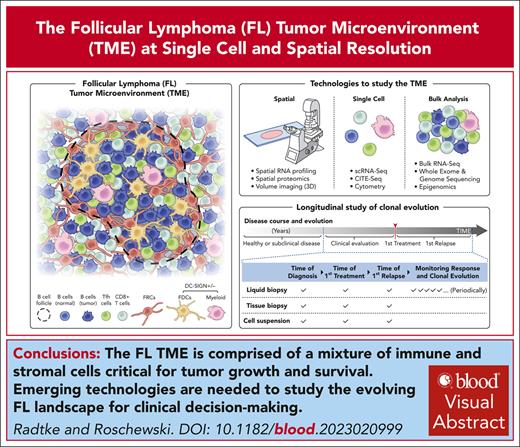
Visual Abstract
Follicular lymphoma (FL) is a generally incurable malignancy that originates from developmentally blocked germinal center B cells residing, primarily, within lymph nodes (LNs). During the long natural history of FL, malignant B cells often disseminate to multiple LNs and can affect virtually any organ. Nonmalignant LNs are highly organized structures distributed throughout the body, in which they perform functions critical for host defense. In FL, the malignant B cells “re-educate” the lymphoid environment by altering the phenotype, distribution, and abundance of other cells such as T cells, macrophages, and subsets of stromal cells. Consequently, dramatic anatomical changes occur and include alterations in the number, shape, and size of neoplastic follicles with an accompanying attenuation of the T-cell zone. Ongoing and dynamic interactions between FL B cells and the tumor microenvironment (TME) result in significant clinical heterogeneity observed both within and across patients. Over time, FL evolves into pathological variants associated with distinct outcomes, ranging from an indolent disease to more aggressive clinical courses with early death. Given the importance of both cell-intrinsic and -extrinsic factors in shaping disease progression and patient survival, comprehensive examination of FL tumors is critical. Here, we describe the cellular composition and architecture of normal and malignant human LNs and provide a broad overview of emerging technologies for deconstructing the FL TME at single-cell and spatial resolution. We additionally discuss the importance of capturing samples at landmark time points as well as longitudinally for clinical decision-making.
Overview of FL
Follicular lymphoma (FL) is a malignancy of germinal center (GC) B-cell origin with a wide range of clinical behavior. Most patients experience an indolent clinical course characterized by a relapsing and remitting disease over many years or decades. Approximately 15% to 20% of patients present with a more aggressive clinical course characterized by early disease progression after therapy, histologic transformation, and premature death from lymphoma.1-4 The biologic basis underlying this broad range of clinical behavior is largely unknown but is postulated to involve a dynamic interplay between intrinsic factors such as tumor genetics and epigenetics with extrinsic factors including the tumor microenvironment (TME) and immune surveillance. No available clinical tools reliably predict disease behavior.
The long natural history of FL further contributes to the diversity of clinical behavior observed because the disease perpetually changes over time due to clonal evolution and the selective pressure of multiple therapies. Indeed, patients with an initial indolent disease course can undergo rapid transformation to a more aggressive disease without a clear inciting event. The study of FL is challenged by the need to account for this diversity across patients but also to longitudinally assess changes within patients over time.
FL is typically characterized by clonal proliferation of B cells with a t(14;18) chromosomal alteration, a genetic hallmark and founding lesion that appears in >85% of FL cases.4,5 The stepwise evolution of t(14;18)-positive FL begins with ectopic overexpression of the antiapoptotic protein BCL2 and follows a multistep pathogenesis involving GC re-entry, antigen stimulation, and accumulation of additional genetic and epigenetic alterations to facilitate malignant transformation.6 The accumulation of advantageous genetic events and the expansion of select clones over time contributes to the distinct clinical outcomes observed across patients with FL.1-3,7,8 Beyond t(14;18) translocations, a high frequency of mutations in epigenetic regulators including KMT2D, CREBBP, EZH2, and EP300 control GC transcriptional programs and contribute to immune escape and apoptosis resistance.4,9-11 In addition to these cell-intrinsic factors, TME is critical for supporting the survival and evolution of malignant clones.12,13 Furthermore, surface immunoglobulin is universally retained among FL B cells, indicating a strong selective pressure for B-cell receptor (BCR) engagement in the TME.14 Finally, several lines of evidence demonstrate that FL B cells “re-educate” the TME through the recruitment and reprogramming of diverse cell types to support malignant growth and survival.10
The general outlook for patients with FL continues to improve, and the expected survival for patients with FL has increased with each decade owing to improvements in diagnostic and monitoring tools along with the emergence of novel therapies.15 Still, the most common cause of death for patients with FL is lymphoma related, and the efficacy of therapy diminishes over time.16,17 In parallel, several methods for studying FL have emerged, including single cell RNA sequencing (scRNA-seq) and imaging methods that can be applied across patients and sequentially over time to better characterize the biology of disease progression.
Architecture of normal LNs
Although FL often disseminates to other organs including the bone marrow and gastrointestinal system, the most common form of FL is primarily nodal.4,6 Lymph nodes (LNs) are highly organized structures responsible for the generation of innate and adaptive immune responses, filtering of lymphatic fluid, and concentrating peripheral tissue antigens into discrete foci.18-20 Humans are estimated to have 500 to 600 LNs distributed throughout the body along a systemic network of lymphatic vessels.21 The cellular composition and functional properties of LNs vary depending on anatomical location and disease status22-24; however, major anatomical structures are conserved and can be subdivided into the capsule, subcapsular sinus, outer cortex, paracortex, and medulla.25The capsule is the dense connective tissue stroma surrounding the LN parenchyma. The subcapsular sinus is within the anatomic space between the capsule and outer cortex and serves to transport lymphatic fluid from the afferent lymphatic vessels to the medullary sinus in the medulla. Primary and secondary B-cell follicles are located in the outer cortex layer and are characterized by having large concentrations of B cells and follicular dendritic cells (FDCs), specialized stromal cells that trap and retain antigens for long periods of time.26 The paracortex or T-cell zone is enriched with T cells, dendritic cells (DCs), and fibroblastic reticular cells (FRCs). Lastly, the medulla contains a rich plexus of large blood vessels, sinuses, and medullary cords populated by antibody-secreting plasma cells, macrophages, and reticular cells.27 Lymph is filtered through the medullary and cortical sinuses before exiting the LN and returning to the lymphatic system through the efferent lymphatic vessels.28
Lymph node stromal cells, FRCs and FDCs, are critical for establishing the macroanatomical structures of the LN as well as local microenvironments for T-cell priming, tolerance, and the generation of humoral immunity.29-31 Within the paracortex of normal LNs, FRCs form a scaffold for T-cell migration and an adhesive substrate for DCs sampling lymph-borne molecules transported by the conduit network,32 thus ensuring that rare antigen-bearing DCs encounter their cognate T cells.20,29,33 In contrast, FDCs support B-cell survival, localization, and tingible body macrophage-mediated apoptosis through the secretion of B-cell activating factor, the chemokine CXCL13, and the milk fat globule epidermal growth factor 8 protein (MFGE8), respectively.34-36 During an immune response, light zone (LZ) FDCs display antigens to maturing B cells and are characterized by high expression of CXCL13 and several complement and Fc receptors, for example, CR1 (CD35), CR2 (CD21), and FcεRII (CD23).26 Dark zone (DZ) FDCs, also known as CXCL12-expressing reticular cells, support the GC reaction by establishing local chemokine gradients with CXCL13+ LZ FDCs.31,37,38 FDCs play an integral role in B-cell selection by retaining antigen in the form of immune complexes that can be captured by B cells and subsequently presented to T follicular helper (Tfh) cells in proportion to their BCR affinity.
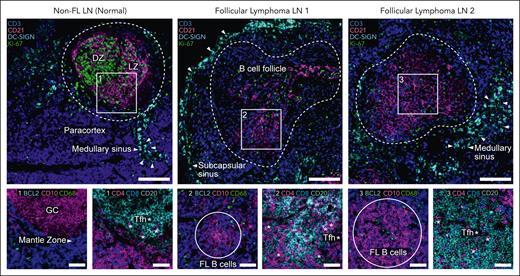
GCs are dynamic structures that facilitate the evolution of antigen-specific B cells and plasma cells through coordinated interactions between B cells, Tfh cells, tingible body macrophages, and FDCs.20 Optimal B-cell responses are achieved through iterative rounds of mutation, expansion, and selection within the GC. During this process, B cells alter the structure of their immunoglobulin through 2 distinct mechanisms: class switch recombination and somatic hypermutation. The organization and function of the GC has been extensively reviewed elsewhere.39,40 Here, we only introduce the GC as a well-defined structure in normal LNs, consisting of a LZ and DZ (Figure 1), that is altered by FL tumor B cells over the course of disease.
Spatial organization and cellular composition of non-FL and FL LNs from human patients. Cell DIVE41 imaging platform and IBEX42 dye inactivation protocol were combined for high parameter imaging (Cell DIVE-IBEX). Changes in the organization and cellular composition of FL LNs as compared with non-FL (normal) LNs. B-cell follicle outlined by dotted line. Arrows denote medullary or subcapsular sinus enriched with DC-SIGN+ myeloid cells. Scale bar, 150 μm (large images) and 50 μm (small images). LZ and DZ of GC. Example Tfh cells marked by asterisks. IBEX, iterative bleaching extends multiplexity.
Spatial organization and cellular composition of non-FL and FL LNs from human patients. Cell DIVE41 imaging platform and IBEX42 dye inactivation protocol were combined for high parameter imaging (Cell DIVE-IBEX). Changes in the organization and cellular composition of FL LNs as compared with non-FL (normal) LNs. B-cell follicle outlined by dotted line. Arrows denote medullary or subcapsular sinus enriched with DC-SIGN+ myeloid cells. Scale bar, 150 μm (large images) and 50 μm (small images). LZ and DZ of GC. Example Tfh cells marked by asterisks. IBEX, iterative bleaching extends multiplexity.
Much knowledge of the architecture and cellular composition of LNs has been obtained from mouse studies; however, the last few years have seen significant investment in international mapping efforts focused on characterizing healthy human tissues, including parenchymatous organs of the complex lymphatic system.43 One of these consortia, the Human BioMolecular Atlas Program (HuBMAP),44 has created versioned, publicly available tables cataloging the anatomical structures, cell types, and biomarkers of diverse human tissues. The human LN table reports 34 anatomical structures and 45 cell types based on data derived from traditional pathology, scRNA-seq, and advanced multiplexed imaging.45
Changes in the architecture and cellular composition of FL LNs
In contrast to healthy human LNs, FL LNs are disorganized, show complete or partial effacement of normal LN architecture with neoplastic follicles, and exhibit an attenuation of anatomical structures normally found in nonmalignant LNs, for example, T-cell zone (paracortex) and mantle zone (Figure 1).46 Despite these well-described changes, the FL TME retains remnants of normal lymphoid tissue architecture. GCs in neoplastic follicles are typically unpolarized, no LZ or DZ, and often display reduced numbers of tingible body macrophages and FDC networks as the disease progresses10 (Figure 1). Malignant LNs mostly comprise tumor B cells expressing BCL2 and CD10, although the tumor cell content is variable depending on the grade.5 Consistent with an indolent natural history, most tumor B cells exhibit low proliferation indices; however, the increased presence of Ki-67+ B cells in biopsies may be associated with a more aggressive disease course.5,47
Beyond these large anatomical changes, tumor LNs vary significantly in their cellular composition compared with control lymphoid tissues.4,10,48 Unsurprisingly, malignant B cells are the dominant cell type of the TME and are typically defined by positive labeling of BCL2, CD10, and classical GC B-cell markers (CD20 and BCL6) by immunohistochemistry with expression of these markers varying by FL grade and LN region, for example, follicle vs interfollicular area.4 In recent years, advanced sequencing and spatial technologies have provided additional insight into the distinct phenotypes and states of tumor B cells in patients with FL.49-51 Together, these studies demonstrate a loss of GC-specific synchronized gene expression patterns in FL B cells52 and reveal the importance of the TME in shaping tumor B-cell heterogeneity with distinct phenotypes mapped to different regions of malignant LNs.51,53 Nevertheless, the phenotype of FL B cells supports their origin as GC B cells4 and propensity to colonize reactive GCs in lymphoid tissues.54
T cells are the next most abundant cell type in the TME comprising >87% of nonmalignant immune cells based on sequencing of dissociated FL samples.55 However, these numbers should be interpreted with caution because they reflect cells that can be readily extracted from the tumor and likely undersample the stromal and myeloid populations of the FL TME.51 These caveats notwithstanding, the FL microenvironment is enriched for CD4+ T-cell subsets including Tfh cells, T regulatory cells (Tregs), T follicular regulatory cells, T effector subpopulations (memory, activated, and exhausted), and cytotoxic CD4+ T cells expressing effector molecules such as perforin and granzymes.50,55 FL-derived Tfh cells have a distinct differentiation pattern from their nonmalignant counterparts, overexpressing genes favoring lymphomagenesis such as TNF, LTA, IL4, and CD40LG.56,57 Importantly, Tfh cells contribute to the resistance of tumor B cells to rituximab-mediated apoptosis through secretion of interleukin-4 (IL-4) and CD40 ligand stimulation in the TME.56,58
In addition to Tfh cells, elevated numbers of Tregs with enhanced suppressive activity are found in FL LNs,59,60 and their enrichment within and around neoplastic follicles has been associated with poor patient survival.61 The FL TME may direct Treg differentiation to T follicular regulatory cells through CXCR5-dependent migration to the follicle, providing a potential mechanism of immunosurveillance suppression.56,59 Conversely, Tfh cells may give rise to a population of IKZF3+ Tregs with strong suppressive capacity as recently demonstrated in a multimodal study evaluating the T-cell landscape of FL and other B-cell lymphomas.62 Lastly, tumor-infiltrating CD8+ T cells represent a functional continuum from naïve, effector, and exhausted states, the latter defined by high expression of inhibitory immune-checkpoint genes (TIGHT, LAG3, and PDCD1 [PD-1]).51,55,62 Despite the presence of Tfh cells (usually CXCR5+PD-1+) and other T-cell populations expressing inhibitory receptors,49,55,62 immune-checkpoint blockade has not been as effective in FL as in other lymphoma subtypes such as Hodgkin lymphoma and primary mediastinal B-cell lymphoma. The underlying reasons for this lack of robust clinical activity with PD-1 inhibitors is likely related to the TME composition, but this requires further study, and other methods for targeting immunosuppressive T cells in FL may be more effective.
Han et al identified 4 major subtypes of FL based on the expression of major histocompatibility complex class II (MHC II) genes and the presence of distinct T-cell subsets in the TME. This work highlights the contribution of tumor MHC II expression on immune exhaustion, with MHC II high tumors exhibiting increased proportions of exhausted CD8+ T cells and cytotoxic CD4+ T cells. In contrast, patients with mutation-driven loss of MHC II expression displayed reduced numbers of activated CD4+ and CD8+ T cells in the TME, reflecting a mechanism of potential immune evasion.55,63 In further support of this idea, patients with high-risk FL have reduced numbers of immune-infiltrating cells including T cells.64
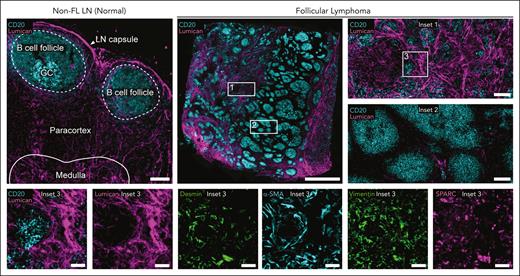
Lymphoid stromal cells (LSCs) of the FL TME have been shown to promote B-cell survival and confer therapeutic resistance.65,66 LSCs may alter the phenotype of tumor-infiltrating CD4+ T cells while also recruiting other cells to the FL TME, for example, neutrophils and monocytes.67-69 FRCs in the FL TME can be characterized by high, albeit heterogeneous, expression of several markers common to myofibroblasts: desmin, vimentin, and α-smooth muscle actin (Figure 2). Using a range of functional, transcriptional, and histological assays, Mourcin et al revealed the presence of CXCL13+ FRCs around neoplastic follicles. These cells were distinguished by the expression of chemokines (CXCL12, CXCL13, CCL19, and CCL21), growth factors (IL7 and BAFF), extracellular matrix (ECM) components, and factors promoting FL-LSC cross talk.70 In another study,51 patients with high-risk FL could be distinguished from other patients based on the enrichment of desmin+vimentin+ fibroblasts and ECM-associated proteins including lumican as well as secreted protein acidic and rich in cysteine (SPARC), a protein expressed by macrophages, endothelial cells, and stromal cells involved in cell-matrix interactions (Figure 2).71 Furthermore, cytokines from tumor B cells and nonneoplastic T cells (IL-4 and IL-13) have been shown to increase the secretion of transglutaminase, a cross-linking enzyme affecting ECM organization.72 Together, the fibroproliferative potential of FRCs contributes to a stiff ECM that may inhibit the transport of nutrients, therapeutic agents, and cells in the TME.73
Stromal remodeling and ECM deposition in FL TME. Cell DIVE-IBEX images of tissue sections from excisional LN biopsies. Non-FL LN, scale bar, 150 μm. FL overview, scale bar, 2 mm. (Insets 1-2) Images depict heterogeneity in the amount of ECM (lumican); scale bars, 150 μm. (Insets 3) Stromal diversity using indicated markers; scale bar, 50 μm. α-SMA, α-smooth muscle actin; IBEX, iterative bleaching extends multiplexity; SPARC, secreted protein acidic and rich in cysteine.
Stromal remodeling and ECM deposition in FL TME. Cell DIVE-IBEX images of tissue sections from excisional LN biopsies. Non-FL LN, scale bar, 150 μm. FL overview, scale bar, 2 mm. (Insets 1-2) Images depict heterogeneity in the amount of ECM (lumican); scale bars, 150 μm. (Insets 3) Stromal diversity using indicated markers; scale bar, 50 μm. α-SMA, α-smooth muscle actin; IBEX, iterative bleaching extends multiplexity; SPARC, secreted protein acidic and rich in cysteine.
Beyond FRCs and FDCs, several subpopulations of lymphatic endothelial cells, blood endothelial cells, and nonendothelial stromal cells in human LNs have been delineated using scRNA-seq.74,75 A comprehensive single-cell transcriptome atlas from 27 normal and lymphoma LNs revealed 30 subclusters of cells.75 A population of nonendothelial stromal cells, termed stromal cells at capsule adventitia, upregulated transcripts associated with the ECM and elastic fiber formation.76 Given their significance in FL, LSCs are the topic of a review in this special collection and require further study to identify specific markers for targeted FL treatment. Strategies for targeting tumor-associated stromal cells include, but are not limited to, preventing stromal cells from entering the TME, blocking communication between tumor and stromal cells, and even exogenous administration of mesenchymal stromal cells as a drug delivery mechanism.77
Lastly, human LNs are populated by a variety of macrophages and DCs, for example, migratory DCs, plasmacytoid DCs, tingible body macrophages, and myeloid cells expressing dendritic cell-specific intercellular adhesion molecule-3–grabbing nonintegrin (DC-SIGN).45,78 In nonmalignant LNs, these cells are strategically positioned in distinct anatomical locations to facilitate protection from lymph-borne pathogens as well as the generation of robust immune responses.20,25 Malignancy dramatically alters the landscape of phagocytic cells, most notably by polarizing macrophages to a more tumor supportive phenotype often through interactions with neighboring stromal cells.69 Previous studies have demonstrated that high levels of IL-4 can polarize LN-resident macrophages to an anti-inflammatory M2 type,4,69 leading to upregulation of DC-SIGN and subsequent engagement with mannosylated surface immunoglobulin on FL cells.14,79,80 In a process known as N-glycosylation, glycans accumulate in the antigen-binding sites of surface immunoglobulin, allowing tumor B cells to interact with lectin-expressing cells in the TME.14 The contributions of DC-SIGN to BCR survival signaling are the focus of another review in this special collection. For this reason, we concentrate on the cells expressing DC-SIGN in the FL TME, which include macrophages and, in a subset of patients with FL, FDCs (Figure 3).51
Location of DC-SIGN+ cells in the FL TME. Cell DIVE-IBEX images depicting location of cell types expressing DC-SIGN in non-FL and FL LNs and IRF4+BCL2+ tumor B cells in close proximity to DC-SIGN+ macrophages outside of the follicles. Scale bar, 50 μm (large images) and 25 μm (insets). Arrows show contact between DC-SIGN+ macrophages and IRF4+ cells. FDCs and DC-SIGN+ macrophages (MΦ). DC-SIGN+ macrophages defined by morphology and location in the LN.
Location of DC-SIGN+ cells in the FL TME. Cell DIVE-IBEX images depicting location of cell types expressing DC-SIGN in non-FL and FL LNs and IRF4+BCL2+ tumor B cells in close proximity to DC-SIGN+ macrophages outside of the follicles. Scale bar, 50 μm (large images) and 25 μm (insets). Arrows show contact between DC-SIGN+ macrophages and IRF4+ cells. FDCs and DC-SIGN+ macrophages (MΦ). DC-SIGN+ macrophages defined by morphology and location in the LN.
We initially found DC-SIGN+ FDC networks in a subset of patients with FL using distinct imaging platforms41,42 and 2 independent antibody clones applied to fixed-frozen81 and formalin-fixed, paraffin-embedded (FFPE) samples.51 Together with Freda Stevenson and Richard Burack, we have found DC-SIGN+ macrophages in the interfollicular area and sinuses of FL LNs; however, DC-SIGN+ FDC networks are only found in a subset of patients with FL and not all meshworks in a particular sample are positive for DC-SIGN. Although the clinical, if any, significance of these findings remains to be determined, it is tempting to speculate that FL B cells may engage with DC-SIGN+ FDCs in early stages of the disease when tumor B cells are more dependent on FDCs, and these networks remain intact.70 Once FL B cells disseminate outside of the follicles, they may become more dependent on intrafollicular DC-SIGN+ macrophages for tonic BCR signaling as suggested by the close proximity of IRF4+ malignant cells and DC-SIGN+ cells in the FL TME (Figure 3).51
Emerging technologies for studying an evolving landscape
High resolution surveys of the TME are needed to obtain a holistic portrait of the genetic, epigenetic, and cellular factors contributing to cancer progression. To this end, the field has seen a rapid expansion of methods empowering granular characterization of tissues across a range of resolutions (x and y coordinates) and volumes (depth z) (Figure 4A-B). Given the promise of these emerging methods and the limited space provided here, we refer the reader to reviews devoted to the wealth of spatial RNA profiling,82-84,88 multiplexed antibody-based imaging,82,85 and 3D volume imaging methods in the field.85,86 Here, we focus on a few examples along with considerations for their adoption to study the FL TME.
Overview of technologies for evaluating the cellular composition of tissues. (A) Spatial technologies organized by resolution (x-axis) and volume of tissue that can be profiled (y-axis). Number of analytes (∗) and resolutions are provided as an estimate only. These numbers are based on the literature44,82-87 and discussions with method developers. Numbers are subject to change as technology improves. Subcellular, <1 μm; single cell, 1-9 μm; multicell, 10-50 μm; and Ecosystem, 500 μm. (B) Simplified depiction of spatial approaches. Antibody-based detection∗ for spatial proteomics denotes that only optical microscopy approaches are represented here not IMC or MIBI-TOF. (C) Broad overview of technologies that can be applied to cell suspensions prepared from dissociated tissues. CITE-Seq, cellular indexing of transcriptomes and epitopes by sequencing; FISH, fluorescence in situ hybridization; H&E, hematoxylin and eosin; IHC, immunohistochemistry.
Overview of technologies for evaluating the cellular composition of tissues. (A) Spatial technologies organized by resolution (x-axis) and volume of tissue that can be profiled (y-axis). Number of analytes (∗) and resolutions are provided as an estimate only. These numbers are based on the literature44,82-87 and discussions with method developers. Numbers are subject to change as technology improves. Subcellular, <1 μm; single cell, 1-9 μm; multicell, 10-50 μm; and Ecosystem, 500 μm. (B) Simplified depiction of spatial approaches. Antibody-based detection∗ for spatial proteomics denotes that only optical microscopy approaches are represented here not IMC or MIBI-TOF. (C) Broad overview of technologies that can be applied to cell suspensions prepared from dissociated tissues. CITE-Seq, cellular indexing of transcriptomes and epitopes by sequencing; FISH, fluorescence in situ hybridization; H&E, hematoxylin and eosin; IHC, immunohistochemistry.
Hematoxylin and eosin, immunohistochemistry, and molecular cytogenic methods such as fluorescence in situ hybridization for t(14;18)(q32;q21) translocation89 are tissue-based assays used in the diagnosis of lymphoma.90 In addition to these low-parameter spatial assays, emerging methods allow for dozens to thousands of RNA or protein molecules to be visualized in tissues (Figure 4A). One class of techniques, spatially resolved transcriptomics, encompass a range of methods that can be subdivided into imaging-based methods that allow subcellular resolution of targeted RNA probes to spatial barcoded techniques that provide whole transcriptome readouts, albeit at lower resolution, using capture arrays and sequencing. Imaging-based methods can be further classified based on the generation of fluorescent signals by in situ sequencing or hybridization (Figure 4A-B).82,84 These technologies are restricted to thin tissue sections (<5-10 μm) and may have limited application to archived clinical samples due to poor RNA quality in heavily fixed samples.82-84 However, the following approaches have demonstrated compatibility with FFPE samples: Visium (10X Genomics), NanoString’s GeoMx Digital Spatial Profiler and CosMx Spatial Molecular Imager, and deterministic barcoding in tissue.84
In parallel, several spatial proteomic methods have been developed (Figure 4A) and can be broadly categorized into targeted approaches capable of detecting >5 to 100+ proteins or untargeted approaches for whole proteome analysis such as deep visual proteomics, an advanced application of laser capture microscopy combined with mass proteomics.91 Targeted approaches can be distinguished by the conjugate of antibody used for protein detection (fluorophore, DNA, or metal) (Figure 4A) as well as the method of immunolabeling and imaging, for example, cyclical or all-in-one as reviewed elsewhere.85 Several multiplexed antibody-based imaging methods have been applied to FFPE tissues from the clinic.41,51,62,92,93 However, antibody validation and panel construction remain significant obstacles for the widespread adoption of these methods with the average panel requiring 6 to 8 months to construct and tens of thousands of US dollars in antibody costs alone.85,94 High fidelity detection of protein biomarkers with antibodies is well-known to be context specific and dependent on several factors such as the tissue preservation method, antigen retrieval conditions, and even clone.85,94 The latter point is especially relevant as certain antibodies directed against BCL2 (clone 124) are known to give a false negative due to a point mutation.46 To this end, “open” methods such as iterative bleaching extends multiplexity,42,81 t-CyCIF,95 and Cell DIVE41 allow for the inclusion of well-characterized clones (clone SP66)46,51 over methods limited to proprietary reagents.
Most spatial methods described here have been applied to thin (<5-10 μm) tissue sections; however, FL grows in a permanent 3D structure with significant heterogeneity observed across a single tissue section (Figure 2). For these reasons, methods that allow for multiparameter 3D imaging (>100 to 2 mm) are promising because they empower the discovery of rare cells and can resolve the cellular composition of entire neoplastic follicles with intact FDC meshworks. Several clearing methods have been developed that allow for systems-wide study of mouse and human tissues.85,86 In general, a chemical clearing solution is applied to tissues, rendering them amenable to fluorescent probe detection and transparent for downstream confocal or light-sheet microscopy. At present, there are several obstacles that prevent widespread use of these methods such as low throughput (2-3 weeks per sample), limited demonstration in FFPE samples, lack of robust segmentation and analysis platforms, and the relatively low number of parameters that can be imaged in a specimen (Figure 4A).
In addition to these emerging spatial methods, single-cell and bulk methods have been applied to suspensions derived from dissociated tissues (Figure 4C). Single-cell technologies such as scRNA-seq and flow cytometry have provided unprecedented insights into the phenotype, clonotypes, and molecular programs of tumor B cells, tumor-infiltrating T cells, and stromal cells of the TME.49-51,55,57,62,70,75,96,97 Cellular indexing of transcriptomes and epitopes by sequencing (CITE-Seq) has the advantage of providing information on the transcriptome and surface proteins of individual cells. By linking RNA profiles with surface protein expression, CITE-Seq overcomes discrepancies in the abundance of RNA and protein molecules in a single cell and, thus, provides greater confidence in cell assignments.62,98 Despite a lack of resolution, bulk RNA-seq, whole-exome sequencing, and other methods offer a cost-effective approach for studying the mutational burden, copy number alterations, and cellular composition of various tumors.99 Additional studies have characterized genomic aberrations and transcriptomic differences in FL B cells shown to correlate with progression.12,96,100
The technical capabilities of each method, sample format, experimental aims, and access to instrumentation and trained personnel will dictate the most suitable platform to use. Nonetheless, several considerations for choosing the optimal spatial transcriptomics101 and multiplexed antibody-based imaging platforms85 have been summarized and include the study size, number of analytes to be investigated, resolution needed, and area of tissue to image. Multimodal approaches have classified tumors into distinct subtypes,55,102 identified cellular communities and spatial patterns associated with inferior prognosis,51,62 and evaluated drug responses of tumor subpopulations.97 Therefore, we recommend the integration of technologies that allow for transcriptomic, genomic, and proteomic profiling of FL LNs. Based on our experience, we favor spatial technologies compatible with whole slide, subcellular imaging of FFPE samples for correlating TME heterogeneity with clinical response.
Studying the FL TME at single-cell and spatial resolution over time
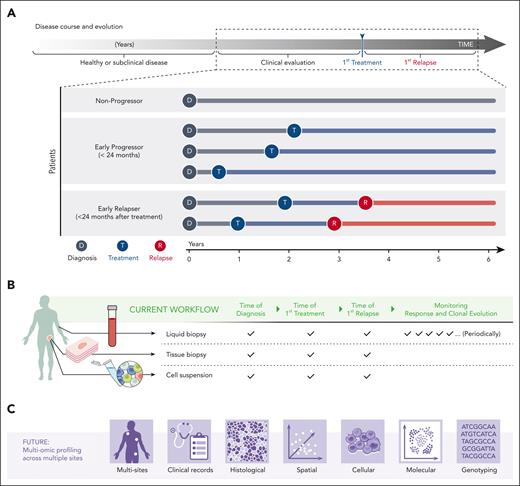
FL is highly heterogeneous as reflected by the number of morphological variants and subtypes identified with distinct pathological and clinical features.4,6,103 Furthermore, clinical outcomes among patients with FL range from an indolent disease with a median overall survival of >15 years to an aggressive course of disease involving histologic transformation into diffuse large B-cell lymphoma.1,4,10,96 Some patients do not need therapy at diagnosis and can be safely monitored for years without disease progression. Those with early progression of disease and those who relapsed early after initial therapy have an increased probability of premature death and are, therefore, considered the greatest risk.1-3,7,8 Importantly, clinical outcome remains largely unpredictable at diagnosis, as graphically depicted in Figure 5A, and risk stratification methods for patients who relapse early are still needed.7
Longitudinal study of clonal evolution. (A) FL has a spectrum of clinical behaviors that evolve over several years. The time from diagnosis to first treatment and to first relapse vary significantly across patients and are unpredictable at diagnosis. (B) Tools to study the biology of FL over time are evolving beyond tissue biopsies and include liquid biopsies at key landmark time points (time of diagnosis, time of first treatment, and time of first relapse). Liquid biopsies additionally empower longitudinal monitoring of the clinical response and clonal evolution. Excisional biopsies provide material for histological studies (tissue biopsy) and dissociated single cell and sequencing methods (cell suspension). (C) Future workflows may include profiling multiple LNs via multiomic technologies and integrating this data to obtain a holistic portrait of cell-intrinsic and extrinsic factors governing clinical outcome.
Longitudinal study of clonal evolution. (A) FL has a spectrum of clinical behaviors that evolve over several years. The time from diagnosis to first treatment and to first relapse vary significantly across patients and are unpredictable at diagnosis. (B) Tools to study the biology of FL over time are evolving beyond tissue biopsies and include liquid biopsies at key landmark time points (time of diagnosis, time of first treatment, and time of first relapse). Liquid biopsies additionally empower longitudinal monitoring of the clinical response and clonal evolution. Excisional biopsies provide material for histological studies (tissue biopsy) and dissociated single cell and sequencing methods (cell suspension). (C) Future workflows may include profiling multiple LNs via multiomic technologies and integrating this data to obtain a holistic portrait of cell-intrinsic and extrinsic factors governing clinical outcome.
Although dependent on study design, it is now possible to interrogate the FL TME at landmark time points, which include the time of diagnosis, first treatment, and first relapse, to obtain a comprehensive survey of the biology of pathogenesis and response to therapy (Figure 5B). Foundational to these efforts is access to sufficient patient material prepared in a manner compatible with downstream assays, for example, excisional LN biopsies banked as single-cell suspensions and FFPE blocks.90 To this end, we constructed a multiscale, multiomic atlas of the FL TME from patient samples collected before treatment.51 Using a variety of advanced sequencing and imaging methods, we demonstrated the upregulation of BCR signaling pathways in tumor B cells along with extensive stromal remodeling and changes to the follicular growth pattern of patients who experienced early relapse.
Detailed analysis at landmark time points provides only “static snapshots” and do not adequately address tumor evolution between sites and over time. To this point, whole-exome sequencing of spatially segregated tumors revealed intrapatient clonal diversity.104 Using scRNA-seq and flow cytometry, Haebe et al demonstrated site-to-site evolutionary divergence in the genetic and phenotypic profiles of patients with FL.50 Therefore, integration of multiomic data collected from different LNs, coupled with longitudinal profiling via liquid biopsies (Figure 5B-C), offer a holistic portrait of FL, a frequently disseminated disease. The approach outlined here ushers in an era of personalized medicine in which therapeutic interventions are targeted to and evolve with a patient’s unique TME, for example, immune augmentation to restore antigen presentation in MHC II low tumors or checkpoint inhibitors to reinvigorate T cells in MHC II high tumors.55,63 Beyond therapies known to enhance tumor-killing mechanisms, we additionally imagine therapies that inhibit tumor-promoting events such as DC-SIGN–mediated BCR signaling and stromal-tumor cross talk in the TME. Next generation sequencing and machine learning deconvolution algorithms105 have shown promise in the classification of diverse tumors into 4 distinct TME subtypes to aid with oncology clinical decision-making.102 In closing, we are optimistic that the parallel advancement of technologies and treatments applied to the FL TME will translate to improved clinical outcomes.
Acknowledgments
The authors are tremendously grateful for the resources and support provided by Ronald N. Germain, intellectual support from outstanding collaborators in the National Institutes of Health, National Cancer Institute (Louis Staudt, Elaine Jaffe, and Stefania Pittaluga) and BostonGene (Ekaterina Postovalova, Arina Varlamova, Alexander Bagaev, Anna Sharun, Daniil Wiebe, and Krystle Nomie), and constructive feedback from Danny Jonigk. The authors are indebted to Melinda Angus-Hill, Michael Smith, Rick Heil-Chapdelaine, and Prachi Bogetto of Leica Microsystems and Anne Wiblin of Abcam for their support with multiplexed imaging. The authors thank Li Yao for creating Figures 4 and 5.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases and National Cancer Institute.
Authorship
Contribution: A.J.R. and M.R. wrote the manuscript and outlined the figures together; A.J.R. created Figures 1-3 from resources provided to the laboratory of Ronald N. Germain, National Institutes of Health; and Figures 4 and 5 were created by Li Yao based on direction provided by A.J.R.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea J. Radtke, Lymphocyte Biology Section and Center for Advanced Tissue Imaging, Laboratory of Immune System Biology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 9000 Rockville Pike, Building 4, Room 407, Bethesda, MD 20892; email: andrea.radtke@nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal