Introduction: Fever is a common adverse event associated with chimeric antigen receptor-T cell (CAR-T cell) therapy and serves as the hallmark of cytokine release syndrome (CRS). However, limited data are available on competing and contributing etiologies of fever. Thus, we aimed to investigate the causes, risk factors, and implications of fever in CAR-T cell recipients in the period with the most profound immunosuppression: between lymphodepletion (LD) and day 30 after infusion.
Methods: In this single-center retrospective analysis, we studied 304 non-Hodgkin lymphoma patients (80% large B-cell lymphoma, 11% mantle cell lymphoma, 4% follicular lymphoma) treated with CD19-CAR-T cells (48% axi-cel, 23% tisa-cel, 23% liso-cel, 6% brexu-cel) between 2016 and 2023. First fever was defined as a temperature of 38°C or higher between LD and day 30 after CD19-CAR-T cells. Patients experiencing fever were routinely cultured, following institutional guidelines. Additional workup, including respiratory virus PCR panel, stool gastrointestinal PCR panel, Clostridioides difficile PCR, urine culture, chest x-ray, and fungal infection assessment, was done at the medical team's discretion. A contributing infectious source for the fever was considered if there was a positive test in the workup within 72 hours of the initial fever.
Results: Fever occurred in 232/304 (76%) of patients between LD and day 30 after CAR-T cell infusion. The median time from LD to first fever was 5 days (range 2-13) and from CAR-T infusion to first fever was 2 days (range: LD initiation to 16). Of patients who had not developed a fever up to the day of infusion (n=271), 199 (73%) experienced a fever between infusion (day 0) and day 30.
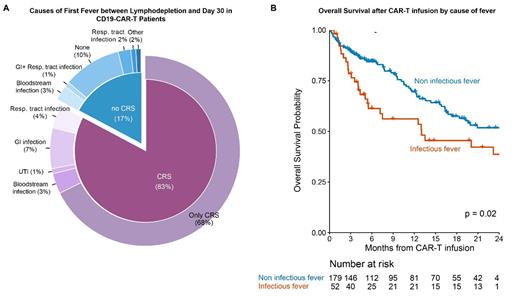
The primary etiology of fever between LD and day 30 was CRS (n=159/232, 68.5%), with 54% experiencing grade 2 or higher. An infectious source was identified in 52/232 (22%) of all patients and 35/194 (18%) of the subgroup developing CRS. Leading infectious etiologies were gastrointestinal (GI), bloodstream, and respiratory infections in 20 (38%), 14 (27%), and 11 (21%) of patients, respectively (Figure A). The most frequent pathogens identified were Clostridioides difficile for GI tract infections (10/20), Enterobacteriaceae for bloodstream infections (8/14), and common upper respiratory viruses, such as Rhinovirus and Parainfluenzavirus (10/16).
Importantly, in patients developing fever after infusion, an infectious source was identified in 42/199 (21%). Among them, 12/42 (29%) were bloodstream infections, and 10/42 (24%) were Clostridioides difficile infections requiring rapid antibiotic management.
Of an array of potential risk factors, only pre-apheresis metabolic tumor volume was associated with an increased risk for an infectious cause of fever in a univariable logistic-regression model (p=0.029). Furthermore, an infectious source of fever, compared to a non-infectious cause, was associated with an increased risk of mortality (HR 1.60, 95%CI 1.02-2.51, p=0.039) in a multivariable Cox regression model adjusted for LDH range before lymphodepletion, age and CAR-T construct type (Figure B).
Conclusions: This study represents one of the first and largest investigations into fever patterns and origins in CAR-T cell therapy recipients. Although fever is common, treatable infectious sources are infrequent. Our findings may have implications for antibiotic stewardship and fever management in CAR-T patients. Finally, the association between infectious fever and inferior survival warrants further investigation on the biological processes driving mortality.
Disclosures
Melica:Pfizer: Honoraria, Research Funding; Janssen: Honoraria; Gilead: Honoraria, Research Funding. Giralt:Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding. Palomba:Seres Therapeutics: Honoraria, Patents & Royalties; GarudaTherapeutics: Honoraria; BMS: Honoraria; Rheos: Honoraria; MustangBio: Honoraria; Cellectar: Honoraria; Novartis: Honoraria; Ceramedix: Honoraria; Synthekine: Honoraria; Kite: Honoraria; Juno: Honoraria, Patents & Royalties; Pluto Immunotherapeutics: Honoraria; Smart Immune: Honoraria; Thymofox: Honoraria. Park:Servier: Consultancy, Research Funding; GC Cell: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Genentech, Inc.: Research Funding; Fate Therapeutics: Research Funding; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Curocell: Consultancy; Kite: Consultancy; Pfizer: Consultancy; Minerva Bio: Consultancy; Sobi: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Bright Pharmacetuicals: Consultancy; Autolus Therapeutics: Research Funding; Be Biopharma: Consultancy; Intella: Consultancy; BeiGene: Consultancy; Artiva Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Affyimmune: Consultancy. Salles:AbbVie: Consultancy, Honoraria; Incyte: Consultancy; Owkin: Current holder of stock options in a privately-held company; ATB Therapeutics: Consultancy; Kite/Gilead: Consultancy; Loxo/Lilly: Consultancy; Janssen: Consultancy, Research Funding; Nurix: Consultancy; EPIZYME: Consultancy; BeiGene: Consultancy; Novartis: Consultancy; Genmab: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Molecular Partners: Consultancy; Merck: Consultancy, Honoraria; BMS/Celgene: Consultancy; Debiopharm: Consultancy; Nordic Nanovector: Consultancy; Ipsen: Consultancy, Research Funding; Orna: Consultancy. Scordo:Amgen, Inc.: Research Funding; Omeros Corporation: Consultancy, Research Funding; Angiocrine Bioscience, Inc.: Research Funding; Medscape, LLC: Honoraria; CancertNetwork (Intellisphere LLC): Honoraria. Shah:Beyond Spring: Research Funding; BMS: Research Funding; Amgen: Research Funding; Janssen: Research Funding; ArcellX: Other: DSMB. Perales:Vor Biopharma: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Caribou: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; Adicet: Honoraria; BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Celgene: Honoraria; Astellas: Consultancy, Honoraria; DSMB: Other; Sellas Life Sciences: Consultancy; Servier: Other; Novartis: Consultancy, Honoraria, Research Funding; Syncopation: Honoraria; Cidara Therapeutics: Consultancy, Other; Medigene: Consultancy, Other; Incyte: Consultancy, Honoraria, Research Funding; Kite: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; NexImmune: Consultancy, Current equity holder in publicly-traded company; Allogene: Research Funding; Miltenyi Biotec: Honoraria; Allovir: Consultancy; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; AbbVie: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal