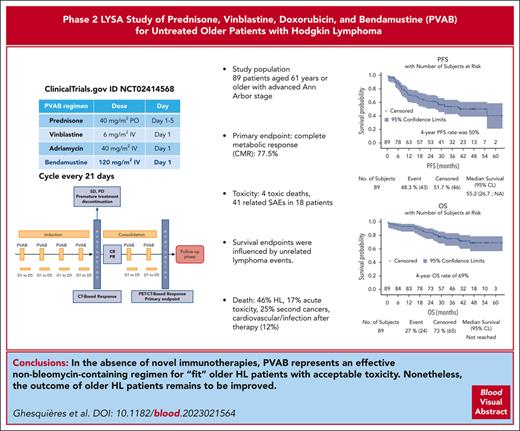
The PVAB regimen yielded a CMR rate of 77.5% as a firstline therapy for older patients with HL, with acceptable toxicity.
The 4-year cumulative risk of events was 35% for progression and relapse, 9% for death from lymphoma, and 6% for nonlymphoma events.
Visual Abstract
Older patients with classical Hodgkin lymphoma (cHL) require more effective and less toxic therapies than younger patients. In this multicenter, prospective, phase 2 study, we investigated a new firstline therapy regimen comprising 6 cycles of prednisone (40 mg/m2, days 1-5), vinblastine (6 mg/m2, day 1), doxorubicin (40 mg/m2, day 1), and bendamustine (120 mg/m2, day 1) (PVAB regimen) every 21 days for patients with newly diagnosed cHL aged ≥61 years with an advanced Ann Arbor stage. A Mini Nutritional Assessment score ≥17 was the cutoff value for patients aged ≥70 years. The primary end point was the complete metabolic response (CMR) rate after 6 cycles. The median age of the 89 included patients was 68 years (range, 61-88 years), with 35 patients (39%) aged ≥70 years. Seventy-eight patients (88%) completed the 6 cycles. The toxicity rate was acceptable, with a 20% rate of related serious adverse events. CMR was achieved by 69 patients (77.5%; 95% confidence interval [CI], 67-86). After a median follow-up of 42 months, 31 patients progressed or relapsed (35%), and 24 died (27%) from HL (n = 11), toxicity during treatment (n = 4), secondary cancers (n = 6), or other causes (n = 3). The 4-year progression-free survival (PFS) and overall survival rates were 50% and 69%, respectively. Multivariate analysis showed that liver involvement (P = .001), lymphopenia (P = .001), CRP (P = .0005), and comedications (P = .003) were independently associated with PFS. The PVAB regimen yielded a high CMR rate with acceptable toxicity. Over long-term follow-up, survival end points were influenced by unrelated lymphoma events. This trial was registered at www.clinicaltrials.gov as #NCT02414568 and at EudraCT as 2014-001002-17.
Introduction
Classical Hodgkin lymphoma (cHL) is an age-specific occurrence, with incidence peaks observed in younger and older adults.1 Large national registries have shown that patients aged ≥60 years with cHL account for ∼30% of all patients with cHL.2 Older patients have specific pathologic and clinical findings that differ from those of younger patients, including an increased rate of the mixed-cellularity subtype, a frequent association with Epstein-Barr virus (EBV), more advanced stage, and B symptoms.3 The results of standard treatments, such as the doxorubicin (Adriamycin), bleomycin, vinblastine, and dacarbazine (ABVD) regimen, are worse for older patients than for younger patients. Evens et al examined a retrospective cohort of 95 older patients who received the ABVD regimen; they reported 5-year progression-free survival (PFS) and overall survival (OS) rates of 44% and 58%, respectively, and the incidence rate of bleomycin lung toxicity was 32%.4 The incidences of toxic death (10%) and secondary cancer (6%) are also high in this age group.5 Other chemotherapy regimens have been developed to decrease the risk of progression and relapse and improve tolerance, for instance, by withdrawal of bleomycin.3,6 Another important finding of previous studies was the necessity for geriatric assessment to identify older patients with cHL with a high risk of toxicity so that alternative therapeutic options can be proposed.7
A prospective trial of patients with relapsed and refractory cHL showed that bendamustine at a dosage of 120 mg/m2 for 2 consecutive days every 28 days yielded an objective response rate of 53% and a favorable safety profile.8 Bendamustine has been integrated into curative treatment regimens for cHL, such as the bendamustine, gemcitabine, and vinorelbine regimen9 or in combination with brentuximab-vedotin (BV).10
We developed a new combination chemotherapy comprising Adriamycin, vinblastine, bendamustine, and prednisone (PVAB regimen) for patients with newly diagnosed advanced-stage cHL aged ≥61 years. In this study, a specific geriatric scale and a Mini Nutritional Assessment (MNA) for older patients (≥70 years) were used to determine patient eligibility. We report the final analysis of the multicenter, prospective, phase 2 PVAB study.
Methods
Study design and participants
This prospective, multicenter, phase 2 study was designed by the Lymphoma Study Association and conducted at 34 centers in Belgium and France. All inclusion and exclusion criteria are described in the supplemental Methods, available on the Blood website, and were, briefly, as follows: first diagnosis of cHL according to the World Health Organization criteria; age ≥61 years; advanced Ann Arbor stages (stage IIB with risk factors and stages III and IV); baseline 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG) positron emission tomography (PET) performed before any treatment revealing at least 1 hypermetabolic lesion; Eastern Cooperative Oncology Group (ECOG) performance status (PS) from 0 to 2; adequate cardiopulmonary function; and an MNA score ≥17 for patients aged ≥70 years. The MNA has a total score of 30 points11; patients with a score <17 and between 17 and 23.5 were considered malnourished and at risk of malnutrition, respectively. We used the MNA questionnaire or patients aged ≥70 years to limit acute toxicity based on the Soubeyran et al study showing that an MNA score <17 was independently associated with early death in older patients with cancer.12 The study was approved by the French and Belgian health authorities, the Lyon Sud-Est IV Ethics Committee, and the institutional review boards in Belgium and performed in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Procedures
Inclusion for each patient was based on the local pathological diagnosis of cHL. A histopathology central review was then performed by at least three 3 experts in hematopathology (A.T.G., D.D., and B.B.). Treatment consisted of administering 4 courses of PVAB every 3 weeks, followed by a computed tomography (CT). Patients with CR or partial response (PR) according to the CT-based Lugano classification response criteria13 received 2 additional cycles of PVAB delivered every 3 weeks. Patients with stable disease and progressive disease (PD) were considered to have treatment failure. The evaluation at the end of treatment after 6 cycles of PVAB was performed according to the Lugano classification criteria (PET-CT–based response).13 Concomitant administration of any other chemotherapy regimen or radiotherapy during the study was not allowed. The PVAB regimen consisted of prednisone (40 mg/m2, days 1-5), vinblastine (6 mg/m2, day 1), doxorubicin (40 mg/m2, day 1), and bendamustine (120 mg/m2, day 1). Mandatory prophylactic measures were subcutaneous granulocyte colony-stimulating factor (G-CSF) from day 6 to day 13 or until neutrophil counts were ≥1.0 g/L; valaciclovir and trimethoprim/sulfamethoxazole or equivalent for preventing herpesvirus infections and Pneumocystis jiroveci pneumonia, respectively. In cases of acute toxicities, a dose-reduction scheme is provided in the full version of the protocol.
Outcomes
The primary end point of this phase 2 study was the complete metabolic response (CMR) rate at the end of the study treatment (after 6 cycles or at premature treatment discontinuation) according to the local review using the Lugano classification (PET-CT–based response).13 Secondary efficacy end points included PFS, OS, and disease-free survival (DFS); a safety profile, including immediate toxicities and nontumor events; tumor response rate by centralized PET review; and feasibility of the geriatric assessment program. Geriatric assessment consisted of a Cumulative Illness Rating Scale for Geriatrics (CIRS-G) score evaluation for all patients.14 Instrumental activities in daily living (IADLs),15 activities of daily living (ADLs),16 MNA (one of the inclusion criteria),11 and the G8 questionnaire17 were evaluated for patients aged ≥70 years. PFS was measured from the date of inclusion to the date of first documented disease progression, relapse, or death from any cause. OS was measured from the date of inclusion to the date of death from any cause. DFS was measured from the time of attainment of CMR to the date of first documented disease progression, relapse, or death from any cause. Patients alive and free of progression were censored at their last disease assessment date. The independent central review of PETs was performed by 2 expert reviewers (S.K. and A.B.-R.) with available imaging, including the evaluation of the initial metabolic tumor volume (using 41% of the maximum of standardized uptake value threshold)18 and the final response according to the Lugano classification (PET-CT–based response).13
The intensity of adverse events (AEs) or serious AEs (SAEs) was graded by the investigator according to the Common Terminology Criteria for Adverse Events grading system version 4.03 in the toxicity categories. An independent data monitoring committee composed of 3 independent members (2 experts in HL and 1 statistician) reviewed the safety data after 25 patients (31 March 2017) had been included and completed treatment.
Statistical analysis
Based on the results with ABVD,4,19 which presented CR rates of 64% to 75%, we selected P0 and P1 to be 70% and 85%, respectively. A significance level α = 0.05 (1-sided) and a power of 80% were used. A 2-stage Simon design was used in this study with an interim analysis for futility.20 In the first stage, 25 patients were enrolled. If ≤18 CMRs (72%) were observed among the 25 patients, the treatment effect was considered inefficient. If at least 19 patients had achieved a CMR after study treatment, the second stage of the study was performed. The results of the first stage were reviewed by the independent data monitoring committee (31 March 2017). If the condition of the first stage was fulfilled, at least 79 patients were analyzed in the second stage, which corresponded to the final analysis for the primary end point. If ≥62 CMRs (78%) were observed after study treatment, the treatment was considered effective. Taking into account an estimated percentage of not evaluable patients of ∼10%, a total of 90 patients were required for the trial. The Kaplan‒Meier method was used to estimate the probability of survival (PFS, DFS, and OS) at any given point in time. The log-rank test was used to compare the survival distributions of ≥2 groups. A Cox proportional hazard model was used to test differences in survival times. For multivariable analysis, factors with a P value ≤ .05 on univariable analysis were added using a backward regression model.21
The data cutoffs were 14 December 2018 for the primary end point and 10 November 2020 for the final analysis. All analyses were performed using SAS version 9.3 and AdClin version 3.3.3.
Results
Patient characteristics
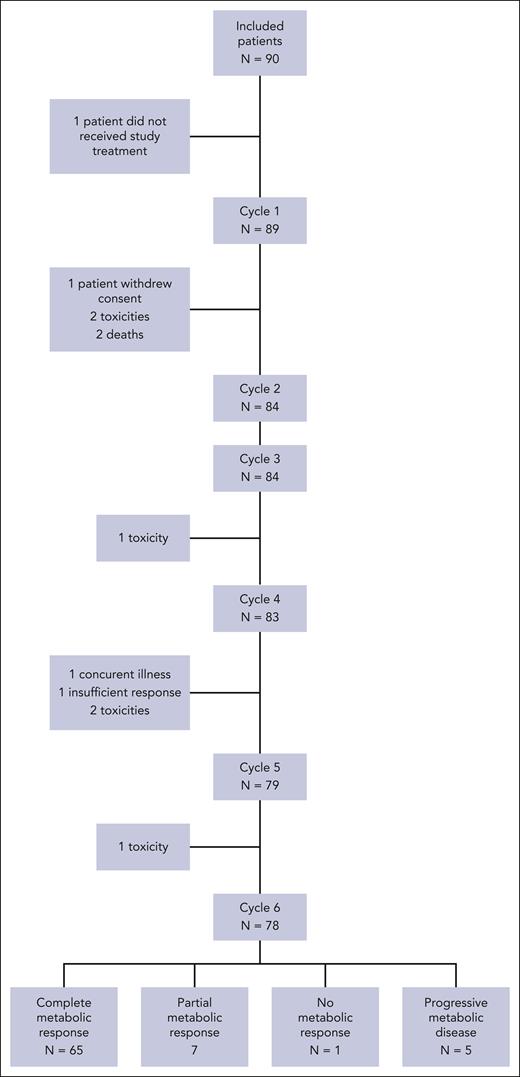
Between 20 July 2015 and 31 July 2018, a total of 90 patients were enrolled in the study, but 1 patient did not receive chemotherapy. The baseline characteristics of the 89 patients are listed in Table 1, and patient disposition is presented in Figure 1. The median age was 68 years (range, 61-88 years), with 35 patients (39%) aged ≥70 years. The ECOG PS ranged from 0 to 1 for 74 patients (73%), and 57% of patients had B symptoms at diagnosis. A majority of patients had Ann Arbor stage III to IV disease (97%). Nodular sclerosis was the most frequent cHL subtype according to local diagnosis (n = 61 [69%]) or after central histological review (n = 56 [63%]). Tumors associated with EBV using latent membrane protein 1 or EBV-encoded RNA staining were found in 26 of 82 (32%) and 33 of 70 (47%) available cases, respectively.
Clinical characteristics
| . | n = 89 . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 68 (61-88) | |
| ≥70 | 35 | 39 |
| Men | 58 | 65 |
| Women | 31 | 35 |
| ECOG PS | ||
| 0 | 33 | 37 |
| 1 | 41 | 46 |
| 2 | 15 | 17 |
| B symptoms | 51 | 57 |
| Ann Arbor stage | ||
| IIB at risk∗ | 3 | 3 |
| III | 30 | 34 |
| IV | 56 | 63 |
| Bone marrow involved† | 18 | 20 |
| Hemoglobin level, median (range), g/dL | 12.4 (7-17) | |
| Albumin level, median (range), g/L | 35 (20-66) | |
| International prognostic score | ||
| 0-2 | 17 | 20 |
| 3-6 | 70 | 80 |
| Missing | 2 | |
| Histological subtype (central review) | ||
| Nodular sclerosis | 56 | 63 |
| Classic, not otherwise specified | 18 | 20 |
| Mixed cellularity | 11 | 12 |
| EBV-positive diffuse large B-cell lymphoma | 1 | 1 |
| Hodgkin lymphocyte-rich | 1 | 1 |
| Insufficient material | 1 | 1 |
| Nodular lymphocyte-predominant Hodgkin lymphoma | 1 | 1 |
| EBV association | ||
| LMP1 positivity‡ | 26 | 32 |
| EBER positivity‡ | 33 | 47 |
| Comorbidity, CIRS-G | ||
| Total score, median | 3 (0–12) | |
| Number of categories endorsed | 2 (0–14) | |
| No grade 3-4 comorbidity | 81 | 91 |
| ≥1 grade 3-4 comorbidity | 8 | 9 |
| IADL (n = 35)§ | ||
| 4 | 21 | 66 |
| ≤3 | 11 | 34 |
| Missing | 3 | |
| ADL (n = 35)§ | ||
| 6 | 25 | 81 |
| ≤5 | 6 | 19 |
| Missing | 4 | |
| MNA (n = 35)§ | ||
| Normal (>23.5) | 18 | 53 |
| At risk of malnutrition (17-23.5) | 15 | 44 |
| Malnourished (<17) | 1‖ | 3 |
| Missing | 1‖ | |
| G8 questionnaire (n = 35)§ | ||
| ≤14 | 27 | 77 |
| >14 | 8 | 23 |
| Median (range) | 11 (3-17) |
| . | n = 89 . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 68 (61-88) | |
| ≥70 | 35 | 39 |
| Men | 58 | 65 |
| Women | 31 | 35 |
| ECOG PS | ||
| 0 | 33 | 37 |
| 1 | 41 | 46 |
| 2 | 15 | 17 |
| B symptoms | 51 | 57 |
| Ann Arbor stage | ||
| IIB at risk∗ | 3 | 3 |
| III | 30 | 34 |
| IV | 56 | 63 |
| Bone marrow involved† | 18 | 20 |
| Hemoglobin level, median (range), g/dL | 12.4 (7-17) | |
| Albumin level, median (range), g/L | 35 (20-66) | |
| International prognostic score | ||
| 0-2 | 17 | 20 |
| 3-6 | 70 | 80 |
| Missing | 2 | |
| Histological subtype (central review) | ||
| Nodular sclerosis | 56 | 63 |
| Classic, not otherwise specified | 18 | 20 |
| Mixed cellularity | 11 | 12 |
| EBV-positive diffuse large B-cell lymphoma | 1 | 1 |
| Hodgkin lymphocyte-rich | 1 | 1 |
| Insufficient material | 1 | 1 |
| Nodular lymphocyte-predominant Hodgkin lymphoma | 1 | 1 |
| EBV association | ||
| LMP1 positivity‡ | 26 | 32 |
| EBER positivity‡ | 33 | 47 |
| Comorbidity, CIRS-G | ||
| Total score, median | 3 (0–12) | |
| Number of categories endorsed | 2 (0–14) | |
| No grade 3-4 comorbidity | 81 | 91 |
| ≥1 grade 3-4 comorbidity | 8 | 9 |
| IADL (n = 35)§ | ||
| 4 | 21 | 66 |
| ≤3 | 11 | 34 |
| Missing | 3 | |
| ADL (n = 35)§ | ||
| 6 | 25 | 81 |
| ≤5 | 6 | 19 |
| Missing | 4 | |
| MNA (n = 35)§ | ||
| Normal (>23.5) | 18 | 53 |
| At risk of malnutrition (17-23.5) | 15 | 44 |
| Malnourished (<17) | 1‖ | 3 |
| Missing | 1‖ | |
| G8 questionnaire (n = 35)§ | ||
| ≤14 | 27 | 77 |
| >14 | 8 | 23 |
| Median (range) | 11 (3-17) |
EBER, EBV-encoded RNA; LMP1, latent membrane protein 1.
Mediastinum/thorax ratio ≥ 0.33 or extranodal localization.
According to PET evaluation.
82 and 70 available cases for EBER and LMP1 staining, respectively.
Geriatric assessment for patients aged ≥70 years.
Without respect to major inclusion criteria.
For geriatric assessments, the median CIRS-G score at baseline for all patients was 3 (range, 0-12), with 8 patients with at least 1 grade 3 to 4 comorbidity (Table 1). The evaluation of the baseline IADL, ADL, MNA, and G8 questionnaire for patients aged ≥70 years are detailed in Table 1. Evaluation of patients (≥70 years) according to the simplified geriatric assessment (sGA) with CIRS-G, ADL, IADL, and age developed by Tucci et al22 showed that 13 (42%), 9 (29%), and 9 (29%) patients were classified as fit, unfit, and frail, respectively (4 missing data points). For the 54 patients aged <70 years, only the CIRS-G scale was used; according to the stratification developed by Tucci et al for CIRS-G scores,22 50 patients were classified as fit, and 4 patients were classified as frail.
Treatment feasibility and efficacy
Of the 89 included patients, 83 (93%) and 78 (88%) patients completed 4 and 6 cycles, respectively, with a median of 6 cycles received (range, 1-6). As expected, patients aged <70 years completed 6 cycles (94%) more frequently than older patients (77%). The administered doses of prednisone, vinblastine, Adriamycin, and bendamustine per cycle were close to the expected doses (Table 2). Delays between the cycles were rare. The median number of days between cycles was 21 days (range, 18-56), with 7 patients with a delay of 1 week and 6 patients with a delay of 29, 31, 31, 35, 36, and 56 days between 2 cycles (Table 2). Predefined dose adjustments according to hematological toxicities or infections were applied for 10 patients. Among the 83 patients who completed the first 4 cycles, 28 patients had CR (34%), 53 patients had PR (65%), and 1 patient had stable disease according to CT evaluation.
Level of exposure to each product and delay between cycles in the PVAB regimen
| . | Cycle 1 (n = 89) . | Cycle 2 (n = 84) . | Cycle 3 (n = 84) . | Cycle 4 (n = 83) . | Cycle 5 (n = 79) . | Cycle 6 (n = 78) . |
|---|---|---|---|---|---|---|
| Total dose taken | ||||||
| Prednisone (200 mg/m2)∗ | ||||||
| Median dose | 199.0 | 200.4 | 200.3 | 198.6 | 198.5 | 199.7 |
| Min to max | 38-241 | 38-241 | 50-243 | 49-244 | 50-243 | 50-241 |
| PPD (%) | 99.5 | 100.2 | 100.1 | 99.3 | 99.3 | 99.8 |
| Min to max | 19-121 | 19-121 | 25-121 | 24-122 | 25-121 | 25-121 |
| Vinblastine (6 mg/m2)∗ | ||||||
| Median dose | 5.9 | 5.9 | 5.9 | 5.8 | 5.9 | 5.9 |
| Min to max | 5-7 | 5-6 | 5-6 | 5-6 | 3-6 | 3-6 |
| PPD (%) | 98.3 | 98.3 | 97.5 | 96.7 | 98.3 | 98.3 |
| Min to max | 78-110 | 78-107 | 75-105 | 75-103 | 50-105 | 45-105 |
| Doxorubicin (40 mg/m2)∗ | ||||||
| Median dose | 39.8 | 39.9 | 39.9 | 39.5 | 39.6 | 39.6 |
| Min to max | 34-44 | 30-44 | 28-44 | 19-43 | 19-43 | 0-43 |
| PPD (%) | 99.5 | 99.8 | 99.6 | 98.8 | 99.0 | 99.0 |
| Min to max | 85-109 | 75-110 | 70-110 | 47-109 | 47-108 | 0-108 |
| Bendamustine (120 mg/m2)∗ | ||||||
| Median dose | 119.2 | 119.6 | 119.4 | 118.6 | 118.4 | 118.7 |
| Min to max | 102-131 | 89-127 | 85-125 | 56-129 | 56-125 | 56-125 |
| PPD (%) | 99.3 | 99.7 | 99.5 | 98.8 | 98.7 | 98.9 |
| Min to max | 85-109 | 74-106 | 71-104 | 47-108 | 47-105 | 47-104 |
| . | Cycle 1 (n = 89) . | Cycle 2 (n = 84) . | Cycle 3 (n = 84) . | Cycle 4 (n = 83) . | Cycle 5 (n = 79) . | Cycle 6 (n = 78) . |
|---|---|---|---|---|---|---|
| Total dose taken | ||||||
| Prednisone (200 mg/m2)∗ | ||||||
| Median dose | 199.0 | 200.4 | 200.3 | 198.6 | 198.5 | 199.7 |
| Min to max | 38-241 | 38-241 | 50-243 | 49-244 | 50-243 | 50-241 |
| PPD (%) | 99.5 | 100.2 | 100.1 | 99.3 | 99.3 | 99.8 |
| Min to max | 19-121 | 19-121 | 25-121 | 24-122 | 25-121 | 25-121 |
| Vinblastine (6 mg/m2)∗ | ||||||
| Median dose | 5.9 | 5.9 | 5.9 | 5.8 | 5.9 | 5.9 |
| Min to max | 5-7 | 5-6 | 5-6 | 5-6 | 3-6 | 3-6 |
| PPD (%) | 98.3 | 98.3 | 97.5 | 96.7 | 98.3 | 98.3 |
| Min to max | 78-110 | 78-107 | 75-105 | 75-103 | 50-105 | 45-105 |
| Doxorubicin (40 mg/m2)∗ | ||||||
| Median dose | 39.8 | 39.9 | 39.9 | 39.5 | 39.6 | 39.6 |
| Min to max | 34-44 | 30-44 | 28-44 | 19-43 | 19-43 | 0-43 |
| PPD (%) | 99.5 | 99.8 | 99.6 | 98.8 | 99.0 | 99.0 |
| Min to max | 85-109 | 75-110 | 70-110 | 47-109 | 47-108 | 0-108 |
| Bendamustine (120 mg/m2)∗ | ||||||
| Median dose | 119.2 | 119.6 | 119.4 | 118.6 | 118.4 | 118.7 |
| Min to max | 102-131 | 89-127 | 85-125 | 56-129 | 56-125 | 56-125 |
| PPD (%) | 99.3 | 99.7 | 99.5 | 98.8 | 98.7 | 98.9 |
| Min to max | 85-109 | 74-106 | 71-104 | 47-108 | 47-105 | 47-104 |
| Delay between 2 cycles . | C1-C2 . | C2-C3 . | C3-C4 . | C4-C5 . | C5-C6 . |
|---|---|---|---|---|---|
| N | N = 84 | N = 84 | N = 83 | N = 79 | N = 78 |
| Median | 21 | 21 | 21 | 21 | 21 |
| Min to max | 20-28 | 19-36 | 18-56 | 19-31 | 18-35 |
| Delay between 2 cycles . | C1-C2 . | C2-C3 . | C3-C4 . | C4-C5 . | C5-C6 . |
|---|---|---|---|---|---|
| N | N = 84 | N = 84 | N = 83 | N = 79 | N = 78 |
| Median | 21 | 21 | 21 | 21 | 21 |
| Min to max | 20-28 | 19-36 | 18-56 | 19-31 | 18-35 |
C1, cycle 1; max, maximum; min, minimum; PPD, percentage of planned dose.
Expected dose per cycle.
At the end of the 6 cycles of PVAB (n = 78) or at discontinuation (n = 11), CMR was achieved by 69 patients (77.5%; 95% confidence interval [CI], 67-86). Partial metabolic response, no metabolic response, and progressive metabolic response were obtained by 8 (9%), 2 (2%), and 5 patients (6%), respectively; 5 patients (6%) could not be evaluated. After independent central review of 79 patients with available PET/CT images, 62 patients had CMR (78%), 14 patients had metabolic PR (18%), and 3 patients had PD (4%; supplemental Table 1).
Toxicity
Eighty-four patients (94%) presented at least 1 grade ≥3 AE, with 346 events declared by investigators (supplemental Table 2). For 7 patients (8%), at least 1 AE led to treatment discontinuation after 1 cycle for 3 patients, 3 cycles for 1 patient, 4 cycles for 2 patients, and 5 cycles for 1 patient. Twenty-eight patients (31.5%) presented at least 1 SAE, for a total of 57 events (median, 1; minimum to maximum, 1-8). Among these SAEs, 41 were considered related SAEs that occurred in 18 patients (Table 3). Ten patients presented with febrile neutropenia (7%) and 9 presented with infections (10%), with 6 documented bacterial infections, 3 nondocumented infections, 1 fungal infection, and 1 septic shock. One patient presented with grade 3 pneumonitis. Four patients presented cardiac disorders (4.5%). The related SAEs were influenced by age, ECOG PS, MNA, and sGA (Table 3). Toxic deaths occurred in 4 patients (4.5%); 3 patients died after cycle 1, including 1 from cardiogenic shock (71 years), 1 from septic shock (70 years), and 1 from brain stem hematoma with grade 4 thrombocytopenia (76 years), and the last patient presented with a fungal infection after cycle 4 (86 years). Two patients with toxic death were incorrectly included based on MNA scores (1 patient’s MNA score was not collected, and 1 patient had a score of 10). Finally, 2 patients with toxic death were classified as frail according to sGA (1 was fit and 1 unclassified for missing data).22
Related SAEs classified into system organ classes (SOCs)
| . | n = 41∗ . | % . |
|---|---|---|
| Blood disorders | 16 | 39 |
| Febrile neutropenia | 7 | |
| Anemia | 4 | |
| Leucopenia | 2 | |
| Thrombocytopenia | 1 | |
| Neutropenia | 1 | |
| Bone marrow failure | 1 | |
| Infections | 11 | 27 |
| Documented bacterial infections | 6 | |
| Nondocumented infections | 3 | |
| Fungal infection | 1 | |
| Septic shock | 1 | |
| Cardiac disorders | 4 | 10 |
| Atrial fibrillation | 2 | |
| Cardiac valve disease | 1 | |
| Cardiogenic shock | 1 | |
| Gastrointestinal disorders | 2 | 5 |
| Occlusion | 1 | |
| Esophagitis | 1 | |
| General disorders | 2 | 5 |
| General physic health | 1 | |
| Deterioration | ||
| Mucositis | 1 | |
| Respiratory disorders | 3 | 7 |
| Acute pulmonary edema | 1 | |
| Acute respiratory distress | 1 | |
| Pneumonitis | 1 | |
| Procedural complications | 1 | 2 |
| Infusion-related reaction | 1 | |
| Nervous system disorders | 1 | 2 |
| Brain stem hematoma | 1 | |
| Renal disorders | 1 | 2 |
| Renal failure | 1 |
| . | n = 41∗ . | % . |
|---|---|---|
| Blood disorders | 16 | 39 |
| Febrile neutropenia | 7 | |
| Anemia | 4 | |
| Leucopenia | 2 | |
| Thrombocytopenia | 1 | |
| Neutropenia | 1 | |
| Bone marrow failure | 1 | |
| Infections | 11 | 27 |
| Documented bacterial infections | 6 | |
| Nondocumented infections | 3 | |
| Fungal infection | 1 | |
| Septic shock | 1 | |
| Cardiac disorders | 4 | 10 |
| Atrial fibrillation | 2 | |
| Cardiac valve disease | 1 | |
| Cardiogenic shock | 1 | |
| Gastrointestinal disorders | 2 | 5 |
| Occlusion | 1 | |
| Esophagitis | 1 | |
| General disorders | 2 | 5 |
| General physic health | 1 | |
| Deterioration | ||
| Mucositis | 1 | |
| Respiratory disorders | 3 | 7 |
| Acute pulmonary edema | 1 | |
| Acute respiratory distress | 1 | |
| Pneumonitis | 1 | |
| Procedural complications | 1 | 2 |
| Infusion-related reaction | 1 | |
| Nervous system disorders | 1 | 2 |
| Brain stem hematoma | 1 | |
| Renal disorders | 1 | 2 |
| Renal failure | 1 |
For patients aged <70 and ≥70 years, 9.3% and 37.1% of patients presented at least 1 related SAE; for patients with ECOG PS 0 to 1 and >1, 14.9% and 46.7% presented at least 1 related SAE; 33% of patients with a normal MNA score had related SAEs compared with 44% of patients with an abnormal MNA score; 23%, 33%, and 56% of patients classified as fit, unfit and frail, respectively, presented at least 1 related SAE.
Number of events.
Survival
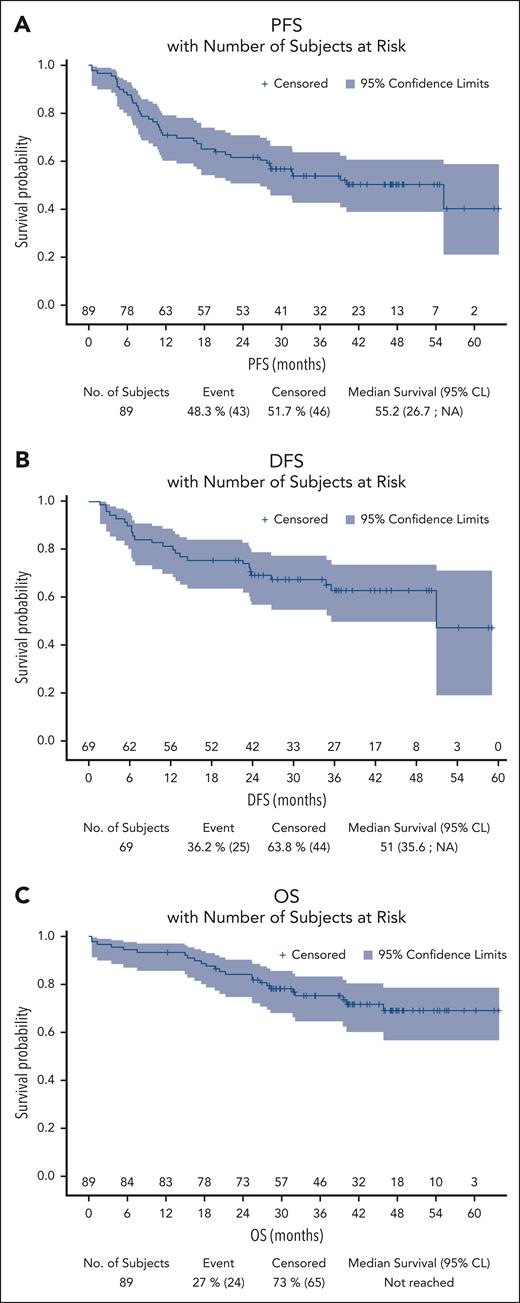
Over a median follow-up of 42 months (95% CI, 40-47), the median PFS was 55.2 months (95% CI, 27 to not reached). The 4-year PFS rate was 50% (95% CI, 39-61) (Figure 2). Events for PFS were progression or relapse for 31 patients (35%) and death from any cause for 12 patients (28%). The 4-year DFS rate was 63% (95% CI, 49-74) for the 69 patients who obtained a CMR at the end of treatment, with 18 relapses (Figure 2). Among the 89 patients, 24 patients (27%) died: 11 (46%) from HL, 4 (17%) from toxicity during treatment, and 6 (25%) from second cancers. Five patients who died of second cancers (colic, esophageal, biliary tract, and lung adenocarcinomas) had CR after the PVAB regimen at the last follow-up. The last patient who died of acute myeloid leukemia relapsed and was treated by BV in combination with cytarabine and oxaliplatin followed by radiotherapy that led to a new CR for HL. The remaining 3 patients (12%) died from other causes after PVAB: 1 patient died from pulmonary infection in CR, 1 patient died from cardiac insufficiency in CR, and 1 patient died from pulmonary embolism in PD at the end of treatment. The median OS was not reached, with a 4-year OS rate of 69% (95% CI, 57-79) (Figure 2).
Kaplan-Meier curves for outcome. (A) PFS; (B) DFS; (C) OS. NA, not applicable.
Survival according to geriatric assessments
As exploratory analyses, PFS was evaluated according to CIRS-G, ADL, IADL, MNA, G8, and sGA scores (supplemental Figure 1). The 4-year PFS rates for patients with CIRS-G ≤6 and CIRS-G >6 were 52% and 40%, respectively (P = .31). Of note, evaluation of CIRS-G using the cutoff of 10 used by Evens et al23 was not feasible, given the low number of patients with CIRS-G >10 (n = 3). The 4-year PFS rates were 33% and 68% (P = .19) for ADL score ≤5 and ADL score >5, respectively; 46% and 65% (P = .19) for IADL score <4 and IADL score ≥4; 77% and 35% (P = .02) for normal and abnormal MNA scores; and 75% and 54% (P = .39) for G8 scores. Finally, the 4-year PFS rates were 76%, 56%, and 44% for fit, unfit, and frail patients, respectively, according to sGA22 (P = .34). The corresponding 4-year OS rates were 76%, 78%, and 67% (P = .86).
Cumulative incidence of events and event decomposition
Because PFS is influenced by related and nonrelated lymphoma events, we estimated the cumulative incidence of each event for PFS.24 At 4 years, the cumulative risks of progression/relapse, death from lymphoma, and death from other cancers were 35% (95% CI, 25-45), 9% (95% CI, 4-16), and 6% (95% CI, 2-13), respectively (Figure 3). The cumulative incidence of death at 4 years from lymphoma, toxicity, and death from other cancers were 13.5% (95% CI, 7-22), 8.7% (95% CI, 4-16), and 8.8% (95% CI, 3-18), respectively (Figure 3). For patients who obtained CMR after the PVAB regimen, the 4-year cumulative risk of relapse and death from lymphoma and nonlymphoma events were 26.5%, 3.9%, and 7.8%, respectively (supplemental Figure 2).
Event description. Cumulative incidence of events for PFS (A) and OS (B).
Prognostic analyses for PFS
Prognostic factors included baseline clinical and biological characteristics, geriatric assessments, and 18F-FDG PET/CT metabolic parameters. Optimal thresholds for some parameters (leucocytes, cutoff at 12 g/L; lymphocytes, cutoff at 0.8 g/L; albumin, cutoff at 30 g/L; and C-reactive protein, cutoff at 88 mg/L) were used after calculation using the X-Tile approach.25 In univariate analysis, altered ECOG PS, B symptoms, Ann Arbor stage IV, >1 extranodal site, bone or medullar involvement, liver involvement, bulky disease (>7 cm), EBV-encoded RNA-positive tumor, CD4+ lymphocyte count (<0.41 g/L), albumin (≤30 g/L), hemoglobin level (<10.5 g/dL), lymphocyte count (<0.8 G/L), leucocyte count (≥12 G/L), B2 microglobulin level (>3 mg/L), C-reactive protein level (>88 mg/L), total metabolic tumor volume (TMTV; >450 mL), and number of medications unrelated to HL (>5) were associated with PFS. In multivariate analysis, liver involvement (hazard ratio [HR], 2.593; 95% CI, 1.28-5.25; P = .0081), lymphopenia (HR, 4.105; 95% CI, 2.15-7.83; P ≤ .0001), C-reactive protein level (HR, 2.602; 95% CI, 1.38-4.90; P = .003), and comedications unrelated to HL (HR, 2.605; 95% CI, 1.37-4.96; P = .0035) were independently associated with PFS (Table 4).
Prognostic factors for PFS
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age, <70 y | 1.428 | 0.75-2.71 | .2752 | |||
| Female | 1.146 | 0.62-2.1 | .6628 | |||
| ECOG PS 2 | 2.378 | 1.19-4.74 | .0138 | |||
| B symptoms | 1.944 | 1.03-3.68 | .0416 | |||
| Ann Arbor stage IV | 2.194 | 1.10-4.36 | .0249 | |||
| Extranodal site >1 | 2.066 | 1.12-3.80 | .0194 | |||
| Medullar and bone involvement | 2.559 | 1.33-4.93 | .0050 | |||
| Liver involvement | 2.595 | 1.31-5.16 | .0065 | 2.593 | 1.28-5.25 | .0081 |
| Lung and pleural involvement | 1.480 | 0.68-3.20 | .3192 | |||
| Mass >7 cm | 2.787 | 1.39-5.60 | .0040 | |||
| Nodular sclerosis subtype | 1.194 | 0.45-3.19 | .7246 | |||
| LMP1 positive | 1.471 | 0.76-2.84 | .2487 | |||
| EBER positive | 2.084 | 1.04-4.19 | .0396 | |||
| CD4+ T cells <0.41 × 109/L∗ | 1.990 | 1.04-3.8 | .0367 | |||
| CD8+ T cells <0.247 × 109/L∗ | 1.293 | 0.69-2.43 | .4257 | |||
| Abnormal LDH level | 1.112 | 0.61-2.04 | .7328 | |||
| Albumin level <30 g/L | 3.608 | 1.94-6.7 | <.0001 | |||
| Hemoglobin level <10.5 g/dL | 2.161 | 1.15-4.07 | .0172 | |||
| Leukocyte count ≥12 × 109/L | 2.111 | 1.13-3.94 | .0190 | |||
| Lymphocyte count <0.8 × 109/L | 3.428 | 1.86-6.32 | <.0001 | 4.105 | 2.15-7.83 | <.0001 |
| B2-microglobulin level >3 g/L | 1.918 | 1.00-3.68 | .0499 | |||
| C-reactive protein level >88 mg/L | 2.625 | 1.42-4.86 | .0022 | 2.602 | 1.38-4.90 | .003 |
| >5 Medications unrelated to lymphoma | 2.436 | 1.30-4.57 | .0055 | 2.605 | 1.37-4.96 | .0035 |
| CIRS-G score ≥7 | 1.465 | 0.70-3.06 | .3085 | |||
| Severity index ≥2 | 1.078 | 0.47-2.46 | .8584 | |||
| TMTV >450 mL | 2.634 | 1.35-5.16 | .0047 | |||
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Age, <70 y | 1.428 | 0.75-2.71 | .2752 | |||
| Female | 1.146 | 0.62-2.1 | .6628 | |||
| ECOG PS 2 | 2.378 | 1.19-4.74 | .0138 | |||
| B symptoms | 1.944 | 1.03-3.68 | .0416 | |||
| Ann Arbor stage IV | 2.194 | 1.10-4.36 | .0249 | |||
| Extranodal site >1 | 2.066 | 1.12-3.80 | .0194 | |||
| Medullar and bone involvement | 2.559 | 1.33-4.93 | .0050 | |||
| Liver involvement | 2.595 | 1.31-5.16 | .0065 | 2.593 | 1.28-5.25 | .0081 |
| Lung and pleural involvement | 1.480 | 0.68-3.20 | .3192 | |||
| Mass >7 cm | 2.787 | 1.39-5.60 | .0040 | |||
| Nodular sclerosis subtype | 1.194 | 0.45-3.19 | .7246 | |||
| LMP1 positive | 1.471 | 0.76-2.84 | .2487 | |||
| EBER positive | 2.084 | 1.04-4.19 | .0396 | |||
| CD4+ T cells <0.41 × 109/L∗ | 1.990 | 1.04-3.8 | .0367 | |||
| CD8+ T cells <0.247 × 109/L∗ | 1.293 | 0.69-2.43 | .4257 | |||
| Abnormal LDH level | 1.112 | 0.61-2.04 | .7328 | |||
| Albumin level <30 g/L | 3.608 | 1.94-6.7 | <.0001 | |||
| Hemoglobin level <10.5 g/dL | 2.161 | 1.15-4.07 | .0172 | |||
| Leukocyte count ≥12 × 109/L | 2.111 | 1.13-3.94 | .0190 | |||
| Lymphocyte count <0.8 × 109/L | 3.428 | 1.86-6.32 | <.0001 | 4.105 | 2.15-7.83 | <.0001 |
| B2-microglobulin level >3 g/L | 1.918 | 1.00-3.68 | .0499 | |||
| C-reactive protein level >88 mg/L | 2.625 | 1.42-4.86 | .0022 | 2.602 | 1.38-4.90 | .003 |
| >5 Medications unrelated to lymphoma | 2.436 | 1.30-4.57 | .0055 | 2.605 | 1.37-4.96 | .0035 |
| CIRS-G score ≥7 | 1.465 | 0.70-3.06 | .3085 | |||
| Severity index ≥2 | 1.078 | 0.47-2.46 | .8584 | |||
| TMTV >450 mL | 2.634 | 1.35-5.16 | .0047 | |||
EBER, EBV-encoded RNA; LMP1, latent membrane protein 1.
Corresponding to the median CD4 and CD8 T-cell counts.
In a second model using only the International Prognostic Score (IPS) and TMTV, IPS was associated with PFS in multivariate analysis with TMTV (>450 mL). The HRs were 2.97 (95% CI, 1.36-6.45; P = .006) and 3.72 (95% CI, 1.62-8.56; P = .002) for patients with IPSs of 4 and ≥5, respectively (reference group IPS, 1-3), and 2.27 (95% CI, 1.12-4.59; P = .02) for TMTV (supplemental Figure 3).
To estimate the cause-specific HR for the event of interest (progression due to HL) in the presence of competing risk events (death from other causes), patients who did not progress and died from other causes were censored. Exploratory univariate and multivariate analyses were performed to obtain cause-specific HRs (supplemental Table 3).26,27 In multivariate analysis, B symptoms (cause-specific HR, 2.829; 95% CI, 1.26-6.349; P = .0117), albumin (cause-specific HR, 3.274; 95% CI, 1.565-6.850; P = .0016), and comedications unrelated to HL (cause-specific HR, 3.140; 95% CI, 1.447-6.815; P = .0038) were independently associated with disease progression in the presence of competing risk events.
Discussion
In this prospective, phase 2 trial, we confirmed the feasibility of a new combination chemotherapy comprising bendamustine, doxorubicin, vinblastine, and prednisone for older patients with newly diagnosed HL with advanced-stage disease, an ECOG PS of 0 to 2, adequate cardiac and renal functions, and a mandatory geriatric assessment with MNA for patients aged ≥70 years to limit acute toxicity.12 Evens et al showed that prospective evaluations of comorbidities and functional status were feasible in a multicenter setting,23 and we confirmed these findings in our study.
The PVAB regimen resulted in a high CMR (77.5%), as confirmed by a blind central review (78.5%). When this trial was designed, there were no prospective trials using PET/CT for treatment evaluation. Our results were close to those of recent prospective trials using PET/CT for response evaluation, with CMR ranging from 65% to 90% (Table 5).
Summary of retrospective and prospective studies for cHL in older patients
| . | N . | Period . | Age (range), y . | Advanced stage . | Treatment . | CR rate . | Toxic death . | Infectious toxicities∗ . | PFS . | OS . |
|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective studies | ||||||||||
| Evens et al4 | 95† | 1999-2009 | 67 60-89 | 64% | ABVD | 75% | 6% | — | 44% at 5 y | 58% at 5 y |
| Stamatoullas et al5 | 147 | 1997-2012 | 68 60-88 | 57% | ABVD | 80% | 10% | — | — | 67% at 5 y |
| Orellana-Noia et al30 | 190‡ | 2010-2018 | 67 60-88 | 64% | ABVD, AVD, BV+AVD, Stanford V | — | 3.3% | — | 67% at 3 y | 87% at 3 y |
| Wahlin et al40 | 691 | 2000-2014 | —61-99 | 51% | ABVD CHOP | — | — | — | — | 75% at 5 y ≈40% at 5 y |
| Cheng et al31 | 401 | 2000-2019 | 70 60-93 | 72% | ABVD, AVD, BV+AVD, Others | — | 5% | — | 50% at 5 y | 54% at 5 y |
| Övergaard et al32 | 1554 | 2000-2021 | 70 60-94 | — | ABVD AVD CHOP Other | 63% at 5 y 64% at 5 y 46% at 5 y 39% at 5 y | ||||
| Prospective studies | ||||||||||
| Böll et al6 | 59 | 2004-2007 | 68 60-75 | 100% | PVAG | 78% | 2% | G3/4 infections: 23% | 58% at 3 y | 66% at 3 y |
| Proctor et al7 | 103 | 2004-2009 | 73 61-85 | 70% | VEPEMB | 65% | 3% | FN: 16% G3/4 infections: 3% | 53% at 3 y | 62% at 3 y |
| Evens et al19 | 45 | 1999-2006 | 65 60-83 | 93% | ABVD vs Stanford V | 64% | 9% | FN: 8% FN: 15% | 48% at 5 y | 58% at 5 y |
| Böll et al29 | 49 | 2015-2017 | 66 60-84 | 100% | BV-CAP | 65%§ | 2% | FN: 27% G3/4 infections: 33% | 74% at 1 y | 92% at 1 y |
| Evens et al23 | 48 | 2012-2016 | 69 60-88 | 81% | BV × 2 AVD × 6 BV × 4 | 93%§ | 2% | FN: 8% | 84% at 2 y | 93% at 2 y |
| Evens et al28 | 186 | 2012-2016 | 67 60-83 | 100% | ABVD vs BV-AVD | 71%§ 74%§ | 4.4% | FN: 17% FN: 37% | 67% at 5 y 62% at 5 y | — |
| Torka et al41 | 33 | — | Nivolumab AVD × 6 | 97%§ | 0% | FN: 8% | 86% at 2 y | 96% at 2 y | ||
| PVAB study | 89 | 2015-2018 | 68 61-88 | 100% | PVAB | 77%§ | 4% | FN: 7% G3/4 infections: 10% | 50% at 4 y | 69% at 4 y |
| . | N . | Period . | Age (range), y . | Advanced stage . | Treatment . | CR rate . | Toxic death . | Infectious toxicities∗ . | PFS . | OS . |
|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective studies | ||||||||||
| Evens et al4 | 95† | 1999-2009 | 67 60-89 | 64% | ABVD | 75% | 6% | — | 44% at 5 y | 58% at 5 y |
| Stamatoullas et al5 | 147 | 1997-2012 | 68 60-88 | 57% | ABVD | 80% | 10% | — | — | 67% at 5 y |
| Orellana-Noia et al30 | 190‡ | 2010-2018 | 67 60-88 | 64% | ABVD, AVD, BV+AVD, Stanford V | — | 3.3% | — | 67% at 3 y | 87% at 3 y |
| Wahlin et al40 | 691 | 2000-2014 | —61-99 | 51% | ABVD CHOP | — | — | — | — | 75% at 5 y ≈40% at 5 y |
| Cheng et al31 | 401 | 2000-2019 | 70 60-93 | 72% | ABVD, AVD, BV+AVD, Others | — | 5% | — | 50% at 5 y | 54% at 5 y |
| Övergaard et al32 | 1554 | 2000-2021 | 70 60-94 | — | ABVD AVD CHOP Other | 63% at 5 y 64% at 5 y 46% at 5 y 39% at 5 y | ||||
| Prospective studies | ||||||||||
| Böll et al6 | 59 | 2004-2007 | 68 60-75 | 100% | PVAG | 78% | 2% | G3/4 infections: 23% | 58% at 3 y | 66% at 3 y |
| Proctor et al7 | 103 | 2004-2009 | 73 61-85 | 70% | VEPEMB | 65% | 3% | FN: 16% G3/4 infections: 3% | 53% at 3 y | 62% at 3 y |
| Evens et al19 | 45 | 1999-2006 | 65 60-83 | 93% | ABVD vs Stanford V | 64% | 9% | FN: 8% FN: 15% | 48% at 5 y | 58% at 5 y |
| Böll et al29 | 49 | 2015-2017 | 66 60-84 | 100% | BV-CAP | 65%§ | 2% | FN: 27% G3/4 infections: 33% | 74% at 1 y | 92% at 1 y |
| Evens et al23 | 48 | 2012-2016 | 69 60-88 | 81% | BV × 2 AVD × 6 BV × 4 | 93%§ | 2% | FN: 8% | 84% at 2 y | 93% at 2 y |
| Evens et al28 | 186 | 2012-2016 | 67 60-83 | 100% | ABVD vs BV-AVD | 71%§ 74%§ | 4.4% | FN: 17% FN: 37% | 67% at 5 y 62% at 5 y | — |
| Torka et al41 | 33 | — | Nivolumab AVD × 6 | 97%§ | 0% | FN: 8% | 86% at 2 y | 96% at 2 y | ||
| PVAB study | 89 | 2015-2018 | 68 61-88 | 100% | PVAB | 77%§ | 4% | FN: 7% G3/4 infections: 10% | 50% at 4 y | 69% at 4 y |
CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; PVAG, prednisone, vinblastine, doxorubicin, and gemcitabine; VEPEMB, vinblastine, cyclophosphamide, procarbazine, prednisolone, etoposide, mitoxantrone, and bleomycin; BV-CAP, brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; FN, febrile neutropenia; G3/4, grade 3 or 4.
Documented for prospective studies.
67 patients treated with ABVD.
244 patients, including 190 patients treated with conventional therapies and 54 with alternative regimens.
Evaluation by PET/CT.
Toxicity was mainly observed in patients aged ≥70 years, with 37% related SAEs compared with 9.3% for younger patients. The MNA was helpful for predicting toxicity; 33% of the patients with a normal MNA score (>23.5) presented with at least 1 related SAE compared with 44% of patients with an abnormal MNA score.12 We also observed a trend for a higher rate of related SAEs among frail (56%) patients than in fit (23%), unfit (33%) patients,22 reinforcing the importance of geriatric assessments to evaluate the risk of toxicity.
In the ECHELON-1 trial, 37% and 17% of older patients treated with BV-AVD (doxorubicin, vinblastine, and dacarbazine) or ABVD, respectively, presented febrile neutropenia, but G-CSF was not mandatory at the beginning of the trial.28 With the brentuximab vedotin, cyclophosphamide, doxorubicine, prednisone (B-CAP) regimen and mandatory G-CSF support, febrile neutropenia was observed in 27% of patients.29 Despite the high dose of bendamustine (120 mg/m2) used in the PVAB regimen, there were 7 events (6 patients [8%]) of febrile neutropenia with the mandatory use of G-CSF and antibioprophylaxis (Table 5).
All studies of patients aged ≥60 years confirmed the problem of deaths attributable to bleomycin lung toxicity with the ABVD regimen, which suggests the use of regimens without bleomycin.30,31 In addition, a large retrospective study showed a similar outcome between ABVD and AVD regimens with less toxicity.32
The in-study death rate for toxicity was close to those of recent trials or contemporary population-based studies, with toxic death in <5% of patients (Table 5). The sequential use of BV followed by AVD was associated with a better tolerability of chemotherapy with low rates of AEs after the first AVD cycle.23 With the PVAB regimen, we observed that 3 of the 4 fatal events occurred after cycle 1, and a significant proportion of SAEs occurred after cycle 1 (17%) and after the last cycle (22%) (supplemental Table 4). As suggested by Evens et al, an initial immunotherapy that allows for disease control and an improvement in disease-related symptoms could increase patient tolerance for subsequent chemotherapy.23
Our prognostic analysis showed that 1 specific disease site (liver) and some biological parameters (C-reactive protein level and lymphocyte count) were associated with PFS. Lymphocyte count is a well-established prognostic parameter of IPS.33 The erythrocyte sedimentation rate, another inflammatory marker, is a well-known risk factor for early-stage HL.34 In multivariate analysis, TMTV was not significant. It is possible that metabolic volume has less prognostic impact in older patients than in younger patients or patients in the early stage, but this needs to be confirmed by further studies.18,35
We confirmed in our prospective trial some specificities observed for older patients with HL with a high rate of nonlymphoma causes of death36 that largely influenced the PFS rate of our trial (50% at 4 years). Event decomposition helped to better evaluate the cumulative incidence of progression/relapse in the presence of competitive risks. Patients in CMR after PVAB had the most favorable outcome, with a 4-year cumulative risk of relapse of 26.5% (supplemental Figure 2). Moccia et al confirmed the importance of the evaluation of multiple survival end points with differences between cause-specific survival (85.5% at 5 years), OS (64%), and PFS (53%) in a series of patients treated with curative intent.37
Several studies have shown the prognostic impact of comorbidities or the functional status of older patients.4,30 Orellana-Noia et al found that 18% of older patients with HL had a total CIRS-G score ≥ 10 (median CIRS-G score, 5) without a prognostic effect on PFS.30 The median CIRS-G score in our study was 3. We found that >5 concomitant treatments not related to lymphoma at baseline was an independent predictive factor for PFS. The multivariate analysis taking into account the presence of competitive risk events confirmed this association (supplemental Table 3). The completion of the CIRS-G scale requires some medical background by the rater, which could explain some discrepancies in scoring between studies. We speculate that medications taken by patients at the date of HL diagnosis could be a surrogate for active comorbidities. One limitation of our study is that geriatric assessment, especially functional scales, was performed only for patients aged ≥70 years. Within the limitation of the number of patients aged ≥70 years, patients with higher scores (ADL, IADL, MNA, G8, and sGA) had a trend for better PFS (supplemental Figure 1).
The choice of standard chemotherapy for older patients with cHL remains important, because alternative therapies or the use of nonanthracycline regimens seems to affect PFS and OS.38-40 In the ECHELON-1 trial for older patients, BV-AVD was not superior to ABVD, but sequential use of BV and AVD seemed to improve tolerance.23,28 In addition, BV is not reimbursed as a firstline therapy in all countries. In the absence of novel drugs, PVAB could be a valuable nonbleomycin regimen that results in high CMR and acceptable toxicity with a favorable outcome, especially in fit patients according to geriatric assessments. However, our trial demonstrated that the outcome for older patients with HL is closed regardless of the chemotherapy regimen used and needs to be improved. Integration of programmed cell death protein 1 (PD-1) inhibitors in the therapeutic armamentarium for older patients with HL may change this prognosis. PD-1 inhibitors with AVD resulted in a high CMR, a good safety profile, and promising outcome results in older patients with HL (Table 5).41 In the prospective phase 3 trial SWOG S1826, subgroup analyses showed favorable outcomes for patients aged ≥60 years treated with anti-PD1 + AVD compared with BV + AVD (HR, 0.27; 95% CI, 0.10-0.76).42 A high inclusion rate for this rarer subgroup of patients with cHL was observed in recent prospective trials of collaborative groups. This could be a great opportunity to design larger prospective trials.
Acknowledgments
The authors acknowledge the Lymphoma Study Association Clinical Research PVAB team and every research team and nurse in the participating centers.
Mundipharma provided bendamustine and funded the trial in part. The funder had no role in trial design, data collection, data analysis, data interpretation, or the writing of the report. Lymphoma Study Association Clinical Research is the sponsor of the PVAB trial.
Authorship
Contribution: H.G. designed the trial in collaboration with F. Morand; H.G., D.K., E.N.-V., A.C.G., S.G., M.T., K.L., F. Morschhauser, C.B., A.W.-R., F.O.-P., M.A., P.Q., and O.C. enrolled and treated patients; M.F. performed the statistical analysis; A.T.-G., D.D., and B.B. performed the pathology review; S.K. and A.B.-R. performed the PET review; H.G., S.K., M.F., F. Morand, A.B.-R., B.B., D.D., A.T.-G., and O.C. analyzed the results; H.G. wrote the first draft of the manuscript; and all authors approved the final version of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: S.K. is the founder of Pixilib (the GaelO platform editor used in this study for PET/CT centralized reading). M.A. reports an advisory role in Takeda, Bristol Myers Squibb, Karyopharm, Gilead, and Incyte; research grants from Roche, Johnson & Johnson, and Takeda; travel grants from Roche, Bristol Myers Squib, Celgene, Gilead, AbbVie, and AstraZeneca. A.W.-R. reports honoraria from Janssen and AbbVie. O.C. reports honoraria from Roche, Takeda, Bristol Myers Squibb, Merck, Gilead, Janssen, Antibody Drug Conjuguates Therapeutics, Incyte, and AstraZeneca; advisory role in Roche, Takeda, Bristol Myers Squibb, Merck, Gilead, Janssen, Antibody Drug Conjuguates Therapeutics, Incyte, and AstraZeneca; research grants from Roche, Takeda, Gilead, and AbbVie; and travel grants from Roche, Takeda, Janssen, and AbbVie. F.O.-P. reports advisory board participation in Bristol Myers Squibb/Celgene and Janssen. The remaining authors report no competing financial interests.
Correspondence: Hervé Ghesquières, Département d’Hématologie, Hôpital Lyon Sud, Hospices Civils de Lyon, 165 Chemin du Grand Revoyet, 69495 Pierre-Bénite, France; email: herve.ghesquieres@chu-lyon.fr.
References
Author notes
Presented at the American Society of Hematology Annual Meeting, Orlando, FL, 7 to 10 December 2019, and Atlanta, GA, 11 to 14 December 2021.
Research data will be shared upon reasonable project outline on request from the corresponding author, Hervé Ghesquières (herve.ghesquieres@chu-lyon.fr).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal