TO THE EDITOR:
Recent World Health Organization (WHO)1 classifications (5th edition), International Consensus Classification (ICC),2 and the European LeukemiaNet (ELN) guidelines3 included new categories for TP53-mutated (TP53mut) myeloid neoplasms (MNs) to acknowledge their uniformly poor outcomes and stimulate clinical research. However, there are critical differences in the details between these classifications, especially regarding single-hit TP53mut status, and their significance relating to therapy-related myeloid neoplasm (t-MN), a rare but often fatal malignancy diagnosed following exposure to cytotoxic therapies, remains unclear. Here, we report an international cohort consisting of t-MN with full characterization of TP53mut allele status and provide compelling evidence of poor outcome of TP53mut t-MN irrespective of the allelic status of TP53.
The WHO defines a single category of myelodysplastic syndrome (MDS) with biallelic TP53 inactivation (MDS-biTP53) irrespective of the blast percentage1 but excludes single-hit TP53mut MDS with bone marrow (BM) blasts <20%. Likewise, in the ICC, single-hit TP53mut MDS with blasts <10% is excluded from the definition of TP53mut MN.2 Similarly, the recent International Prognostic Scoring System-Molecular acknowledged the poor outcome of multi-hit TP53mut but excluded single-hit TP53mut.4 The ICC and ELN guidelines emphasize TP53mut variant allele frequency (VAF) >10% regardless of single- or multi-hit status for MDS/acute myeloid leukemia (AML) and AML.2,3 These critical differences in the classification of the TP53mut MN reveal a lack of consensus among experts that is likely driven by limited evidence or conflicting results.1-3 For example, in a large cohort consisting predominantly (93%) of de novo MDS, single-hit TP53mut had outcomes similar to wild-type TP53 (TP53wt), whereas the association with complex karyotype (CK), high risk of AML transformation, and poor overall survival (OS) were limited to multi-hit TP53mut.5 In contrast, in another study the OS of TP53mut AML and MDS with excess blasts were equally poor irrespective of single- or multi-hit TP53mut.6 Notably, MDS <10% blasts were not included in this study. Similarly, survival of MDS and AML with CK was equally poor irrespective of single- or multi-hit TP53mut status, and the distinction between MDS and AML by blast percentage did not hold any predictive value.7 Thus, the majority of the studies driving changes in classification are derived predominantly from de novo MDS and AML with only a small fraction of t-MN5,7 or were restricted to MDS and AML with CK.7 Overall, this highlights a lack of data in the clinical context of t-MN to resolve the complex interactions between TP53mut single- versus multi-hit status, blast percentage, and VAF.
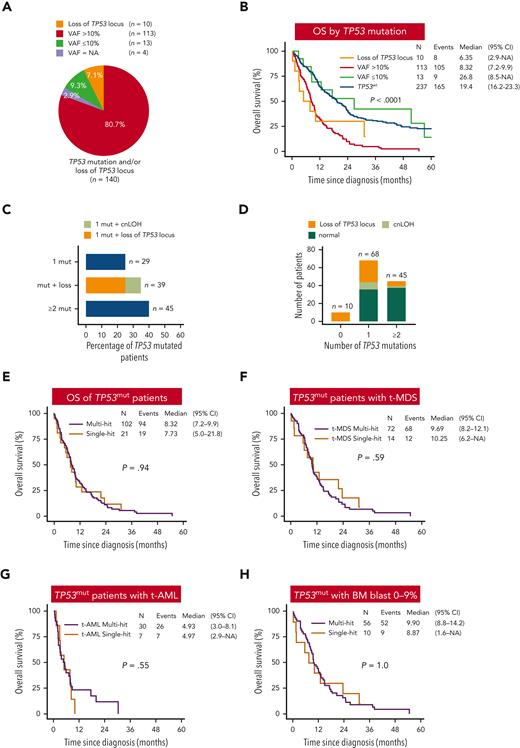
To address these gaps, we performed a comprehensive analysis of an international cohort of 377 t-MN patients that included 245 t-MDS (65%) and 132 t-AML (35%) (supplemental Methods, available at the Blood website). The median age at t-MN diagnosis was 67 years. Somatic mutation analysis identified 185 putative oncogenic mutations in TP53 at VAF ≥2% in 132 (35%) patients (supplemental Figure 1 and Figure 1A). The majority of the TP53mut patients with available information (n = 128; 96.9%) had a VAF >10% (n = 113; 88.2%), and only 15 (11.7%) had VAF ≤10% (supplemental Figure 1). Allelic imbalances overlapping the TP53 locus were detected in 56 (14.8%) patients, including TP53mut VAF >10% (n = 46) and <10% (n = 2) and without TP53mut (n = 8, supplemental Figure 1). In summary, 123 t-MN patients had TP53mut VAF >10% or LOH or cnLOH involving the TP53 locus (supplemental Figure 1). Genomic instability including CK, monosomal karyotype, chromosome 5 aberrancies, and marker chromosomes were enriched in TP53mut VAF >10% and/or LOH of TP53 locus compared with TP53wt t-MN (supplemental Table 1). Median OS was significantly shorter in the TP53mut cases compared with in TP53wt cases (8.3 vs 19.4 months; P < .001) (Figure 1B). The OS in TP53mut t-MN with VAF ≤10% was similar to TP53wt (Figure 1B), although the number of cases with TP53mut VAF ≤10% were limited and requires further validation.
Survival of TP53mut t-MN is poor irrespective of the allelic status or bone marrow blast percentage. (A) Proportion of TP53mut t-MN patients with TP53 VAF ≤10% or >10% or loss of TP53 locus without TP53mut. Of the 15 patients with VAF ≤10%, 13 had VAF ≤10% without loss of heterozygosity (LOH). Two patients had LOH across the TP53 locus and were grouped with “loss of TP53 locus” (n = 10). (B) OS of TP53mut with VAF >10% or loss of TP53 locus was significantly poor compared with TP53wt and TP53mut with VAF ≤10% t-MN. (C) The frequency of TP53mut subgroup within the TP53mut t-MN. TP53mut subgroup were defined as cases with single mutation (1 mut), ≥2 mutations without the loss of chromosome 17p13 across TP53 locus (≥2 mut), mutation(s) plus copy neutral LOH (cnLOH) and/or loss of the TP53 locus (mut + loss). (D) Number of patients with 0, 1, or ≥2 TP53mut. Colors represent the status of chromosome 17 at the TP53 locus, to include cnLOH, loss of TP53 locus, and no detected aberration (normal). Unbalanced translocations leading to loss of TP53 locus are encoded as “loss.” OS of single- vs multi-hit TP53mut in the whole cohort (E), t-MDS (F), t-AML (G), and bone marrow blast 0% to 9% (H).
Survival of TP53mut t-MN is poor irrespective of the allelic status or bone marrow blast percentage. (A) Proportion of TP53mut t-MN patients with TP53 VAF ≤10% or >10% or loss of TP53 locus without TP53mut. Of the 15 patients with VAF ≤10%, 13 had VAF ≤10% without loss of heterozygosity (LOH). Two patients had LOH across the TP53 locus and were grouped with “loss of TP53 locus” (n = 10). (B) OS of TP53mut with VAF >10% or loss of TP53 locus was significantly poor compared with TP53wt and TP53mut with VAF ≤10% t-MN. (C) The frequency of TP53mut subgroup within the TP53mut t-MN. TP53mut subgroup were defined as cases with single mutation (1 mut), ≥2 mutations without the loss of chromosome 17p13 across TP53 locus (≥2 mut), mutation(s) plus copy neutral LOH (cnLOH) and/or loss of the TP53 locus (mut + loss). (D) Number of patients with 0, 1, or ≥2 TP53mut. Colors represent the status of chromosome 17 at the TP53 locus, to include cnLOH, loss of TP53 locus, and no detected aberration (normal). Unbalanced translocations leading to loss of TP53 locus are encoded as “loss.” OS of single- vs multi-hit TP53mut in the whole cohort (E), t-MDS (F), t-AML (G), and bone marrow blast 0% to 9% (H).
Among patients with TP53mut VAF >10% (n = 113), 75% had single TP53mut plus loss of TP53 locus or cnLOH (n = 39; 34.5%) or ≥2 TP53mut (n = 45; 39.8%), whereas 25% (n = 29) had single TP53mut (Figure 1C). Of the 29 patients with single TP53mut, 18 (62%) and 11 (37.9%) patients had VAF >50% and 10% to 50%, respectively (supplemental Figure 1). Additionally, 10 patients had loss of the TP53 locus without evidence of TP53mut (n = 8) or TP53mut VAF ≤10% (n = 2) (Figure 1D and supplemental Figure 1). Loss or cnLOH of TP53 locus was more prevalent in cases with single TP53mut compared with ≥2 TP53mut (57.4% vs 15.5%, P < .0001) (Figure 1D).
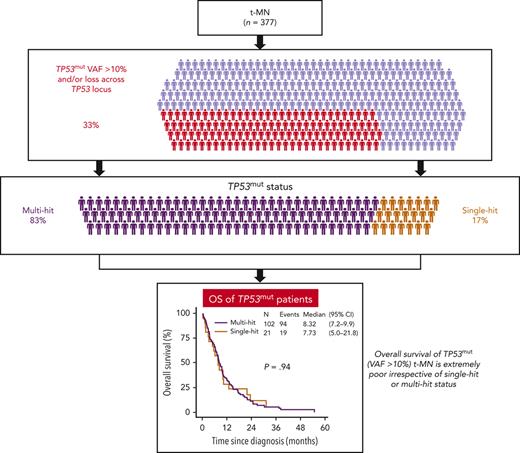
Integrating data from next generation sequencing, copy number, cytogenetic banding, fluorescence in situ hybridization, and single nucleotide polymorphism microarray analyses, TP53mut were classified as multi- or single-hit following ICC2 (supplemental Methods). In total, 21 (17.1%) of the 123 patients with TP53mut and/or loss of TP53 locus were considered single-hit and 102 (82.9%) were considered multi-hit (supplemental Figure 1). The clinical parameters and OS of t-MN with single-hit TP53mut and 17p loss across the TP53 locus were comparable (8.3 vs 6.3 months, P = .58) (supplemental Figure 2A and supplemental Table 2) and were combined for subsequent analyses.
We next compared the clinical features, profiles of genome stability, and patterns of co-mutation for each TP53 state. In contrast to the findings for predominantly de novo MDS,5 no difference in the frequency of structural chromosomal aberrancies including CK, monosomal karyotype, chromosome 5 aberrancy, or comutation pattern between single- and multi-hit TP53mut t-MN were observed (Table 1). Similarly, no significant differences were observed in age, latency, blood counts, BM blast percentage, and cytogenetic aberrancies. Critically, OS was not significantly different between the single- and multi-hit TP53mut in the whole t-MN cohort and for the t-MDS subgroup or the t-AML subgroup or when stratified by blast percent categories (Figure 1E-H and supplemental Figure 2B). There was also no difference in the incidence of progression to AML between single- vs multi-hit TP53mut t-MDS (supplemental Figure 2C). The striking enrichment of CK in single-hit TP53mut t-MN (85.7%) compared with reported frequency of 13% in de novo MDS5 and VAF cut-off >10% vs 2% could partly explain the difference between the 2 studies. Single nucleotide polymorphism microarray analyses and copy number analysis were unavailable for 7 cases with single-hit TP53mut with VAF 10% to 50%, and thus few cases of multi-hit TP53mut could have been missed. However, the OS between single- and multi-hit TP53mut was still not significantly different after excluding these cases (supplemental Figure 2D).
Comparison of genomic instability and other clinical features in single-hit and multi-hit TP53-mutated therapy-related myeloid neoplasms
| Variables . | Multi-hit (n = 102) . | Single-hit (n = 21) . | P value . |
|---|---|---|---|
| Age at t-MN diagnosis, y, median (IQR) | 67.5 (61.3, 74.0) | 67.0 (61.0, 72.0) | .664 |
| Female/Male | 46/56 | 7/14 | .346 |
| Hemoglobin, g/L, median (IQR) | 91.0 (81.0, 105.0) | 87.0 (80.3, 102.0) | .585 |
| WBC ×109/L, median (IQR) | 2.9 (1.9, 4.2) | 2.7 (2.2, 5.3) | .586 |
| ANC ×109/L, median (IQR) | 0.9 (0.5, 1.9) | 1.2 (0.3, 1.8) | .91 |
| Platelets ×109/L, median (IQR) | 54.5 (30.0, 92.5) | 35.0 (25.5, 64.5) | .196 |
| BM blasts %, median (IQR) | 6.0 (2.0, 22.0) | 4.0 (2.0, 29.0) | .88 |
| t-MN phenotype | |||
| t-MDS | 72 (70.6) | 14 (66.7) | .795 |
| t-AML | 30 (29.4) | 7 (33.3) | |
| Cytogenetic changes | |||
| Any cytogenetic aberrancies | 101 (99.0) | 20 (95.2) | .313 |
| Complex karyotype, n (%) | 91 (89.2) | 18 (85.7) | .706 |
| Monosomal karyotype, n (%) | 85 (83.3) | 16 (76.2) | .531 |
| Marker chromosome, n (%) | 59 (57.8) | 9 (42.9) | .235 |
| Ring chromosome, n (%) | 21 (20.6) | 4 (19.0) | 1 |
| Abnormal Chrom 17, n (%) | 31 (30.4) | 10 (47.6) | .136 |
| Abnormal Chrom 5, n (%) | 83 (81.4) | 14 (66.7) | .148 |
| Abnormal Chrom 7, n (%) | 61 (59.8) | 14 (66.7) | .63 |
| Abnormal Chrom 3, n (%) | 33 (32.4) | 4 (19.0) | .3 |
| Trisomy 8, n (%) | 20 (19.6) | 5 (23.8) | .766 |
| Abnormal Chrom 9, n (%) | 21 (20.6) | 4 (19.0) | 1 |
| Abnormal Chrom 11, n (%) | 27 (26.5) | 5 (23.8) | 1 |
| Abnormal Chrom 12, n (%) | 37 (36.3) | 6 (28.6) | .619 |
| Abnormal Chrom 13, n (%) | 36 (35.3) | 6 (28.6) | .622 |
| Abnormal Chrom 16, n (%) | 26 (25.5) | 3 (14.3) | .399 |
| Abnormal Chrom 18, n (%) | 34 (33.3) | 8 (38.1) | .801 |
| Abnormal Chrom 19, n (%) | 27 (26.5) | 7 (33.3) | .594 |
| Abnormal Chrom 20, n (%) | 24 (23.5) | 5 (23.8) | 1 |
| Abnormal Chrom 21, n (%) | 34 (33.3) | 5 (23.8) | .451 |
| Somatic mutations on NGS | |||
| TP53mut VAF, median (IQR) | 42.0 (31.6, 69.0) | 37.70 (20.0, 43.0) | .03 |
| Co-mutations (excluding TP53mut) | |||
| ≥ 2 mutations, n (%) | 18 (17.6) | 7 (33.3) | .06 |
| 1 mutation, n (%) | 26 (25.5) | 8 (38.1) | |
| No mutations, n (%) | 58 (56.9) | 6 (28.6) | |
| ASXL1, n (%) | 7 (6.9) | 3 (14.3) | .372 |
| RAS, n (%) | 2 (2.0) | 2 (9.5) | .135 |
| RUNX1, n (%) | 5 (4.9) | 1 (4.8) | 1 |
| SF3B1, n (%) | 2 (2.0) | 0 (0.0) | 1 |
| SRSF2, n (%) | 4 (3.9) | 2 (9.5) | .272 |
| TET2, n (%) | 7 (6.9) | 2 (9.5) | .65 |
| DNMT3A, n (%) | 11 (10.8) | 5 (23.8) | .148 |
| FLT3, n (%) | 0 (0.0) | 1 (4.8) | .171 |
| IDH2, n (%) | 1 (1.0) | 0 (0.0) | 1 |
| Disease-modifying therapy for t-MN∗ | |||
| No DMT, n (%) | 20 (19.6) | 6 (28.6) | .17 |
| Intensive chemotherapy, n (%) | 14 (13.7) | 4 (19.0) | |
| HMA-based chemotherapy, n (%) | 43 (42.2) | 9 (42.9) | |
| Venetoclax-based therapy, n (%) | 24 (23.5) | 1 (4.8) | |
| Unknown, n (%) | 1 (1) | 1 (4.8) | |
| Allogeneic SCT, n (%) | 16 (15.7) | 3 (14.3) | 1 |
| Months between primary to t-MN, median (IQR) | 103.4 (48.2, 165.5) | 79.4 (43.9, 151.9) | .685 |
| Clinical features at primary disease | |||
| Age at primary disease, y, median (IQR) | 57.0 (49.5, 65.0) | 55.5 (48.3, 60.8) | .464 |
| Hematologic malignancy, n (%) | 61 (59.8) | 14 (66.7) | .777 |
| Solid cancer, n (%) | 34 (33.3) | 7 (33.3) | |
| Other, n (%) | 7 (6.9) | 0 (0.0) | |
| Treatment for primary cancer/disease† | |||
| Chemotherapy alone for primary cancer/disease, n (%) | 53 (52%) | 9 (42.8%) | .603 |
| Chemotherapy plus radiotherapy for primary cancer, n (%) | 35 (34.3%) | 9 (42.8%) | .621 |
| Radiation only for primary cancer, n (%) | 8 (7.8%) | 3 (14.4%) | .602 |
| Immunosuppression, n (%) | 12 (11.8) | 0 (0.0) | .217 |
| Auto SCT for primary cancer, n (%) | 26 (25.5) | 6 (28.6) | .788 |
| Variables . | Multi-hit (n = 102) . | Single-hit (n = 21) . | P value . |
|---|---|---|---|
| Age at t-MN diagnosis, y, median (IQR) | 67.5 (61.3, 74.0) | 67.0 (61.0, 72.0) | .664 |
| Female/Male | 46/56 | 7/14 | .346 |
| Hemoglobin, g/L, median (IQR) | 91.0 (81.0, 105.0) | 87.0 (80.3, 102.0) | .585 |
| WBC ×109/L, median (IQR) | 2.9 (1.9, 4.2) | 2.7 (2.2, 5.3) | .586 |
| ANC ×109/L, median (IQR) | 0.9 (0.5, 1.9) | 1.2 (0.3, 1.8) | .91 |
| Platelets ×109/L, median (IQR) | 54.5 (30.0, 92.5) | 35.0 (25.5, 64.5) | .196 |
| BM blasts %, median (IQR) | 6.0 (2.0, 22.0) | 4.0 (2.0, 29.0) | .88 |
| t-MN phenotype | |||
| t-MDS | 72 (70.6) | 14 (66.7) | .795 |
| t-AML | 30 (29.4) | 7 (33.3) | |
| Cytogenetic changes | |||
| Any cytogenetic aberrancies | 101 (99.0) | 20 (95.2) | .313 |
| Complex karyotype, n (%) | 91 (89.2) | 18 (85.7) | .706 |
| Monosomal karyotype, n (%) | 85 (83.3) | 16 (76.2) | .531 |
| Marker chromosome, n (%) | 59 (57.8) | 9 (42.9) | .235 |
| Ring chromosome, n (%) | 21 (20.6) | 4 (19.0) | 1 |
| Abnormal Chrom 17, n (%) | 31 (30.4) | 10 (47.6) | .136 |
| Abnormal Chrom 5, n (%) | 83 (81.4) | 14 (66.7) | .148 |
| Abnormal Chrom 7, n (%) | 61 (59.8) | 14 (66.7) | .63 |
| Abnormal Chrom 3, n (%) | 33 (32.4) | 4 (19.0) | .3 |
| Trisomy 8, n (%) | 20 (19.6) | 5 (23.8) | .766 |
| Abnormal Chrom 9, n (%) | 21 (20.6) | 4 (19.0) | 1 |
| Abnormal Chrom 11, n (%) | 27 (26.5) | 5 (23.8) | 1 |
| Abnormal Chrom 12, n (%) | 37 (36.3) | 6 (28.6) | .619 |
| Abnormal Chrom 13, n (%) | 36 (35.3) | 6 (28.6) | .622 |
| Abnormal Chrom 16, n (%) | 26 (25.5) | 3 (14.3) | .399 |
| Abnormal Chrom 18, n (%) | 34 (33.3) | 8 (38.1) | .801 |
| Abnormal Chrom 19, n (%) | 27 (26.5) | 7 (33.3) | .594 |
| Abnormal Chrom 20, n (%) | 24 (23.5) | 5 (23.8) | 1 |
| Abnormal Chrom 21, n (%) | 34 (33.3) | 5 (23.8) | .451 |
| Somatic mutations on NGS | |||
| TP53mut VAF, median (IQR) | 42.0 (31.6, 69.0) | 37.70 (20.0, 43.0) | .03 |
| Co-mutations (excluding TP53mut) | |||
| ≥ 2 mutations, n (%) | 18 (17.6) | 7 (33.3) | .06 |
| 1 mutation, n (%) | 26 (25.5) | 8 (38.1) | |
| No mutations, n (%) | 58 (56.9) | 6 (28.6) | |
| ASXL1, n (%) | 7 (6.9) | 3 (14.3) | .372 |
| RAS, n (%) | 2 (2.0) | 2 (9.5) | .135 |
| RUNX1, n (%) | 5 (4.9) | 1 (4.8) | 1 |
| SF3B1, n (%) | 2 (2.0) | 0 (0.0) | 1 |
| SRSF2, n (%) | 4 (3.9) | 2 (9.5) | .272 |
| TET2, n (%) | 7 (6.9) | 2 (9.5) | .65 |
| DNMT3A, n (%) | 11 (10.8) | 5 (23.8) | .148 |
| FLT3, n (%) | 0 (0.0) | 1 (4.8) | .171 |
| IDH2, n (%) | 1 (1.0) | 0 (0.0) | 1 |
| Disease-modifying therapy for t-MN∗ | |||
| No DMT, n (%) | 20 (19.6) | 6 (28.6) | .17 |
| Intensive chemotherapy, n (%) | 14 (13.7) | 4 (19.0) | |
| HMA-based chemotherapy, n (%) | 43 (42.2) | 9 (42.9) | |
| Venetoclax-based therapy, n (%) | 24 (23.5) | 1 (4.8) | |
| Unknown, n (%) | 1 (1) | 1 (4.8) | |
| Allogeneic SCT, n (%) | 16 (15.7) | 3 (14.3) | 1 |
| Months between primary to t-MN, median (IQR) | 103.4 (48.2, 165.5) | 79.4 (43.9, 151.9) | .685 |
| Clinical features at primary disease | |||
| Age at primary disease, y, median (IQR) | 57.0 (49.5, 65.0) | 55.5 (48.3, 60.8) | .464 |
| Hematologic malignancy, n (%) | 61 (59.8) | 14 (66.7) | .777 |
| Solid cancer, n (%) | 34 (33.3) | 7 (33.3) | |
| Other, n (%) | 7 (6.9) | 0 (0.0) | |
| Treatment for primary cancer/disease† | |||
| Chemotherapy alone for primary cancer/disease, n (%) | 53 (52%) | 9 (42.8%) | .603 |
| Chemotherapy plus radiotherapy for primary cancer, n (%) | 35 (34.3%) | 9 (42.8%) | .621 |
| Radiation only for primary cancer, n (%) | 8 (7.8%) | 3 (14.4%) | .602 |
| Immunosuppression, n (%) | 12 (11.8) | 0 (0.0) | .217 |
| Auto SCT for primary cancer, n (%) | 26 (25.5) | 6 (28.6) | .788 |
ANC, absolute neutrophil count; Chrom, chromosome; DMT, disease modifying therapy; HMA, hypomethylating agents; IQR, interquartile range; NGS, next-generation sequencing; SCT, stem cell transplant; WBC, white blood count.
First line of therapy only.
Six patients with muti-hit did not receive chemotherapy and/or radiotherapy. They had only immunosuppression.
These findings are highly relevant considering the changes proposed in the recent classifications. The ICC2 and the ELN3 removed the subcategory of “therapy-related,” substituting it with diagnostic qualifiers instead. The WHO1 has grouped t-MN with secondary MN and renamed it as “myeloid neoplasm post-cytotoxic therapy,” with the assertion that the majority of MDS and AML occurring post–cytotoxic therapy have TP53mut and that multi-hit TP53mut have poor outcome compared with single-hit.1 The underlying assumption of these changes is that TP53mut MNs, regardless of the underlying etiology, have similar genomic characteristics and outcomes.
Our results provide compelling evidence that neither the allelic status nor the BM blast percentage of t-MDS provides meaningful prognostic information and that the TP53 VAF of 10% is a clinically useful threshold to identify patients with poor survival. Underestimation of the poor prognosis of single-hit TP53mut t-MDS, by exclusion from TP53mut MN, has significant implications on patient management such as consideration for allogeneic transplantation and exclusion from clinical trials. Our findings, therefore, warrant reconsideration of the allelic status in TP53mut t-MN.
Acknowledgments
The authors thank their patients and their families.
The authors gratefully acknowledge the support of the South Australia Cancer Research Biobank (SACRB).
This work was supported by Paul Calabresi Program in Clinical/Translational Research at Mayo Clinic (K12CA090628), Leukemia Research Foundation New Investigator Award, Bridget Kiely Clinician Career Development in Transplant Research, and research funding by Astellas, Celgene, and Marker Therapeutics (M.V.S.); by a National Health and Medical Research Council (NHMRC)/Medical Research Future Fund (MRFF) Investigator Grant (MRF1195517), Cancer Australia, and Leukemia Foundation Australia (D.H.); by a NHMRC Investigator Grant (GNT2007739) (S.K.); and by a CSL Centenary Fellowship, Medical Research Futures Fund, and Leukemia-Lymphoma Translational Research Program funding (D.T.).
Authorship
Contribution: D.H. designed the study, contributed the patient data, analyzed the data, and wrote the manuscript; E.N.H.T. and R.C. collated the data, analyzed the data, and edited the manuscript; C.H.K. performed statistical analysis and edited the manuscript; C.H., E.N.H.T., A. Brown, and H.S. contributed to variant annotation; D.L. contributed to cytogenetic analysis; P.W. contributed to variant annotation and CNA analysis; S.K. contributed DDR expertise and edited the manuscript; N.S. reviewed the manuscript; A. Brown collected the data and contributed patients; A.A., H.A., T.B., M.R.L., A.M., W.J.H., N.G., M.P., K.B., A. Beligaswatte, R.H., P.G., M.K., D.M.R., D.S., N.S., P.B., D.Y., P.G., C.L., A.Y., N.H., R.G., I.S.W., A.W., and A.T. contributed patients and edited manuscript; D.T. edited the manuscript; M.V.S. designed the study, contributed the patient data, and edited the manuscript; and all authors agreed to the final version of the manuscript.
Conflict-of-interest disclosure: D.H. is a member of the board of directors or advisory committees of AbbVie and Novartis. N.S. receives honoraria support from Novartis. A.A. provides research support to Novartis and Astex. M.P. is a member of the board of directors or advisory committees of Stemline Therapeutics and receives research funding from Kura Oncology. P.G. is a member of the advisory board of AbbVie. D.M.R. receives research funding from Novartis and BMS and honoraria from Novartis, BMS, Keros, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Devendra Hiwase, Royal Adelaide Hospital and South Australian Health and Medical Research Institute, Adelaide, SA 5000, Australia; e-mail: devendra.hiwase@sa.gov.au; and Mithun Vinod Shah, Mayo Clinic, Rochester, 200 1st Street SW, Rochester, MN 55906; e-mail: shah.mithun@mayo.edu.
References
Author notes
Data are available on request from the corresponding authors.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal