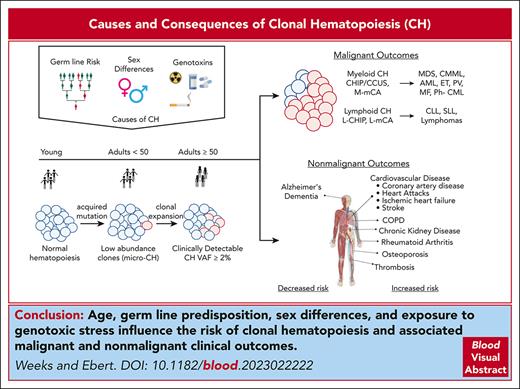
Visual Abstract
Clonal hematopoiesis (CH) is described as the outsized contribution of expanded clones of hematopoietic stem and progenitor cells (HSPCs) to blood cell production. The prevalence of CH increases dramatically with age. CH can be caused by somatic mutations in individual genes or by gains and/or losses of larger chromosomal segments. CH is a premalignant state; the somatic mutations detected in CH are the initiating mutations for hematologic malignancies, and CH is a strong predictor of the development of blood cancers. Moreover, CH is associated with nonmalignant disorders and increased overall mortality. The somatic mutations that drive clonal expansion of HSPCs can alter the function of terminally differentiated blood cells, including the release of elevated levels of inflammatory cytokines. These cytokines may then contribute to a broad range of inflammatory disorders that increase in prevalence with age. Specific somatic mutations in the peripheral blood in coordination with blood count parameters can powerfully predict the development of hematologic malignancies and overall mortality in CH. In this review, we summarize the current understanding of CH nosology and origins. We provide an overview of available tools for risk stratification and discuss management strategies for patients with CH presenting to hematology clinics.
Introduction
The acquisition of somatic mutations in healthy human cells is ubiquitous, increases with age, and underlies the development of cancer and nonmalignant disease.1,2 Somatic mutagenesis of stem cells leads to genetically divergent clonal populations of cells and decreased stem cell pool diversity.3 Although anatomical constraints challenge our ability to fully capture the genetic diversity in solid organs, circulating blood cells are easily sampled and represent the genetic diversity of the entire hematopoietic system. The study of clonal hematopoiesis (CH; clonally expanded hematopoietic stem and progenitor cells [HSPCs] with acquired selective growth advantage)4-6 has allowed for an in-depth assessment of mechanisms underlying somatic genetic variation and related malignant and nonmalignant outcomes. Early descriptions of CH came from observations of biased X chromosome inactivation7,8 and the clonal origins of chronic myelogenous leukemia.9 In the decades since these discoveries, CH has gained relevance as a hematologic malignancy precursor, analogous to monoclonal gammopathy of uncertain significance and monoclonal B-cell lymphocytosis (MBL), and established precursor conditions for plasma cell and lymphoid malignancies.10 CH investigations provide insights into the mechanisms and kinetics of leukemogenesis and the role of environmental factors in the selection and maintenance of cancer-causing HSPC clones. CH is associated with significant clinical outcomes, including an increased risk of hematologic cancers,11-13 increased all-cause mortality,4 and various nonmalignant conditions.14 We review CH nosology, relative fitness, and associated clinical outcomes, concluding our review with a discussion of the management of patients with CH.
CH nosology
Myeloid CH: CHIP, CCUS, and M-mCA
Among the various types of CH (Figure 1), 2 entities have been formally defined by the detection of acquired pathogenic variants in ≥1 genes known for or suspected of promoting clonal expansion of HSPCs.15-17 CH of indeterminate potential (CHIP) refers specifically to CH possessing somatic mutations in leukemia driver genes at a variant allele fraction (VAF) of ≥2% in the absence of diagnosed blood disorder or cytopenia. Clonal cytopenia of undetermined significance (CCUS) describes CHIP in the presence of persistent, unexplained cytopenia in which dysplastic features of myelodysplastic syndrome (MDS) are absent.16,17 CHIP and CCUS are detected in 10% to 20% of individuals aged >70 years4-6,15 and are rare in individuals aged <50 years.5 The epigenetic modifiers DNMT3A, ASXL1, and TET2 are mutated in 87% of CHIP/CCUS cases. Mutations in JAK2, TP53, SF3B1, and SRSF2 are commonly observed in the remaining cases.4,5,12 Although the genetic drivers overlap, the genetics of CH is largely distinguishable from that of overt malignancy.6,15,18
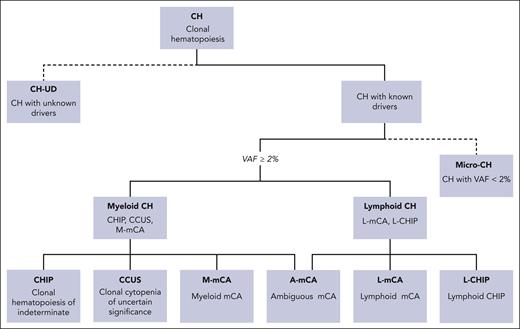
Nosology of CH. CH is the expansion of a clonal population of HSPCs. Many instances of CH are caused by unknown drivers and can be referred to as CHUD (dotted branch). When molecular drivers of CH are known, they can be further subclassified as initiating events of myeloid malignances (myeloid CH) or lymphoid malignancies (lymphoid CH). Myeloid CH is classified as CH caused by somatic mutations in myeloid malignancy-driver genes at VAF ≥ 2%. The term CHIP is used in the absence of cytopenias, and CCUS is used when cytopenias are present. Mosaic chromosomal alterations (mCAs) can also be drivers of myeloid CH (m-MCA). Lymphoid CH is subdivided into CH caused by mutations in drivers of lymphoid malignancy with a VAF ≥ 2% (L-CHIP) or mCA that reflect chromosomal abnormalities driving lymphoid malignancies (L-mCA). Ever-improving sensitivity of next-generation sequencing has led to an increasing identification of low-abundance (VAF < 2%) clones, which some have referred to as micro-CH. The clinical significance of these low-abundance clones remains to be fully elucidated.
Nosology of CH. CH is the expansion of a clonal population of HSPCs. Many instances of CH are caused by unknown drivers and can be referred to as CHUD (dotted branch). When molecular drivers of CH are known, they can be further subclassified as initiating events of myeloid malignances (myeloid CH) or lymphoid malignancies (lymphoid CH). Myeloid CH is classified as CH caused by somatic mutations in myeloid malignancy-driver genes at VAF ≥ 2%. The term CHIP is used in the absence of cytopenias, and CCUS is used when cytopenias are present. Mosaic chromosomal alterations (mCAs) can also be drivers of myeloid CH (m-MCA). Lymphoid CH is subdivided into CH caused by mutations in drivers of lymphoid malignancy with a VAF ≥ 2% (L-CHIP) or mCA that reflect chromosomal abnormalities driving lymphoid malignancies (L-mCA). Ever-improving sensitivity of next-generation sequencing has led to an increasing identification of low-abundance (VAF < 2%) clones, which some have referred to as micro-CH. The clinical significance of these low-abundance clones remains to be fully elucidated.
Somatic copy number abnormalities occur in ∼2% of individuals aged >70 years in the absence of any other manifestation of hematologic malignancy.19,20 These mosaic chromosomal alterations (mCAs) are another subtype of CH and include amplifications, deletions, and copy-neutral loss of heterozygosity.21-25 Like CHIP/CCUS, mCAs accumulate with age and are associated with subsequent hematologic malignancy.12,21-23 Niroula et al used the differential frequency of mCAs detected in individuals with prevalent hematologic malignancy to identify mCAs associated with a near-exclusive risk of myeloid malignancy (myeloid mCA [M-mCA]) and those associated with both myeloid and lymphoid malignancies (ambiguous mCA [A-mCA]).12 As expected, M-mCAs and A-mCAs occurred in the chromosomal loci of known drivers of myeloid malignancy.12 Although mCAs may be observed without comutations,21 co-occurrence of M-mCAs and mutations in candidate drivers occur in a minority of CH cases,12,21 mimicking the genetic interactions of chromosomal alterations and somatic mutations observed in overt myeloid malignancy.26,27
Lymphoid CH: L-CHIP and L-mCA
Lymphoid-CHIP (L-CHIP) refers to CHIP with mutations in genetic drivers of lymphoid malignancies.12 Like myeloid CHIP/CCUS, the prevalence of L-CHIP increases with age though it is less common than its myeloid counterpart. There is a more balanced distribution of genotypes in L-CHIP, with some L-CHIP mutations, such as NOTCH1,12 also recurrently observed in the lymphoid malignancy precursor state, MBL.28 Chromosomal aberrations that drive lymphoid malignancy (lymphoid mCAs [L-mCAs]) are the most commonly observed mCAs. As with M-mCAs, in some cases, L-mCAs reveal biallelic targeting, involving the chromosomal loci of L-CHIP genes.12
CHUD
A substantial fraction of CH lacks detectable mutations or chromosomal alterations affecting known driver genes.5,29,30 CH with unknown drivers (CHUD) may represent outgrowth of hematopoietic clones caused by acquired variants in genes that are yet to be characterized, noncoding or epigenetic alterations, or genetic drift of stem cell populations.31,32 Emergence of CHUD may be observed within the first 2 decades of life, with expansion rates similar to those of CH with known drivers.33 CHUD is also associated with increased risk of hematologic malignancy and greater all-cause mortality though this risk of adverse outcomes in CHUD is likely lower than the risk associated with CH with known molecular drivers.5,29,34 However, dramatic instances of CHUD without malignancy have also been reported.35
Micro-CH
The lower limit for VAF that defines CHIP/CCUS16 is based on sequencing error rates in early studies.4,15,34 Contemporary targeted error–corrected sequencing detects less abundant clones, remarkably demonstrating ubiquity of CH with mutations in DNMT3A and TET2 in adults aged >50 years.36 The term micro-CH has been proposed for these low-abundance clones (VAF < 2%).37 In 1 study investigating the prevalence of 15 different hot spot mutations associated with acute myeloid leukemia (AML), including mutations in IDH1/2, FLT3, and DNMT3A-R882, ultradeep sequencing identified CH with VAF as low as 0.8% in individuals aged < 60 years.6
Contextualizing CH: origins and fitness
CH origins and dynamics
Somatic mutations in HSCs that cause CH can be acquired early in life.38-40 Because mutations steadily accumulate with age, they can be considered as a molecular clock to map the long-term dynamics of both healthy and premalignant HSCs.30,39,41 For most individuals, somatic events do not involve known driver genes.30,39 Sequencing for both driver and passenger mutations has been used to derive Bayesian logistic growth models that reconstruct hematopoietic lineage hierarchies using input from sequencing of serial blood samples33 or individually isolated HSC colonies.39 More recently, Weinstock et al introduced passenger-approximated clonal expansion rate, an approach that uses the acquisition rate of passenger mutations identified through whole genome sequencing to estimate the relative fitness of CH clones using material from a single blood draw.42
CH origins and subsequent dynamics are genotype specific. For instance, decelerated growth is observed for some clones,33,43 and spontaneous attrition of less fit CH clones over one’s life span is probable. Relative to other genotypes of CH, DNMT3A-mutant clones are less likely to display clonal growth.43DNMT3A clones likely have faster clonal outgrowth at younger ages, followed by deceleration in older ages. This decelerated clonal expansion with age is further suggested by the fact that TET2-mutated CH becomes the most prevalent CH driver in older adults.33 Splicing factor–mutated CH emerges at later ages,6,33 with greater growth rate and relative fitness than those of other common CH genotypes.44 CH clones that reach the VAF threshold for CHIP/CCUS (VAF ≥ 2%) are more commonly linked to malignant and nonmalignant outcomes.43 The clinical consequences of micro-CH are less certain but may also be driver gene specific and best estimated based on change in clone size over time.44
Clonal fitness and context-dependent selection
Clone fitness is context dependent. Intrinsic and extrinsic selection pressures influence genotype-dependent clonal expansion or attrition (Figure 2). Chemotherapy and radiation are clear selection pressures with major influence on clonal dominance and ascendency.45-50 Cytotoxic chemotherapy exposure is associated with an increased rate of TP53- and PPM1D-mutant CH, particularly in patients treated with platinum-based chemotherapy and radiation.46,47,49 Deep sequencing before and after chemotherapy shows that CHIP, micro-CH, and mCAs driving therapy-related myeloid malignancies may precede chemotherapy exposure.25,47,48 Importantly, this finding suggests that rather than directly causing CH through DNA damage, chemotherapy exposure, in many cases, drives selection and transformation of pre-existing CH clones.47,51 Clonal outgrowth is faster for TP53, PPM1D, and CHEK2 than for other CH mutations in the presence of chemotherapy and/or radiation.49,51 Although the strength of association is moderate, there is also a clear causal relationship between tobacco exposure and ASXL1-mutated CH suggesting more broadly that toxin-related cellular stresses at least partially contribute to the development and expansion of CH in a genotype-specific manner.52,53
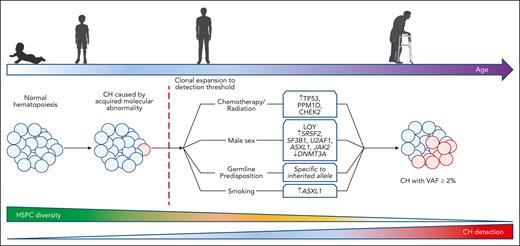
Context-dependent expansion of CH genotypes. Several lines of evidence suggest molecular abnormalities driving CH are acquired early in life. Detection of CH is rare for individuals aged <50 years. The ability to detect CH increases with age, corresponding to a decline in HSPC diversity. Male sex, inherited germ line predisposition, systemic inflammation, chemotherapy/radiation therapy exposure, and smoking each select for distinct CH genotypes.
Context-dependent expansion of CH genotypes. Several lines of evidence suggest molecular abnormalities driving CH are acquired early in life. Detection of CH is rare for individuals aged <50 years. The ability to detect CH increases with age, corresponding to a decline in HSPC diversity. Male sex, inherited germ line predisposition, systemic inflammation, chemotherapy/radiation therapy exposure, and smoking each select for distinct CH genotypes.
Sex differences in peripheral blood counts have been observed in population cohorts,54 and male sex is associated with worse outcomes in myeloid malignancy.55 In CH, mutations in splicing factors and ASXL1 are more common in males with CHIP than in females, whereas DNMT3A mutations are enriched in females.56-58 The biological rationale for these observed sex differences in CH has not been fully resolved, but similar genotype-specific sex biases are observed in myeloid malignancies.
Although germ line and somatic mutations in inflammatory regulators are uncommon in CH and hematologic malignancy, inflammation may directly affect HSC proliferation and renewal and influence CH selection.59 An extreme case is aplastic anemia, in which specific CH genotypes appear to have a competitive advantage in the setting of cytotoxic T-cell–mediated autoreactive HSC destruction.60-63 In murine models, Dnmt3a-mutant HSCs outcompete healthy cells in the context of chronic infection and interferon gamma signaling,64 and Tet2 loss causes myeloproliferation in the presence of gut microbial stimuli.65 In humans, expansion of CH clones may also be a consequence of inflammaging, the age-related chronic sterile low-grade systemic inflammation implicated in the pathogenesis of age-related chronic illnesses.66,67 However, data demonstrating clonal outgrowth as a direct result of inflammation are lacking. Increased prevalence of CHIP has been documented in people with HIV infection68 and autoimmune disease,69,70 but the directionality of these associations is not clear. Although anti-inflammatory compounds may mitigate the risks of some inflammation-associated comorbidity in CHIP,71 it is unclear whether targeting inflammatory signaling pathways will affect clonal fitness.
Germ line predisposition to CH
Inherited bone marrow failure syndromes (IBMFSs) are characterized by ineffective hematopoiesis and an increased risk of MDS/AML. In the absence of phenotypic bone marrow failure, germ line mutations in CEBPA, DDX41, GATA2, RUNX1, and SAMD9/9L are associated with familial predisposition to MDS/AML.72 CH is detected at increased rates in IBMFSs and many familial predisposition syndromes, highlighting a relationship between germ line variants and selective pressure for the acquisition of specific somatic mutations (Figure 2).72 Somatic compensation can lead to CH in IBMFSs, involving the acquisition of molecular abnormalities that augment expression of the unmutated allele or otherwise bypass the deleterious effects of the mutated allele.72 In Schwachman Diamond Syndrome, CH is characterized by recurrent mCA (isocromosome 7q and deletions at 20q) or inactivating mutations in EIF6,73 which functionally correct inherited defects in translational efficiency and aberrant p53 activation. In severe congenital neutropenia, CSF3R-mutant CH is a mechanism of compensatory myeloid hyperproliferation that predicts leukemic transformation.74 CH may also arise as a form of somatic reversion, correcting or removing the germ line molecular defect. This is the most commonly observed cause of CH in telomeropathies, in which in addition to CH caused by skewed X chromosome inactivation, mCAs, and CHUD,75 acquired mutations in the promoter of the TERT allele possessing the inherited germ line variant are observed.76 Somatic compensation has also been observed in Fanconi anemia and in patients with germ line SAMD9/9L (reviewed previously72). Specific patterns of CHIP, such as GATA2 deficiency, may be observed in other rare but high-penetrance inherited alleles.77
Genome-wide association studies have identified a set of common germ line variants that predispose to CH. Both rare and common variants have been identified, with rare variants having higher penetrance for CH and common variants having less penetrance.78 An 8-bp intronic deletion in TERT was associated with a 1.37-fold increase in CH driver mutations, suggesting telomere maintenance may play a role in predisposition to CH.29 More common coding variants in TERT associate with JAK2-mutant myeloproliferative neoplasms but not with JAK2-mutant CH: a discrepancy that may indicate a short or nonexistent CH phase when JAK2 is mutated.79 A genome-wide association study performed to identify germ line variants influencing clonal expansion rates determined by passenger-approximated clonal expansion rate identified a germ line variant in the core promoter of TCL1A associated with slowed growth of TET2-mutant CH and significantly reduced odds of CHIP involving mutations in TET2, ASXL1, SF3B1, and SRSF2 relative to DNMT3A.42 This TCL1A variant is also associated with mosaic loss of chromosome Y.80 Functional studies identified an aberrant expression of TCL1A in CHIP-mutant HSCs that drives clonal expansion.42 This same TCL1A variant was separately associated with a modest increase in DNMT3A-mutant CHIP but not other genotypes,81 consistent with the observation that TCL1A-mediated reduction in fitness for certain HSC clones specifically increases the likelihood of DNMT3A clones.42 Other germ line variants including a frameshift variant in CHEK2 that confers a 2.2-fold increased risk of CHIP78 and an intergenic variant near TET2 observed in people of African ancestry that confers a 2.4-fold increased risk of CHIP have been observed.81 These findings underscore the need to study CH and germ line susceptibility to CH in cohorts of individuals from diverse genetic ancestral backgrounds.
CH-associated malignant outcomes
Hematologic malignancy
Molecular drivers of myeloid CH are common initiators of myeloid malignancy, and CHIP, CCUS, and M-mCA are associated with an increased risk of subsequent myeloid malignancy (Figure 3).4,5,11,29,36 Although the relative risk of myeloid malignancy is increased, the absolute risk remains small, with an estimated rate of malignant transformation from ∼0.5% to 1% per year in CHIP/CCUS.4,82
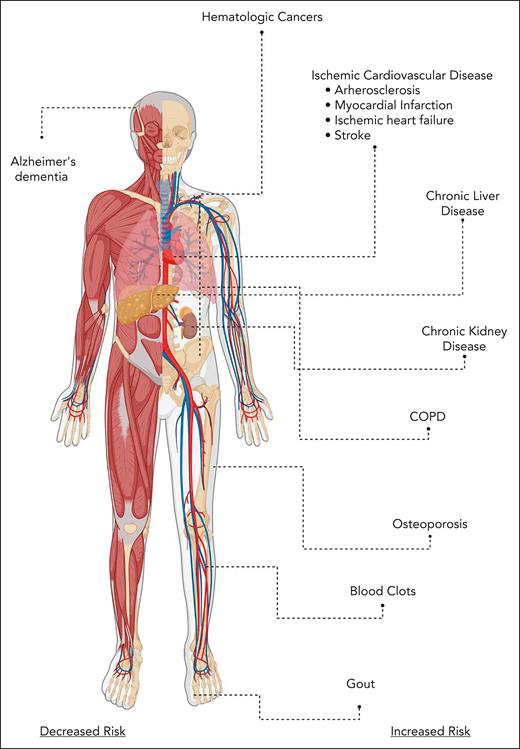
Malignant and nonmalignant consequences of CH. COPD, chronic obstructive pulmonary disease.
Malignant and nonmalignant consequences of CH. COPD, chronic obstructive pulmonary disease.
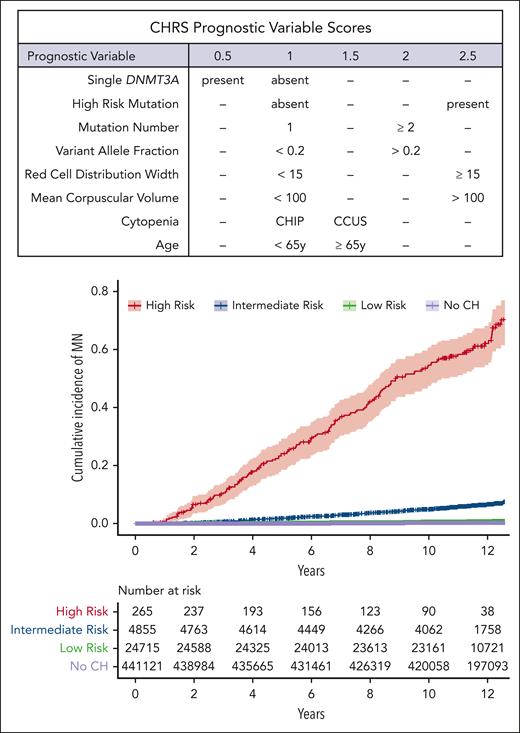
The risk of transformation to myeloid malignancy is determined by the presence of specific molecular and hematologic features. CHIP/CCUS caused by a single mutation in DNMT3A has the lowest risk of progression compared with all other CHIP/CCUS genotypes.83 In contrast, CHIP/CCUS with mutations in splicing factor genes, TP53, IDH1, IDH2, and RUNX1 have the highest risk of evolution to myeloid neoplasia.11,13,49,83 Clone size, as indicated by VAF4,11,12 and clonal composition, including the number of molecular abnormalities,21,83-85 are also prognostic. Large-scale longitudinal analyses of micro-CH with serial sequencing are needed to clarify the significance of low-abundance clones. The clonal hematopoiesis risk score (CHRS) was developed as a clinical tool to estimate the risk of progression to myeloid malignancy in CHIP/CCUS based on the presence of molecular and hematologic features.83 CHRS characterizes CHIP/CCUS into high-, intermediate-, and low-risk groups based on the presence or absence of 8 features: mutations in high-risk genes (splicing factors JAK2, TP53, IDH1, IDH2, RUNX1, or FLT3); single mutation in DNMT3A; VAF ≥20%; presence of ≥2 mutations; mean corpuscular volume ≥100 femtoliters; red cell distribution width ≥ 15%; presence of CCUS vs CHIP; and age ≥ 65 years (Figure 4).83 Features not included in the CHRS may also have predictive value. MN-predict, a recently published risk algorithm, combines serum chemistries and body mass index in addition to molecular features to predict individual risk of specific myeloid neoplasms.86 Additionally, cytogenetic abnormalities may initiate myeloid malignancy, and mCAs independently predict malignant transformation in CHIP/CCUS.21,83 Molecular evaluation of CH that considers mCAs in conjunction with somatic mutations may be beneficial.12,21,83
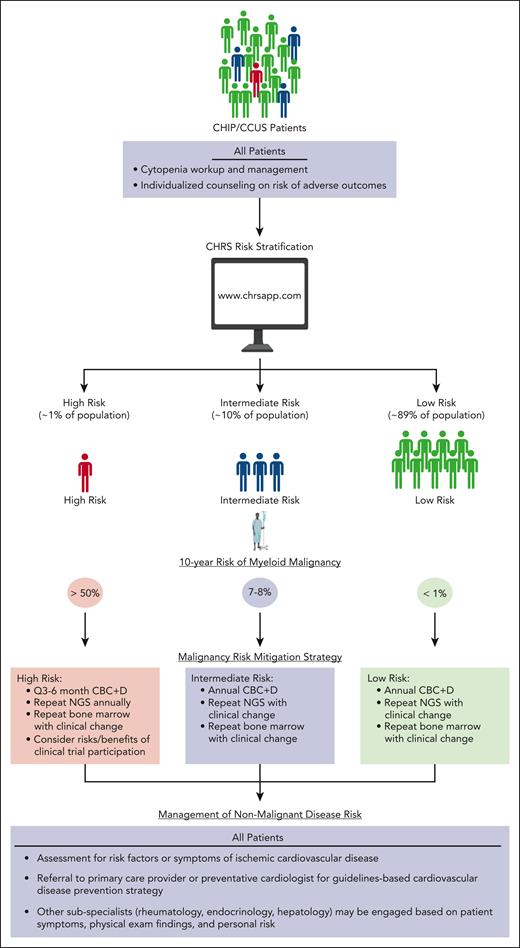
Current management strategies for CH. Patients with CHIP/CCUS are typically identified incidentally as routine screening for CHIP/CCUS is not currently recommended outside of the context of a well-designed clinical research study. We advise the use of the CHRS to risk stratify patients with CHIP/CCUS into high-, intermediate-, and low-risk groups. All patients with cytopenia should be offered a bone marrow biopsy and cytogenetic profile to rule out underlying MDS. Patients at high risk may be followed up every 3 to 6 months depending on cytopenia burden and the rate of clinical change. Bone marrow biopsies and NGS should be repeated for clinical changes that may indicate progression. These patients are most likely to derive benefit from therapeutic intervention clinical trials designed to prevent malignant transformation and, if interested, may be considered for these studies. Less frequent monitoring is indicated for patients at intermediate and low risk. Bone marrow biopsies should not be performed outside of initial workup of cytopenia or to investigate clinical changes that may be indicative of progression. These patients are statistically unlikely to derive benefit from therapeutic clinical trials designed to prevent malignant transformation, and these patients should not be routinely considered as candidates for these studies. All patients may derive benefit from healthy lifestyle modifications such as smoking cessation, reduction of visceral fat burden, and exercise. Patients with CVD risk factors may derive benefit from preventive cardiology evaluation and/or enrollment on clinical trials to prevent CVD outcomes. A complete review of symptoms should be performed on all patients with CCUS to evaluate for systemic immune and autoinflammatory disease, including VEXAS syndrome. CBC + D, complete blood count with differential; NGS, next-generation sequencing.
Current management strategies for CH. Patients with CHIP/CCUS are typically identified incidentally as routine screening for CHIP/CCUS is not currently recommended outside of the context of a well-designed clinical research study. We advise the use of the CHRS to risk stratify patients with CHIP/CCUS into high-, intermediate-, and low-risk groups. All patients with cytopenia should be offered a bone marrow biopsy and cytogenetic profile to rule out underlying MDS. Patients at high risk may be followed up every 3 to 6 months depending on cytopenia burden and the rate of clinical change. Bone marrow biopsies and NGS should be repeated for clinical changes that may indicate progression. These patients are most likely to derive benefit from therapeutic intervention clinical trials designed to prevent malignant transformation and, if interested, may be considered for these studies. Less frequent monitoring is indicated for patients at intermediate and low risk. Bone marrow biopsies should not be performed outside of initial workup of cytopenia or to investigate clinical changes that may be indicative of progression. These patients are statistically unlikely to derive benefit from therapeutic clinical trials designed to prevent malignant transformation, and these patients should not be routinely considered as candidates for these studies. All patients may derive benefit from healthy lifestyle modifications such as smoking cessation, reduction of visceral fat burden, and exercise. Patients with CVD risk factors may derive benefit from preventive cardiology evaluation and/or enrollment on clinical trials to prevent CVD outcomes. A complete review of symptoms should be performed on all patients with CCUS to evaluate for systemic immune and autoinflammatory disease, including VEXAS syndrome. CBC + D, complete blood count with differential; NGS, next-generation sequencing.
Chronic lymphocytic leukemia (CLL) is often initiated by copy number abnormalities, and L-mCAs including trisomy 12, deletion of 13q, and copy-neutral loss of heterozygosity at the NOTCH1 locus are associated with subsequent CLL or small lymphocytic lymphoma.12 Niroula et al also demonstrated a 20.5-fold increase in the risk of CLL for patients with L-CHIP relative to that of controls. The clinical significance of L-CHIP or L-mCA relative to MBL is unclear. Although CHIP/CCUS more commonly precedes myeloid malignancy, lymphoid malignancy is also possible.4,12 That DNMT3A mutations are detectable in multipotent HSCs and lymphocytes87 suggests certain CH genotypes occurring in common hematopoietic progenitors could drive myeloid or lymphoid malignancy outcomes.
CH and HSCT
In hematopoietic stem cell transplantation (HSCT), CH detected in the donor or recipient can influence outcomes. Patients with lymphoma and myeloma with CHIP at the time of autologous transplantation had inferior survival and increased risk of therapy-related myeloid malignancy.88,89 In keeping with this, an investigation of 81 individuals undergoing autologous stem cell transplantation noted that in most individuals, CHIP mutations detected after transplantation were present in pretransplant specimen, albeit as less abundant micro-CH clones. Preferential expansion of clones with CHIP-causing mutations in this milieu may suggest a reconstitution advantage for mutant HSCs.90
In allogeneic HSCT, donor-derived CHIP engrafts in recipients,91 but the consequences are genotype dependent.92 An increased risk of donor-derived leukemias is observed for TP53-mutant or splicing factor gene–mutant donor CH.93 The reduced risk of relapse and higher rates of graft-versus-host disease observed for DNMT3A-mutant donor CH suggests increased graft-versus-leukemia effect driven by DNMT3A-mutant donor–derived T cells.93,94
CH-associated nonmalignant outcomes
iCVD
CHIP/CCUS is causally linked to ischemic cardiovascular disease (iCVD), with the strongest associations observed for TET2- and JAK2-mutant CHIP/CCUS.14 Animal studies clarified a causal role of CH in iCVD outcomes, demonstrating a clear acceleration of atherosclerotic plaque formation in mice that underwent transplantation with bone marrow with heterozygous or homozygous deficiency in Tet2.14,95 These experiments also revealed inflammasome activation in Tet2-deficient mice, including increased expression of interleukin-1β (IL-1β), IL-6, and other proinflammatory molecules.95 Data from human experiments link IL-6 receptor gene expression to reduced levels of the inflammatory marker, C-reactive protein, and decreased coronary artery disease.96 Consistent with this finding, exploratory analysis of the canakinumab anti-inflammatory thrombosis outcomes study showed that the IL-1β inhibitor, canakinumab, reduced cardiovascular events among individuals with TET2-mutant CH.71 In murine97 and human data,98 activation of the absent in melanoma (AIM2) inflammasome is noted in JAK2 V617F macrophages. Together, these data point to anti-inflammatory compounds, particularly inflammasome inhibitors, as potential strategies to mitigate iCVD risk in CH. Among the other subtypes of CH, iCVD is also associated with the loss of chromosome Y99,100 but not autosomal mCAs24 or L-CHIP.12
The strength of the association between CHIP/CCUS and iCVD varies depending on the cohort examined. In cohorts enriched for individuals with CVD risk factors, CHIP/CCUS is associated up to approximately twofold increased risk of coronary artery disease, myocardial infarction, and ischemic stroke,14 a similar level of risk conferred by smoking, hyperlipidemia, and diabetes.14,95 In the UK Biobank healthier cohort, the association with iCVD was mild101 or absent58,78 when CHIP/CCUS genotypes were evaluated as a composite, and stronger associations were observed when JAK2- and TET2-mutant CHIP/CCUS were analyzed separately.101 Individuals in the UK Biobank with established atherosclerotic CVD and CHIP/CCUS were also noted to have a higher rate of secondary ischemic events,102 consistent with data reporting enhanced mortality in ischemic heart failure in patients with CHIP.103 Clone size, a strong predictor of myeloid malignancy risk, is also prognostic of iCVD outcomes in CHIP/CCUS, such that individuals with VAF ≥ 10% had a greater likelihood of developing incident iCVD than those with smaller clones.14
Other age-related inflammatory diseases
Other age-related inflammatory diseases have been associated with CHIP, including chronic liver disease,104 chronic kidney disease (CKD),105-107 gout,108 chronic obstructive pulmonary disease,109 and osteoporosis (Figure 3).110 Of these associations, the most robust observation is that CHIP with a VAF ≥ 10% doubles the risk of chronic liver disease, nonalcoholic hepatic steatosis, and cirrhosis.104 This is consistent with the observation of a higher prevalence of nonalcoholic hepatic steatosis among individuals who have MDS/CMML than in controls without hematologic malignancy.111 Like iCVD, the association between chronic liver disease and CHIP is powerfully genotype dependent, with a 17.6-fold increase in chronic liver disease for JAK2-mutant CH, a 5.4-fold increase in chronic liver disease with TET2-mutant CH, and a low risk for DNMT3A mutations. Causality was inferred by Mendelian randomization in human population cohorts and in Tet2-deficient mice, which had increased liver fat accumulation and enrichment of transcriptional programs associated with steatohepatitis and liver fibrosis.104
Proinflammatory cytokine signaling contributes to several other observed phenotypes in CHIP. A higher incidence of gout in CHIP/CCUS is driven by the exaggerated IL-1β signaling in response to urate crystal exposure in Tet2-deficient compared with in Tet2-proficient animals.108 In CKD, CHIP is also a potential biomarker for worse outcomes in humans105,107 because individuals with known CKD have a doubled risk of end-stage CKD at 5 years.105 The osteoclastogenesis and accelerated bone loss observed in Dnmt3a-deficient mouse models is also linked to proinflammatory cytokines, offering a potential mechanism for the association between osteoporosis and CHIP in humans.110 Other models have demonstrated exacerbated age- and obesity-associated insulin resistance with parallel enhancement of proinflammatory signaling in Tet2-deficient mice, suggesting that CHIP increases the risk of type 2 diabetes mellitus (T2DM).112 However, despite a modest association between CHIP and T2DM in the initial report of adverse outcomes for CHIP,4 causality has been difficult to establish for this association because T2DM diagnosis often precedes sample collection in sequenced CH cohorts.
Autoimmune diseases and chronic infections
There is growing evidence pointing to a shared pathophysiology for hematologic malignancy and diseases of immune dysregulation. In addition to aberrant inflammatory signaling in CH, CHIP is detected in ∼30% of individuals with antineutrophil cytoplasmic antibody–associated vasculitis.70 Additionally, overt myeloid malignancy risk is higher in people with autoimmune diseases113 and autoinflammatory phenotypes are common in certain genotypes of myeloid malignancy.114,115 However, although rheumatoid arthritis is prevalent in myeloid malignancy,111 no observed differences in the gene distribution or rate of CHIP has been observed.116 More recently, VEXAS (vacuoles, E1 enzyme, X-linked, autoimmune, somatic) syndrome was described as a clonal hematopoietic disorder driven by mutations in UBA1 and characterized by multisystem, adult-onset autoinflammatory disease and MDS in 24% to 50% of cases.117,118 Mutations in UBA1 can emerge independently or from existing CH with typical CHIP/CCUS mutations.119 AML transformation is rare in VEXAS syndrome, and treatment goals are centered around prevention of morbidity and mortality related to systemic inflammation.
In some cases, lymphoid CH may drive autoimmune disease through the hyperinflammatory activity of terminally differentiated T or B cells with CH mutations. Sequencing of CD8+ T-cell isolates from patients with multiple sclerosis demonstrated mutations recurrent in L-CHIP12 and in STAT3, a known driver of large granular lymphocytosis.120 L-mCAs carry an increased risk of incident infection,121 reflecting immune dysfunction, which is also observed in MBL122 and CLL.123 Chronic or severe infections may be associated with other forms of CH including CHIP, although these associations are less clear.68,124
Alzheimer dementia
Epidemiologic data demonstrate that unlike cardiovascular and metabolic diseases, neurodegenerative diseases are less prevalent among individuals with myeloid malignancy than among controls.111 Consistent with this finding, recent data demonstrate a reduced risk of Alzheimer dementia in CHIP.125 This association could be due to altered phagocytic activity of bone marrow–derived microglial-like cells, which are enriched in the brains of individuals with CHIP compared with those in controls.
CH clinics: current approaches and priorities for clinical research
Several cancer centers have established “CHIP clinics” to counsel and monitor patients with CH.126,127 Patients in these clinics are primarily being identified incidentally when CH is detected on next-generation sequencing ordered to work up cytopenia, evaluate solid tumors, diagnose germ line cancer predisposition syndromes, or investigate suspected donor cell leukemia after HSCT (Figure 5). Clinical management in CH involves work up and monitoring of any blood count abnormalities and counseling patients at risk of CH-associated adverse outcomes. For CHIP/CCUS in particular, the CHRS estimates myeloid malignancy risk83 and may be used to guide risk-specific management. Patients with high-risk CHIP/CCUS may be monitored more frequently and considered for inclusion in clinical trials focused on preventing myeloid malignancy, whereas frequent monitoring and investigational therapeutic intervention is inappropriate for patients at low risk. Risk-stratification tools are needed for other CH subtypes.
CHRS: a tool to predict risk of myeloid malignancy in CHIP/CCUS. (Top) Table of prognostic features and corresponding scores assigned in CHRS, and (bottom) cumulative incidence of hematologic malignancy among 470 960 participants in the UK Biobank stratified based on CHRS status. Top panel: From Weeks et al. Prediction of Risk for Myeloid Malignancy in Clonal Hematopoiesis. New England Journal of Medicine Evidence. 2023;2(5). Copyright ©(2023) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.83
CHRS: a tool to predict risk of myeloid malignancy in CHIP/CCUS. (Top) Table of prognostic features and corresponding scores assigned in CHRS, and (bottom) cumulative incidence of hematologic malignancy among 470 960 participants in the UK Biobank stratified based on CHRS status. Top panel: From Weeks et al. Prediction of Risk for Myeloid Malignancy in Clonal Hematopoiesis. New England Journal of Medicine Evidence. 2023;2(5). Copyright ©(2023) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.83
Collaboration between hematologists and other subspecialists (eg, cardiologists or rheumatologists) may be helpful, given the risk of nonmalignant comorbidity accompanying various CH subtypes. In CHIP/CCUS, comorbidity risk correlates with CHRS risk groups suggesting that CHRS may also help guide risk-specific recommendations for nonmalignant comorbidity risk mitigation.83 Age-appropriate screening, prevention, and management strategies for nonmalignant comorbidities are likely to provide universal benefit and should be used in patients with CH. Ongoing research efforts will determine whether additional benefit is derived from CH-specific lifestyle modifications128 or therapies.71 For patients with CH with solid tumors, there may soon be sufficient data to guide selection or timing of potentially genotoxic therapies and mitigate the risk of subsequent therapy-related MDS/AML (t-MDS/AML). Prospective randomized investigations of the differential risk of t-MDS/AML in CH patients with solid tumors treated with standard vs alternative therapies are needed.
Conclusions
Groundbreaking discoveries in CH have transformed our understanding of hematopoietic aging, leukemogenesis, and the role of bone marrow–derived immune cells in the etiology of age-related inflammatory disease. We now recognize CH as a collection of distinct subtypes, each with a risk of adverse outcomes that depends on genotype and clinical context. The accelerated pace of CH research is made possible by the availability of large genomic data sets, openly shared with long-term follow-up and detailed clinical annotation. In parallel, experimental model systems have allowed for basic understanding of cellular dependencies that etiologically link CH to adverse malignant and nonmalignant outcomes. Increasing clinical indications for next-generation sequencing has led to an exploding population of patients with incidentally diagnosed CH. The field is primed for rigorous prospective clinical investigations to validate and refine risk stratification in diverse clinical contexts and identify therapeutic interventions that counter the risk of malignant and nonmalignant outcomes in CH.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01HL082945, P01CA066996, P50CA206963, and R35CA253125), Adelson Medical Research Foundation, Howard Hughes Medical Institute, and Fondation Leducq award (B.L.E.) and from the Edward P Evans foundation for MDS, the American Society of Hematology/Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program, and the Wood Foundation (L.D.W.).
Authorship
Contribution: L.D.W. and B.L.E. conceived the study design and wrote and approved the manuscript; and L.D.W. designed the figures.
Conflict-of-interest disclosure: B.L.E. has received research funding from Celgene, Deerfield, and Novartis and consulting fees from GRAIL; and serves on the scientific advisory boards for Skyhawk Therapeutics, Exo Therapeutics, Neomorph, and TenSixteen Bio, all unrelated to this work. L.D.W. has received consulting fees from AbbVie, Vertex, and Sobi, all unrelated to this work.
Correspondence: Benjamin L. Ebert, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, D1610A Boston, MA 02215; email: benjamin_ebert@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal