Key Points
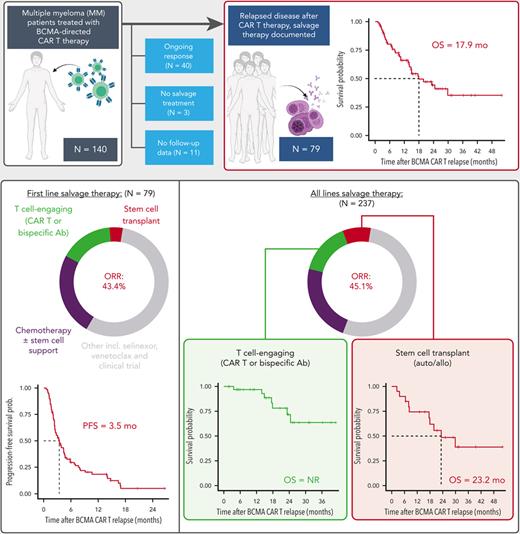
In a cohort of 79 patients relapsing after BCMA-directed CAR T, multiple lines of salvage therapy led to a median overall survival of 17.9 months.
In 35 patients who received a subsequent T-cell-engaging therapy (CAR T or bispecific antibody), the response rate was 91.4% and median overall survival was not reached.
Abstract
B-cell maturation antigen (BCMA)–directed chimeric antigen receptor T-cell (CAR T) therapy has demonstrated remarkable efficacy in patients with relapsed/refractory multiple myeloma, and now there are two US Food and Drug Administration–approved BCMA-directed CAR T products. However, despite high initial response rates, most patients eventually relapse. The outcomes of patients with disease recurrence after BCMA-directed CAR T have not been comprehensively studied, and such an analysis would help define optimal treatment strategies. We analyzed the salvage treatments and outcomes of 79 patients with multiple myeloma from two academic institutions, who had progression of disease after treatment with BCMA-directed CAR T. A total of 237 post–CAR T salvage treatment lines were used, and patients received a median of 2 (range, 1-10) treatment lines. The median overall survival from the date of relapse post-CAR T therapy was 17.9 months (95% confidence interval [CI], 14.0 non-estimable). The overall response rate to the first salvage regimen was 43.4%, with a median progression-free survival of 3.5 months (CI, 2.5-4.6). Thirty-five patients (44.3%) received a T-cell–engaging therapy (bispecific antibody or subsequent CAR T) as salvage treatment. The overall survival in patients who received subsequent T-cell–engaging therapy was not reached after a median follow up of 21.3 months. Patients with multiple myeloma who relapse after BCMA-directed CAR T have a limited prognosis but can be potentially treated with multiple lines of salvage therapy. T-cell–engaging therapies appear to maintain pronounced clinical activity in this setting.
Introduction
The emergence of B-cell maturation antigen (BCMA)–directed chimeric antigen receptor T-cell (CAR T) therapy is one of the most significant advancements in the treatment landscape of relapsed/refractory multiple myeloma (RRMM) over the last decade.1 There are now two US Food and Drug Administration–approved BCMA-directed CAR T products in triple-class–exposed RRMM after at least 4 prior lines of therapy.2,3 Despite the high response rates seen with these therapies, relapses are common.
In the pivotal KarMMa study of idecabtagene vicleucel (ide-cel), the median progression-free survival (PFS) and overall survival (OS) were 8.8 months and 19.4 months, respectively.2 In the CARTITUDE-1 study of ciltacabtagene autoleucel, the 27-month PFS and OS rates were 54.9% and 70.4%, respectively.4 Patients with RRMM after BCMA-directed CAR T therapy pose significant management challenges with limited data for prognosis and effective therapeutics, and no standard of care has been established. In a small, single-center analysis of 7 patients with MM who progressed after ide-cel, the overall response rate (ORR) to the subsequent antimyeloma regimen was only 29%.5 Furthermore, the median PFS and OS to the subsequent antimyeloma regimen were dismal at 2 and 5 months, respectively.5 In a larger study of 68 patients who progressed after receiving ide-cel on the KarMMA study, the median duration of the subsequent regimen was only 44 days, and the time to second disease progression (ie, from ide-cel treatment) was 13.6 months.6
The detailed clinical outcomes and subsequent treatments from a large series of patients with progression to various BCMA-directed CAR T therapies has not been reported. With the recent approval of two different BCMA-directed CAR T-cell therapies and the increasing use of these treatments in patients with RRMM, understanding the natural course of patients with post–CAR T progression will enable a better navigation of treatment options for these patients. A thorough evaluation of patient outcomes post–BCMA-directed CAR T therapy is also vital to establish a benchmark for clinical trials and to help define the optimal strategy for treatment sequencing in this setting. We performed a comprehensive analysis of the clinical characteristics, post–CAR T treatments, and outcomes of patients with MM who progressed after BCMA-directed CAR T cell therapies from two academic centers.
Methods
Clinical data collection
All patients evaluated in this study had RRMM and were treated with an autologous BCMA-directed CAR T therapy on phase 1 dose-escalation or phase 2 clinical trials at one of the two participating academic institutions and had evidence of progressive disease (PD) to the BCMA-directed CAR T therapy. PD was defined as per the International Myeloma Working Group (IMWG) response criteria.7–9 Patients with ongoing responses to the BCMA-directed CAR T and those that did not receive any post–CAR T antimyeloma therapy were not included in this analysis. Patients were treated with BCMA-directed CAR T at the Tisch Cancer Institute (TCI), Mount Sinai Hospital, New York between 24 April 2017 and 26 January 2022 and at the Memorial Sloan Kettering Cancer Center (MSKCC) between 22 March 2017 and 03 May 2021.
We retrospectively collected the demographics, baseline disease characteristics, pre– and post–CAR T treatment regimen(s), responses to post–CAR T salvage therapy, and clinical outcomes of the patients who progressed after the BCMA-directed CAR T therapy. Post study data was collected until the cutoff dates of 27 May 2022 for TCI and 14 January 2022 for MSKCC. The time point at which patients had PD to the BCMA-directed CAR T therapy was defined as time point zero (T0). If reinfusion of the CAR T was attempted as part of the clinical trial protocol (n = 13, all because of suboptimal response or disease progression), T0 was defined as the time of PD after attempted reinfusion. Disease responses to salvage treatments were assessed by the treating physician according to the IMWG criteria.7–9 The initial study protocols and this retrospective analysis were reviewed and approved by the institutional review boards at MSKCC and the Icahn School of Medicine at Mount Sinai, and the research was conducted according to the declaration of Helsinki.
Statistical analysis
Patient demographics and disease characteristics are shown as median (range) for continuous variables and counts (percentages) for categorical variables, unless otherwise specified. Group comparisons of continuous variables were performed using Wilcoxon rank-sum tests and of categorical variables using the χ2 test or Fisher exact test, as appropriate. Survival analyses and the follow-up duration were calculated using the reverse Kaplan-Meier method.10 Distributions for the survival outcome metrics were estimated using the Kaplan-Meier method with comparisons between patient groups made by the log-rank test. All statistical analyses were performed using R version 4.0.2. Hypothesis testing was 2-sided and conducted at the 5% level of significance.
Results
Outcomes after relapse to BCMA-directed CAR T therapy
Between March 2017 and January 2022, a total of 140 patients with MM were treated with BCMA-directed CAR T therapy on a clinical trial at TCI and MSKCC. Of the 140 treated patients, 79 patients (56.4%) were evaluable in this analysis, 40 were not evaluable because of ongoing responses to CAR T therapy, 7 patients were not evaluable because they passed away during the clinical trial, 3 patients only received palliative care and no antimyeloma therapy, and 11 were not evaluable for salvage treatment because patients were lost to follow up after CAR T, although OS data were available for most of them (supplemental Figure 1, available on the Blood website). Demographics and disease characteristics of the 79 patients included in the downstream analysis are summarized in Table 1. Demographics (age, gender) and disease characteristics (including presence of high-risk cytogenetic characteristics, time since MM diagnosis, and number of prior treatment lines) were comparable between the 2 participating institutions.
Clinical and disease characteristics of the study cohort
| . | Salvage therapy . | |
|---|---|---|
| N = 79 . | ||
| Demographics | ||
| Age (y) | 60 | (35-78) |
| Male gender | 47 | 59.5% |
| Disease characteristics | ||
| IgG | 41 | 51.9% |
| IgA | 16 | 20.3% |
| Light chain disease only | 22 | 27.8% |
| Kappa | 49 | 62.0% |
| Lambda | 29 | 36.7% |
| Cytogenetics | ||
| Hyperdiploidy | 43 | 54.4% |
| t(11;14) | 14 | 17.7% |
| t(4;14) | 4 | 5.1% |
| t(14;16) | 5 | 6.3% |
| del17p | 12 | 15.2% |
| 1q gain | 55 | 69.6% |
| All high-risk | 64 | 81.0% |
| Time since diagnosis (mo) | 74 | (22-282) |
| Number of treatment lines before CAR T | 5 | (1-18) |
| Prior treatment exposure | ||
| Auto-SCT | 75 | 94.9% |
| Lenalidomide | 79 | 100.0% |
| Pomalidomide | 67 | 84.8% |
| Bortezomib | 75 | 94.9% |
| Carfilzomib | 70 | 88.6% |
| CD38 mAb | 76 | 96.2% |
| Elotuzumab | 23 | 29.1% |
| Alkylating agent∗ | 77 | 97.5% |
| Combination chemotherapy† | 28 | 35.4% |
| Venetoclax | 13 | 16.5% |
| Selinexor | 11 | 13.9% |
| Belantamab | 0 | 0.0% |
| Non-BCMA CAR T | 1 | 1.3% |
| BCMA-directed bispecific Ab | 0 | 0.0% |
| Non-BCMA-directed bispecific Ab | 5 | 6.3% |
| Prior treatment refractoriness | ||
| Lenalidomide | 68 | 86.1% |
| Pomalidomide | 60 | 75.9% |
| Bortezomib | 50 | 63.3% |
| Carfilzomib | 62 | 78.5% |
| CD38 mAb | 71 | 89.9% |
| Triple-class refractory‡ | 66 | 83.5% |
| Penta-drug refractory§ | 30 | 38.0% |
| Relapse characteristics | ||
| Biochemical only | 40 | 50.6% |
| Imaging (bone only) ± biochemical | 16 | 20.3% |
| Imaging (EMD) ± biochemical | 23 | 29.1% |
| Anemia, any grade | 55/77 | 71.4% |
| Anemia, grade ≥ 3 | 5/77 | 6.5% |
| Leukopenia, any grade | 48/77 | 62.3% |
| Leukopenia, grade ≥ 3 | 4/77 | 5.2% |
| Neutropenia, any grade | 22/76 | 28.9% |
| Neutropenia, grade ≥ 3 | 4/76 | 5.3% |
| Thrombocytopenia, any grade | 49/77 | 63.6% |
| Thrombocytopenia, grade ≥ 3 | 17/77 | 22.1% |
| . | Salvage therapy . | |
|---|---|---|
| N = 79 . | ||
| Demographics | ||
| Age (y) | 60 | (35-78) |
| Male gender | 47 | 59.5% |
| Disease characteristics | ||
| IgG | 41 | 51.9% |
| IgA | 16 | 20.3% |
| Light chain disease only | 22 | 27.8% |
| Kappa | 49 | 62.0% |
| Lambda | 29 | 36.7% |
| Cytogenetics | ||
| Hyperdiploidy | 43 | 54.4% |
| t(11;14) | 14 | 17.7% |
| t(4;14) | 4 | 5.1% |
| t(14;16) | 5 | 6.3% |
| del17p | 12 | 15.2% |
| 1q gain | 55 | 69.6% |
| All high-risk | 64 | 81.0% |
| Time since diagnosis (mo) | 74 | (22-282) |
| Number of treatment lines before CAR T | 5 | (1-18) |
| Prior treatment exposure | ||
| Auto-SCT | 75 | 94.9% |
| Lenalidomide | 79 | 100.0% |
| Pomalidomide | 67 | 84.8% |
| Bortezomib | 75 | 94.9% |
| Carfilzomib | 70 | 88.6% |
| CD38 mAb | 76 | 96.2% |
| Elotuzumab | 23 | 29.1% |
| Alkylating agent∗ | 77 | 97.5% |
| Combination chemotherapy† | 28 | 35.4% |
| Venetoclax | 13 | 16.5% |
| Selinexor | 11 | 13.9% |
| Belantamab | 0 | 0.0% |
| Non-BCMA CAR T | 1 | 1.3% |
| BCMA-directed bispecific Ab | 0 | 0.0% |
| Non-BCMA-directed bispecific Ab | 5 | 6.3% |
| Prior treatment refractoriness | ||
| Lenalidomide | 68 | 86.1% |
| Pomalidomide | 60 | 75.9% |
| Bortezomib | 50 | 63.3% |
| Carfilzomib | 62 | 78.5% |
| CD38 mAb | 71 | 89.9% |
| Triple-class refractory‡ | 66 | 83.5% |
| Penta-drug refractory§ | 30 | 38.0% |
| Relapse characteristics | ||
| Biochemical only | 40 | 50.6% |
| Imaging (bone only) ± biochemical | 16 | 20.3% |
| Imaging (EMD) ± biochemical | 23 | 29.1% |
| Anemia, any grade | 55/77 | 71.4% |
| Anemia, grade ≥ 3 | 5/77 | 6.5% |
| Leukopenia, any grade | 48/77 | 62.3% |
| Leukopenia, grade ≥ 3 | 4/77 | 5.2% |
| Neutropenia, any grade | 22/76 | 28.9% |
| Neutropenia, grade ≥ 3 | 4/76 | 5.3% |
| Thrombocytopenia, any grade | 49/77 | 63.6% |
| Thrombocytopenia, grade ≥ 3 | 17/77 | 22.1% |
Ab, antibody; DCEP, dexamethasone-cyclophosphamide-etoposide-cisplatin; EMD, extramedullary disease; Ig, immunoglobulin; mAb, monoclonal antibody; PACE, cisplatin-doxorubicin-cyclophosphamide-etoposide.
Any treatment with intravenous or oral alkylating agents (including melphalan, cyclophosphamide, bendamustine, carmustine, and cisplatin).
Treatment with DCEP or PACE.
Defined as refractory to ≥1 immunomodulatory drug, ≥1 proteasome inhibitor, and ≥1 anti-CD38 mAb.
Defined as refractory to ≥2 immunomodulatory drugs, ≥2 proteasome inhibitors, and anti-CD38 mAb therapy.
The 79 patients had a median age of 60 years (range, 37-78 years) at the time of PD after BCMA-directed CAR T therapy (T0), and 59.5% were male. Cytogenetics by fluorescent in situ hybridization was available for 77 patients, and 64 (83.1%) had documented high-risk cytogenetic characteristics, defined as gain of chromosome 1q21, del17p, t(4;14), t(14;16), or t(14;20).11 There was a median of 74 months (22-282 months) between the time of MM diagnosis and T0. Patients received a median of 5 treatment lines (range, 1-18) before receiving BCMA-directed CAR T infusion. Prior treatment exposure included autologous stem cell transplant (auto-SCT) in 94.9% of patients. Prior treatment exposure and refractoriness for commonly used MM drug regimens are shown in Table 1. Of the 79 patients, 66 (83.5%) were triple-class refractory (defined as refractory to ≥1 immunomodulatory drug, ≥1 proteasome inhibitor, and ≥1 anti-CD38 mAb). Furthermore, 54 patients (68.4%) were penta-drug exposed, and 30 (38%) were penta-drug refractory (ie, exposed/refractory to ≥2 immunomodulatory drugs, ≥2 proteasome inhibitors, and anti-CD38 mAb therapy). The characteristics of patients at the time of relapse (including the presence of extramedullary disease and cytopenias) are highlighted in Table 1.
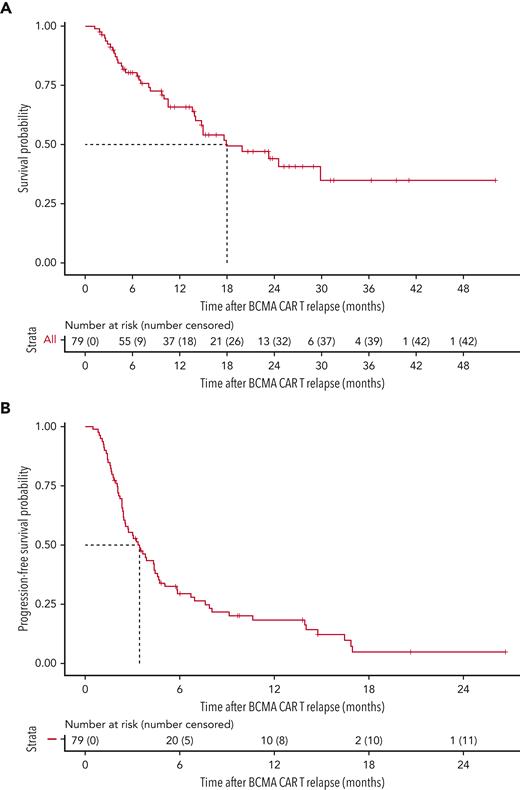
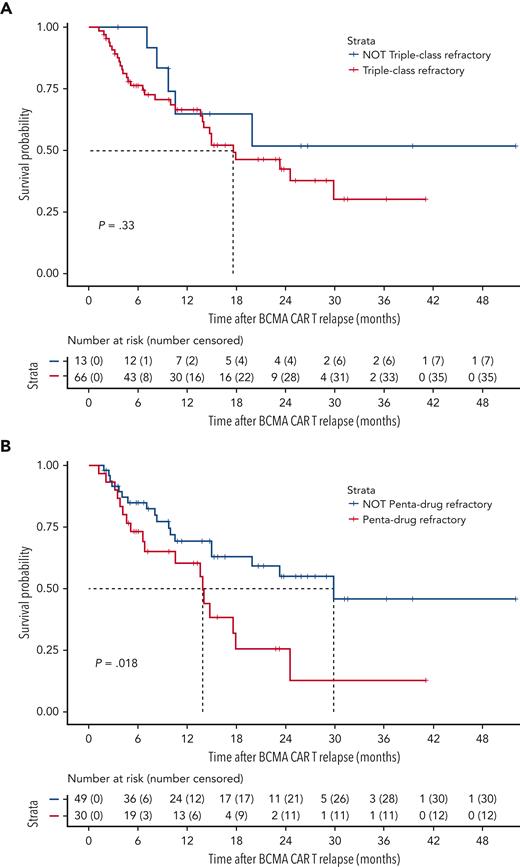
The median follow-up time at the data cutoff was 21.3 months (649 days, range 37-1585 days). The median OS from T0 for the cohort of 79 patients was 17.9 months (95% confidence interval [CI] 14.0-non-estimable [NE]) (Figure 1A). The OS according to prior antimyeloma treatment refractoriness is shown in Figure 2. The median OS for patients who were triple-class refractory was not significantly different from that of patients who were not triple-class refractory. The median OS for patients who were penta-drug refractory (13.9 months, 95% CI 6.8-NE) was significantly lower (P = .018) than for patients who were not penta-drug refractory (29.9 months, 95% CI 15.0-NE). Although there was a trend toward longer OS in patients without high-risk cytogenetics vs in those with high-risk cytogenetics, this was not statistically significant and may be limited by the relatively small number of patients in this group (n = 15) (supplemental Figure 2A). The median OS of the overall cohort did not change when the 3 patients who only received palliative end-of-life care were included in the analysis (17.9 months, 95% CI 13.9-NE).
OS after BCMA-directed CAR T relapse and PFS of the first line of salvage therapy. (A) OS curve. (B) PFS curve for first-line salvage therapy.
OS after BCMA-directed CAR T relapse and PFS of the first line of salvage therapy. (A) OS curve. (B) PFS curve for first-line salvage therapy.
OS after BCMA-directed CAR T relapse stratified by disease characteristics. (A) OS curve, stratified by triple-class refractory status (defined as refractory to ≥1 immunomodulatory drug, ≥1 proteasome inhibitor, and ≥1 anti-CD38 monoclonal antibody). (B) OS curve, stratified by penta-drug refractory status (defined as refractory to ≥2 immunomodulatory drugs, ≥2 proteasome inhibitors, and ≥1 anti-CD38 mAb).
OS after BCMA-directed CAR T relapse stratified by disease characteristics. (A) OS curve, stratified by triple-class refractory status (defined as refractory to ≥1 immunomodulatory drug, ≥1 proteasome inhibitor, and ≥1 anti-CD38 monoclonal antibody). (B) OS curve, stratified by penta-drug refractory status (defined as refractory to ≥2 immunomodulatory drugs, ≥2 proteasome inhibitors, and ≥1 anti-CD38 mAb).
Treatment landscape after relapse on BCMA CAR T
The 79 patients received a median of 2 salvage treatment lines (range, 1-10) after T0. The median PFS for the first attempted salvage therapy was 3.5 months (105 days, 95% CI 2.5-4.6 months) (Figure 1B). The characteristics of the first line of salvage therapy are summarized in Table 2. For the first regimen after T0, the depth of response could be evaluated in 76 patients. The ORR among these patients was 43.4%, with 7 patients (9.2%) achieving a (stringent) complete response (CR), 9 patients (11.8%) achieving a very good partial response (VGPR), and 17 patients (22.4%) achieving a partial response (PR). Achieving an objective response (PR or better) to the first line of salvage therapy was associated with a significantly longer median OS of 29.9 months (95% CI 23.3-NE) compared with patients not achieving an objective response (median OS, 14.6 months; 95% CI, 10.0-NE; P = .028) (supplemental Figure 2B). Overall, a total of 237 salvage treatment lines were used among the 79 patients at any time post–CAR T relapse. The characteristics of all these therapies, including the associated ORR and rate of responses ≥ VGPR, are summarized in Table 2. We did not find the ORR to decrease significantly up to beyond the fifth line of salvage therapy.
Characteristics and response rate of first and subsequent salvage treatments
| Treatment group . | First line of salvage treatment . | All lines of salvage treatment . | ||||||
|---|---|---|---|---|---|---|---|---|
| N . | % used . | N ≥ PR ORR . | N ≥ VGPR % . | N . | % used . | N ≥ PR ORR . | N ≥ VGPR % . | |
| Allo-SCT | 0 | 0.0% | 0/0 N/A | 0/0 N/A | 7 | 3.0% | 4/4 100.0% | 2/4 50.0% |
| Auto-SCT | 3 | 3.8% | 1/3 33.3% | 1/3 33.3% | 14 | 5.9% | 10/14 71.4% | 7/14< 50.0% |
| BCMA ADC | 1 | 1.3% | 0/1 0.0% | 0/1 0.0% | 9 | 3.8% | 2/8 25.0% | 2/8 25.0% |
| Bispecific trial | 11 | 13.9% | 7/10 70.0% | 5/10 50.0% | 32 | 13.5% | 17/29 58.6% | 12/29 41.4% |
| BCMA-directed bispecific trial | 2 | 2.5% | 1 out of 2 50.0% | 0 out of 2 0.0% | 9 | 3.8% | 4 out of 9 44.4% | 3 out of 9 33.3% |
| Non-BCMA–directed bispecific trial | 9 | 11.4% | 6 out of 8 75.0% | 5 out of 8 62.5% | 23 | 9.7% | 13 out of 20 65.0% | 9 out of 20 45.0% |
| CAR T trial | 2 | 2.5% | 2 out of 2 100.0% | 1 out of 2 50.0% | 6 | 2.5% | 5 out of 6 83.3% | 3 out of 6 50.0% |
| Chemotherapy with or without stem cell support | 20 | 25.3% | 11 out of 19 57.9% | 4 out of 19 21.1% | 53 | 22.4% | 29 out of 51 56.9% | 12 out of 51 23.5% |
| Doublet/triplet/quadruplet combination of approved agents | 23 | 29.1% | 7 out of 22 31.8% | 2 out of 22 9.1% | 56 | 23.6% | 15 out of 53 28.3% | 4 out of 53 7.5% |
| Selinexor-based therapy | 5 | 6.3% | 2 out of 5 40.0% | 2 out of 5 40.0% | 15 | 6.3% | 3 out of 14 21.4% | 3 out of 14 21.4% |
| Venetoclax-based therapy | 3 | 3.8% | 2 out of 3 66.7% | 1 out of 3 33.3% | 14 | 5.9% | 5 out of 14 35.7% | 2 out of 14 14.3% |
| Other combinations (including MAPKi, checkpoint inhibitor or other trial) | 11 | 13.9% | 1 out of 11 9.1% | 0 out of 11 0.0% | 31 | 13.1% | 12 out of 31 38.7% | 1 out of 31 3.2% |
| All treatment groups | 79 | 100.0% | 33 out of 76 43.4% | 16 out of 76 21.1% | 237 | 100.0% | 101 out of 224 45.1% | 48 out of 224 21.4% |
| Total N = 79 | Total N = 76 | Total N = 237 | Total N = 224 | |||||
| Treatment group . | First line of salvage treatment . | All lines of salvage treatment . | ||||||
|---|---|---|---|---|---|---|---|---|
| N . | % used . | N ≥ PR ORR . | N ≥ VGPR % . | N . | % used . | N ≥ PR ORR . | N ≥ VGPR % . | |
| Allo-SCT | 0 | 0.0% | 0/0 N/A | 0/0 N/A | 7 | 3.0% | 4/4 100.0% | 2/4 50.0% |
| Auto-SCT | 3 | 3.8% | 1/3 33.3% | 1/3 33.3% | 14 | 5.9% | 10/14 71.4% | 7/14< 50.0% |
| BCMA ADC | 1 | 1.3% | 0/1 0.0% | 0/1 0.0% | 9 | 3.8% | 2/8 25.0% | 2/8 25.0% |
| Bispecific trial | 11 | 13.9% | 7/10 70.0% | 5/10 50.0% | 32 | 13.5% | 17/29 58.6% | 12/29 41.4% |
| BCMA-directed bispecific trial | 2 | 2.5% | 1 out of 2 50.0% | 0 out of 2 0.0% | 9 | 3.8% | 4 out of 9 44.4% | 3 out of 9 33.3% |
| Non-BCMA–directed bispecific trial | 9 | 11.4% | 6 out of 8 75.0% | 5 out of 8 62.5% | 23 | 9.7% | 13 out of 20 65.0% | 9 out of 20 45.0% |
| CAR T trial | 2 | 2.5% | 2 out of 2 100.0% | 1 out of 2 50.0% | 6 | 2.5% | 5 out of 6 83.3% | 3 out of 6 50.0% |
| Chemotherapy with or without stem cell support | 20 | 25.3% | 11 out of 19 57.9% | 4 out of 19 21.1% | 53 | 22.4% | 29 out of 51 56.9% | 12 out of 51 23.5% |
| Doublet/triplet/quadruplet combination of approved agents | 23 | 29.1% | 7 out of 22 31.8% | 2 out of 22 9.1% | 56 | 23.6% | 15 out of 53 28.3% | 4 out of 53 7.5% |
| Selinexor-based therapy | 5 | 6.3% | 2 out of 5 40.0% | 2 out of 5 40.0% | 15 | 6.3% | 3 out of 14 21.4% | 3 out of 14 21.4% |
| Venetoclax-based therapy | 3 | 3.8% | 2 out of 3 66.7% | 1 out of 3 33.3% | 14 | 5.9% | 5 out of 14 35.7% | 2 out of 14 14.3% |
| Other combinations (including MAPKi, checkpoint inhibitor or other trial) | 11 | 13.9% | 1 out of 11 9.1% | 0 out of 11 0.0% | 31 | 13.1% | 12 out of 31 38.7% | 1 out of 31 3.2% |
| All treatment groups | 79 | 100.0% | 33 out of 76 43.4% | 16 out of 76 21.1% | 237 | 100.0% | 101 out of 224 45.1% | 48 out of 224 21.4% |
| Total N = 79 | Total N = 76 | Total N = 237 | Total N = 224 | |||||
PR, partial response; VGPR, very good partial response.
Salvage with T-cell–engaging therapies after CAR T is feasible and can lead to durable responses
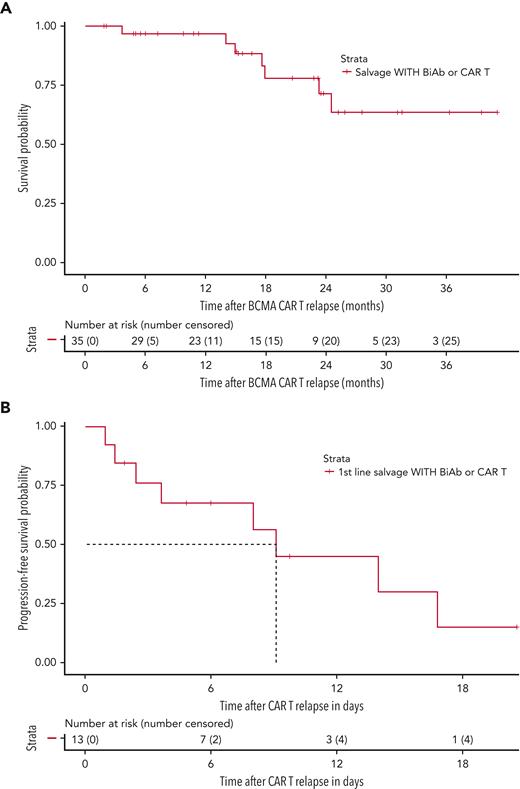
Thirty-five patients (44.3%) received salvage therapy with a T-cell–engaging agent (ie, CAR T or bispecific antibody therapy) at any time point after relapse to BCMA-targeted CAR T therapy; all were treated on clinical trials. There were 32 instances of salvage therapy with a bispecific antibody therapy among 29 patients (36.7%), of which, 9 were BCMA-directed and 23 were non–BCMA-directed bispecific antibody constructs; 3 patients received 2 different bispecific antibody treatments. Six patients (7.6%) received salvage treatment with a G protein-coupled receptor class C group 5 member D–directed (GPRC5D-directed) CAR T at any point after CAR T relapse.12 The ORR for T-cell–engaging therapies at any point after CAR T relapse was 91.4% (32/35 evaluable instances). These patients, who were eligible for and treated with a T-cell–engaging agent as a salvage therapy after T0, demonstrated a median OS that was not reached (95% CI 24.6-NE) (Figure 3A). The 13 patients (16.4%) who received a T-cell–engaging therapy immediately after relapse on CAR T (ie, as the first salvage therapy), had an ORR of 75.0% (9/12 evaluable instances) and a median PFS of 9.1 months (95% CI 3.6-NE) (Figure 3B), highlighting the feasibility and efficacy of T-cell–engaging therapies as an option after CAR T relapse.
After BCMA-directed CAR T relapse, use of subsequent T-cell–engaging therapies can lead to durable responses. (A) OS curve for the 35 patients who received at least 1 instance of subsequent T-cell–engaging therapy (ie, CAR T and/or bispecific antibodies) at any time point after relapse on BCMA-directed CAR T. (B) PFS curve of first-line salvage therapy for the 13 patients who immediately transitioned onto a T-cell–engaging therapy after relapse to BCMA-directed CAR T therapy.
After BCMA-directed CAR T relapse, use of subsequent T-cell–engaging therapies can lead to durable responses. (A) OS curve for the 35 patients who received at least 1 instance of subsequent T-cell–engaging therapy (ie, CAR T and/or bispecific antibodies) at any time point after relapse on BCMA-directed CAR T. (B) PFS curve of first-line salvage therapy for the 13 patients who immediately transitioned onto a T-cell–engaging therapy after relapse to BCMA-directed CAR T therapy.
Transplant-based approaches show efficacy in the post–CAR T setting
Stem cell transplant is another potential salvage strategy for eligible patients, in particular for those patients with autologous stem cells available. Among the 79 patients included, there were 21 instances of stem cell transplantation after relapse to BCMA–directed CAR T therapy. Fourteen patients received only auto-SCT, and 7 patients received allogeneic SCT (allo-SCT) (3 of whom received auto-SCT as a bridge to allo-SCT). One patient received both auto- and allo-SCT as a salvage treatment. The observed ORR across all salvage treatment lines was 100% for allo-SCT (4 out of 4 evaluable instances) and 71.4% for auto-SCT (10/14 evaluable instances). The median OS for all patients that received at least 1 stem cell transplant was 23.2 months (95% CI 17.6–NE, supplemental Figure 3A).
Other salvage approaches can be used with variable efficacy based on patient characteristics
The availability of various other newer treatment options also contributed to the observed OS within the cohort. Although doublet/triplet/quadruplet combinations of approved agents and combination chemotherapy were most commonly used (with 56 and 53 instances at any time point, respectively), we specifically note the use of selinexor-based regimens (with an observed ORR 40.0% [2 out of 5 evaluable instances] as a first-line salvage treatment and 21.4% [3 out of 14 evaluable instances] at any line of salvage treatment). Furthermore, there were 14 instances of using venetoclax-based treatments (among 12 patients [15.2%], 6 of whom had a confirmed t(11;14)), with an observed ORR of 66.7% (2 out of 3 instances) as a first-line salvage treatment and 35.7% (5 out of 14 instances) at any line of salvage treatment. The ORR in the patients with confirmed t(11;14) was 50% (3 out of 6 instances).
Discussion
We demonstrate that patients with MM who progress after BCMA-directed CAR T therapies can be salvaged with additional therapies, including on clinical trials. As expected, patients in this cohort were heavily pretreated (median 5 previous regimens), had disease that was refractory to most available therapies (84% were triple-class refractory and 38% penta-drug refractory), and most (83%) had high-risk cytogenetic abnormalities. The only pretherapy risk factor associated with inferior survival at relapse after CAR T was penta-drug refractoriness, emphasizing the fact that penta-drug refractory disease continues to represent an adverse prognosis, even in the post–BCMA-directed CAR T setting.13
In the absence of a standard-of-care approach for relapse post–BCMA-directed CAR T, a wide range of management strategies were used as the subsequent antimyeloma regimen. The 79 patients in our cohort received a median of 2 salvage regimens (range 1-10), with a total of 237 salvage treatment lines used, which highlights the multiple potential options available for patients progressing after CAR T therapies. The ORR for the first salvage regimen was 43.4%, whereas the median PFS was 3.5 months.
The median OS from the time of BCMA-directed CAR T relapse was 17.9 months. We observed that the use of subsequent T-cell–engaging therapies (CAR T and/or bispecific antibody therapy) as a salvage regimen was associated with a median OS that was not reached after a median follow up of more than 21 months. Furthermore, patients who received treatment with a T-cell–engaging therapy as the first line of salvage treatment (ie, immediately after relapse) had a median PFS of more than 9 months. This suggests that for eligible patients who progress after BCMA-directed CAR T, additional T-cell–engaging therapies contribute meaningfully to survival and that T-cell activation is feasible down the line. This is consistent with studies suggesting that the mechanisms of BCMA-directed CAR T failure are likely multifactorial, including BCMA downregulation and the subsequent antigenic escape in a subset of patients.14–16 However, these results must be interpreted cautiously, as all patients receiving T-cell–engaging therapies were treated on clinical trials, for which they had to be eligible, and many required washout periods. Therefore, it is conceivable that these patients may have a different disease biology and prognosis compared with patients who may not meet the eligibility requirements of such trials.
Notably, patients received both BCMA-directed and non–BCMA-directed T-cell–engaging therapies in the post–CAR T setting, and responses were noted with both approaches. This is consistent with recent results demonstrating activity of BCMA-directed bispecific antibodies teclistamab and elranatamab in patients with prior BCMA-directed therapies and showing consistent response rates between patients with and without prior BCMA-directed drug exposure.17,18 Targeting alternative myeloma tumor antigens (such as GPRC5D and Fc receptor-homolog 5) may be a potential strategy to overcome BCMA antigenic loss.19,20 GPRC5D- and Fc receptor-homolog 5–directed therapies are also being studied in the setting of prior BCMA-directed therapy exposure.21,22 A subset of patients in this cohort were treated with GPR5CD-directed CAR T. Indeed, it was recently reported that 7 out of 10 patients who relapsed after BCMA-directed CAR T therapy responded to GPRC5D-directed CAR T in a phase I, first-in-class clinical trial.12 Future studies should evaluate factors that may a priori identify patients who will be most responsive to T-cell–engaging therapies, including additional BCMA-directed treatments in the post–CAR T setting.
Another important question is whether there may be a role for salvage auto-SCT in the setting of relapse after CAR T therapy, particularly given the fact that some consensus guidelines recommend that at the time of the first auto-SCT enough hematopoietic stem cells be collected to perform 2 auto-SCTs.23 In this cohort, we noted responses with both auto- and allo-SCT. The 20 patients who were able to receive at least 1 SCT after relapse had a median OS of almost 2 years. However, prospective clinical trials are required to evaluate the role of salvage auto- and allo-SCT in the post–CAR T setting. We noted responses, albeit with limited response rates, using available doublet, triplet, or quadruplet regimens, which reflects the fact that a large majority of patients had triple-class refractory disease before receiving CAR T.
Despite modest response rates and/or duration for each individual salvage treatment option (especially outside the context of clinical trials), the availability and sequencing of multiple available treatment options for patients with RRMM after relapse to BCMA-directed CAR T contributes to an observed OS of almost 18 months. These results compare favorably to previous reports of patients with relapse after CD38-targeted mAb therapy in which the OS in the whole cohort was only 11.2 months and in patients who were penta-drug refractory, only 5.6 months.24 Despite this progress, patients with MM refractory to BCMA-directed CAR T therapy have a limited prognosis. This study hopes to provide a direction for possible standard-of-care and investigational treatment options for these patients and potential future directions.
There are several limitations to this study, including the retrospective nature of this analysis. At the time of data analysis, 100 of the 140 patients treated with BCMA-directed CAR T at our institutions had relapsed. It is therefore possible that our data set is biased toward those patients with earlier relapse, which might represent a cohort with intrinsically more challenging biological characteristics. Given the fact that BCMA-directed CAR T therapy has only recently become commercially available, all patients included in this study were enrolled on one of several clinical trials. Potential trial selection criteria might have introduced a deviation between enrolled patients and the real-world population. Similarly, at the time of relapse, T-cell–engaging therapies were only available in a clinical trial setting, which again might have been a source of selection bias. The choice of subsequent therapy is likely to be influenced by multiple factors, including the time of relapse, clinical phenotype at relapse, cytopenias, physician preference, and the distance from the treating center. Differences between the numerous BCMA-directed CAR T products may have also affected responses to subsequent lines of therapy. Because patient characteristics are not homogeneous across treatment strategies, no strong conclusions can be drawn from directly comparing different treatment categories after the relapse to BCMA-directed CAR T based on this data set. Prospective studies in this context can help answer this important clinical question.
In conclusion, to our knowledge, this is the largest analysis of patients with relapse post–BCMA-directed CAR T therapies. We demonstrate that patients with progression after BCMA-directed CAR T therapies can receive additional therapies and have a median OS of 17.9 months. Although numerous treatment regimens, including auto- and allo-SCT, were used and patient selection should be taken into consideration, T-cell–engaging therapies appear to have the most pronounced clinical activity with high response rates and durable responses in this setting. The findings of this study can serve as a benchmark for future prospective clinical studies that intend to improve the outcomes of patients who progress after CAR T therapy. Further biological insights regarding the mechanisms of CAR T therapy failure are also required to guide subsequent treatment strategies.
Acknowledgments
The authors thank all participants for their generosity and willingness to participate in longitudinal research studies. The authors acknowledge the clinical and research staff at the Tisch Cancer Institute at Mount Sinai and at Memorial Sloan Kettering Cancer Center and also the support of the Center of Excellence for Multiple Myeloma at Mount Sinai Philanthropy.
Authorship
Contribution: U.A.S., S.M., and S.P. were responsible for study conceptualization; O.V.O., K.N., T.H.M., A.A., D.T.M., Y.G.-P., T.F., G.L.S., A.L., S.G., S.T., A.R., C.R., L.S., J.R., S.R., H.J.C., A.C., S.Z.U., S.J., U.A.S., S.M., and S.P. contributed to data collection and data curation; O.V.O., K.N., A.A., D.T.M., Y.G.-P., and T.F. contributed to data analysis and visualization; and O.V.O., K.N., U.A.S., S.M., and S.P. wrote the original draft of the manuscript. All authors provided review and edits and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.H.M. reports advisory board fees from Legend Biotech; G.L.S. receives research funding from Janssen, Amgen, and Beyond Spring; A.L. reports grant funding from Genmab, Amgen, Bristol Myers Squibb, and Janssen, has served on an advisory panel for Trillium Therapeutics, Pfizer, and Bristol Myers Squibb, and has received research funding from Janssen Oncology, Trillium Therapeutics, Genentech, Bristol Myers Squibb, Sanofi, and Pfizer; S.G. receives research funding from Miltenyi Biotec, Takeda Pharmaceutical, Celgene, Amgen, Sanofi, Johnson & Johnson, Actinium Pharmaceuticals, and Omeros, and is a member on the advisory boards for Kite Pharma, Celgene, Sanofi, Novartis, Johnson & Johnson, Amgen, Takeda Pharmaceutical, Jazz Pharmaceuticals, Janssen, Actinium Pharmaceuticals, and Spectrum Pharma; A.C. reports research support from Amgen, Array Biopharma, Celgene, GSK, Janssen, Millennium/Takeda, Novartis Pharmaceuticals, Oncoceutics, Pharmacyclics, and Seattle Genetics, consultancy fees from Amgen, Bristol Myers Squibb, Celgene, Millennium/Takeda, Janssen, and Karyopharm, and membership on the scientific advisory board for Amgen, Celgene, Millennium/Takeda, Janssen, Karyopharm, Sanofi, and Seattle Genetics; S.Z.U. reports grants/personal fees from Amgen, Celgene, Sanofi, Seattle Genetics, Janssen, Takeda, Skyline DX, Merck, and GSK, grant funding from BMS and Pharmacyclics; and personal fees from AbbVie, MundiPharma, Gilead, Genentech, and Oncopeptides; S.J. reports consulting fees for Bristol Myers Squibb (Celgene), Janssen, Karyopharm Therapeutics, Merck, Sanofi, and Takeda Pharmaceuticals; U.A.S. reports consultancy fees from Janssen, grant funding from the Parker Institute for Cancer Immunotherapy, the International Myeloma Society, Paula and Rodger Riney Foundation, Allen Foundation Inc, HealthTree Foundation, Janssen, Celgene/BMS, and the MSK Paul Calabresi Career Development Award for Clinical Oncology K12CA184746 and payment for lectures including service on speaker bureaus from ACCC and MJH Life Sciences; S.M. has received consulting fees from Evicore, Optum, BioAscend, Janssen Oncology, and Legend Biotech; T.F., G.L.S., A.L., S.G., S.U., U.A.S., and S.M. report funding support from the National Cancer Institute Memorial Sloan Kettering Core Grant (P30 CA008748); Memorial Sloan Kettering Cancer Center receives research funding from the National Cancer Institute, Janssen Oncology, Bristol Myers Squibb, Allogene Therapeutics, Fate Therapeutics, and Takeda Oncology for the research conducted by S.M. He has received honoraria from OncLive, Physician Education Resource, MJH Life Sciences, and Plexus Communications; S.P. is supported by NCI R01 CA244899, R01 CA252222, P30 CA196521 and research funding from Bristol Myers Squibb (Celgene), Karyopharm, and Amgen; The remaining authors declare no competing financial interests.
Correspondence: Sham Mailankody, Myeloma and Cellular Therapy Services, Department of Medicine, Memorial Sloan Kettering Cancer Center, Koch Center: 530 E 74th St, New York, NY 10021; e-mail: mailanks@mskcc.org; and Samir Parekh, Icahn School of Medicine at Mount Sinai, 1470 Madison Ave, New York, NY 10029; e-mail: samir.parekh@mssm.edu.
References
Author notes
∗O.V.O. and K.N. are joint first authors.
∗∗S.M. and S.P. are joint senior authors.
Data are available on request from the corresponding authors, Samir Parekh (samir.parekh@mssm.edu) or Sham Mailankody (mailanks@mskcc.org).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal