Visual Abstract
Escape from immune surveillance is a hallmark of cancer. Immune deregulation caused by intrinsic and extrinsic cellular factors, such as altered T-cell functions, leads to immune exhaustion, loss of immune surveillance, and clonal proliferation of tumoral cells. The T-cell immune system contributes to the pathogenesis, maintenance, and progression of myelodysplastic syndrome (MDS). Here, we comprehensively reviewed our current biological knowledge of the T-cell compartment in MDS and recent advances in the development of immunotherapeutic strategies, such as immune checkpoint inhibitors and T-cell– and antibody–based adoptive therapies that hold promise to improve the outcome of patients with MDS.
Introduction
T cells play a key role in adaptive immunity by orchestrating immune responses against foreign antigens while preserving self-tolerance. T cells maintain immune homeostasis by overcoming pathogens, arresting the clonal expansion of cancer cells, and preventing the development of autoimmune (AI) diseases. T-cell differentiation and functional specialization are tightly regulated processes, which are essential for effective immune responses.
T cells play a key role in tumor surveillance by identifying and eliminating tumoral cells. T cells’ aberrant differentiation and function can disrupt immune surveillance mechanisms and foster an immunosuppressive tumor microenvironment, which promotes cancer initiation and progression. Moreover, deregulated T-cell homeostasis can affect clinical outcomes in patients with cancer by modulating therapy responses.1 Regulated immunity prevents or delays the appearance of malignant clones through innate antitumor activity or specific recognition of neoantigens. Given that tumor immunity arises from a balance between immunosurveillance and immune escape, errors in the immune regulatory pathways can lead to malignant clone expansion in many cancers,2,3 including those affecting hematopoietic stem cells (HSCs).4
Mounting experimental evidence demonstrates that immune deregulation in the hematopoietic niche and chronic inflammation due to aberrant secretion of cytokines by immune cells have prominent roles in the pathogenesis and progression of myelodysplastic syndromes (MDS). Progression of MDS to acute myeloid leukemia (AML) is associated with an increased inflammatory signature, which significantly affects the composition of the immune microenvironment and impairs immune cells’ function.4,5
T lymphocytes are key effectors of cell-mediated immune responses against tumor cells, and these cells’ alterations contribute to immune dysfunction in MDS and expansion of malignant clones.6,7 Here, we comprehensively overview: (1) how T-cell subtypes’ composition and functional alterations contribute to MDS initiation, maintenance, and progression; (2) whether T cells are biomarkers of response to treatment; and (3) the recent strides toward the development of novel T-cell–based therapeutic approaches to treat MDS.
T-cell subtypes and immune deregulation in MDS
CD4+ helper T (Th), CD8+ cytotoxic T, and regulatory T (Treg) cells play distinct functional roles in regulating the immune response. Immune-mediated cell death of pathogens and cancer cells is mainly mediated by CD8+ and CD4+ T cells, whereas Treg cells regulate immune tolerance and modulate immune responses (Figure 1).7 The absolute number of peripheral blood (PB) CD4+ T cells is reduced in patients with MDS compared with that of healthy donors (HDs), which results in a lower PB CD4+/CD8+ T-cell ratio.8 Indeed, the age-adjusted CD4/CD8 ratio is deceased in patients with MDS with both lower- and higher-risk MDS when compared with HDs, due to a decreased numbers of CD4+ T cells in these patients rather than an expansion of CD8+ T cells.9 Given that CD4/CD8 ratio is a conventional measure of immune function and response,10 these studies suggest that the immune system is severely impaired in MDS.
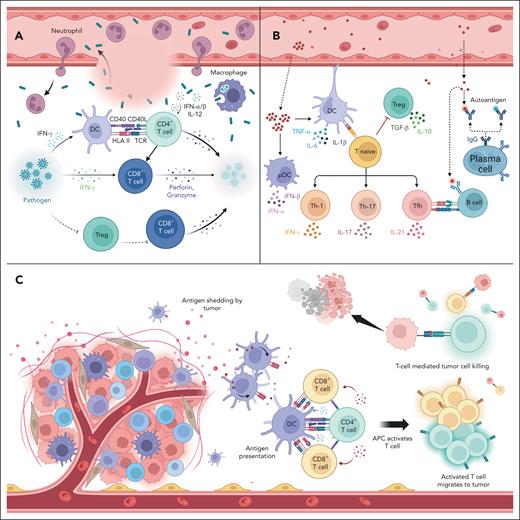
Role of T cells in infection, autoimmunity, and cancer. (A) During infection, CD8+ T cells directly recognize and eliminate pathogenic peptides presented on the surfaces of infected cells. (B) T cells do not react against self-antigens owing to central and peripheral immunogenic tolerance. Failure of the checkpoints described in panels A and B can cause uncontrolled expansion of self-reactive T cells, which leads to the development of AI diseases. (C) In steady-state, T cells survey, patrol, identify, and destroy abnormal cells, including cancer cells. pDC, plasmacytoid DC.
Role of T cells in infection, autoimmunity, and cancer. (A) During infection, CD8+ T cells directly recognize and eliminate pathogenic peptides presented on the surfaces of infected cells. (B) T cells do not react against self-antigens owing to central and peripheral immunogenic tolerance. Failure of the checkpoints described in panels A and B can cause uncontrolled expansion of self-reactive T cells, which leads to the development of AI diseases. (C) In steady-state, T cells survey, patrol, identify, and destroy abnormal cells, including cancer cells. pDC, plasmacytoid DC.
CD4+ T cells
CD4+ T cells are highly functionally heterogeneous and have a central role in tumor immunity by enhancing the effect of CD8+ cytotoxic T cells, mediating humoral responses, and secreting effector cytokines, such as interferon gamma (IFN-γ) and tumor necrosis factor-α (TNF-α).11
CD4 is a glycoprotein located on the surface of CD4+ T cells, dendritic cells (DCs), monocytes, and macrophages.12 CD4 serves as a coreceptor for the T-cell receptor (TCR), which engages with antigenic peptides presented by the HLA class II molecules, and facilitates communication with antigen-presenting cells (APCs), such as DCs (Figure 1).13
CD4+ T cells activate cytotoxic T cells, B lymphocytes, innate immune cells (eg, DCs, basophils, and neutrophils), and nonimmune cells.13 Upon activation of the TCR, antigen naïve CD4+ T cells differentiate into 6 distinct functional subtypes, namely, Th-1, Th-2, Th-17, Th-22 cells, T follicular helper (Tfh) cells, and Treg cells, each characterized by the secretion of specific cytokines, which are essential to functionally activate APCs and CD8+ T cells (Figure 1).14
Differentiated CD4+ T cells are classically divided in 2 groups, Th-1 and Th-2 cells, based on the cytokines they release.15 Th-1 cells secrete IFN-γ, TNF-α, and interleukin-2 (IL-2), which promote cell-mediated immunity and control infections induced by intracellular pathogens.16,17 Th-2 cells secrete IL-4, IL-5, IL-10, and IL-13, which mediate humoral immune responses and resistance to external pathogens.15 Under physiological conditions, the differentiation of Th-1/Th-2 cells is balanced, which leads to a tight regulation of the cellular and humoral immune response.
However, the Th-1/Th-2 ratio is altered in MDS because the number of Th-1 cells is lower than that in HDs. Significantly decreased Th-1 cell counts are inversely correlated with higher blast counts in the BM of patients with MDS.18 Moreover, a high level of IL-4, a cytokine that is mainly produced by Th-2 cells, is an independent factor that predicts shorter overall survival in patients with intermediate- to higher-risk MDS.19 Interestingly, T cells cultured in the presence of MDS-derived monocytes are significantly skewed toward Th-2 differentiation,20 which supports the hypothesis that Th-2 cells rely on Th-1 cells’ effective anticancer immunity instead of driving tumor evasion by their own polarization.21,22
Th-17 cells
Th-17 cells are a subset of CD4+ T cells that mainly secrete the proinflammatory cytokines IL-17 and IL-23 and protect the body from bacterial and fungal infections (Figure 1).23 IL-17 facilitates and induces an inflammatory cytokine environment, and abnormal expression of IL-17 has been reported in patients with AI diseases and cancers.24 IL-17–induced inflammatory mediators, such as granulocyte-colony stimulating factor, IL-6, and C-X-C motif Chemokine Ligand 1 (CXCL1) stimulate the expansion and recruitment of dysfunctional myeloid cells and establish a proangiogenic and immune suppressive tumor environment that enhances tumor growth and clonal expansion. Notably, IL-17 enhances the development and progression of a wide array of malignancies.25
Patients with lower-risk MDS have significantly higher Th-17 cell counts and IL-17 levels in the PB and BM than those with higher-risk MDS (Figure 2). Additionally, PB cells from patients with lower-risk MDS have significantly increased expression of the RAR-related orphan receptor gene family, which encode key transcription factors inducing Th-17 lineage commitment.26 In patients with lower-risk MDS, Th-17 cells stimulate secretion of several inflammatory-associated cytokines (eg, IL-6, IL-21, IL-22, and IL-23), which results in a proinflammatory milieu and leads to increased HSC apoptosis and ineffective BM hematopoiesis (Figure 2).27,28
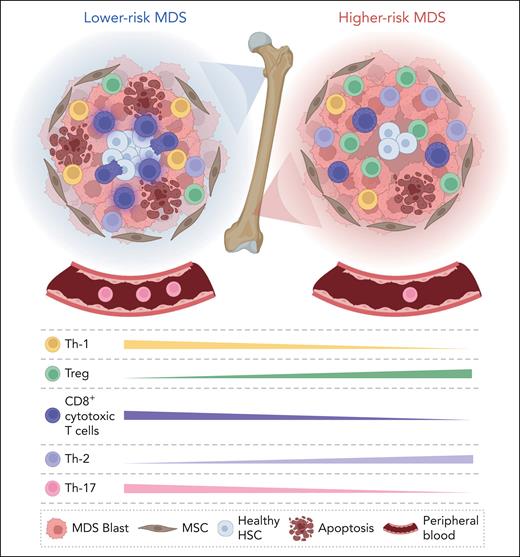
Immune deregulation in lower-risk MDS and higher-risk MDS. (Left panel) Lower-risk MDS are characterized by the hyperfunction of immune cells. CD8+ T cells are increased in number and functionally activated. (Right panel) Higher-risk MDS are characterized by an immunosuppressive environment that causes immune escape. CD8+ T cells are significantly decreased, whereas the number of Treg cells is increased. MSC, mesenchymal stem cell.
Immune deregulation in lower-risk MDS and higher-risk MDS. (Left panel) Lower-risk MDS are characterized by the hyperfunction of immune cells. CD8+ T cells are increased in number and functionally activated. (Right panel) Higher-risk MDS are characterized by an immunosuppressive environment that causes immune escape. CD8+ T cells are significantly decreased, whereas the number of Treg cells is increased. MSC, mesenchymal stem cell.
Th-22 cells
Th-22 cells are a subset of Th cells that mainly secrete IL-22, IL-13, and TNF-α.29 Naïve CD4+ T cells differentiate into Th-22 cells after IL-6 and TNF-α stimulation.30 IL-22, a member of the IL-10 family, protects tissues from inflammation but can also elicit proinflammatory effects, contributing to disease pathogenesis.31 Indeed, IL-22 has a pathogenic role in several AI diseases.32
In MDS, the number of Th-22 cells is increased in the PB of late-stage patients compared with that of early-stage patients and correlates with TNF-α and IL-6 levels.33 This observation suggests that exacerbation of inflammatory cytokine signaling during MDS progression may polarize and expand Th-22 cells, thereby further contributing to immune escape and clonal evolution (Figure 2).
Tfh cells
Tfh cells represent a subpopulation of CD4+Th cells, characterized by the surface expression of CXC Receptor 5 (CXCR5), inducible costimulatory molecule (ICOS), programmed cell death protein 1 (PD-1), and transcription factor B-cell lymphoma 6.34 Tfh cells mainly secrete IL-21, which induces the proliferation and differentiation of B cells into antibody-producing cells (Figure 1).35 The binding of CXCL13 to its receptor CXCR5 regulates lymphocyte infiltration within the tumor microenvironment, thus affecting responsiveness to immune- and cytotoxic-targeted therapies.36
In MDS, patients with lower-risk disease have significantly decreased PB Tfh cell counts compared with HDs.37 These findings were supported by preclinical studies in the NUP98-HOXD13 mouse MDS model that showed reduced PB and BM numbers and aberrant function of Tfh cells. Further in vitro experiments using this system demonstrated that Tfh cells’ reduction and dysfunction hindered antibody production by B cells, which suggests that Tfh cells have a role in regulating humoral immunity.38,39 Moreover, patients with MDS with AI diseases have a higher number of PB Tfh cells than that of those without AI diseases,40 but whether these cells can mediate immune deregulation in this setting remains to be defined.
Treg cells
Treg cells have an established role in suppressing abnormal/excessive immune responses to self- and nonself-antigens to maintain immune homeostasis. However, Treg cells can also play an active role in inhibiting tumor-specific immunity, thus facilitating immune evasion of cancer cells.41 Treg cells are divided into different subsets, such as naïve cells, central memory cells, effector memory (emTreg) cells, and effector Treg cells based on these cells’ differentiation state and immunosuppressive potential.42 Treg cells modulate immune reactions and affect immune surveillance43 by secreting immunosuppressive cytokines (eg, IL-10 and TGF-β; Figure 1).44 Thus, elevated Treg counts and function result in defective immune activation and compromised antitumor immunity.45
In MDS, the number of Treg cells in the PB and BM of patients with higher-risk MDS is increased compared with that in lower-risk patients,46,47 possibly because the expression of tumor-associated antigens on MDS cells during disease evolution. Treg cells decrease after response to therapy but increase again at therapy failure.48 These findings suggest that aberrant expansion of Treg cells drives immune surveillance suppression in MDS, thereby contributing to disease progression (Figure 2).46,49,50
CD8+ cytotoxic T cells
CD8+ cytotoxic T cells directly kill tumor cells and are the most powerful effectors of surveillance and immune defense against cancer cells.51,52 The CD8 glycoprotein is located on the membrane of CD8+ T cells, which recognize specific antigens bound to the HLA class I molecules on the surface of APCs. Upon recognition of pathogens, CD8+ T cells activate different mechanisms to kill infected or malignant cells by secreting proinflammatory cytokines such as TNF-α and IFN-γ, releasing cytotoxic mediators (eg, perforin and granzymes), or activating the Fas/FasL pathway (Figure 1).53 CD8+ T cells also regulate HSC pool dynamics in the BM milieu.54 Exhaustion and functional impairment of CD8+ T cells in response to the tumor microenvironment or chronic antigenic stimulation52 is a hallmark of many cancers.53
In MDS, patients with lower-risk disease have higher CD8+ T-cell counts.47,55 However, while suppressing the malignant clone, CD8+ T cells also affect normal hematopoiesis, resulting in the apoptosis observed in the BM of these patients (Figure 2).56 In higher-risk MDS, CD8+ T cells are decreased, have lower cytotoxicity capability and overexpress the PD-1/PD ligand 1 (PD-L1), which enhances the ability of tumor cells to evade the host immune surveillance57 by reducing TCR-induced redirected toxicity.58
γδ T cells
Although γδ T cells are a minor subset of T cells (1%-10% of circulating PB T cells59), they constitute an important component of innate immunity and play a key role in the rapid response to pathogens and tumoral cells.60,61 γδ T cells display distinct TCR γ and δ (γδ TCR) chains that are heterogeneous in structure and function.62 Specifically, γδ T-cell subsets characterized by the expression of Vδ1 and Vδ2 chains exhibit distinct tissue tropisms. The Vδ1+ subset is preferentially enriched in mucosal tissues, whereas the Vδ2+ subset is frequently encountered in the PB and lymphoid organs.63 These findings suggest that γδ T cells are functionally specialized to execute specific immune surveillance functions in different tissue environments.
γδ T cells are reduced in patients with lower-risk MDS, mainly in patients with associated AI diseases.64 Additionally, in vitro studies showed that, independently of any MDS risk stratification, γδ T cells do not expand in response to bromohalohydrin pyrophosphate (a potent stimulator of human γδ T cells) and do not proliferate after IL-2 stimulation,64 which suggests these cells’ irreversible functional impairment.
A summary of the quantitative and qualitative changes in T-cell subsets among different MDS genetic subgroups is included in supplemental Tables 1 and 2 (available on the Blood website), respectively.
Inflammation and the immune system in MDS
Inflammation, a pivotal driver of MDS pathogenesis, significantly induces progressive dysfunction in the hematopoietic niche4,65,66 and affects the immune response.67-69 The MDS proinflammatory milieu attracts regulatory and suppressive immune cells, thereby inhibiting immune surveillance of malignant clones through the elevated production of inflammatory cytokines such as TNF-α, IFN-γ, IL-6, IL-1β, and IL-8.14 Several clinical studies are currently investigating the potential to inhibit dysregulated inflammatory signaling in MDS by targeting key hub mediators such as IRAK1 and IRAK4, as well as ligands and receptors, including S100A9, CD33, IL-1β, IL1RAP, and TGF-β.
A better understanding of the molecular and cellular mechanisms through which the inflammatory environment contributes to immune system dysfunction could allow the development of new therapeutic strategies, particularly in patients with lower-risk MDS.
TCR repertoire in patients with MDS
The TCR plays a fundamental role as a transmembrane glycoprotein in the immunological synapse. The TCR is a heterodimeric protein that is formed by the combination of either α and β (αβ TCR) or γδ TCR chains. Expression of either αβ or γδ TCRs on T cells are crucial for antigen recognition and immune responses.70
The populations of cells with unique TCR sequences are known as the TCR repertoire (Figure 3). Development of the TCR repertoire is a dynamic process that occurs over a lifetime. However, the diversity of the TCR repertoire dramatically decreases during the seventh and eighth decades of life, which affects the recognition of a wide range of antigenic targets and immune functions (Figure 3).71
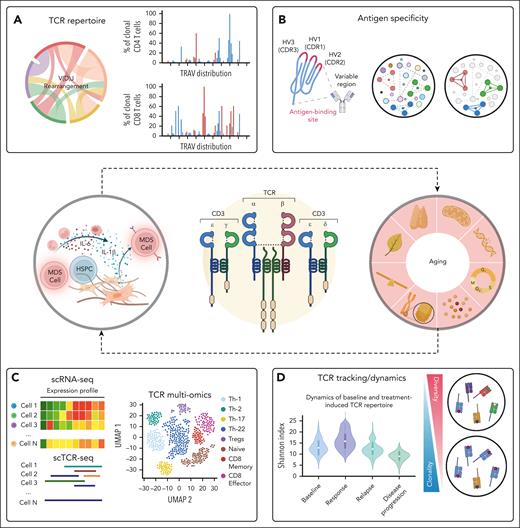
Integrative approach dissecting TCR complexity. TCR repertoire is regulated during thymocyte development and drives the generation of αβ and γδ T lymphocytes. Chronic inflammatory states or physiological aging can hinder TCR development and function. (A) TCR clonotype frequency distribution. (B) Prediction of TCR binding specificity and putative antigens. (C) Gene expression profile of clonotypes within a TCR cluster. (D) Comparison of clonotype tracking and repertoire diversity across different groups of patients with varying treatment responses. HV, hypervariable region; HSPC, hematopoietic stem and progenitor cell; TRAV, T-cell receptor alpha variable region.
Integrative approach dissecting TCR complexity. TCR repertoire is regulated during thymocyte development and drives the generation of αβ and γδ T lymphocytes. Chronic inflammatory states or physiological aging can hinder TCR development and function. (A) TCR clonotype frequency distribution. (B) Prediction of TCR binding specificity and putative antigens. (C) Gene expression profile of clonotypes within a TCR cluster. (D) Comparison of clonotype tracking and repertoire diversity across different groups of patients with varying treatment responses. HV, hypervariable region; HSPC, hematopoietic stem and progenitor cell; TRAV, T-cell receptor alpha variable region.
Analysis of the TCR repertoire provides a holistic representation of the extensive versatility and breadth of the immune T-cell compartment. Clonal T cells can be identified by analyzing the third complementarity determining region (CDR3) of the TCR, a hypervariable domain that directly binds to antigenic cell-surface peptides, named HLA proteins. In normal T-cell homeostasis, a restricted number of T cells are activated and undergo intermittent clonal expansion that is triggered by foreign antigens. However, viral infections, AI diseases and clonal T-cell malignancies induce an excessive clonal T-cell expansion.72-74
In MDS, the diversity of the TCR repertoire correlates with response to various therapeutic approaches (Figure 3). As an example, sequential analyses of the αβ TCR repertoire identified a significant group of T cells that shared identical CDR3 lengths and involvement of variable TCR β chains which declined in patients with clinical responses to immunosuppression therapies.75-78 Moreover, although significant skewness in CDR3 length is detected in patients with MDS compared with that in HDs,79 treatment with hypomethylating agents (HMAs) increased the TCR diversity.80 In addition, patients with MDS whose disease responds to HMA treatment show TCR clonotype expansion, whereas patients with MDS whose disease is refractory to HMA therapy exhibited TCR clonotype contraction.81 These data suggest that the TCR clonotype diversity contributes to response to HMA therapy. Our unpublished data also show that patients whose disease respond to venetoclax-based therapy have a higher count of T-cell clonotypes and T-cell diversity, whereas reduction of these cells’ clonotypes predicts disease progression. Together, these results highlight the potential role of adoptive immunotherapy strategies to enhance therapy efficacy in improving the survival of patients with MDS.
However, to date, it is not yet known whether the increase in TCR diversity or the emergence of new TCR clonotypes in patients whose disease responds to therapy is directly related to the therapeutic effect of the treatment (ie, increased release of neoantigens) or is induced by the reduction in tumor burden, which leads to the restoration of hematopoiesis. Further randomized clinical trials evaluating the differential impact of each therapy on the immune microenvironment may provide insights into T-cell adaptive immunity and establish a causal relationship between TCR dynamics and the pathogenesis and progression of MDS.
T-cell evasion in MDS
Immune evasion is a hallmark of cancer that enables malignant cell clones to expand and overpopulate healthy tissues.82 Immune evasion is especially relevant in patients with MDS whose aged immune system is vulnerable.83 The overexpression of immune checkpoint proteins, such as PD-1/PD-L1 and cytotoxic T lymphocyte–associated protein 4 (CTLA-4) on T cells, leads to T-cell exhaustion and transition toward an immune-evading tumor microenvironment (Figure 4).84
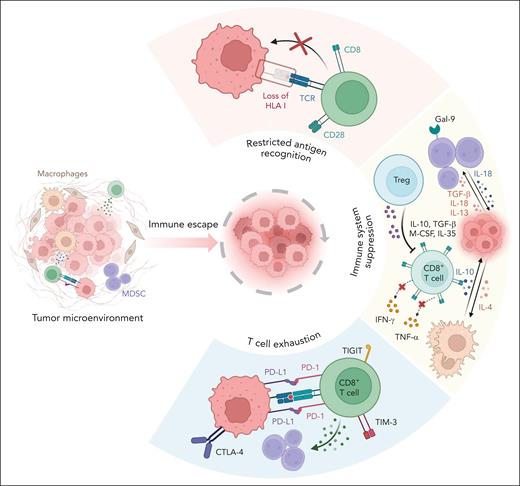
Mechanisms of tumor evasion in MDS. MDS cells escape immune responses and bypass immune-mediated attacks by restricting their antigen recognition, dysregulating several immune checkpoints, and inducing T-cell exhaustion. Gal-9, galectin-9; MDSC, myeloid-derived suppressor cell.
Mechanisms of tumor evasion in MDS. MDS cells escape immune responses and bypass immune-mediated attacks by restricting their antigen recognition, dysregulating several immune checkpoints, and inducing T-cell exhaustion. Gal-9, galectin-9; MDSC, myeloid-derived suppressor cell.
The interaction of PD-1 with its ligand PD-L1 (Figure 5) suppresses TCR-mediated T-cell proliferation and the release of cytokines that regulate immune activation, thus compromising immune response. In MDS, PD-1/PD-L1 expression is significantly altered. MDS CD34+ hematopoietic stem and progenitor cells overexpress PD-L1, effector T cells and Treg cells upregulate PD-1.57,85-88 Secreted inflammatory cytokines, such as IFN-γ, TNF-α, and S100A9, which are present at high levels in the BM microenvironment of patients with MDS,89-91 induce PD-1 and/or PD-L1 upregulation on MDS cells, thus facilitating MDS cells’ escape from immune surveillance.
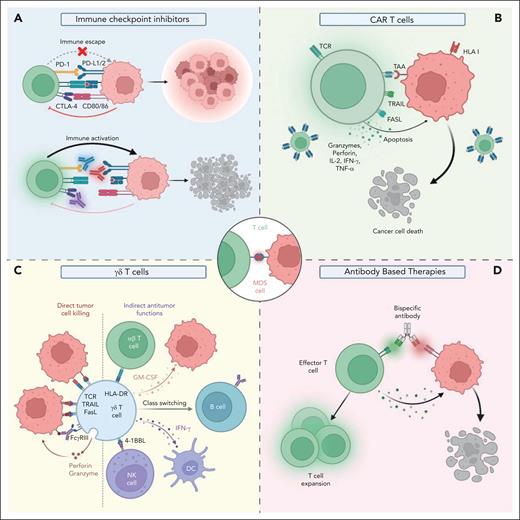
Emerging T-cell–based therapies in MDS. T-cell–based treatment strategies, such as immune checkpoint inhibitors, CAR T-cell therapies, expanded/activated γδ T cells, and BiTEs hold promise to achieve robust antileukemic activities in MDS while avoiding T-cell cytotoxicity against healthy tissues. (A) Immune checkpoint inhibitors. (B) CAR T cells. (C) γδ T cells. (D) Antibody-based therapies. FcyRIII, low-affinity IgG receptor type 3; GM-CSF, granulocyte-macrophage colony-stimulating factor; TAA, tumor-associated antigen; TRAIL, TNF-related apoptosis inducing ligand.
Emerging T-cell–based therapies in MDS. T-cell–based treatment strategies, such as immune checkpoint inhibitors, CAR T-cell therapies, expanded/activated γδ T cells, and BiTEs hold promise to achieve robust antileukemic activities in MDS while avoiding T-cell cytotoxicity against healthy tissues. (A) Immune checkpoint inhibitors. (B) CAR T cells. (C) γδ T cells. (D) Antibody-based therapies. FcyRIII, low-affinity IgG receptor type 3; GM-CSF, granulocyte-macrophage colony-stimulating factor; TAA, tumor-associated antigen; TRAIL, TNF-related apoptosis inducing ligand.
CTLA-4 is a costimulatory receptor that delivers a potent inhibitory signal to T cells, leading to restriction of immune responses.92 Thus, CTLA-4 expression on tumor cells induces an immunosuppressive state and allows tumor growth (Figure 4). In MDS, CTLA-4 levels correlate with disease stage and the risk of progression to AML (being overexpressed in higher-risk MDS compared with lower-risk MDS).93
Other co-inhibitory receptors, such as the T-cell immunoglobulin and mucin domain 3 (TIM-3), and the T-cell immunoglobulin and immunoreceptor tyrosine–based inhibitory motif (ITIM) domain (TIGIT) are also associated with immune evasion (Figure 4). TIM-3 is a checkpoint receptor that was initially identified on terminally differentiated Th-1 and CD8+ T cells. TIM-3 is also expressed on other immune cells, such as Treg and natural killer (NK) cells. TIM-3 inhibits Th-1 cells’ responses by inducing Th-1 cell-mediated apoptosis and regulates the expression of cytokines, such as TNF-α and IFN-γ.94,95
Immune evasion, mainly mediated by the overexpression of immune checkpoints, plays a crucial role in the development and progression of MDS. Th-1, CD8+ and Treg cells have high TIM-3 expression, which further increases over the course of the disease.94-96 The overexpression of TIM-3 in MDS CD8+ T cells is correlated with reduced levels of perforin and granzyme B, and upregulation of the death receptor CD95, which affects these cells’ cytotoxicity and killing capabilities and leads to their susceptibility to cell death, respectively, thus facilitating immune escape.94 Importantly, TIM-3 is also overexpressed in MDS/AML leukemic stem cells and blasts, which suggests that monoclonal antibodies targeting TIM-3 may have a dual anticancer effect by directly depleting the leukemic clone while potentiating the immune response.97
TIGIT levels are increased in patients with higher-risk MDS85 and lead to CD4+ T, CD8+ T, and NK cells’ hypo-responsiveness upon stimulation and these cells’ decreased secretion capability of effector cytokines (eg, CD107a, IFN-γ and TNF-α), which results in malignant clonal expansion and tumor escape.85
T-cell–based therapeutic approaches in MDS
Current treatment options for patients with MDS are mainly based on supportive care or HMA-based therapies. Except for allogeneic stem cell transplantation, which is limited to eligible patients, no new curative treatments have been developed for MDS in the last 10 years.98
Several exploratory clinical trials targeting signaling pathways, cell death regulators or immune cell dysfunction are currently under development.99 Harnessing the power of immune cells, especially T cells, to increase antitumor responses has emerged as a promising approach in the management of hematologic malignancies.3 Immune checkpoint inhibitors, chimeric antigen receptor (CAR) T-cell therapies, and novel approaches based on expanded/activated γδ T cells and bispecific T-cell engagers (BiTEs) are promising therapeutic options to treat patients with MDS (Figure 5; Table 1).
Select targeted T-cell therapies currently in MDS clinical trials
| Therapy . | Target . | Phase . | Enrolled patients . | Response rate OS rate and mOS . | AEs grade ≥3 . | NCT number/reference . |
|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors | ||||||
| Pembrolizumab | PD-1 | 1b | N = 28 lower- and higher-risk MDS (HMA failure) | Overall ORR: 0% SD: 44% mOS: 6 mo 2-y OS (overall): 17% 2-y OS (intermediate-1): 46% 2-y OS (intermediate-2 and higher-risk): 0% | Gastro-enteritis: 4% Pain: 4% TLS: 4% | NCT01953692100 |
| Pembrolizumab + azacytidine | PD-1 | 2 | N = 37 20 HMA failure n = 17 frontline | HMA-refractory ORR: 25% CR: 5% mCR: 10% HI: 10% mOS: 5.8 mo Frontline cohort ORR: 76% CR: 18% mCR: 29% HI: 6% mOS: not reached (mFU: 12.8 mo) | Neutropenia: 32% Pneumonia: 24% FN: 18% Anemia: 12% | NCT03094637101 |
| Nivolumab and/or ipilimumab ± azacytidine | PD-1 (Nivo) CTLA-4 (Ipi) | 2 | N = 76 n = 35 HMA-failure n = 41 frontline | ORR: 13% (Nivo; HMA-failure) ORR: 35% (Ipi; HMA-failure) ORR: 75% (Nivo + Aza; frontline) ORR: 71% (Ipi + aza; frontline) CR/CRp: 15% (Nivo; HMA-failure) CR/CRp: 0% (Ipi; HMA-failure) CR/CRp: 50% (Nivo + Aza; frontline) CR/CRp: 38% (Ipi + Aza; frontline) mOS: 8 mo (Ipi or Nivo; HMA-failure) mOS: not reached (Ipi + Aza; frontline) mOS: 12 mo (Nivo + Aza; frontline) | NS | NCT02530463102 |
| Nivolumab and ipilimumab ± azacytidine | PD-1 (Nivo) CTLA-4 (Ipi) | Basket exploratory phase 2 | N = 26 n = 11 HMA-failure n = 15 frontline | Ipi + Nivo (HMA-refractory cohort) ORR: 36% CR: 9%; CRi: 9% HI: 18% mPFS: 7.1 mo mOS: 11.4 mo Ipi + Nivo + Aza (frontline cohort) ORR: 67% CR: 5% HI: 5% mPFS: 10 mo mOS: 12 mo | Infection: 55% FN: 46% Rash: 24% Elevated AST/ALT: 24% | NCT02530463103 |
| Sabatolimab + HMAs | TIM-3 | 1b | 53 MDS | ORR: 57% mDOR: 16.1 mo | Neutropenia: 47% Thrombocytopenia: 43% FN: 36% Anemia: 28% | NCT03066648104 |
| Sabatolimab + HMAs | TIM-3 | 2 | N = 65 higher-risk MDS | Overall ORR: 68% CR: 22% mOS: 22.8 mo mPFS: 11.1 mo | Neutropenia: 56% Thrombocytopenia: 37% FN: 35% Anemia: 23% Leukopenia: 23% | NCT03946670105 |
| Adoptive T-cell therapies | ||||||
| CYAD-01 | NKG2D-based CAR T cells | 1 | 22 total 1 R/R MDS | Patient achieved mCR | CRS: 31% Lymphopenia: 19% | NCT03018405106 |
| PRGN-3006 Ultra CAR T cells | CD33 | 1/1b | N = 24 n = 3 R/R MDS n = 1 CMML n = 20 R/R AML | ORR MDS/CMML: 0% ORR (AML): 30% (1 CRi, 1 CRh, 1 PR) | Transient CRS grade 3 | NCT03927261107 |
| Bispecific antibodies | ||||||
| Flotetuzumab | CD3 + CD123 | 1/2 | N = 5 R/R MDS | 1 evaluable patient: PD | No results reported | NCT02152956108,109 |
| APVO436 | CD3 + CD123 | 1b | N = 46 n = 39 R/R AML n = 7 R/R MDS | patients with MDS NE: 1 SD: 3 mCR: 3 | CRS: 8.7% Anemia: 4.3% IRR: 4.3% | NCT03647800110 |
| Vibecotamab | CD3 + CD123 | 2 | 23 total 9 R/R MDS 12 AML 2 CMML | mCR + HI: 44% HI: 11% CRL: 56% | Infusion reaction: 4% | NCT05285813111 |
| JNJ-67571244 | CD3 + CD33 | 1 | N = 68 R/R HR-MDS R/R AML | No results reported | No results reported | NCT03915379112 |
| AMV564 | CD3 + CD33 | 1 | N = 14 | No reported results | No results reported | NCT03516591113 |
| Therapy . | Target . | Phase . | Enrolled patients . | Response rate OS rate and mOS . | AEs grade ≥3 . | NCT number/reference . |
|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors | ||||||
| Pembrolizumab | PD-1 | 1b | N = 28 lower- and higher-risk MDS (HMA failure) | Overall ORR: 0% SD: 44% mOS: 6 mo 2-y OS (overall): 17% 2-y OS (intermediate-1): 46% 2-y OS (intermediate-2 and higher-risk): 0% | Gastro-enteritis: 4% Pain: 4% TLS: 4% | NCT01953692100 |
| Pembrolizumab + azacytidine | PD-1 | 2 | N = 37 20 HMA failure n = 17 frontline | HMA-refractory ORR: 25% CR: 5% mCR: 10% HI: 10% mOS: 5.8 mo Frontline cohort ORR: 76% CR: 18% mCR: 29% HI: 6% mOS: not reached (mFU: 12.8 mo) | Neutropenia: 32% Pneumonia: 24% FN: 18% Anemia: 12% | NCT03094637101 |
| Nivolumab and/or ipilimumab ± azacytidine | PD-1 (Nivo) CTLA-4 (Ipi) | 2 | N = 76 n = 35 HMA-failure n = 41 frontline | ORR: 13% (Nivo; HMA-failure) ORR: 35% (Ipi; HMA-failure) ORR: 75% (Nivo + Aza; frontline) ORR: 71% (Ipi + aza; frontline) CR/CRp: 15% (Nivo; HMA-failure) CR/CRp: 0% (Ipi; HMA-failure) CR/CRp: 50% (Nivo + Aza; frontline) CR/CRp: 38% (Ipi + Aza; frontline) mOS: 8 mo (Ipi or Nivo; HMA-failure) mOS: not reached (Ipi + Aza; frontline) mOS: 12 mo (Nivo + Aza; frontline) | NS | NCT02530463102 |
| Nivolumab and ipilimumab ± azacytidine | PD-1 (Nivo) CTLA-4 (Ipi) | Basket exploratory phase 2 | N = 26 n = 11 HMA-failure n = 15 frontline | Ipi + Nivo (HMA-refractory cohort) ORR: 36% CR: 9%; CRi: 9% HI: 18% mPFS: 7.1 mo mOS: 11.4 mo Ipi + Nivo + Aza (frontline cohort) ORR: 67% CR: 5% HI: 5% mPFS: 10 mo mOS: 12 mo | Infection: 55% FN: 46% Rash: 24% Elevated AST/ALT: 24% | NCT02530463103 |
| Sabatolimab + HMAs | TIM-3 | 1b | 53 MDS | ORR: 57% mDOR: 16.1 mo | Neutropenia: 47% Thrombocytopenia: 43% FN: 36% Anemia: 28% | NCT03066648104 |
| Sabatolimab + HMAs | TIM-3 | 2 | N = 65 higher-risk MDS | Overall ORR: 68% CR: 22% mOS: 22.8 mo mPFS: 11.1 mo | Neutropenia: 56% Thrombocytopenia: 37% FN: 35% Anemia: 23% Leukopenia: 23% | NCT03946670105 |
| Adoptive T-cell therapies | ||||||
| CYAD-01 | NKG2D-based CAR T cells | 1 | 22 total 1 R/R MDS | Patient achieved mCR | CRS: 31% Lymphopenia: 19% | NCT03018405106 |
| PRGN-3006 Ultra CAR T cells | CD33 | 1/1b | N = 24 n = 3 R/R MDS n = 1 CMML n = 20 R/R AML | ORR MDS/CMML: 0% ORR (AML): 30% (1 CRi, 1 CRh, 1 PR) | Transient CRS grade 3 | NCT03927261107 |
| Bispecific antibodies | ||||||
| Flotetuzumab | CD3 + CD123 | 1/2 | N = 5 R/R MDS | 1 evaluable patient: PD | No results reported | NCT02152956108,109 |
| APVO436 | CD3 + CD123 | 1b | N = 46 n = 39 R/R AML n = 7 R/R MDS | patients with MDS NE: 1 SD: 3 mCR: 3 | CRS: 8.7% Anemia: 4.3% IRR: 4.3% | NCT03647800110 |
| Vibecotamab | CD3 + CD123 | 2 | 23 total 9 R/R MDS 12 AML 2 CMML | mCR + HI: 44% HI: 11% CRL: 56% | Infusion reaction: 4% | NCT05285813111 |
| JNJ-67571244 | CD3 + CD33 | 1 | N = 68 R/R HR-MDS R/R AML | No results reported | No results reported | NCT03915379112 |
| AMV564 | CD3 + CD33 | 1 | N = 14 | No reported results | No results reported | NCT03516591113 |
AEs, adverse effects; Aza, azacytidine; CMML, chronic myelomonocytic leukemia; CRh, complete response with complete cytogenetic remission; CRi, complete response with incomplete count recovery; CRp, complete remission with incomplete platelet recovery; CRS, cytokine release syndrome; DOR, duration of response; FN, febrile neutropenia; HI, hematological improvement; HR, higher-risk; Ipi, ipilimumab; IRR, infusion-related reaction; mCR, marrow complete response; mFU, median follow-up; mOS, median overall survival; PR, partial response; mPFS, median progression-free-survival; NA, not available; NE, not evaluable; Nivo, nivolumab; ORR, overall response rate; OS, overall survival; PD, progressive disease; RFS, relapse-free survival; SD, stable disease; TLS, tumor lysis.
Checkpoint inhibitors are monoclonal antibodies that reactivate the immune system against malignant cells by blocking the interactions of immune function inhibitory receptors with their ligands (Figure 5). To date, treatments targeting PD-1, PD-L1 or CTLA-4-mediated interactions showed modest response rates in MDS.100,101 Indeed, pembrolizumab (MK3475), a humanized IgG4 monoclonal antibody that blocks the interaction of PD-1 with PD-L1, showed no clinical activity as single agent in patients with intermediate-1/2 and higher-risk MDS whose disease previously failed HMA therapy (KEYNOTE-013 study; NCT01953692)100 and pembrolizumab in combination with azacytidine only modestly improved these patients’ overall response rate (25%) (NCT03094637; Table 1).101 These studies suggest that checkpoint inhibitors targeting the PD-1-mediated signaling pathway cannot overcome the poor outcomes of patients with MDS whose disease failed HMA therapy. However, a phase 2 basket clinical trial of PD-1 and CTLA-4 inhibitors (nivolumab and ipilimumab, respectively) alone or in combination with azacytidine (NCT02530463) showed clinical activity and a tolerable safety profile in patients with frontline and HMA therapy-refractory MDS, respectively (Table 1).103
A phase 1b clinical trial (NCT03066648) of the immune checkpoint inhibitor sabatolimab (a humanized monoclonal antibody that targets TIM-3) in combination with HMA therapy showed antileukemic activity and emerging response durability in patients with higher-risk MDS (Table 1).104 However, another clinical trial of sabatolimab in combination with HMA therapy (NCT03946670; STIMULUS-MDS1) did not show any significant improvement in complete remission (CR) or progression-free-survival when compared with HMA therapy alone.105
The STIMULUS-MDS2 study, a phase 3, randomized trial evaluating the clinical effects of sabatolimab alone or in combination with azacytidine in higher-risk MDS (NCT04266301) is currently ongoing and aims to provide definitive evidence of the potential long-term benefits of sabatolimab in combination with HMA therapy in patients with higher-risk MDS97.
CAR T-cell therapies have revolutionized the treatment in lymphoid malignancies (Figure 5).114-116 However, the identification of CAR T-cell–specific antigenic targets in MDS remains challenging.117 Current CAR T-cell therapies in MDS target the myeloid CD123 and CD33 antigens which are concomitantly expressed on normal HSCs, thereby resulting in off-target toxicities with profound myeloablation.117 Recent preclinical studies based on the administration of MDS-derived CAR T cells against CD123 or CD33 in primary MDS/AML-derived xenograft models showed significant efficacy in depleting leukemic clones.118,119
A phase 1 clinical trial (NCT03018405) in patients with AML, multiple myeloma, and MDS after HMA failure assessed the efficacy of the autologous CAR T product CYAD-01 based on the natural killer group 2D (NKG2D) receptor.106 NKG2D is an activating immunoreceptor, which plays a pivotal role in antitumor immunity by binding to numerous and highly diversified MHC class I-like self-molecules.120 The expression of NKG2D ligands is largely absent on healthy cells but elevated in hematological malignancies.121,122 The study, which mainly enrolled patients with relapsed or refractory (R/R) AML and multiple myeloma (only 1 MDS patient was included and achieved a marrow CR; Table 1) showed that the treatment with CYAD-01 was well tolerated and had an antileukemic activity.106
The quality of T cells from patients with MDS who previously received many other therapies hinders the efficacy of autologous CAR T in MDS. Thus, the feasibility of treatments based on the administration of “off-the-shelf” products or allogeneic CAR T cells generated from HDs is currently under investigation. Allogeneic products do not require patient-specific manufacturing, which lowers the costs and reduces the time to infusion,123 the latter being particularly problematic in patients with higher- risk features who may experience disease progression before autologous CAR T-cell treatments are available. However, side effects induced by graft-versus-host disease and risk of host immune rejection still remain challenges to overcome before successfully implementing allogeneic CAR T cells into clinical practice.124
γδ T cells represent an appealing treatment for MDS in light of these cells’ favorable safety profile, potent and wide-ranging antitumor capabilities, and their potential for allogeneic administration (Figure 5).125 Currently, several ongoing clinical trials based on expanded/activated γδ T cells after allogeneic stem cell transplantation (NCT03533816, NCT03849651) aim to maximize the antitumor response and minimize graft-versus-host disease in patients with AML and patients with MDS. However, a better understanding of the mechanisms underlying the antitumor activities of γδ T cells and their interactions with the tumor microenvironment remains a crucial point to be addressed before developing effective γδ T-cell–based therapies in MDS.
Bispecific antibodies (BITEs) are recombinant antibodies designed to recognize and bind 2 different antigens or 2 different epitopes on the same antigen (Figure 5). BITEs target CD3 and tumor-specific antigens simultaneously, thereby promoting T-cell–induced cytotoxicity.126
An increasing number of BITEs against tumor-specific antigens are under evaluation in R/R MDS and AML, including those targeting the CD123 (NCT02152956, NCT03647800, NCT05285813) and CD33 (NCT03915379, NCT03516591) antigens (Table 1).127 As an example, an open-label phase 2 trial of the dual CD3-CD123 inhibitor vibecotamab (NCT05285813) is actively accruing patients with R/R MDS and AML harboring at least 20% of aberrant myeloblasts with CD123 expression. However, another ongoing multicenter phase 1b clinical trial of the dual CD3-CD123 inhibitor APVO436 (NCT03647800) is showing modest efficacy in R/R MDS and AML patients (Table 1).110 These findings, although preliminary, highlight the need to better understand which cohort of patients with MDS might benefit from this immune approach and when, during disease stages, these bispecific antibodies might be effective to improve patient survival.
Immunosuppressive therapy (IST) in MDS
In a subset of patients with lower-risk MDS, PB cytopenias are caused by hyperactive T cells that suppress hematopoiesis through the direct attack on BM cells or the release of a variety of inflammatory cytokines, such as IFN-γ, TNF-α, and IL-17, as also observed in aplastic anemia.128 This cohort of patients may benefit of IST. The most used IST involves the administration of cyclosporine (CsA) or antithymocyte globulin (ATG), either as monotherapy or in combination. CsA is a calcineurin inhibitor, which effectively suppresses CD4+ T cells, enhances cytotoxic lymphocyte function, and inhibits the release of TNF-α.129 ATG is a mixture of purified polyclonal IgG derived from rabbits or horses immunized with human thymocytes that induces immune modulation mainly through T cells’ complement-dependent lysis and apoptosis.130 Based on National Comprehensive Cancer Network guidelines, IST is indicated as a treatment option for symptomatic anemia in lower-risk, non-del(5q) MDS, particularly in patients younger than 60 years old, with ≤5% blasts in the BM, hypocellular BM, paroxysmal nocturnal hemoglobinuria, or STAT3 mutant cytotoxic T-cell clones.131
CsA and ATG combination therapy have shown response rates of up to 51% in patients with lower-risk MDS.132 A recent systematic review and meta-analysis of patients with MDS treated with IST that includes 9 prospective cohort studies and 13 clinical trials showed an overall response rate of 42.5%, including a CR rate of 12.5% and red blood cell transfusion independence rate of 33.4%.133 Future randomized clinical trials are critically warranted to definitively determine the impact of IST on response and survival in patients with MDS.133
Conclusions
Impaired immune functions in the MDS microenvironment enable tumoral cell immune escape, which contributes to disease initiation and maintenance. Further T-cell alterations during MDS progression induce autoimmunity, aberrant release of cytokines, and attenuation or loss of immune surveillance, which results in the proliferation of the malignant clone.
Dysregulation of immune checkpoints (ie, PD-1/PD-L1, CTLA-4, TIM-3, and TIGIT) on T cells is a key mechanism of immune evasion. Thus, emerging T-cell–based therapies, including immune checkpoint inhibitors, CAR T-cell therapy, expanded/activated γδ T-cell injections, and BiTEs offer promising avenues to target dysfunctional T-cell populations and enhance antitumor responses in MDS (Figure 5).
A better understanding of how different T-cell subtypes and MDS cells interact during disease evolution and how T-cell subpopulations dynamically change after therapy remains a future challenge for the development of more effective therapeutic combinations for improving the outcome of patients with MDS.
Acknowledgments
The authors thank Helen T. Chifotides and Kelly A. Soltysiak for editing the manuscript. Figures were created using Biorender.com.
This work was supported by philanthropic contributions to The University of Texas MD Anderson Cancer Center’s myelodysplastic syndrome/acute myeloid leukemia Moon Shot, by the Umberto Veronesi Foundation, and by the Edward P. Evans Foundation. J.J.R.-S. is the recipient of the MDACC Odyssey fellowship.
Authorship
Contribution: J.J.R.-S. and S.C. wrote the manuscript; and J.J.R.-S. created the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juan Jose Rodriguez-Sevilla, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: jrsevilla@mdanderson.org; and Simona Colla, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: scolla@mdanderson.org.
References
Author notes
The online version of this article contains a data supplement.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal