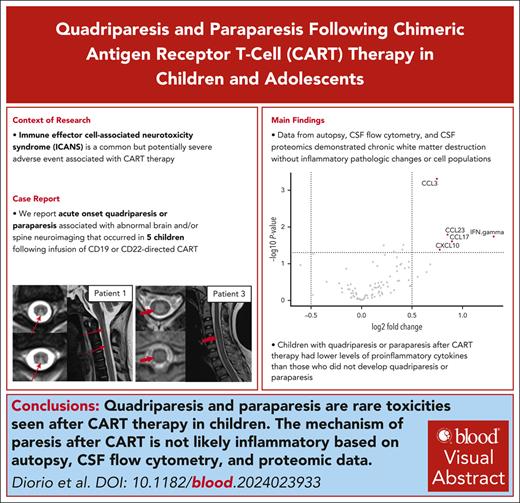
Key Points
Quadriparesis and paraparesis are rare toxicities seen after CART therapy in children.
The mechanism of post-CART paresis is likely complex but not clearly inflammatory based on autopsy, CSF flow cytometry, or proteomic data.
Visual Abstract
Immune effector cell–associated neurotoxicity syndrome (ICANS) is a common but potentially severe adverse event associated with chimeric antigen receptor T-cell (CART) therapy, characterized by the development of acute neurologic symptoms following CART infusion. ICANS encompasses a wide clinical spectrum typified by mild to severe encephalopathy, seizures, and/or cerebral edema. As more patients have been treated with CART, new ICANS phenomenology has emerged. We present the clinical course of 5 children who developed acute onset of quadriparesis or paraparesis associated with abnormal brain and/or spine neuroimaging after infusion of CD19- or CD22-directed CART, adverse events not previously reported in children. Orthogonal data from autopsy studies, cerebrospinal fluid (CSF) flow cytometry, and CSF proteomics/cytokine profiling demonstrated chronic white matter destruction, but a notable lack of inflammatory pathologic changes and cell populations. Instead, children with quadriparesis or paraparesis post-CART therapy had lower levels of proinflammatory cytokines, such as interferon gamma, CCL17, CCL23, and CXCL10, than those who did not develop quadriparesis or paraparesis. Taken together, these findings imply a noninflammatory source of this newly described ICANS phenomenon in children. The pathophysiology of some neurologic symptoms following CART may therefore have a more complex etiology than exclusive T-cell activation and excessive cytokine production.
Introduction
Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) are the most common severe adverse events associated with chimeric antigen receptor T-cell (CART) therapy.1 Although effective targeted therapies exist for CRS, the primary treatment for ICANS is corticosteroids, with variable efficacy.2 The pathophysiology of ICANS is not well understood, but it is thought to be inflammation based, related to excessive cytokine production in the setting of activated CART, and in particular interleukin-1 (IL-1) production.3-5 Limited pathobiological understanding has precluded the identification of effective targeted therapies.
Most ICANS is mild and self-limited; however, some patients develop severe symptoms, including seizures, encephalopathy, focal neurologic deficits, and fatal cerebral edema. Acute onset of quadriparesis or paraparesis has not been previously reported following CART infusion in children or adolescents. Here, we present 5 patients with B-cell acute lymphoblastic leukemia (B-ALL) who received CD19- or CD22-directed CART (CART19 or CART22) therapy with postinfusion courses complicated by acute paresis associated with abnormal brain and/or spine neuroimaging. We present autopsy findings and detailed immunophenotyping, including cerebrospinal fluid (CSF) flow cytometry and proteomics. We aim to inform clinicians of this rare presentation, to generate mechanistic hypotheses regarding potential neuropathophysiology, and to expand the reported phenomenology of ICANS in pediatric patients.
Methods
Study approvals
This study was conducted in accordance with the Declaration of Helsinki and received approval from the Institutional Review Board at Children’s Hospital of Philadelphia.
Sample collection
Four of the patients included were treated on CART clinical trials (NCT02650414, NCT03792633, and NCT02906371) and had ≈5 mL of CSF collected with day 28 (D28) procedures after infusion and cryopreserved for subsequent analyses. In 2 patients (patients 2 and 3), fresh CSF was immediately processed for flow cytometry from lumbar punctures (LPs) performed with D28 procedures and on D11, respectively. One patient (patient 4) was treated with commercial tisagenlecleucel and had no samples available.
Serum CART quantitation
CARTs were measured by quantitative polymerase chain reaction (PCR), as previously described.6-8
CSF correlative analyses
Supernatant was removed from CSF, and cells were stained for multiparameter flow cytometry with 16 antibodies (supplemental Table 1 [available on the Blood website]). Samples were analyzed on a BD LSR Fortessa (BD Biosciences, Franklin Lakes, NJ). BD FACSDiva and FlowJo software was used for analysis.
Results
Five patients developed acute paresis following CART for B-ALL. Clinical details are presented in Table 1.
Demographic and preinfusion clinical characteristics of included patients
| Characteristic . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Clinical history before infusion | |||||
| Age at infusion, y | 7 | 14 | 13 | 2.5 | 7 |
| Sex | F | M | M | M | M |
| Cancer genetics | T21 related | IGH::CRLF2 fusion, IZKF1 deletion | Hyperdiploid | KMT2A-R | TCF3 rearrangement, ETV6 deletion |
| No. of relapses | 3 | 2 | 1 | 1 | 1 |
| Prior CART products received | Tisacel (2 y prior), CART22-65s (1 y prior) | Tisacel (8 mo prior) | None | None | None |
| Prior blinatumomab | No | Yes (10 mo and >1 y prior) | No | No | Yes (4 mo prior) |
| Highest CNS status over course of previous treatment | 2 | 1 | 2 | 3 | 1 |
| History of cranial or craniospinal radiation | Yes | No | No | Yes (optic nerve) | No (XRT to mandibular mass) |
| History of previous ICANS | ICANS grade 4 (tisacel) | ICANS grade 3 (tisacel) | No | No | No |
| History of chronic neurologic disorder | None | None | None | CNS involvement of leukemia | Peripheral neuropathy (VCR) |
| Previous HSCT | Yes | No | No | Yes | No |
| Disease status before infusion | |||||
| Bone marrow blasts at infusion, % | 64 | 99 | 26 | 0 | 93 |
| CNS status based on CSF at CART infusion | 1 | N/A (LP deferred; CNS1 several weeks prior) | 1 | 1 | 1 |
| CART treatment information | |||||
| CART product | CART22-65s | CART22-65s | huCART19 | Tisacel | Investigational CTL019 |
| Lymphodepleting chemotherapy | Flu/Cy | Flu/Cy | Flu/Cy | Flu/Cy | Flu/Cy |
| Mortality | Yes | Yes | No | Yes | Yes |
| CNS status at day 28 after CAR infusion | 1 | 3 | 1 | 1 | 1 |
| Characteristic . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Clinical history before infusion | |||||
| Age at infusion, y | 7 | 14 | 13 | 2.5 | 7 |
| Sex | F | M | M | M | M |
| Cancer genetics | T21 related | IGH::CRLF2 fusion, IZKF1 deletion | Hyperdiploid | KMT2A-R | TCF3 rearrangement, ETV6 deletion |
| No. of relapses | 3 | 2 | 1 | 1 | 1 |
| Prior CART products received | Tisacel (2 y prior), CART22-65s (1 y prior) | Tisacel (8 mo prior) | None | None | None |
| Prior blinatumomab | No | Yes (10 mo and >1 y prior) | No | No | Yes (4 mo prior) |
| Highest CNS status over course of previous treatment | 2 | 1 | 2 | 3 | 1 |
| History of cranial or craniospinal radiation | Yes | No | No | Yes (optic nerve) | No (XRT to mandibular mass) |
| History of previous ICANS | ICANS grade 4 (tisacel) | ICANS grade 3 (tisacel) | No | No | No |
| History of chronic neurologic disorder | None | None | None | CNS involvement of leukemia | Peripheral neuropathy (VCR) |
| Previous HSCT | Yes | No | No | Yes | No |
| Disease status before infusion | |||||
| Bone marrow blasts at infusion, % | 64 | 99 | 26 | 0 | 93 |
| CNS status based on CSF at CART infusion | 1 | N/A (LP deferred; CNS1 several weeks prior) | 1 | 1 | 1 |
| CART treatment information | |||||
| CART product | CART22-65s | CART22-65s | huCART19 | Tisacel | Investigational CTL019 |
| Lymphodepleting chemotherapy | Flu/Cy | Flu/Cy | Flu/Cy | Flu/Cy | Flu/Cy |
| Mortality | Yes | Yes | No | Yes | Yes |
| CNS status at day 28 after CAR infusion | 1 | 3 | 1 | 1 | 1 |
Cy, cyclophosphamide; F, female; Flu, fludarabine; HSCT, hematopoietic stem cell transplant; M, male; N/A, not available; tisacel, tisagenlecleucel; VCR, vincristine; XRT, radiation therapy.
Cases with spinal cord involvement
Patient 1
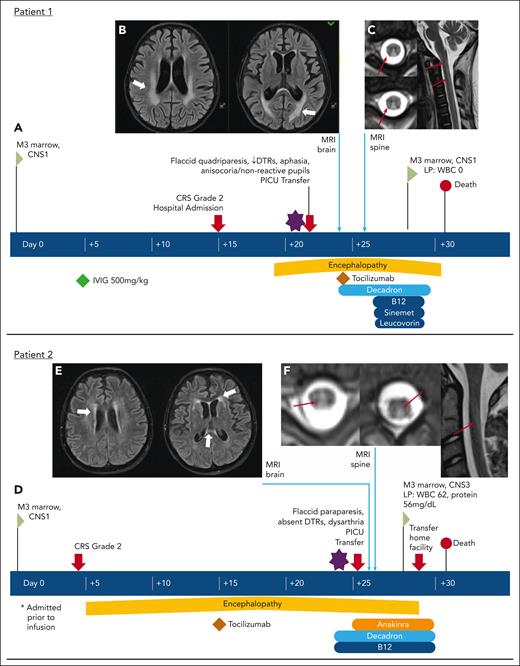
A 7-year-old girl with trisomy 21 and multiply relapsed B-ALL received CART22-65s. Previous treatment included standard chemotherapy and tisagenlecleucel (CART19) 2 years prior, which was complicated by grade 4 CRS and grade 3 ICANS (seizures and encephalopathy). One year prior, she received CART22-65s (grade 1 CRS, no ICANS) followed by a cranial (600 cGy) and total body irradiation (1200 cGy) containing consolidative matched sibling donor hematopoietic stem cell transplant (HSCT). Four months after HSCT, she experienced another relapse, prompting retreatment with newly manufactured CART22-65s. Before the second CART 22-65S infusion, she had 64% bone marrow blasts, central nervous system (CNS) 1 (Table 1). Screening magnetic resonance imaging (MRI) of the brain before first CART22-65s infusion demonstrated volume loss and T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities in the periventricular and subcortical white matter (WM), thought to be treatment related. She did not have repeat brain or spine imaging before the second CART22-65s infusion.
The second CART22-65s postinfusion course is summarized in Figure 1A and Table 2. Briefly, on D15, she was admitted for CRS. On D19, she was noted to have new mild encephalopathy (sleepy, irritable). On D22, she was sleepy. although she opened her eyes to voice. She demonstrated new global aphasia, inability to visually fix or track, intermittently nonreactive anisocoric pupils, and areflexic flaccid quadriparesis (grimaced but no withdrawal to noxious stimuli in all extremities). Computed tomography of the head (HCT) at that time demonstrated no acute abnormalities, and there were no seizures on electroencephalogram (EEG). MRI of the brain on D24 (Figure 1B) demonstrated new nonenhancing confluent areas of T2/FLAIR hyperintensity and restricted diffusion in the bilateral deep cerebral WM, extending from the medulla into the visualized cervical cord. She was treated with dexamethasone and received tocilizumab to mitigate CRS. MRI of the spine on D26 (Figure 1C) demonstrated diffuse T2 hyperintensity predominantly in the central and midline posterior cord, involving almost the entire spinal cord. Leucovorin and vitamin B12 were started. Levodopa/carbidopa therapy was given based on experience with patient 5 (see below). D28 LP demonstrated 0 CSF white blood cells (WBCs), 0 red blood cells, and no leukemic blasts; protein was not measured. CSF Gram stain and culture were negative. However, bone marrow biopsy (BMBx) demonstrated significant residual disease, so the family elected to transition to comfort care measures only. She died on D31 from progressive leukemia.
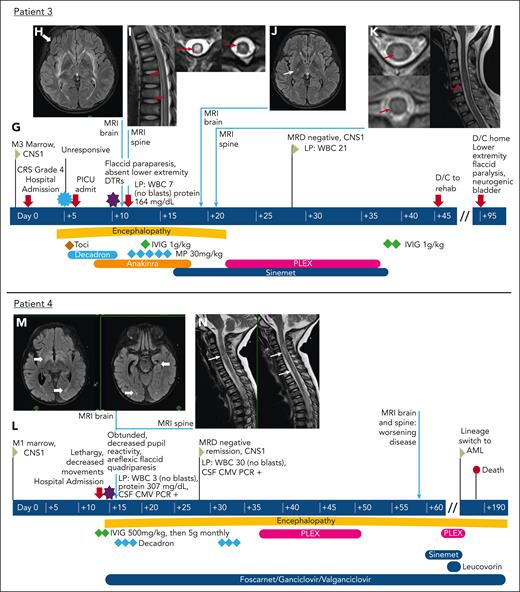
Visual vignettes describing development of paresis in 4 patients with spinal cord abnormalities on neuroimaging. Patient 1: (A) Time line of clinical course. (B) Axial FLAIR images from MRI of the brain showing bilateral, symmetric white matter changes (thick white arrows) with restricted diffusion (diffusion-weighted images not shown). (C) Axial (left) and sagittal (right) T2 images from MRI of the spine showing increased T2 signal in dorsal columns through entire cord (thin red arrows), without enhancement (enhanced images not shown). Patient 2: (D) Time line of clinical course. (E) Axial FLAIR images from MRI of the brain showing changes in the subcortical, deep, and periventricular cerebral white matter (thick white arrows). (F) Axial (left) and sagittal (right) T2 images from MRI of the spine showing increased T2 signal in dorsal columns through entire cord (thin red arrows), without enhancement (enhanced images not shown). Patient 3: (G) Time line of clinical course. (H) Axial FLAIR images from MRI of the brain showing symmetric foci of T2 hyperintensity involving supratentorial white matter (thick white arrow) and brainstem. (I) Axial (left) and sagittal (right) T2 images from MRI of the spine showing diffuse hyperintensity throughout the entire cord, from the brainstem to the conus (thin red arrows), without enhancement (gadolinium-enhanced images not shown). (J) Axial FLAIR images from MRI of the brain showing interval reduction in T2 signal abnormalities from prior study (thin white arrow). (K) Axial (left) and sagittal (right) T2 images from MRI of the spine showing interval progression of mildly expansile T2 hyperintense signal involving most of the spinal cord. Patient 4: (L) Time line of clinical course. (M) Axial FLAIR images from MRI of the brain showing nonspecific nonenhancing white matter T2/FLAIR hyperintensities (thick white arrows). (N) Sagittal T2 image from MRI of the spine showing extensive, patchy, nonenhancing multifocal T2 lesions, some of which were expansile (thin white arrows; contrast images not shown). D/C, discharge; DTR, deep tendon reflex; IVIG, intravenous immunoglobulin; PICU, pediatric intensive care unit; rehab, rehabilitation; WBC, white blood cell.
Visual vignettes describing development of paresis in 4 patients with spinal cord abnormalities on neuroimaging. Patient 1: (A) Time line of clinical course. (B) Axial FLAIR images from MRI of the brain showing bilateral, symmetric white matter changes (thick white arrows) with restricted diffusion (diffusion-weighted images not shown). (C) Axial (left) and sagittal (right) T2 images from MRI of the spine showing increased T2 signal in dorsal columns through entire cord (thin red arrows), without enhancement (enhanced images not shown). Patient 2: (D) Time line of clinical course. (E) Axial FLAIR images from MRI of the brain showing changes in the subcortical, deep, and periventricular cerebral white matter (thick white arrows). (F) Axial (left) and sagittal (right) T2 images from MRI of the spine showing increased T2 signal in dorsal columns through entire cord (thin red arrows), without enhancement (enhanced images not shown). Patient 3: (G) Time line of clinical course. (H) Axial FLAIR images from MRI of the brain showing symmetric foci of T2 hyperintensity involving supratentorial white matter (thick white arrow) and brainstem. (I) Axial (left) and sagittal (right) T2 images from MRI of the spine showing diffuse hyperintensity throughout the entire cord, from the brainstem to the conus (thin red arrows), without enhancement (gadolinium-enhanced images not shown). (J) Axial FLAIR images from MRI of the brain showing interval reduction in T2 signal abnormalities from prior study (thin white arrow). (K) Axial (left) and sagittal (right) T2 images from MRI of the spine showing interval progression of mildly expansile T2 hyperintense signal involving most of the spinal cord. Patient 4: (L) Time line of clinical course. (M) Axial FLAIR images from MRI of the brain showing nonspecific nonenhancing white matter T2/FLAIR hyperintensities (thick white arrows). (N) Sagittal T2 image from MRI of the spine showing extensive, patchy, nonenhancing multifocal T2 lesions, some of which were expansile (thin white arrows; contrast images not shown). D/C, discharge; DTR, deep tendon reflex; IVIG, intravenous immunoglobulin; PICU, pediatric intensive care unit; rehab, rehabilitation; WBC, white blood cell.
Postinfusion clinical, radiographic, and treatment data
| Variable . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Age at infusion, y | 7 | 14 | 13 | 2.5 | 7 |
| CART product | CART22-65s | CART22-65s | huCART19 | Tisacel | Investigational CTL019 |
| Acute clinical course | |||||
| Limb weakness onset day after infusion | D22 | D25 | D5-D10 | D15 | D36 |
| Pertinent MRI brain findings | ↑T2/FLAIR and restricted diffusion (nonenhancing) in bilateral deep cerebral white matter (D24) | ↑T2/FLAIR and restricted diffusion (nonenhancing) in subcortical, deep, and periventricular cerebral white matter (D25) | ↑T2/FLAIR (nonenhancing, no restricted diffusion) in supratentorial white matter and brainstem (D10) | ↑T2/FLAIR (nonenhancing, no restricted diffusion) in the cerebral white matter (D15) | ↑T2/FLAIR in corona radiata, centrum semiovale, corpus callosum, and periventricular white matter (D39) |
| Pertinent MRI spine findings | ↑T2 in central and midline posterior cord, with slight edema in anterior midline cervical cord (D26) | ↑T2 (nonenhancing) in dorsal columns of the cord (D25) | ↑T2 (nonenhancing) and mild expansion in central portion of the entire cord (D10) | Extensive, patchy, nonenhancing multifocal T2 lesions, some expansile (D16) | Normal |
| Day of death | D31 | D30 | — | D189 | D72 |
| Pertinent autopsy findings | Spinal cord: White matter pallor throughout the cord, few macrophages, extremely rare T lymphocytes, absent B lymphocytes | — | — | — | Spinal cord: normal Brain: cerebral cortex white matter pallor, with vacuolation and gliosis, axonal swelling |
| Concomitant CRS and CRS and ICANS therapies received | |||||
| Maximum CRS | Grade 2 | Grade 2 | Grade 4 | Grade 1 | Grade 4 |
| Tocilizumab | Yes | Yes | Yes | No | Yes |
| Corticosteroids | Yes | Yes | Yes | Yes | Yes |
| Plasmapheresis | No | No | Yes | Yes | No |
| IVIG | No | No | Yes | Yes | Yes |
| Sinemet | Yes | No | Yes | Yes | Yes |
| Anakinra | No | Yes | Yes | No | No |
| Other anticytokine therapy | No | No | No | No | No |
| Variable . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Age at infusion, y | 7 | 14 | 13 | 2.5 | 7 |
| CART product | CART22-65s | CART22-65s | huCART19 | Tisacel | Investigational CTL019 |
| Acute clinical course | |||||
| Limb weakness onset day after infusion | D22 | D25 | D5-D10 | D15 | D36 |
| Pertinent MRI brain findings | ↑T2/FLAIR and restricted diffusion (nonenhancing) in bilateral deep cerebral white matter (D24) | ↑T2/FLAIR and restricted diffusion (nonenhancing) in subcortical, deep, and periventricular cerebral white matter (D25) | ↑T2/FLAIR (nonenhancing, no restricted diffusion) in supratentorial white matter and brainstem (D10) | ↑T2/FLAIR (nonenhancing, no restricted diffusion) in the cerebral white matter (D15) | ↑T2/FLAIR in corona radiata, centrum semiovale, corpus callosum, and periventricular white matter (D39) |
| Pertinent MRI spine findings | ↑T2 in central and midline posterior cord, with slight edema in anterior midline cervical cord (D26) | ↑T2 (nonenhancing) in dorsal columns of the cord (D25) | ↑T2 (nonenhancing) and mild expansion in central portion of the entire cord (D10) | Extensive, patchy, nonenhancing multifocal T2 lesions, some expansile (D16) | Normal |
| Day of death | D31 | D30 | — | D189 | D72 |
| Pertinent autopsy findings | Spinal cord: White matter pallor throughout the cord, few macrophages, extremely rare T lymphocytes, absent B lymphocytes | — | — | — | Spinal cord: normal Brain: cerebral cortex white matter pallor, with vacuolation and gliosis, axonal swelling |
| Concomitant CRS and CRS and ICANS therapies received | |||||
| Maximum CRS | Grade 2 | Grade 2 | Grade 4 | Grade 1 | Grade 4 |
| Tocilizumab | Yes | Yes | Yes | No | Yes |
| Corticosteroids | Yes | Yes | Yes | Yes | Yes |
| Plasmapheresis | No | No | Yes | Yes | No |
| IVIG | No | No | Yes | Yes | Yes |
| Sinemet | Yes | No | Yes | Yes | Yes |
| Anakinra | No | Yes | Yes | No | No |
| Other anticytokine therapy | No | No | No | No | No |
Dashes denote not applicable (patient 3 did not die; no autopsy findings available on patients 2, 3 or 4). IVIG, intravenous immunoglobulin.
Patient 2
A 14-year-old patient with Philadelphia chromosome–like, multiply relapsed B-ALL received CART22-65s. Previous treatment included standard chemotherapy, 2 courses of blinatumomab (CRS grade 2, no ICANS), and tisagenlecleucel 8 months prior. Tisagenlecleucel course was complicated by grade 4 CRS (hypotension, respiratory failure, and renal failure) and ICANS grade 3 (encephalopathy and agitation). Brain MRI was without acute abnormality. Two months following tisagenlecleucel, a CNS relapse (CNS3) occurred with progression to aggressive CD19-negative disease (99% bone marrow blasts) (Table 1). He was enrolled on the CART22-65s trial. While receiving lymphodepleting chemotherapy, he developed fever and was admitted, requiring intensive care for severe tumor lysis syndrome. Because of severe tumor lysis, he did not have a routine D1 LP; however, CSF studies at D31 demonstrated CNS1. Screening MRI of the brain on D27 demonstrated multifocal microhemorrhages, an old lacunar stroke in the left caudate nucleus, and T2/FLAIR hyperintensities in the periventricular and subcortical WM with volume loss, thought to be treatment related. He did not have spine imaging before his CART22-65s infusion.
His postinfusion course is summarized in Figure 1D and Table 2. Briefly, he developed CRS on D4, followed by encephalopathy on D8, characterized by somnolence and difficulty answering questions. He was treated on D15 with tocilizumab for worsening CRS, with subsequent clinical improvement. On D25, he developed worsened dysarthria and a new areflexic flaccid lower-extremity paraparesis, moving legs briefly/rarely in the plane of the bed only, with associated decreased sensation to light touch (not pain) in the right greater than left leg. He was sleepy, but able to awaken and otherwise interact appropriately. HCT demonstrated no acute abnormalities. MRI of the brain (Figure 1E) demonstrated new nonenhancing areas of T2/FLAIR hyperintensity and restricted diffusion in the subcortical, deep, and periventricular cerebral WM. MRI of the spine (Figure 1F) demonstrated nonenhancing T2/FLAIR hyperintensity within the dorsal columns of the cord, extending from the midcervical spine to just above the conus medullaris. He was treated with dexamethasone, anakinra, and B12. D28 routine LP and BMBx demonstrated significant residual CNS and marrow disease, so family elected to return home for comfort care. CSF Gram stain, culture, and enterovirus PCR were negative. He died on D30 from progressive leukemia.
Patient 3
A 13-year-old patient with hyperdiploid ALL received humanized CART19 (huCART19) for first, late medullary relapse refractory to chemotherapy. Previous treatment included standard chemotherapy with no prior immunotherapy. Before CART infusion, he had 26% bone marrow blasts, CNS1 (Table 1). MRI of the lumbar spine 5 years before huCART19 demonstrated leukemic involvement of multiple thoracic and lumbar vertebral bodies, but no spinal cord abnormalities. He did not have further brain or spine imaging before his huCART19 infusion.
His postinfusion course is summarized in Figure 1G and Table 2. Briefly, he was admitted on D1 with fever progressing to grade 4 CRS. On D4, he developed a progressive encephalopathy (confusion), progressing to unresponsiveness on D5, requiring intubation. HCT demonstrated no acute abnormalities. EEG demonstrated generalized rhythmic delta activity without seizures, indicating an encephalopathic state. He was treated with tocilizumab, dexamethasone, and anakinra. As his mental status improved, a new areflexic flaccid lower-extremity paraparesis with associated loss of global sensation became apparent. MRI of the brain on D10 (Figure 1H) demonstrated nonenhancing, nondiffusion restricting T2/FLAIR hyperintensities of the supratentorial WM and brainstem, of uncertain chronicity given lack of preinfusion imaging. MRI of the spine (Figure 1I) demonstrated a diffuse, central, nonenhancing T2/FLAIR hyperintensity extending from the brainstem to the conus. LP on D11 demonstrated CSF WBC 7 cells/μL, elevated protein, and a marked elevation in soluble IL-2-receptor, IL-6, and IL-13; other cytokines were within normal limits. CSF enterovirus, varicella-zoster virus, cytomegalovirus (CMV), and human herpesvirus-6 PCRs were all negative. Serum herpes simplex virus PCR and hepatitis B/C serology was negative. Nasal swab severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR was negative. He was treated with high-dose methylprednisolone and intravenous immunoglobulin (IVIG), and anakinra dose was increased. The patient subsequently had minor improvements in his examination, including renewed sensation to pain in the legs. Levodopa/carbidopa therapy was transiently trialed based on experience with patient 5 (see below), but weaned off because of lack of benefit.
Repeat MRI of the brain on D19 (Figure 1J) demonstrated marked interval reduction of previously noted T2/FLAIR signal abnormalities. Repeat MRI of the spine on D21 (Figure 1K) demonstrated interval progression of diffuse mildly expansile T2 hyperintensity involving most of the spinal cord, as well as new smooth enhancement of nerve roots of the cauda equina. Given persistent symptoms and imaging progression, plasmapheresis was started on D22, followed by repeat IVIG. D28 LP and BMBx demonstrated CNS and marrow remission. Follow-up MRI of the brain and spine 3 months later demonstrated near complete resolution of brain signal abnormalities, interval improvement (but not resolution) in cord T2 signal abnormality and enhancement, but new focal volume loss and cystic malacic changes within the cord at the level of T1 to T2. At last neurologic follow up, >15 months after infusion, areflexic flaccid lower-extremity paraparesis persisted, with associated absent light touch and temperature sensation up to the midchest.
Patient 4
A 34-month-old boy with multiply relapsed B-ALL and persistent CMV viremia received tisagenlecleucel (CART19). Previous treatment included standard chemotherapy and a paternal haploidentical HSCT 8 months before CART. Posttransplant asymptomatic CMV viremia was treated with valganciclovir. Four months before CART, a marrow and CNS relapse (CNS3) occurred, which was treated with chemotherapy, followed by right optic nerve radiation and inotuzumab. He had progressive lethargy, generalized weakness, and pain after radiation. MRI of the spine demonstrated a normal cord. Symptoms were thought to be due to CNS leukemia and deconditioning. Screening MRI of the brain at D26 demonstrated pachymeningeal, ependymal, optic chiasm, and optic nerve enhancement. However, before CART19, there were no marrow blasts, and he was CNS1 on D5 (Table 1). Lethargy and generalized weakness had resolved before CART infusion. CSF CMV PCR was not performed before CART19 infusion; serum CMV PCR was positive (1855 IU/mL or 3.27 log IU/mL on D1; dynamic range, 1300-1 billion IU/mL). Valganciclovir dose was increased at this time.
His postinfusion course is summarized in Figure 1L and Table 2. Briefly, he was admitted on D14 for lethargy and decreased movement. Respiratory failure and encephalopathy (sleepy, irritable, and repetitive) developed shortly after admission. On D15, the patient became obtunded with decreased pupil reactivity and an areflexic flaccid quadriparesis. MRI of the brain at that time (Figure 1M) demonstrated nonspecific nonenhancing WM T2/FLAIR hyperintensities. LP demonstrated CSF WBC 3 cells/μL, protein 307 mg/dL, and a positive CSF CMV PCR. CSF herpes simplex virus, enterovirus, and human herpesvirus-6 PCRs were negative. Serum CMV PCR remained positive. MRI of the spine on D16 (Figure 1N) demonstrated new, extensive, patchy, nonenhancing multifocal T2 lesions throughout nearly the entire length of his spinal cord. Some of these, particularly in the cervical cord, were expansile. Differential diagnosis for these lesions included a new leukemic infiltrate, CART-mediated inflammation, or CMV myelitis. The patient was treated with dexamethasone, IVIG, and foscarnet, and valganciclovir was transitioned to ganciclovir with subtle improvement in examination. Follow-up MRI of the brain and spine on D20 showed improvement without resolution. D28 LP and BMBx demonstrated CNS and marrow remission. CSF WBC was elevated at 30 cells/μL (no blasts) and CSF CMV PCR remained positive. Serum CMV PCR peak copy number was 36 331 IU/mL (4.56 log IU/mL) on D24.
On D28, the child developed decreased spontaneous movement again and no withdrawal to painful stimuli. Encephalopathy was ongoing. Follow-up MRI of the brain and spine on D30 again showed interval improvement. Treatment with dexamethasone was started on D32 without improvement, followed by a course of plasmapheresis starting on D37 with minimal improvement in level of consciousness. Given concern for a CMV-related Guillain-Barre–like syndrome causing weakness, nerve conduction studies and electromyography were performed and demonstrated decreased motor units, unclear if anterior horn cell, nerve root, nerve, or muscle in origin. Muscle ultrasound was unrevealing.
On D58, repeat MRI of the brain and spinal cord demonstrated interval worsening of brain enhancement and spine T2 signal abnormalities. Carbidopa/levodopa was given starting on D59, given prior experience with patient 5. A second round of plasmapheresis was started on D66. Over several weeks, the patient regained the ability to fixate, nod yes/no, and withdraw his right leg antigravity. Routine laboratory findings on D156 demonstrated lineage switch to acute myeloid leukemia, and the family elected to transition to comfort care. The patient died on D189 following compassionate withdrawal of care.
Case without spinal cord involvement
Patient 5
A 7-year-old boy with early medullary relapsed B-ALL received investigational CTL019 (CART19). Previous treatment included standard chemotherapy augmented by blinatumomab (uncomplicated) and radiation to a mandibular chloroma. Before CART19, he had an M3 marrow and was CNS1 (Table 1). Screening HCT on D7 demonstrated scattered foci of mineralization and some hypodensity in the periventricular WM, thought to be long-term and treatment related.
His postinfusion course is summarized in Figure 2A and Table 2. Briefly, he developed anaphylaxis following CART infusion and was admitted; he received tocilizumab after developing high, persistent fevers as part of a study of preemptive tocilizumab administration (NCT02906371). On D5, he progressed to grade 4 CRS and new encephalopathy (awake but minimally responsive, aphasic). He was treated with methylprednisolone and tocilizumab. HCT on D6 was unchanged from prior findings. On D11, he developed new-onset refractory generalized status epilepticus, which was treated with multiple abortive antiseizure medications and a midazolam infusion. HCT at this time (Figure 2B) was unchanged from prior findings. He improved over the course of weeks, without return to baseline. D28 routine LP and BMBx demonstrated CNS and marrow remission.
Visual vignette describing development of paresis in 1 patient with brain abnormalities only on neuroimaging. Patient 5: (A) Time line of clinical course. (B) Head computed tomography showing mild lateral ventricle prominence (thin white arrow), but no short-term change. (C) Axial FLAIR images from MRI of the brain showing extensive bilateral areas of abnormal T2 signal intensity involving the white matter of the centrum semiovale, superior corona radiata, and periventricular white matter (thick white arrows), associated with restricted diffusion centrally, but no enhancement (diffusion-weighted and gadolinium-enhanced imaging not shown). (D) Sagittal T2 image from MRI of the spine with normal findings. DTR, deep tendon reflex; PICU, pediatric intensive care unit.
Visual vignette describing development of paresis in 1 patient with brain abnormalities only on neuroimaging. Patient 5: (A) Time line of clinical course. (B) Head computed tomography showing mild lateral ventricle prominence (thin white arrow), but no short-term change. (C) Axial FLAIR images from MRI of the brain showing extensive bilateral areas of abnormal T2 signal intensity involving the white matter of the centrum semiovale, superior corona radiata, and periventricular white matter (thick white arrows), associated with restricted diffusion centrally, but no enhancement (diffusion-weighted and gadolinium-enhanced imaging not shown). (D) Sagittal T2 image from MRI of the spine with normal findings. DTR, deep tendon reflex; PICU, pediatric intensive care unit.
On D30, encephalopathy worsened with decreased interaction and verbal output. He was treated with dexamethasone and received routine post-CART IVIG. On D36, he became alternatingly lethargic and irritable, crying to noxious stimuli, but otherwise not engaging with surroundings. He demonstrated a new global aphasia, new inability to visually fix or track, and a new spastic/dystonic quadriparesis, with rigid bilateral lower extremities at rest and diffuse hyperreflexia. MRI of the brain on D39 (Figure 2C) demonstrated diffuse confluent T2/FLAIR WM abnormalities, involving the corona radiata, centrum semiovale, corpus callosum, and periventricular WM, with sparing of the U-fibers. MRI of the spine was unremarkable (Figure 2D). LP demonstrated CSF WBC of 2 cells/μL and low protein (27 mg/dL). CSF Gram stain, culture, and JC virus PCR were negative. EEG did not demonstrate seizures. He was treated with leucovorin, diazepam, and high-dose methylprednisolone.
Over the coming weeks, he became more awake but remained aphasic with a spastic/dystonic quadriparesis. On D57, CSF neurotransmitters resulted and demonstrated a low tetrahydrobiopterin (<5; normal range, 9-40), so carbidopa/levodopa therapy was started. He was thought to have slight improvement in wakefulness and spontaneous movements with this therapy. However, he developed relapsed leukemia with peripheral blasts on D70, and he was transitioned to comfort care. No BMBx or LP was performed. He died on D72 from progressive leukemia.
Autopsy findings
Postmortem examination was performed on 2 patients (patients 1 and 5) and was remarkable in both cases for (1) leukoencephalopathy/leukomyelopathy and (2) absence of lymphocytic infiltrates.
Autopsy was limited to the spinal cord in patient 1 and showed significant pallor throughout all WM tracts (Figure 3A-F). There was extensive vacuolation (Figure 3B) of WM with numerous axonal spheroids indicative of axonal damage (Figure 3C). Gray matter was preserved. There was an absence of inflammatory findings with only scattered macrophages (Figure 3D), rare T lymphocytes (Figure 3E), and no B lymphocytes (Figure 3F).
Autopsy findings from the spinal cord (A-F) of patient 1 treated with CART22 and the brain and spinal cord (G-J) of patient 5 treated with CTL019, with both samples notable for white matter damage and an absence of lymphocytic inflammation. (A) Spinal cord showing pallor throughout all the white matter tracts (hematoxylin and eosin [H&E], 1× objective). (B) White matter of the cord showed extensive vacuolation, whereas the gray matter (bottom right) was relatively well preserved (H&E, 10× objective). (C) Numerous axonal spheroids (white arrows) were seen throughout white matter of the cord, indicating axonal injury (neurofilament immunostain, 20× objective). (D) Few macrophages were present within the white matter (CD68 immunostain, 10× objective). (E) Only extremely rare T lymphocytes (black arrow) were present within cord parenchyma (CD3 immunostain, 10× objective). (F) B lymphocytes were absent within the cord (CD79a immunostain, 10× objective). (G) White matter of the spinal cord was well preserved (H&E, 1× objective). (H) Cerebral cortex showing a region of pallor within the white matter (black arrow) (H&E, 1× objective). (I) Cortical white matter with vacuolation and gliosis (H&E, 20× objective). (J) Axonal swellings were seen within the white matter of the cerebral cortex, indicating axonal injury (β-APP immunostain, 10× objective).
Autopsy findings from the spinal cord (A-F) of patient 1 treated with CART22 and the brain and spinal cord (G-J) of patient 5 treated with CTL019, with both samples notable for white matter damage and an absence of lymphocytic inflammation. (A) Spinal cord showing pallor throughout all the white matter tracts (hematoxylin and eosin [H&E], 1× objective). (B) White matter of the cord showed extensive vacuolation, whereas the gray matter (bottom right) was relatively well preserved (H&E, 10× objective). (C) Numerous axonal spheroids (white arrows) were seen throughout white matter of the cord, indicating axonal injury (neurofilament immunostain, 20× objective). (D) Few macrophages were present within the white matter (CD68 immunostain, 10× objective). (E) Only extremely rare T lymphocytes (black arrow) were present within cord parenchyma (CD3 immunostain, 10× objective). (F) B lymphocytes were absent within the cord (CD79a immunostain, 10× objective). (G) White matter of the spinal cord was well preserved (H&E, 1× objective). (H) Cerebral cortex showing a region of pallor within the white matter (black arrow) (H&E, 1× objective). (I) Cortical white matter with vacuolation and gliosis (H&E, 20× objective). (J) Axonal swellings were seen within the white matter of the cerebral cortex, indicating axonal injury (β-APP immunostain, 10× objective).
Patient 5 underwent a complete autopsy, including examination of the brain and spinal cord. No histologic abnormalities were observed within the WM of the spinal cord (Figure 3G), consistent with imaging findings. However, WM abnormalities were present in the brain, including pallor (Figure 3H), vacuolation (Figure 3I), and axonal damage, as evidenced by β-amyloid precursor protein (APP) staining (Figure 3J). No significant lymphocytic infiltrates were observed, and the gray matter of both the brain and spinal cord was preserved.
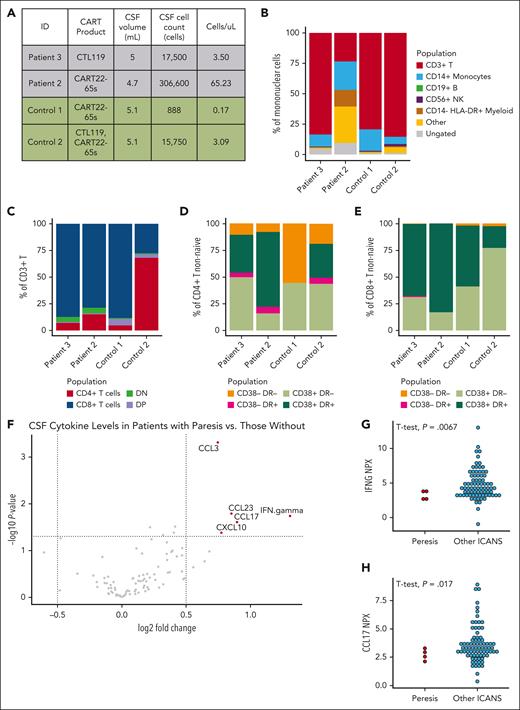
CSF correlative analyses
Clinical data from CSF are presented in Table 3. We performed flow cytometry on CSF samples from patients 2 and 3 and compared them with control patients who received CART without ICANS symptoms. Total cell counts are presented in Figure 4A. Patient 2 had high cellularity, with a significant proportion of “other” cells, likely blasts (Figure 4B). Lineage distributions were otherwise similar between patients with paresis and controls. Similarly, T-cell population distribution was similar across all patients (Figure 4C), with similar patterns of CD4 (Figure 4D) and CD8 activation (Figure 4E). As with autopsy findings, we were unable to identify an association between an inflammatory lymphocyte population and development of paresis.
Pertinent laboratory data following CART infusion
| Variable . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Day after infusion | D24 | D28 | D10 | D15 | D38 |
| CSF WBC (cells/uL) | 0 | 68 | 3 | 3 | 2 |
| CSF blasts, % | 0 | 95 | 0 | 0 | 0 |
| CSF protein (mg/dL) | N/A | 56 | 164 | 307 | 27 |
| CSF BH4 (nmol/L) | N/A | N/A | N/A | N/A | <5 |
| CSF qPCR at day 28, copies/μg DNA | 26 562 | 10 237 | 22 853 | N/A | 88 040 |
| Peripheral WBC at time of LP (k/μL) | 0.2 | 0.1 | 4.7 | 1.8 | 0.3 |
| Highest serum CART qPCR, copies/μg DNA | 82 198 (D28) | 412 318 (D28) | 113 502 (D7) | N/A | 136 002 (D10) |
| Serum B12, pg/mL (normal range) | 406 (247-1174) | 3022 (214-865) | 1396 (214-865) | 549 (264-1216) | N/A |
| Serum copper (normal range) (ug/dL) | N/A | N/A | 82.1 (57-129) | 122 (75-153) | N/A |
| Serum zinc (nornal range) (ug/dL) | N/A | N/A | 98.5 (60-120) | 116 (60-120) | N/A |
| Variable . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Day after infusion | D24 | D28 | D10 | D15 | D38 |
| CSF WBC (cells/uL) | 0 | 68 | 3 | 3 | 2 |
| CSF blasts, % | 0 | 95 | 0 | 0 | 0 |
| CSF protein (mg/dL) | N/A | 56 | 164 | 307 | 27 |
| CSF BH4 (nmol/L) | N/A | N/A | N/A | N/A | <5 |
| CSF qPCR at day 28, copies/μg DNA | 26 562 | 10 237 | 22 853 | N/A | 88 040 |
| Peripheral WBC at time of LP (k/μL) | 0.2 | 0.1 | 4.7 | 1.8 | 0.3 |
| Highest serum CART qPCR, copies/μg DNA | 82 198 (D28) | 412 318 (D28) | 113 502 (D7) | N/A | 136 002 (D10) |
| Serum B12, pg/mL (normal range) | 406 (247-1174) | 3022 (214-865) | 1396 (214-865) | 549 (264-1216) | N/A |
| Serum copper (normal range) (ug/dL) | N/A | N/A | 82.1 (57-129) | 122 (75-153) | N/A |
| Serum zinc (nornal range) (ug/dL) | N/A | N/A | 98.5 (60-120) | 116 (60-120) | N/A |
BH4, tetrahydrobiopterin; N/A, not available; qPCR, quantitative PCR.
Correlative studies of cellular populations and proteins from CSF demonstrate a marked absence of inflammatory signature. (A) CSF volume and cell counts for 2 patients with spinal paresis after CART at the time of symptoms and 2 patients who received CART but did not develop symptoms of ICANS. Proportion of cell types determined by flow cytometry of each lineage distribution (B) and T-cell distribution (C) were similar between all 4 patients. Proportion of CD4 (D) and CD8 (E) cells with flow cytometry evidence of activation were similar between all 4 patients. (F) Volcano plot (adjusted P > .05, log fold change >0.5) demonstrating differentially expressed proteins measured from D28 CSF in patients who developed quadriparesis or paraparesis (N = 4) and patients treated with CART19 or CART22 who did not (N = 150). The top 2 differentially expressed proteins were interferon gamma (G) and CCL17 (H), and were lower in patients who developed paresis (N = 4) than in those who developed any other phenomenology of ICANS (N = 78). ID, identifier.
Correlative studies of cellular populations and proteins from CSF demonstrate a marked absence of inflammatory signature. (A) CSF volume and cell counts for 2 patients with spinal paresis after CART at the time of symptoms and 2 patients who received CART but did not develop symptoms of ICANS. Proportion of cell types determined by flow cytometry of each lineage distribution (B) and T-cell distribution (C) were similar between all 4 patients. Proportion of CD4 (D) and CD8 (E) cells with flow cytometry evidence of activation were similar between all 4 patients. (F) Volcano plot (adjusted P > .05, log fold change >0.5) demonstrating differentially expressed proteins measured from D28 CSF in patients who developed quadriparesis or paraparesis (N = 4) and patients treated with CART19 or CART22 who did not (N = 150). The top 2 differentially expressed proteins were interferon gamma (G) and CCL17 (H), and were lower in patients who developed paresis (N = 4) than in those who developed any other phenomenology of ICANS (N = 78). ID, identifier.
Cryopreserved CSF from D28 was available on patients 1 to 3 and 5, and was compared with other patients treated with CART19 and CART22 (N = 150). Patients with paresis had lower levels of proinflammatory cytokines, such as interferon gamma, CCL17, CCL23, and CXCL10, than patients who received CART but did not develop those symptoms (Figure 4F). The 2 most highly differentially expressed cytokines, interferon gamma (Figure 4G) and CCL17 (Figure 4H), are shown in comparison to patients who developed other symptoms of ICANS (N = 78) and are notably lower in the patients with paresis (N = 4; P = .067 and P = .017, respectively; t-test).
Discussion
We present 5 children who developed quadriparesis/paraparesis following infusion with CART22 or CART19, of ≈500 treated at our center to date. Two children developed this phenomenon following CART22 and had similar clinical and neuroimaging findings. Three children developed quadriparesis or paraparesis following CART19 infusion, with more variable clinical and neuroimaging findings. In 1 child, an alternative cause of possible CMV myelitis was identified11; however, no cause was identified despite exhaustive investigations in the others. Multiple treatment strategies were trialed without evidence of significant improvement. On the basis of CSF findings proximal to the time of symptom onset (Table 3), available autopsy findings (Figure 3), and neuroimaging patterns, we do not feel these findings can be attributable to leukemic progression alone.
The 2 children who developed paresis following CART22-65s had similar clinical and neuroimaging findings with signal abnormalities most prominent in the spinal dorsal columns. This distribution was unexpected, given the dorsal columns contain sensory tracks for vibration and proprioception rather than motor neurons. However, autopsy findings in patient 1 demonstrated more diffuse abnormalities, including of the corticospinal tracts, than seen on imaging. Corticospinal tract abnormalities would account for motor findings. Rates of ICANS following other CD22-directed CART products have been low.12 The 3 children who received CART19 had more heterogeneous clinical and neuroimaging findings: Patient 3 had a diffuse central longitudinally extensive transverse myelitis, patient 4 had a multifocal expansile spinal cord lesion, and patient 5 had extensive supratentorial abnormalities but a normal spinal cord. With the exception of patient 4, whose spinal cord changes were thought to be likely related to CMV infection, the cause of the other neuroimaging findings remains occult despite extensive evaluation. Given the proximity to CART infusion, we hypothesized that these findings would be inflammatory in nature, consistent with known ICANS pathophysiology. However, there was a notable absence of inflammation on CSF flow cytometry, proteomics, and autopsy studies. Two patients did have significant leukopenia at symptom onset, and all patients received corticosteroids for their acute symptoms before D28 CSF sampling, which may temper measurement of a potential inflammatory response. Given the degree of clinical injury even in the setting of leukopenia and corticosteroid therapy, we would expect to see some evidence of inflammation if it were the primary driver of this phenomenon. Several of the patients had the onset of paraparesis in the setting of persistent disease, and it is possible that the combination of CART activation and active disease may have contributed to the clinical findings. The mechanism by which this occurred is an important area for future research. Unlike typical ICANS, symptom onset was later than D20 in 3 of the 4 cases.
Quadriparesis and paraparesis have not previously been reported in children following CART infusion. There is 1 prior report of acute quadriparesis in 2 adults with lymphoma who received axicabtagene ciloleucel (axi-cel) on the ZUMA-1 trial (1 flaccid, 1 spastic; NCT02348216).13 Both patients improved with tocilizumab and corticosteroids. In contrast to our patients, CART expansion was higher and inflammatory cytokines were elevated compared with others on the ZUMA trial, implicating an inflammation-driven process. In our pediatric patients, expansion was typical (Table 2), and there was not evidence of excessive inflammation.
The differential diagnosis for the spinal cord imaging abnormalities found in the first 3 patients is broad. The longitudinally extensive dorsal column involvement in the CART22-65s patients (patients 1 and 2) is consistent with subacute combined degeneration. Although this pattern is often caused by vitamin B12 deficiency, both patients had normal B12 levels (Table 3). This type of longitudinal tract involvement can also be suggestive of a metabolic predisposition, toxic exposure,14 or paraneoplastic process.15 Patient 2 had a high number of blasts in the CSF at the time, raising suspicion for leukemic involvement; however, longitudinal tract involvement with short-term onset of flaccid paralysis is not a typical manifestation of CNS leukemia, especially in the absence of a focal lesion, such as a chloroma. The autopsy findings of severe leukomyelopathy for patient 1 could support a metabolic or toxic process. Furthermore, both patients had a history of CART exposure. It is unknown if this could also be a predisposing factor. Patient 3 had a unique clinical presentation with flaccid paralysis of the lower extremities with central cord involvement. This clinical picture could be consistent with an infectious myelitis, although the observed CSF flow cytometry profile was not markedly distinct from that of control patients without paresis, and no infectious etiology was identified. MRI findings could be consistent with infarction given the lack of inflammation and worsening of cord swelling over time; however, there was no clear clinical precipitating event.16
Patient 5 was unique in the presentation of spastic compared with flaccid paresis and was the only patient with normal spinal cord imaging. Notably, he had a low CSF tetrahydrobiopterin, an essential cofactor in dopamine metabolism. Given concern that a depressed tetrahydrobiopterin may reflect downstream dopamine deficiency contributing to his symptoms, he was treated with carbidopa/levodopa. His presentation differed from recent reports of Parkinson-like symptoms in adults who received B-cell maturation antigen–CART for multiple myeloma, who presented with weeks to months of slowing, limb rigidity, micrographia, and tremor.17,18 Patient 5’s symptoms were acute and isolated to spastic weakness. He was thought to have slight improvement in wakefulness and spontaneous movements with carbidopa/levodopa, although improvements were subtle. Patients 1, 3, and 4 were empirically treated with carbidopa/levodopa after this given unclear mechanism of symptoms, without clear impact on their symptoms.
The etiology of this newly reported post-CART ICANS phenomenology remains uncertain but may be the result of a complex interaction between host diathesis, tumor activation, and CART. Consistently obtaining preinfusion neurologic histories, examinations, and neuroimaging studies, and banking CSF from multiple time points before and after infusion for later study, will help us better understand host risk factors in the future. A better understanding of potential toxic and metabolic sequelae evoked by CART/tumor interactions, a more nuanced approach to ICANS by clinical phenotype, and indications for more detailed neuroimaging are all essential areas for future research. These findings imply that the underlying mechanism of different manifestations of ICANS may be distinct and may be more complex than exclusive T-cell activation and excessive cytokine production alone.
Acknowledgments
The authors gratefully acknowledge the assistance of the Translational and Correlative Studies Laboratory at the University of Pennsylvania.
C.D. was supported by the Abramson Cancer Center (5K12CA076931-24) K12 Award, a Canadian Institute for Health Research Fellowship Award, and a Children’s Hospital of Philadelphia Cell and Gene Therapy Award.
Authorship
Contribution: C.D., L.H.-M., B.L.B., R.M.M., D.A.E., and J.L.M. designed and conceptualized the study; C.D., L.H.-M., D.A.E., A.B.-O., A.M.D., A.B.L., Z.M., R.M.M., S.E.H., S.R.R., D.T.T., A.N.V., L.M.W., S.L.M., S.A.G., and J.L.M. performed data acquisition and analysis; C.D., L.H.-M., R.M.M., D.A.E., J.L.M., and A.N.V. drafted the manuscript and figures; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: D.T.T. serves on advisory boards for Jazz, Servier, BEAM Therapeutics, and Sobi; receives research funding from BEAM Therapeutics and NeoImmune Tech; and has multiple patents/patents pending on chimeric antigen receptor T-cell therapy. S.R.R. is a consultant for Pfizer and Jazz. S.L.M. has received clinical trial support from Novartis and Wugen, has served on advisory and study steering committees for Wugen and Novartis, and has a patent pending and licensed to Novartis Pharmaceuticals without royalty for PCT/US2017/044425: Combination Therapies of Car and PD-1 Inhibitors. A.B.-O. has received personal fees for advisory board participation and/or consulting from Abata, Accure, Atara Biotherapeutics, Biogen, Bristol Myers Squibb/Celgene/Receptos, GlaxoSmithKline, Gossamer, Horizon Therapeutics, Immunic, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, Sangamo, Sanofi-Genzyme, and Viracta; and grant support to the University of Pennsylvania from Biogen Idec, Roche/Genentech, Merck/EMD Serono, and Novartis. S.E.H. receives consulting fees from Bristol Myers Squibb and receives salary support from the US Centers for Disease Control and Prevention for activities related to acute flaccid myelitis (AFM) surveillance. B.L.B. reports a grant from the National Multiple Sclerosis Society; personal compensation for consulting from Roche, Sanofi, Novartis, and UCB; and serves on the American Academy of Neurology Board of Directors and the International Medical and Scientific Advisory Board for the Multiple Sclerosis International Federation. S.A.G. receives clinical research funding from Novartis, Cellectis, Kite, Vertex, and Servier; consults for Novartis, Eureka, Adaptive, and Jazz Pharmaceuticals; and has advised for Novartis, Adaptimmune, Kyttaro, Vertex, Allogene, Jazz Pharmaceuticals, and Cabaletta. The remaining authors declare no competing financial interests.
Correspondence: Caroline Diorio, The Children’s Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, 3501 Civic Center Blvd, Philadelphia, PA 19104; email: diorioc@chop.edu.
References
Author notes
C.D. and L.H.-M. contributed equally to this work.
Written requests for access to a decoded version of the data reported in this article can be submitted to the corresponding author, Caroline Diorio (diorioc@chop.edu), for review with appropriate regulatory approval and signed data use agreement.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Autopsy findings from the spinal cord (A-F) of patient 1 treated with CART22 and the brain and spinal cord (G-J) of patient 5 treated with CTL019, with both samples notable for white matter damage and an absence of lymphocytic inflammation. (A) Spinal cord showing pallor throughout all the white matter tracts (hematoxylin and eosin [H&E], 1× objective). (B) White matter of the cord showed extensive vacuolation, whereas the gray matter (bottom right) was relatively well preserved (H&E, 10× objective). (C) Numerous axonal spheroids (white arrows) were seen throughout white matter of the cord, indicating axonal injury (neurofilament immunostain, 20× objective). (D) Few macrophages were present within the white matter (CD68 immunostain, 10× objective). (E) Only extremely rare T lymphocytes (black arrow) were present within cord parenchyma (CD3 immunostain, 10× objective). (F) B lymphocytes were absent within the cord (CD79a immunostain, 10× objective). (G) White matter of the spinal cord was well preserved (H&E, 1× objective). (H) Cerebral cortex showing a region of pallor within the white matter (black arrow) (H&E, 1× objective). (I) Cortical white matter with vacuolation and gliosis (H&E, 20× objective). (J) Axonal swellings were seen within the white matter of the cerebral cortex, indicating axonal injury (β-APP immunostain, 10× objective).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/13/10.1182_blood.2024023933/2/m_blood_bld-2024-023933-gr3.jpeg?Expires=1765897111&Signature=CyK2nHr9jbm7VxSs6hMpTdinMlpBylmt9pXEuwus1Jp-meOxxoNfcspgmEX-vyOEdSvfJZY0FiNjSvimle8KHEc5ikHzuPVcR4wsRMlgq9rumQTJdDQO7LSUcudbeMqPQY68218byCHDdhKfPajDBLcLSktC4H2guIK8GhbyZaGyW2O~ZlBV4RyoKLrrSyZo4vQtpIbagyMu5SHMxQpSOoPta54D5VZgOjUlc2lTwElWECKKHTx~DbL9y8ftLXad7Cy9bhewPUBgcd2e9d1AqxM-YYZJHf2Ulbvh9a3cvz0q5aENYdGdgULvSgk53CEKYxkZbwH16si~FKqCqFMSSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal