapy (time from axi-cel infusion to initiation of new anticancer therapy, including CAR T-cell retreatment and excluding SCT, or death from any cause), and disease-specific survival (time from axi-cel infusion to death due to progressive disease). PFS and OS were assessed by response at 3, 6, 12, and 24 months. The association between median EFS and key baseline patient and disease characteristics was assessed. In addition, association between DOR and achievement of a CR at, or after, the week 4 assessment was assessed.
Disease assessments and safety monitoring have been previously reported.6,13 Briefly, response was assessed per protocol by the investigator and by an independent central review committee at month 1 and every 3 months between months 3 and 24, after which assessments only occurred as clinically indicated per institutional standard-of-care. AEs monitored between 3 and 24 months after infusion included only select AEs of clinical interest. Cytokine release syndrome (CRS) was graded based on Lee et al.14 The severity of all AEs was graded per the NCI CTCAE, version 4.03. Biomarker assessments are detailed in the supplemental Data, available on the Blood website, and have been described previously.15
Statistical analyses
Efficacy, safety, and biomarker assessments included all patients treated with axi-cel in cohorts 1 and 2 of phase 2 of ZUMA-1 and were performed after patients had ≥5 years of follow-up. Per protocol, no formal statistical hypotheses were assessed in this analysis. Descriptive statistics were used to summarize baseline characteristics and incidence of AEs. Two-sided 95% confidence intervals (CIs) for response rates were assessed using the Clopper-Pearson method. Time-to-event outcomes were assessed using Kaplan-Meier methodology.
Results
Patients
As reported previously, 111 patients with LBCL were enrolled and leukapheresed between 19 May 2015 and 15 September 2016.1 Of those, 101 patients were treated with axi-cel. Baseline patient and disease characteristics have been detailed previously.1,6 The median age of treated patients was 58 years (range, 23-76; Table 1). Most patients (84%) had lactate dehydrogenase levels above the upper limit of normal, and median tumor burden by sum of product diameters was 3723 mm2. Among 42 patients with pretreatment tumor samples, 33 (79%) had poor prognostic markers, including 6 with high-grade B-cell lymphoma and 27 with double-expressor lymphoma (Table 1).
Patient baseline characteristics
| . | N = 101 . |
|---|---|
| Age, median (range), y | 58 (23-76) |
| ≥65 y, n (%) | 24 (24) |
| Male sex, n (%) | 68 (67) |
| Disease type, n (%) | |
| DLBCL | 77 (76) |
| PMBCL | 8 (8) |
| TFL | 16 (16) |
| Prognostic marker per central laboratory, n (%) | |
| HGBL – double hit | 3 (3) |
| HGBL – triple hit | 1 (1) |
| HGBL-NOS | 2 (2) |
| Double-expressor lymphoma | 27 (27) |
| No HGBL/tested negative | 7 (7) |
| N/A | 2 (2) |
| Not tested | 59 (58) |
| Previous therapies, n (%) | |
| ASCT | 25 (25) |
| Platinum based | 90 (89) |
| Response to most recent prior therapy, n (%)∗ | |
| SD | 13 (13) |
| PD | 67 (66) |
| Lymphoma present in bone marrow, n (%) | 8 (8) |
| LDH > ULN, n (%) | 85 (84) |
| Sum of product diameters, median (range), mm2 | 3723 (171-23 297) |
| . | N = 101 . |
|---|---|
| Age, median (range), y | 58 (23-76) |
| ≥65 y, n (%) | 24 (24) |
| Male sex, n (%) | 68 (67) |
| Disease type, n (%) | |
| DLBCL | 77 (76) |
| PMBCL | 8 (8) |
| TFL | 16 (16) |
| Prognostic marker per central laboratory, n (%) | |
| HGBL – double hit | 3 (3) |
| HGBL – triple hit | 1 (1) |
| HGBL-NOS | 2 (2) |
| Double-expressor lymphoma | 27 (27) |
| No HGBL/tested negative | 7 (7) |
| N/A | 2 (2) |
| Not tested | 59 (58) |
| Previous therapies, n (%) | |
| ASCT | 25 (25) |
| Platinum based | 90 (89) |
| Response to most recent prior therapy, n (%)∗ | |
| SD | 13 (13) |
| PD | 67 (66) |
| Lymphoma present in bone marrow, n (%) | 8 (8) |
| LDH > ULN, n (%) | 85 (84) |
| Sum of product diameters, median (range), mm2 | 3723 (171-23 297) |
ASCT, autologous SCT; HGBL, high-grade B-cell lymphoma; LDH, lactate dehydrogenase; N/A, not available; NOS, not otherwise specified; PD, progressive disease; PMBCL, primary mediastinal B-cell lymphoma; SD, stable disease; TFL, transformed follicular lymphoma; ULN, upper limit of normal.
Patients included those who did not relapse after ASCT.
Efficacy
The data cutoff date for this analysis was 11 August 2021, and patients treated with axi-cel had a median of 63.1 months of follow-up (range, 58.9-68.4) from infusion. Among the 101 patients who received axi-cel, the investigator-assessed ORR was 83% (n = 84; 95% CI, 74-90; Table 2), and 58% (n = 59) achieved a CR. Among all treated patients, median DOR was 11.1 months (95% CI, 4.2-51.3). At data cutoff, 31 patients (31%) had an ongoing objective response and 30 (30%) had an ongoing CR. Concordantly, the median duration of CR was 62.2 months, whereas the median duration of partial response was 1.9 months (Table 2). Among patients who achieved a CR (n = 59), 37 (62.7%) had a CR by the week-4 assessment and 22 (37.3%) reached CR after the week-4 assessment. The median DOR was 34.7 months (95% CI, 7.8-not estimable [NE]) in those who had a CR by week 4 and was not reached (95% CI, 26.9-NE) in those who achieved a CR after week 4 (supplemental Figure 1).
Investigator-assessed response
| . | N = 101 . |
|---|---|
| Best response, n (%, 95% CI) | |
| Objective response | 84 (83, 74-90) |
| CR | 59 (58, 48-68) |
| PR | 25 (25, 17-34) |
| SD | 10 (10, 5-17) |
| PD | 5 (5, 2-11) |
| Not done | 2 (2, 0-7) |
| Ongoing response, n (%) | 31 (31) |
| CR | 30 (30) |
| PR | 1 (1) |
| DOR (95% CI) | |
| Median DOR, mos | 11.1 (4.2-51.3) |
| Median duration of CR, mos | 62.2 (12.9-NE) |
| Median duration of PR, mos | 1.9 (1.3-2.1) |
| . | N = 101 . |
|---|---|
| Best response, n (%, 95% CI) | |
| Objective response | 84 (83, 74-90) |
| CR | 59 (58, 48-68) |
| PR | 25 (25, 17-34) |
| SD | 10 (10, 5-17) |
| PD | 5 (5, 2-11) |
| Not done | 2 (2, 0-7) |
| Ongoing response, n (%) | 31 (31) |
| CR | 30 (30) |
| PR | 1 (1) |
| DOR (95% CI) | |
| Median DOR, mos | 11.1 (4.2-51.3) |
| Median duration of CR, mos | 62.2 (12.9-NE) |
| Median duration of PR, mos | 1.9 (1.3-2.1) |
PD, progressive disease; PR, partial response; SD, stable disease.
Median EFS was 5.7 months (95% CI, 3.1-13.9; Figure 1), and the estimated 5-year EFS rate was 30.3% (95% CI, 21.5-39.6). Medians for EFS were largely consistent across subgroups of key baseline and disease characteristics (supplemental Figure 2), although patients who were aged ≥65 years and those with a tumor burden below the mean had numerically longer median EFS than those aged <65 years (12.5 months vs 5.6 months) and those with tumor burden above the median (15.0 months vs 3.1 months), respectively. Median PFS was 5.9 months (95% CI, 3.3-15.0), and the 5-year PFS estimate was 31.8% (95% CI, 22.9-41.1). After the 2-year follow-up analysis (data cutoff date, 11 August 2018), 9 deaths and 4 disease progression events occurred; all of those who progressed after the 2-year analysis were alive by the data cutoff of this analysis. Median PFS appeared consistent with response at months 3, 6, 12, and 24 (supplemental Table 1). Among the 8 patients with lymphoma present in the bone marrow, 5 (62.5%) had disease progression by data cutoff. Median time to progression was 6.1 months (95% CI, 4.4-29.7; Figure 1) and median time to next therapy was 8.7 months (95% CI, 6.9-34.9; supplemental Figure 3).
EFS, PFS, and time to progression. Kaplan-Meier estimates of (A) EFS, (B) PFS, and (C) time to progression by investigator assessment among the 101 patients with LBCL treated with axi-cel in cohorts 1 and 2 of phase 2.
EFS, PFS, and time to progression. Kaplan-Meier estimates of (A) EFS, (B) PFS, and (C) time to progression by investigator assessment among the 101 patients with LBCL treated with axi-cel in cohorts 1 and 2 of phase 2.
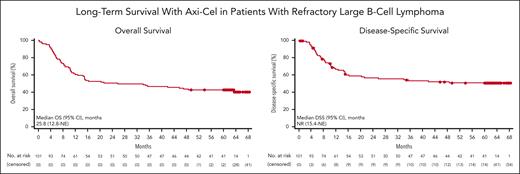
Median OS in patients who received treatment was 25.8 months (95% CI, 12.8-NE), and the 5-year OS rate was 42.6% (95% CI, 32.8-51.9; Figure 2). Among those who achieved a CR, median OS was not reached (95% CI, 63.4 months-NE), and the 5-year OS rate was 64.4% (95% CI, 50.8-75.1). Among patients with (n = 57) and without (n = 44) an EFS event by 12 months, 5-year OS rates were 5.3% (95% CI, 1.4-13.2) and 90.9% (95% CI, 77.6-96.5), respectively; among those with (n = 62) and without (n = 39) an EFS event by 24 months, 5-year OS rates were 11.3% (95% CI, 5.0-20.5) and 92.3% (95% CI, 78.0-97.5), respectively (supplemental Figure 4). As of the data cutoff date, 42% of patients who received treatment (42/101) remained alive, including 63% of patients who achieved a CR. Of 11 patients who remained alive but had not achieved sustained ongoing response, all had disease progression before data cutoff; 8 of 11 patients received subsequent therapy including 2 who were retreated with axi-cel. Other regimens after axi-cel included rituximab, rituximab + lenalidomide, tafasitamab + lenalidomide, pembrolizumab + lenalidomide + rituximab, bendamustine + rituximab + polatuzumab, investigational therapy, autologous SCT, romidepsin + gemcitabine + oxaliplatin + rituximab, radiation, and lenalidomide monotherapy. Median disease-specific survival was not yet reached (95% CI, 15.4 months-NE; Figure 2), with a 5-year disease-specific survival rate of 51.0% (95% CI, 40.4-60.6).
OS and disease-specific survival. Kaplan-Meier estimates of (A) OS and (B) disease-specific survival among 101 patients with LBCL treated with axi-cel in cohorts 1 and 2 of phase 2. One patient’s event time for OS was updated from month 42 to month 39 after data cutoff and is not reflected in this figure. DSS, disease specific survival.
OS and disease-specific survival. Kaplan-Meier estimates of (A) OS and (B) disease-specific survival among 101 patients with LBCL treated with axi-cel in cohorts 1 and 2 of phase 2. One patient’s event time for OS was updated from month 42 to month 39 after data cutoff and is not reflected in this figure. DSS, disease specific survival.
Safety
The safety profile of axi-cel after 5 years of follow-up was largely consistent with prior reports.1,6 CRS occurred in 94 patients (93%), with grade ≥3 cases in 11 patients (11%).1 Neurologic events occurred in 65 patients (64%) with grade ≥3 events in 30 patients (30%). For the management of CRS and/or neurologic events, 43% of patients received tocilizumab and 26% received corticosteroids. No new safety signals were reported in patients treated with axi-cel (n = 101), and no new serious AEs related to axi-cel were reported after the 2-year analysis. The 2 grade 3 cytopenias (anemia and neutropenia) reported within the 2-year analysis resolved before data cutoff for this analysis. Since the 2-year analysis, immunoglobulin therapy was administered to 3 patients (2 for prophylaxis and 1 because of grade 2 immunoglobulin G decrease related to axi-cel). No secondary malignancies related to axi-cel have been reported thus far on-study (supplemental Table 2).
Among all patients who received treatment, 59 (58%) have died (Table 3), largely because of progressive disease (n = 45), and most of the deaths occurred within the first year after infusion (n = 40). As previously reported, 4 patients on-study died because of an AE (2 related to axi-cel, 2 with no causal relationship),6 and no deaths due to AEs were reported after the 2-year analysis data cutoff. Since the 2-year analysis, 1 death due to myelodysplastic syndrome that was related to prior therapy and/or lymphodepleting chemotherapy was reported; this patient was in CR for LBCL.
Deaths by year
| n (%) . | N = 101 . | ||||||
|---|---|---|---|---|---|---|---|
| Total . | Year 1 . | Year 2 . | Year 3 . | Year 4 . | Year 5 . | Year >5 . | |
| Patients who died | 59 (58) | 40 (40) | 10 (10) | 4 (4) | 3 (3) | 1 (1) | 1 (1) |
| Primary cause of death | |||||||
| Progressive disease∗ | 45 (45) | 32 (32) | 9 (9) | 3 (3) | 0 | 1 (1) | 0 |
| AE† | 4 (4) | 3 (3) | 1 (1) | 0 | 0 | 0 | 0 |
| Secondary malignancy | 1 (1) | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| Other‡ | 9 (9) | 5 (5) | 0 | 1 (1) | 3 (3) | 0 | 0 |
| n (%) . | N = 101 . | ||||||
|---|---|---|---|---|---|---|---|
| Total . | Year 1 . | Year 2 . | Year 3 . | Year 4 . | Year 5 . | Year >5 . | |
| Patients who died | 59 (58) | 40 (40) | 10 (10) | 4 (4) | 3 (3) | 1 (1) | 1 (1) |
| Primary cause of death | |||||||
| Progressive disease∗ | 45 (45) | 32 (32) | 9 (9) | 3 (3) | 0 | 1 (1) | 0 |
| AE† | 4 (4) | 3 (3) | 1 (1) | 0 | 0 | 0 | 0 |
| Secondary malignancy | 1 (1) | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| Other‡ | 9 (9) | 5 (5) | 0 | 1 (1) | 3 (3) | 0 | 0 |
MDS, myelodysplastic syndrome.
After year 2, 4 patients with DLBCL who had a best response of a CR later had progressive disease on days 99, 184, 266, and 546 after infusion, respectively. During ongoing safety monitoring after the data cutoff, 1 event of central nervous system lesion, which was not amenable to biopsy, was reported. Treatment for presumed progressive disease for DLBCL was initiated by the investigator.
Two events had no causal relationship (sepsis and pulmonary embolism), and 2 events were related to axi-cel (brain injury due to cardiac arrest, and hemophagocytic lymphohistiocytosis).
Events included infection (n = 3), cardiac arrest (n = 2), pulmonary nocardiosis (n = 1), sepsis (n = 1), complications of allogeneic transplantation for previous treatment–related MDS not related to axi-cel (n = 1), and unknown (n = 1).
Biomarker analysis
Among patients who had received treatment who had evaluable samples (n = 97), the median peak CAR T-cell level appeared higher in patients whose response was ongoing at month 60 after infusion (65.76 cells per μL) than in those who relapsed (35.27 cells per μL) or did not have a response (12.08 cells per μL; Figure 3). A similar trend was observed with CAR T-cell area under the curve between days 0 and 28 after infusion (Figure 3), as well as with peak CAR T-cell levels normalized to tumor burden. Consistent with previous reports, B-cell aplasia and recovery were observed in patients with ongoing response at 60 months (supplemental Table 3).6 In an analysis of evaluable patients in ongoing response 3 years after infusion, 91% (21 of 23) demonstrated polyclonal B-cell recovery and diversity, measured by immunoglobulin κ (Igκ) and Ig λ, with a median Igκ:Igλ ratio of 1.6 (supplemental Figure 5).
Early CAR T-cell expansion correlated with ongoing response. (A) Peak CAR T-cell levels and (B) AUC between days 0 and 28 among patients who received treatment who had evaluable samples who had an ongoing response after axi-cel infusion, along with those who relapsed after responding and those who did not respond to axi-cel. Ongoing response was defined as responders (CR or PR) who did not have PD or die by the data cutoff date. Four patients did not have evaluable postinfusion samples to allow for determination for CAR T-cell peak or AUC. The median is represented by the horizontal line within each box, and the 25th and the 75th percentiles are represented by the lower and upper borders, resepctively, of each box. AUC, area under the curve; PD, progressive disease, PR, partial response.
Early CAR T-cell expansion correlated with ongoing response. (A) Peak CAR T-cell levels and (B) AUC between days 0 and 28 among patients who received treatment who had evaluable samples who had an ongoing response after axi-cel infusion, along with those who relapsed after responding and those who did not respond to axi-cel. Ongoing response was defined as responders (CR or PR) who did not have PD or die by the data cutoff date. Four patients did not have evaluable postinfusion samples to allow for determination for CAR T-cell peak or AUC. The median is represented by the horizontal line within each box, and the 25th and the 75th percentiles are represented by the lower and upper borders, resepctively, of each box. AUC, area under the curve; PD, progressive disease, PR, partial response.
Discussion
This updated 5-year analysis of the pivotal cohorts in phase 2 of ZUMA-1 demonstrates continued durability of response and long-term survival in patients with refractory LBCL, with no new safety signals. At 5 years, estimated OS was 43% among all patients who had received treatment and 64% among those achieving a CR. Importantly, the 5-year disease-specific survival rate was 51%, supporting the curative potential of axi-cel in a substantial proportion of patients.
The median OS of 25.8 months reported with axi-cel in refractory LBCL appeared favorable compared with other approved noncellular therapies for DLBCL after ≥2 lines of prior therapy. Median OS with polatuzumab vedotin in combination with bendamustine and rituximab was 12.4 months, and median OS with single agents selinexor and loncastuximab tesirine were 9.1 and 9.9 months, respectively16-18; however, the limited follow-up for trials supporting these approvals and varying patient populations make cross-trial comparisons difficult. Consistent with current and prior observations of association between response status at 3 months and PFS,6 patients in ZUMA-1 who had a CR at the week-4 assessment had a shorter DOR than those who achieved an initial CR after the week-4 assessment; however, overall, patients who achieved a CR as best response had a durable response. Of note, 11 patients whose disease progressed before data cutoff remained alive, although contributions of axi-cel to their survival are unclear. It should be noted that beyond 24 months, disease assessments were per institutional standard-of-care rather than protocol-mandated procedures. Thus, this change in assessment practice could potentially affect the long-term analyses.
The safety profile of axi-cel was similar to previous reports, and no serious events related to axi-cel were observed with beyond the 2-year analysis.1,6 The pharmacokinetic profile of axi-cel remained consistent with prior reports, including the observation that 5-year ongoing response was associated with early CAR T-cell expansion.6 Moreover, patients with 3-year ongoing responses showed evidence of polyclonal B-cell restoration. The median Igκ:Igλ ratio and relative levels of key B-cell subsets, including memory and naive B-cell immunophenotypes, suggested reconstitution of the B-cell repertoire to that similar to healthy individuals, consistent with earlier observations that protracted functional CAR T cells and B-cell aplasia were not a prerequisite of durable response in this histology.6,19,20
Results from ZUMA-1 and other studies support the use of anti-CD19 CAR T-cell therapy for aggressive B-cell malignancies with curative intent. An updated report from an early CAR T-cell trial from the National Cancer Institute demonstrated long-term remission in patients with DLBCL or primary mediastinal B-cell lymphoma, with median duration of ongoing responses of 50 months and median OS not reached.21 In the 3-year analysis of the JULIET trial assessing tisagenlecleucel, median PFS and OS were not reached in patients who achieved a CR at 3 or 6 months (overall medians, 2.9 and 11.1 months, respectively).22 Survival outcomes in the 2-year analysis of the TRANSCEND-NHL-001 study of lisocabtagene maraleucel were comparable with those in ZUMA-1, with a median PFS of 6.8 months and median OS of 27.3 months.23 No new safety signals were reported with longer follow-up in either trial.22,23 Collectively, these long-term durable remissions reported with anti-CD19 CAR T-cell therapy in R/R B-cell lymphomas posit that the therapy may be curative for a subset of patients, especially those who achieve a CR as best response. Comparatively, further analyses are needed to determine whether newly emerging bispecific antibodies are likely to result in long-term remission, because follow-up of patients off-treatment is limited, although response and survival are favorable.24,25 In addition, although allogeneic SCT may also offer long-term survival in some patients with R/R DLBCL, risks of nonrelapse mortality and limited patient eligibility compared with CAR T-cell therapy have contributed to decreased clinical use.26
The application of CAR T-cell therapy for aggressive lymphomas has evolved beyond treatment of patients who are heavily pretreated, supported by analyses of axi-cel and others in earlier lines of treatment. The randomized, phase 3 ZUMA-7 study demonstrated superiority of axi-cel vs standard-of-care in the second line with manageable safety,27 and similar findings were reported with second-line lisocabtagene-maraleucel in the TRANSFORM trial.28 Furthermore, a recent report demonstrated the feasibility of axi-cel in the first-line for patients with high-risk LBCL.29 Responses with axi-cel in the first- and second-lines were comparable with those in ZUMA-1, with higher CR rates observed in the first- and second- vs third-line (78% and 65% vs 58%, respectively), suggesting favorable efficacy with CAR T-cells in earlier lines.
In summary, this updated 5-year analysis of the multicenter ZUMA-1 study demonstrated continued benefit of axi-cel for overall and disease-specific survival in patients with refractory LBCL with no new safety signals. Importantly, long-term results from the ZUMA-1 trial support the curative potential of axi-cel for a large proportion of patients with aggressive lymphomas.
Acknowledgments
The authors thank the patients who participated in this trial and their families, caregivers, and friends; the trial investigators, coordinators, and health care staff at each site; Allen Xue, Ashwini Noronha-Jackson, Trishna Dhobi, Andrew Lee, Deborah Mirjah, John Rossi, and Susan Cunningham, of Kite, for support of statistical analysis, operations, safety, translational analyses, and data management, respectively; and Danielle Fanslow, of Nexus Global Group Science, for medical writing assistance.
This study was funded by Kite, a Gilead Company.
Authorship
Contribution: S.S.N. and F.L.L. designed the trial; S.S.N., C.A.J., A.G., D.B.M., L.J.L., O.O.O., Y.L., I.B., B.T.H., J.M.T., A.D., P.M.R., P.S., I.W.F., U.F., A.H.G., P.A.M., J.M., T.S., J.C.C., A.F.H., N.L.B., and F.L.L. provided study materials; S.S.N., C.A.J., A.G., D.B.M., L.J.L., O.O.O., Y.L., I.B., B.T.H., J.M.T., A.D., P.M.R., P.S., I.W.F., U.F., A.H.G., P.A.M., J.M., T.S., J.C.C., A.F.H., N.L.B., and F.L.L. collected and assembled data; S.S.N., R.R.S., A.A.B., J.D., K.S., C.S., R.K., H.M., J.J.K., Y.Z., and F.L.L. analyzed and interpreted the data; and all authors contributed to the writing and final approval of the manuscript.
Conflict-of-interest disclosure: S.S.N. received consulting fees or honoraria from Kite, Merck, Bristol-Myers Squibb (BMS), Novartis, Allogene Therapeutics, Cell Medica/Kuur/Athenex, Incyte, Legend Biotech, Adicet Bio, bluebird bio, Sellas Life Sciences, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, MorphoSys, Medscape, Aptitude Health, Bio Ascend, and MJH Life Sciences; grants, contracts, or research funding from Kite, BMS, Poseida, Cellectis, Unum Therapeutics (Cogent Biosciences), Allogene, Precision BioSciences, and Adicet Bio; stock options from Longbow Immunotherapy; and patents, royalties, or other intellectual property from Takeda Pharmaceuticals, related to cell therapy. C.A.J. received honoraria from Kite, Novartis, BMS/Celgene, Instill Bio, ImmPACT Bio, Lonza, Ipsen, Epizyme, bluebird bio, and Daiichi-Sankyo; performed a consulting/advisory role for Kite, Novartis, BMS/Celgene, Instill Bio, ImmPACT Bio, Lonza, Ipsen, Epizyme, bluebird bio, and Daiichi-Sankyo; and received research funding from Kite and Pfizer. A.G. performed a consulting/advisory role for Amgen, Atara, Celgene, Kite, and Wugen Inc; received research funding from Amgen and Kite; and honoraria from Kite. D.B.M. received honoraria and research funding from Janssen; performed a consulting/advisory role for Janssen, Adaptive Biotechnologies, Kite, BMS, and Miltenyi; received patents, royalties, other intellectual property as a cGVHD patent holder for ibrutinib as cGVHD therapy with no compensation; and travel and other support from Janssen. O.O.O. performed a consultancy role and participated in an advisory board for Pfizer, Kite, Gilead Sciences, AbbVie, Janssen, TGR Therapeutics, ADC, Novartis, Epizyme, Curio Science, Nektar, and Syncopation; and received honoraria from Pfizer and Gilead Sciences. Y.L. performed a consulting/advisory role for Kite, BMS, bluebird bio, Janssen, Legend BioTech, Gamida Cells, Novartis, Iovance, Takeda, Fosun Kite, and Pfizer; and received research funding from Kite, BMS, bluebird bio, Janssen, Legend Biotech, Merck, Takeda, and Boston Scientific. B.H. received honoraria from, performed a consulting/advisory role for, and received research funding and travel support from Kite. J.M.T. performed a consulting/advisory role for Kite; and received research funding from BMS, Kite, Merck, and Spectrum. A.D. performed a consulting/advisory role for Adicet, Janssen, and Kite. P.M.R. performed a consulting/advisory role for Kite; and received research funding from Genentech and Seagen. P.S. received honoraria from and performed a consulting/advisory role for MorphoSys and CRISPR Therapeutics; and received research funding from Amgen, Gamida Cell, Pfizer, Karyopharm, Gilead Sciences, Incyte, Seagen, and Cellectar. I.W.F. performed a consulting/advisory role for AbbVie, AstraZeneca, BeiGene, Century Therapeutics, Genentech, Genmab, Gilead Sciences, Great Point Partners, Hutchison MediPharma, Iksuda Therapeutics, InnoCare Pharma, Janssen, Juno Therapeutics, Kite, MorphoSys, Novartis, Nurix Therapeutics, Pharmacyclics, Roche, Seagen, Servier Pharmaceuticals, Takeda, TG Therapeutics, Unum Therapeutics, Verastem, Vincerx Pharma, and Yingli Pharmaceuticals; and received research funding from AbbVie, Acerta Pharma, Agios, ArQule, AstraZeneca, BeiGene, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Curis, Forma Therapeutics, Forty Seven, Genentech, Gilead Sciences, IGM Biosciences, Incyte, Infinity Pharmaceuticals, Janssen, Juno Therapeutics, Karyopharm Therapeutics, Kite, Loxo, Merck, MorphoSys, Novartis, Pfizer, Pharmacyclics, Portola Pharmaceuticals, Rhizen Pharmaceuticals, Roche, Seagen, Takeda, Teva, TG Therapeutics, Trillium Therapeutics, Triphase Research and Development Corp, Unum Therapeutics, and Verastem. U.F. received honoraria from Kite; performed a consulting/advisory role for MorphoSys; and received research funding from Checkmate Pharma. A.G. was employed with Regional Cancer Care Associates and OMI; had a leadership role with COTA Healthcare and Genomics Testing Cooperative LLC; had stock or other ownership in COTA Healthcare, Genomics Testing Cooperative LLC, Alloplex, and Resilience; performed a consulting/advisory role for Kite, Pharmacyclics, Janssen, Clinical Advances in Hematology & Oncology, Michael J. Hennessey Associates, Inc, Physicians Education Resource; received research funding from Acerta, AstraZeneca, BMS, Celgene, Constellation, Genentech, Genentech-Hoffman, Roche, Infinity, Infinity Pharmaceuticals, Verastem, Janssen, Karyopharm, Kite, MorphoSys, Pharmacyclics, Seagen, and Verastem; travel support from Physcians Education Resource; participated in scientific advisory boards with BMS, Alloplex, and Vincerx; and participated in steering committees with AstraZeneca, Pharmacyclics, and Janssen. P.A.M. received honoraria from, performed a consulting/advisory role for, and participated in a speakers' bureau for Kite; and research funding from AlloVir, AutolusTherapeutics, Kite, and Novartis. J.M. had a consulting/advisory role for Pharmacyclics/AbbVie, Bayer, Kite, Pfizer, Janssen, Juno/Celgene, BMS, Kyowa, Alexion, Fosunkite, Innovent, Seagen, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, BeiGene, Servier, Novartis, MorphoSys/Incyte, Mei Pharma, and Zodiac; received research funding from Bayer, Kite, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seagen, Janssen, and Millennium; honoraria from Targeted Oncology, OncView, Curio, Kyowa, Physicians’ Education Resource, Dava, Global Clinical Insights, MJH, Shanghai Youyao, and Seagen; and participated in a speakers’ bureau with Kite, Kyowa, Bayer, Pharmacyclics/Janssen, Seagen, Acrotech/Aurobindo, BeiGene, Verastem, AstraZeneca, Celgene/BMS, and Genentech/Roche. T.S. performed a consultancy or advisory role for AbbVie, AstraZeneca, BeiGene, Celgene, Juno, Kite, and PCYC; participated in a speakers’ bureau with AstraZeneca, BeiGene, and BMS; and received institutional research funding from Ascentage Pharma, AstraZeneca, BeiGene, BMS, Celgene, Juno, Kite, Oncternal, PCYC, and TG Therapeutics. J.C.C. received honoria from BeiGene, AstraZeneca, and Epyzime; performed a consulting/advisory role for AbbVie, Kite, Novartis, BMS, TG Therapeutics, and TeneBio; and received research funding from Adaptive, Merck, Janssen, ADC Therapeutics, and AstraZeneca. A.F.H. performed a consulting/advisory role for ADC Therapeutics, AstraZeneca, BMS, Genentech, Karyopharm, Merck, Seagen, Takeda, and Tubulis; and received research funding from ADC Therapeutics, AstraZeneca, BMS, Genentech, Gilead Sciences, Karyopharm, Kite, Merck, and Seagen. N.L.B. performed a consulting/advisory role for ADC Therapeutics, Roche/Genentech, and Seagen; and received research funding from ADC Therapeutics, Autolus, BMS, Celgene, Forty Seven, Genentech, Janssen, Kite, Merck, Millennium, Pharmacyclics, and Seagen. A.A.B. was a former employee with Kite; currently employed with Capstan Therapeutics; with stock or other ownership with Capstan Therapeutics and Gilead Sciences; performed a consulting/advisory role for Cero Therapeutics and Elicio; and provided expert testimony for Gilead Sciences. R.R.S. is employed with, and has stock or other ownership at Kite; and received patents, royalties, and other intellectual property from Atara and Kite. J.D. is employed at Kite; with stock or other ownership in Gilead Sciences; performed a consulting/advisory role for GliaCure; and received patents, royalties, or other intellectual property from Patent US8598141 (3 December 2013). K.S., J.J.K., and Y.Z. have been employed with and stock or other ownership at Kite. H.M. has been employed at Kite and Takeda; with stock or ownership in Kite and Gilead Sciences. F.L.L. performed a consulting/advisory role for Allogene, Amgen, bluebird bio, BMS, Calibr, Cellular Biomedicine Group, Cowen, ecoR1, Emerging Therapy Solutions Gerson Lehman Group, GammaDelta Therapeutics, Iovance, Janssen, Kite, Legend Biotech, Novartis, Umoja, and Wugen; received research funding from Allogene, Kite, and Novartis; and the institution holds patents, royalties, other intellectual property from several patents in author’s name (unlicensed) in the field of cellular immunotherapy. The remaining authors declare no competing financial interests.
Correspondence: Sattva S. Neelapu, Division of Cancer Medicine, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, #429, Houston, TX 77030; e-mail: sneelapu@mdanderson.org.
References
Author notes
∗S.S.N. and F.L.L. contributed equally to this study.
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal