Abstract
With aging, hematopoietic stem cells (HSCs) have an impaired ability to regenerate, differentiate, and produce an entire repertoire of mature blood and immune cells. Owing to dysfunctional hematopoiesis, the incidence of hematologic malignancies increases among elderly individuals. Here, we provide an update on HSC-intrinsic and -extrinsic factors and processes that were recently discovered to contribute to the functional decline of HSCs during aging. In addition, we discuss the targets and timing of intervention approaches to maintain HSC function during aging and the extent to which these same targets may prevent or delay transformation to hematologic malignancies.
Introduction
Aging is a major risk factor for the development of hematologic malignancies. In 2020, blood cancers were diagnosed worldwide in more than 600 000 adults aged ≥65.1 Although not fully understood, the relationship between aging and the development of blood cancers is partly a consequence of functional decline of the hematopoietic system. Because hematopoietic stem cells (HSCs) are primarily responsible for sustaining the production of all hematopoietic and immune cells throughout life, understanding how aging affects HSCs is necessary to understand how and why hematopoietic function declines with increase in age. Much effort has focused on the comprehensive identification of the hallmarks of HSC aging, including transcriptional and epigenetic changes, altered inflammatory cytokine signaling, myeloid bias, impaired autophagy, mitochondrial dysfunction, and impaired regenerative capacity.2 These HSC aging phenotypes overlap mechanistically with the processes that cause transformation and hematologic malignancy. Whether interventions to prevent HSC aging or rejuvenate the functionality of aged HSCs can effectively prevent or delay the development of leukemia remaina a long-standing question. Here, we provide an updated synthesis of recent discoveries and a perspective on intervention opportunities.
HSC function in young vs middle-aged vs elderly adults
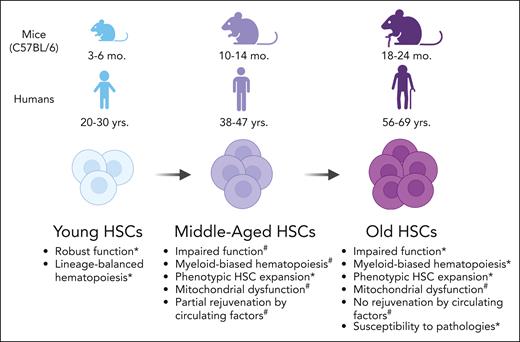
Extensive literature describes the phenotypic and functional differences between cell surface marker–defined HSCs isolated from young mice (age, 3-6 months) and old mice (age, 18-24 months), typically studied in mice of a C57BL/6 inbred strain background. These studies reported that old HSCs have impaired hematopoiesis,3 myeloid bias at the transcriptome and functional levels,4 dysfunctional mitochondria,5 decreased polarity of Cdc42,6 increased reactive oxygen species (ROSs),7 increased γH2.AX caused by ineffective H2.AX dephosphorylation rather than sustained DNA damage,8 altered DNA methylation,9 changes in histone modification patterns10 (which are also observed in aged human hematopoietic stem and progenitor cells [HSPCs]),11 and increased transformation to leukemia.2 An important nuance revealed by single-cell transplantation assays and inducible lineage tracing systems is that old mice contain a greater number of functional HSCs compared with young mice, although on a per HSC basis, they have reduced mature hematopoietic cell output.12,13 Recently, integrative analysis of transcriptome and chromatin accessibility of old HSCs revealed selective accessibility of enhancers with stress-responsive transcription factor motifs, suggesting chronic or historical exposure to external stress may be inscribed at an epigenetic level in old HSCs.14
Whether the aforementioned numerous alterations coincide or occur in a particular order or sequence over time and age is unknown because longitudinal studies collecting phenotypic data on a cohort of animals, from young adulthood into old age, have yet to be reported. We can glean insights from cross-sectional studies, including the midage point(s) between young and old animals. Many phenotypes considered to be hallmarks of aged hematopoiesis are observed in C57BL/6 mice at 9 or12 months of age,15 estimated to be equivalent to ∼37 to 47 years for humans16 (Figure 1). These phenotypes include increased frequency and number of cell surface marker–defined HSCs and myeloid bias in the HSC compartment and have been described in both mice and humans.15,17 These observations are consistent with the mathematical modeling of hematopoietic aging within C57BL/6 mice from young age (10-16 weeks old) to middle age (40-54 weeks old).18 This approach found a rapid decrease in the ratio of short-term HSCs to long-term HSCs during middle age that remained constant into old age (>86 weeks old),18 suggesting impairment in the first steps of differentiation from HSCs to their progeny approximately at middle age. A stress signaling pathway mediated by p38MAPK, known to be activated in old mouse HSCs,7 was also recently found to be more active in middle-aged HSCs than in younger HSCs.19 In humans, HSCs isolated from middle-aged individuals (42-61 years old) have increased expression of aging-associated myeloid lineage genes such as P-selectin (SELP) and HOXA9 compared with that in young individuals.17 Although there are many molecular similarities between middle-aged HSCs and old HSCs, transplantation experiments using mouse models have revealed that middle-aged HSCs, but not old HSCs, can be functionally rejuvenated by a young bone marrow (BM) microenvironment.20 This work suggests that there may be a therapeutic window of opportunity at or before middle age to effectively intervene to prevent functional HSC decline, which is discussed in detail later in this review.
Phenotypic alterations in adult HSCs with increasing age in C57BL/6 mice and humans. ∗Supporting studies in mice and humans. #Supporting studies in mice. Illustration created using BioRender.com.
Phenotypic alterations in adult HSCs with increasing age in C57BL/6 mice and humans. ∗Supporting studies in mice and humans. #Supporting studies in mice. Illustration created using BioRender.com.
Aging-associated CH: friend or foe?
Age-associated clonal hematopoiesis (CH) is a natural consequence of somatic mutations in the human hematopoietic stem and/or progenitor cells. A subset of acquired somatic mutations can confer a selective advantage to HSCs and their progeny such that they clonally expand and become overrepresented within the total pool of hematopoietic cells. The most common mutations found in human CH are the epigenetic regulatory genes DNA methyltransferase 3a (DNMT3A), Tet methylcytosine dioxygenase 2 (TET2), and additional sex combs like 1 (ASXL1).21,22 Although CH is not a disease, it is associated with a modestly enhanced risk of hematologic malignancy.21 Using genetically engineered mouse models, the functions of DNMT3A, TET2, and ASXL1 in HSCs and hematopoiesis have been well described.23-25 However, the manner and reason for human hematopoietic cells with these mutations to undergo positive selection during aging, leading to clonal expansion and leukemic transformation remains to be determined. Some hints have emerged regarding the relevant mechanisms from related studies, using stress and inflammation as selective pressures.
Allogeneic HSC transplantation is a context in which, the impact of stress on CH can be directly studied in humans by comparing donors and recipients. Recent reports have found a larger clone size in transplantation recipients with donor-engrafted CH than that in donors.26,27 Similarly, in C57BL/6 mice, mutations in recurrent human CH genes were detectable only after transplantation.28 Together, these data support that transplantation stress contributes to positive clonal selection/expansion. Importantly, the latter study suggests that the lifetime of healthy laboratory mice is not sufficient to allow most clones with mutations in human CH-relevant genes to expand to a level detectable via the current methodology.28 Although this may preclude modeling of human CH in aging wild-type C57BL/6 mice, it does support the study of aging mice with genetically engineered human CH mutations to reveal mechanisms relevant to humans.
Studies using Mycobacterium avium infection of genetically engineered Dnmt3a-mutant mouse models have found that interferon gamma–mediated inflammation promotes expansion of Dnmt3a-mutant HSCs.29 Tumor necrosis factor α (TNF-α) also promotes the selective advantage of mouse Dnmt3a-mutant HSCs, and specific targeting of TNF receptor 1 but not TNF receptor 2 selectively impaired Dnmt3a-mutant HSC fitness.30 TNF-α has also been found to favor mouse Tet2-mutant hematopoiesis.31 Not only do CH-mutant HSCs have differential responses to proinflammatory cytokine signaling, new work also suggests that CH-mutant hematopoietic cells can induce and maintain a proinflammatory state. In a zebrafish model of a human ASXL1 mutation, mature mutant myeloid cells produce proinflammatory cytokines, whereas mutant HSPCs remain resistant to this inflammatory environment via the expression of immunomodulatory genes.32 Inflammation also affects the transformation of CH to hematologic malignancy. In Tet2-mutant mice, activation of inflammatory signaling is an essential trigger of chronic myelomonocytic leukemia–like disease.31 Together, literature primarily using mouse models supports the concept that inflammatory processes provide selective pressure favoring survival of HSPCs with CH mutations, leading to clonal expansion and contributing to transformation to hematologic malignancies. This will need to be critically evaluated in future human studies. Because chronic low-grade elevation of circulating proinflammatory cytokines has been widely reported during aging,33 this is a potential mechanistic link between aging, CH, and hematologic malignancy.
The risks and benefits of age-associated CH continue to be explored. CH has been associated with an increased risk of nonhematologic diseases, such as atherosclerosis34 and osteoporosis.35 In contrast, evidence supports that specific CH mutations may enhance adaptive immune cell functionality. For example, improved overall survival and reduced risk of relapse after BM transplantation were reported in transplantation recipients of DNMT3A-mutant donor hematopoietic cells,36 which could be related to the enhanced function of DNMT3A-mutant T lymphoid cells discovered in mouse models.37 Dissecting mechanisms by which CH can have a protective benefit in the context of certain diseases yet accelerate or positively contribute to the development of others, particularly within the hematopoietic system, will be critical in considering appropriate intervention strategies.
Update on HSC-intrinsic mechanisms causing hematopoietic aging and initiation of hematologic malignancies
Here, we provide an update on recent literature and emerging genes and mechanisms responsible for HSC aging and hematologic malignancy initiation (Table 1).
Genes and processes recently revealed to have roles in both HSC aging and transformation
| Gene . | Expression in old vs young HSCs . | HSC phenotype . | Hematologic malignancy phenotype . | Function . |
|---|---|---|---|---|
| Selp | HSC aging (RNA and protein38) | Knockout increases hematopoietic regeneration39 | Knockout accelerates leukemogenesis in CML39,40 | Cell adhesion molecule produced by platelets and endothelial cells for leukocyte adhesion38 |
| Nupr1 | HSC aging (RNA38) | Knockout increases HSC quiescence and engraftment potency41 | Targeted inhibition reduces the growth of AML, T-cell ALL, lymphoma, and multiple myeloma cell lines42,43 | High-mobility group protein family member involved in apoptosis, stress response, and cancer progression41 |
| Sema4a | HSC aging (RNA38) | Knockout increases myeloid-biased HSC proliferation and impairs regenerative capacity44 | Knockout impairs multiple myeloma cell growth45 | Binds to surface receptor Plexin-D1, essential for HSC self-renewal and protection from stress44 |
| Cited2 | HSC aging (RNA38) | Knockout depletes functional HSCs in young mice46 | Knockdown decreases AML pathogenesis and induces apoptosis47 | Binding partner of the acetyltransferase CBP/p300 in transcriptional regulation46 |
| Hsf1 | HSC aging (protein48) | Dispensable in young HSCs; knockout impairs hematopoietic regeneration by middle age48 | Knockout impairs initiation and maintenance of AML and T-cell ALL49,50 | Maintains proteostasis and self-renewal in response to stress48 |
| Igf2bp2 | HSC aging (RNA51) | Knockout impairs HSC function in young mice51 | Knockdown inhibits the growth of AML cell lines52 | RNA-binding protein that regulates messenger RNA stability and translation51 |
| Egr1 | HSC aging (RNA53) | Knockout increases HSC cycling and mobilization54 | Tumor suppressor in ALL, CML, and AML55 | Transcription factor that regulates cell growth, differentiation, and depolarization55 |
| Twist1 | HSC aging (RNA56) | Knockout reduces HSC self-renewal and causes myeloid-biased hematopoiesis56 | Knockdown reduces AML cell line proliferation and sensitizes to decitabine57 | Transcription factor essential for embryonic mesoderm development56 |
| Gata2 | No change (RNA38) | Haploinsufficiency promotes HSC proliferation and functional decline58 | Haploinsufficiency causes long latency but aggressive MDS and AML59 | Essential transcription factor for fetal and adult hematopoiesis60 |
| Gene . | Expression in old vs young HSCs . | HSC phenotype . | Hematologic malignancy phenotype . | Function . |
|---|---|---|---|---|
| Selp | HSC aging (RNA and protein38) | Knockout increases hematopoietic regeneration39 | Knockout accelerates leukemogenesis in CML39,40 | Cell adhesion molecule produced by platelets and endothelial cells for leukocyte adhesion38 |
| Nupr1 | HSC aging (RNA38) | Knockout increases HSC quiescence and engraftment potency41 | Targeted inhibition reduces the growth of AML, T-cell ALL, lymphoma, and multiple myeloma cell lines42,43 | High-mobility group protein family member involved in apoptosis, stress response, and cancer progression41 |
| Sema4a | HSC aging (RNA38) | Knockout increases myeloid-biased HSC proliferation and impairs regenerative capacity44 | Knockout impairs multiple myeloma cell growth45 | Binds to surface receptor Plexin-D1, essential for HSC self-renewal and protection from stress44 |
| Cited2 | HSC aging (RNA38) | Knockout depletes functional HSCs in young mice46 | Knockdown decreases AML pathogenesis and induces apoptosis47 | Binding partner of the acetyltransferase CBP/p300 in transcriptional regulation46 |
| Hsf1 | HSC aging (protein48) | Dispensable in young HSCs; knockout impairs hematopoietic regeneration by middle age48 | Knockout impairs initiation and maintenance of AML and T-cell ALL49,50 | Maintains proteostasis and self-renewal in response to stress48 |
| Igf2bp2 | HSC aging (RNA51) | Knockout impairs HSC function in young mice51 | Knockdown inhibits the growth of AML cell lines52 | RNA-binding protein that regulates messenger RNA stability and translation51 |
| Egr1 | HSC aging (RNA53) | Knockout increases HSC cycling and mobilization54 | Tumor suppressor in ALL, CML, and AML55 | Transcription factor that regulates cell growth, differentiation, and depolarization55 |
| Twist1 | HSC aging (RNA56) | Knockout reduces HSC self-renewal and causes myeloid-biased hematopoiesis56 | Knockdown reduces AML cell line proliferation and sensitizes to decitabine57 | Transcription factor essential for embryonic mesoderm development56 |
| Gata2 | No change (RNA38) | Haploinsufficiency promotes HSC proliferation and functional decline58 | Haploinsufficiency causes long latency but aggressive MDS and AML59 | Essential transcription factor for fetal and adult hematopoiesis60 |
An integrative analysis of 16 distinct transcriptomics studies of murine HSC aging from different research groups worldwide has provided a unified perspective on aged HSCs and a centralized resource for the field.38 This work has identified altered expression of genes in physiological HSC aging, including Selp, nuclear protein 1, Semaphorin 4a (Sema4a), and Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain 2 (Cited2). Selp expression at the transcriptional and protein levels in old HSCs has been found to contribute to myeloid-biased hematopoiesis and HSC activation phenotypes.38 In a BCR/ABL-induced chronic myelogenous leukemia (CML) model, knockout of Selp accelerated leukemogenesis, increased cell surface marker–defined leukemia stem cells, and altered adhesion of myeloid progenitors to the marrow stroma.39,40 Taken together, more studies are needed to fully elucidate the contribution of SELPhi vs SELPlo old HSCs to leukemogenesis and to determine whether fine-tuning levels of Selp would facilitate the rejuvenation of old HSCs without increasing susceptibility to hematologic malignancies.
Nupr1, encoding a stress response factor, is elevated at the transcript level in old HSCs. In young mice, conditional knockout of Nupr1 caused HSCs to exit quiescence and conferred a competitive repopulation advantage.41 In hematologic malignancy, Nupr1 is significantly higher in multiple myeloid cell lines and primary multiple meyloid BM samples than in healthy BM, and short hairpin RNA knockdown of Nupr1 resulted in reduced proliferation, induction of apoptosis, and arrest of cell cycle.42 A modified small molecule capable of targeting NUPR1 has shown dose-dependent growth inhibition of multiple cancer cell lines, including acute myeloid leukemia (AML) (THP-1), lymphoma (Daudi), and acute T-cell leukemia (Jurkat).43 Further investigation is needed to determine whether Nupr1 is an effective target to both deplete dysfunctional old HSCs and inhibit hematologic malignancy.
Sema4a is elevated at the transcript level in old HSCs. In a recent nonpeer-reviewed preprint, knockout of Sema4a resulted in proliferation and expansion of myeloid-biased HSCs at the expense of their regenerative capacity.44 Surprisingly, this was noncell-autonomous because the transplantation of wild-type myeloid-biased HSCs into Sema4a-deficient recipient mice also resulted in excessive proliferation and engraftment failure in the long term. In hematologic malignancy, cell surface expression of SEMA4A was found to be essential for myeloma cell growth, and a potent antibody-drug conjugate has been engineered for therapeutic targeting of myeloma based on SEMA4A expression.45 Further studies will be critical to define Sema4a-cell context dependencies for aging and malignancies, both with hematopoietic and nonhematopoietic cell fractions of the BM, to assess the potential therapeutic index for SEMA4A targeting.
Levels of Cited2, encoding a binding partner of the acetyltransferase CBP/p300, are also elevated in old HSCs at the transcript level. Knockout of Cited2 in young mice reduced HSCs but had no effect on steady-state hematopoiesis.46 Mice survived normally, and the HSC pool failed to expand upon aging. In contrast, Cited2 is necessary for the functional regenerative capacity after HSC transplantation. In hematologic malignancies, RNA interference–mediated knockdown of Cited2 in AML cell lines decreased their pathogenicities in vivo and increased apoptosis.47 Taken together, targeting Cited2 in steady-state hematopoiesis may prevent the expansion of phenotypic HSCs with aging and achieve selective targeting of AML.
Heat shock factor 1 (Hsf1) levels increased in old HSCs at the protein level, maintain proteostasis and are critical for HSC regenerative capacity.48 A similar function for Hsf1 has been recently reported in AML. Deletion of Hsf1 reduced the initiation and maintenance of murine MLL-AF9–induced AML, and knockdown or small molecule inhibition of HSF1 reduced the growth of primary human AML cells.49 Because nuclear HSF1 expression is increased in human AML samples relative to that in healthy BM controls and is correlated with disease status, it has been suggested to be used as a biomarker as well as a therapeutic target.49 This is consistent with elevated Hsf1 in T-cell acute lymphoblastic leukemia (ALL), in which Hsf1 ablation was found to suppress the growth of both mouse and human T-cell ALL cells.50 A multitude of small molecules and naturally derived compounds to inhibit HSF1 have been developed, but most show off-target effects, precluding further clinical testing.61 Additional studies are needed to determine whether HSC rejuvenation strategies can reduce the dependence of old HSCs on Hsf1, or whether a sufficient therapeutic window exists between the reliance of malignant and healthy, old HSCs on Hsf1, enabling this to be a targetable factor in the treatment of hematologic malignancies.
In old HSCs, Igf2bp2 has been found to be decreased in expression at the transcript level.51Igf2bp2 encodes an RNA-binding protein that regulates messenger RNA stability and translation. The role of Igf2bp2 in HSC aging is multifaceted. In younger individuals, Igf2bp2 contributes to aging-associated HSC expansion and myeloid lineage bias, whereas age-related loss of Igf2bp2 impairs the growth and repopulation capacity of old HSCs.51Igf2bp2-deficient mice did not exhibit aging-associated phenotypes, such as increase in phenotypic HSCs or myeloid-biased hematopoiesis. Notably, IGF2BP2 is found to be increased in expression in adult AML, and such increased expression is associated with poor prognosis.52 Together, despite a natural decline in Igf2bp2 levels in old HSCs, targeting this molecule could achieve beneficial effects with respect to reducing old HSC attributes and their transformation into hematologic malignancies.
The transcriptional factors early growth response factor 1 (Egr1), Twist-related protein 1 (Twist1), and Gata binding protein 2 (Gata2) have also recently been implicated, or reimplicated, in HSC aging and transformation. Egr1, an immediate-early response transcription factor, is increased in expression in old HSCs.53 Mice lacking Egr1 have expansion in steady-state levels of cycling HSCs and spontaneous mobilization.54 In contrast, in a variety of studies conducted for human AML, chronic lymphocytic leukemia, and ALL cell lines, EGR1 has been identified as a tumor suppressor.55Twist1, an essential regulator of mesoderm development, is increased in old HSCs, at the transcript level. Unexpectedly, Twist1 knockout mice have been reported exhibit aging-associated phenotypes of reduced HSC self-renewal and myeloid-biased hematopoiesis.56 In AML cell lines, knockdown of TWIST1 was found to reduce proliferation and increase sensitivity to the hypomethylating agent decitabine.57Gata2, known to be essential for proliferation, maintenance, and function of HSCs, is not reported to be altered in transcript expression with aging. In hematologic malignancy, overexpression of GATA2 is observed in myelodysplastic syndrome (MDS) and AML.60 In addition, gain-of-function mutations in GATA2 are correlated with poor prognosis in CML.59 In contrast, individuals with germ line or acquired GATA2 haploinsufficiency also have a high propensity to develop MDS or AML, typically preceded by immunodeficiency. A recent report provides evidence that Gata2 haploinsufficiency in mice exacerbates the decline in HSC function and decreases lymphoid progenitor cell production during aging,58 providing a mechanistic link between aging and propensity for the transformation. In an independent study, Gata2 haploinsufficiency was found to delay leukemia onset in a mouse model of human inv(16) AML (Cbfb-MYH11 knock in) but paradoxically resulted in a more aggressive leukemia phenotype.62 Taken together, these data suggests that Egr1, Twist1, and Gata2 may not be ideal targets for effective intervention strategies based on their complex roles.
Update on HSC-extrinsic mechanisms causing hematopoietic aging and leukemia initiation
Numerous changes have been described in aging BM microenvironments, including vascular remodeling, BM mesenchymal stromal cell dysfunction, altered adrenergic signaling, proinflammatory cytokine production, and frequent senescence.63 The aging BM microenvironment can induce HSC aging phenotypes and contribute to the pathogenesis of hematologic malignancies. Moreover, leukemia cells can remodel the BM microenvironment and accelerate aging phenotypes in a feed-forward loop. Here, we provide an update on the recent literature in this area.
Acute and chronic inflammation
Inflammation influences both HSCs and the BM microenvironment, and increasing evidence suggests that this can permanently change the HSC pool. Young adult mice exposed to acute inflammatory challenges show accelerated HSC aging and impaired hematopoietic regeneration capacity.64 Increased levels of interleukin 1β (IL-1β) produced by old and damaged endosteum65 reduces HSC self-renewal66 and increases myeloid cell proliferation.67 Pharmacologic inhibition of IL-1 mitigates myeloid-biased hematopoietic output from HSCs,67 reverses deterioration of the aging BM microenvironment, and restores HSC regenerative function.65 In contrast, pharmacologic inhibition of IL-6 increases the frequency and functionality of aging erythroid progenitor cell populations rather than directly affecting aging HSCs.68 This observation highlights that pharmacologic strategies to broadly ameliorate hematopoietic aging phenotypes may require an expanded viewpoint to replenish critical factors for both HSC and progenitor cell function.
Chronic inflammation has been shown to promote the growth of mutant HSC clones, leading to leukemic transformation. Proinflammatory IL-6 signaling drives progression of MDS to AML, and the knockout of IL-6 was sufficient to extend survival in murine models.69 Increased IL-1β predicts poor prognosis in CML, and inhibition of IL-1 signaling in combination with a tyrosine kinase inhibitor enhanced targeting of CML stem cells.70 IL-1 has also been shown to affect disease progression in AML through p38MAPK.71 Together, this work suggests an intriguing possibility that persistent exposure to inflammation early in adulthood may influence the process and timing of aging HSC phenotypes, and continuous elevation of inflammation later in life may increase the susceptibility to hematologic malignancies.
The gut microbiome is a source of inflammatory signaling that affects hematopoiesis and HSC function. In old germ-free mice, the HSC pool remains lineage balanced compared with specific pathogen-free mice with expanded myeloid-biased HSCs.72 Old mice have increased levels of microbial compounds stimulating IL-1 production,67 which reduces HSC self-renewal and increases myeloid cell proliferation, as described earlier. Fecal microbiota transplantation from young mice into old mice rejuvenated HSC function, mitigated inflammatory signaling, and restored lymphoid differentiation capacity of aging HSCs.73 In young mice genetically predisposed to develop precursor B-cell ALL, antibiotic treatment accelerates leukemia development.74 Antibiotic treatment also accelerates leukemia development in a mouse model of AML driven by MLL-AF9, which can be reversed by fecal microbiota transplantation.75 Autologous fecal microbiota transfer has been shown to be safe in patients with AML in a recent phase 2 clinical trial.76 These observations supports alterations in gut microbiota and intestinal barrier damage during aging dysregulates HSC function and provides a therapeutic option for restoring HSC function and preventing hematologic malignancies.
BM microenvironment senescence
Senescent cells accumulate with aging, and recent evidence suggests that senescence can be induced throughout the body of young mice by the transfer of blood from old mice.77 In the BM microenvironment, senescent cells have been found to directly affect HSC function. In a model of induced senescence, Terc knockout BM stromal cells (BMSCs) impair HSC functional regenerative capacity and accelerate myelopoiesis.78 Aging human BMSCs have been found to impair the clonogenic potential of young human CD34+ HSPCs and induce a proinflammatory gene expression program.79 Interventions targeting senescent cells using senolytics have shown effects on both nonhematopoietic and hematopoietic cells within the BM microenvironment. An important nuance is that senescence has been shown not to be a major mechanism intrinsically regulating HSC aging,80 therefore, studies targeting senescence in the rejuvenation of HSC function are likely to occur through non–cell-autonomous mechanisms. For example, aging mice treated with the senolytic drug ABT263 (Navitoclax) induced apoptosis of HSCs and non-HSCs, expressing markers of senescence and resulting in rejuvenated HSC function.81 In addition, a senolytic cocktail of dasatinib and quercetin has recently been shown to improve the osteogenic capacity of aging BMSCs in vitro and in vivo,82 which has the potential to improve aging HSC function in a non–cell-autonomous manner.
In hematologic malignancy, AML cells have been shown to contribute to the induction of BMSC senescence, and the resulting senescence-associated secretory phenotype promotes leukemia proliferation and survival.83 Targeting the senescent microenvironment by deleting p16INK4a-expressing BMSCs slows AML progression and extends survival.83 Because the senesecent microenvironment appears to be a vital component in the progression of hematologic malignancy, it warrants further investigation for therapeutic intervention.
Adipogenesis and metabolic changes in the BM microenvironment
BM adipocyte tissue volume increases with aging in humans.84 In mouse models, assessments of adipocyte abundance with aging show varying conclusions, and this abundance may be influenced by diet.65,85,86 A further dissent in the field is with respect to the role of BM adipocytes in regulating HSC function. Multiple studies have suggested that increased mature adipocytes negatively regulate HSC number and function.86,87 Although accumulation of BM adipocytes due to the loss of Bmi1 expression was reported to reduce HSC proliferation and number, these alterations were insufficient to broadly cause HSC aging phenotypes,88 suggesting that they may cooperate with other aging-associated changes in the BM microenvironment, causing a decline in HSC function. In contrast, a distinct report has robustly determined that adipocytes are a critical source of stem cell factors required to support hematopoietic regeneration. Deletion of Scf using an Adipoq-Cre/ER mouse model impaired hematopoietic regeneration, depleted HSCs, and reduced mouse survival after irradiation or 5-fluorouracil treatment.89 Before moving toward therapeutic intervention, further studies are needed to clarify the effect of adipocyte populations on HSC function in distinct contexts of steady-state, regenerative, and aging hematopoiesis.
Age-related adipocyte expansion clearly plays a role in the promotion of hematologic malignancies. ALL cells have been shown to use free fatty acids released from adipocytes as a fuel source to enhance leukemia progression.90 Additionally, AML cells stimulate lipolysis of BM adipocytes to release free fatty acids taken up by blast cells. This process enhances AML proliferation by increasing mitochondrial fatty acid β-oxidation.91 Leukemia cells use mitochondrial respiration to meet their metabolic demands, and inhibition of amino acid metabolism by venetoclax can target human leukemia stem cells and is effective in combination with azacytidine for extending survival in patients with AML.92-94 It has been reported that human AML generates reduced NAD phosphate oxidase to promote mitochondrial transfer from BMSCs to AML cells through tunneling nanotubes.95 Recent studies have shown that inhibiting this mitochondrial transfer decreases AML metabolic capacity and inhibits leukemia proliferation.96,97
New insights into therapeutic interventions for hematopoietic aging: is rejuvenation of aged HSCs possible?
Recent literature generated by independent groups strongly supports that HSCs in old mice resist functional rejuvenation by circulating factors present in young blood.20,98 Old HSCs did not show functional rejuvenation upon plasma transfer from young animals, caloric restriction, exercise, parabiosis with young animals,98 or transplantation into unconditioned young recipient mice.20 Surprisingly, transplantation into unconditioned young recipient mice restored the transcriptome, but not DNA methylation patterns, in old HSCs such that they closely resemble young HSCs.20 This suggests a disconnect between transcriptional, epigenetic, and functional rejuvenation at the HSC level. In addition to DNA methylation,20 HSCs in old mice have posttranscriptional alterations in autophagy and metabolism,99 micro RNA regulation, protein translation,100 and proteostasis,48 which represent potential mechanisms by which old HSCs are not functionally restored despite the reset transcriptional profiles. Of the remaining transcriptional signatures that were not restored upon transplantation of old HSCs into young recipient mice, these were enriched for proteasome, calcium ion transport, and specific metabolic (phosphate and phosphorus) processes,20 further pointing to specific processes that may be essential for functional rejuvenation.
In contrast to old HSCs, transplantation of HSCs from middle-aged donor mice into young mice could, in part, functionally rejuvenates middle-aged HSCs.20 Our group has performed transcriptional comparisons between middle-aged and old C57BL/6 HSCs and have found key differences, including that middle-aged HSCs have higher fatty acid metabolism, oxidative phosphorylation, and ribosomal protein translation initiation and less inflammatory signaling (mediated by TNFα, IL-6, and interferon gamma, IL-2), myeloid transcriptional network activation, and ROS signatures compared with old HSCs.15 Upon examining hematopoietic-extrinsic contributions toward aging and rejuvenation, reduction of insulin-like growth factor 1 in the middle-aged BM microenvironment causes HSCs aging phenotypes, including myeloid bias and expansion of phenotypic HSCs.15 Stimulation of middle-aged HSCs with insulin-like growth factor 1 expanded lymphoid-biased HSCs and restored lymphoid cell output from HSCs.15 In addition, a report describing the use of mitoquinol to enhance the mitochondrial membrane potential of aged HSCs found that supplementing drinking water of mice, starting at middle age (14 months old), delayed the onset of myeloid-biased hematopoiesis, prevented the onset of anemia, and reduced phenotypic HSC expansion over a 5-month period.101 Taken together, targeting HSCs at middle age may be a window of opportunity for rejuvenation. Beyond the transcriptome, investigation of biomarkers of functional HSC rejuvenation is needed to enable high-throughput screening approaches and clinical assessment of the success of rejuvenation factors.
The question “can interventions to prevent HSC aging or rejuvenate the functionality of aged HSCs effectively prevent or delay the development of leukemia?” remains unanswered. However, recent findings of HSC-intrinsic and -extrinsic mechanisms contributing to hematopoietic aging and leukemogenesis (Figure 2) provide compelling potential intervention mechanisms, including targeting the proteasome and translational processes, resetting metabolism and chromatin/epigenome patterning, chronic inflammation, senescence, and senescence-associated secretory phenotype.
Potential targetable mechanisms to rejuvenate aged HSCs and prevent hematologic malignancy. Phenotypes and biological mechanisms implicated in HSC aging, hematologic malignancy, or both are shown on the left. As overlapping mechanisms represent the most compelling targets for intervention, the inset table outlines the potential targetable mechanisms discussed in detail in this review. Illustration created using BioRender.com.
Potential targetable mechanisms to rejuvenate aged HSCs and prevent hematologic malignancy. Phenotypes and biological mechanisms implicated in HSC aging, hematologic malignancy, or both are shown on the left. As overlapping mechanisms represent the most compelling targets for intervention, the inset table outlines the potential targetable mechanisms discussed in detail in this review. Illustration created using BioRender.com.
Emerging research directions to pursue in HSC aging and hematologic malignancy
In a forward-thinking manner of emerging research directions, intriguing new findings that HSCs express major histocompatibility complex II, act as antigen-presenting cells to protect the integrity of the HSC pool, and delay the development of leukemia102 have revealed new biological aspects to be explored during aging. If the antigen-presentation capacity is compromised in aging HSCs, restoring this capacity could be a valuable therapeutic strategy to decrease the risk of hematologic malignancies, in the context of aging.
It has very recently been discovered that macrophages groom the HSC compartment during development, depleting HSCs high in ROSs, and stimulating proliferation and expansion of HSCs passing through this checkpoint.103 During aging, HSCs with high ROSs accumulate and are associated with myeloid-biased hematopoiesis.104 Exploring HSC grooming in adults and the elderly may open new avenues of exploration and intervention or rejuvenation strategies.
A historical challenge in this field is in the translation of the findings from animal models into human interventions. The development of an innovative new human BM organoid platform from induced pluripotent stem cells enables healthy and malignant human hematopoietic cell engraftment and survival.105 The adaptation of this technology offers a means to evaluate the efficacy of small molecules or other therapeutic strategies in the prevention or reversal of human HSC aging phenotypes and transformation to malignancy.
Acknowledgments
The authors thank the members of Trowbridge laboratory for their helpful discussions and critical comments.
This work was supported by National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK118072 (J.J.T.) and NIH National Institute on Aging grants R01AG069010 and U01AG077925 (J.J.T.). J.J.T. is a Scholar of the Leukemia & Lymphoma Society. J.J.M. is supported by a scholar award from The Jackson Laboratory.
Authorship
Contribution: All authors wrote and edited the manuscript.
Conflict-of-interest disclosure: J.J.T. receives royalties for patent licensing from Fate Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Jennifer J. Trowbridge, The Jackson Laboratory, 600 Main St, Bar Harbor, ME 04609; e-mail: jennifer.trowbridge@jax.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal