Key Points
Asciminib monotherapy leads to high rates of early and major molecular response in newly diagnosed chronic-phase CML.
Safety and tolerance of asciminib were excellent.
Visual Abstract
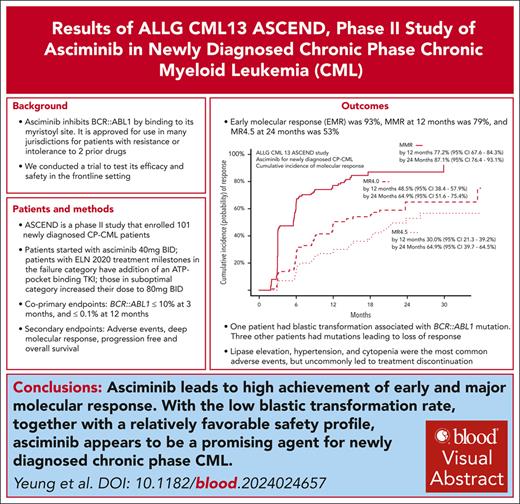
Asciminib is a myristoyl site BCR::ABL1 inhibitor approved for patients with chronic-phase chronic myeloid leukemia (CP-CML) failing ≥2 prior lines of therapy. The Australasian Leukaemia and Lymphoma Group conducted the Asciminib Evaluation in Newly Diagnosed CML study to assess efficacy of asciminib for newly diagnosed CP-CML. Patients commenced asciminib 40 mg twice daily. Patients with treatment failure, defined as BCR::ABL1 of >10% at 3 or 6 months, or >1% at 12 or 18 months, received either imatinib, nilotinib, or dasatinib in addition to asciminib. In patients with suboptimal response, defined as levels of 1% to 10% at 6 months, >0.1% to 1% at 12 months, or >0.01% to 1% at 18 months, the asciminib dose was increased to 80 mg twice daily. With a median follow-up of 21 months (range, 0-36), 82 of 101 patients continue asciminib. Most common reasons for treatment discontinuation were adverse events (6%), loss of response (4%), and withdrawn consent (5%). There were no deaths; 1 patient developed lymphoid blast crisis. The coprimary end points were early molecular response (BCR::ABL1 of ≤10% at 3 months), achieved in 93% (96% confidence interval [CI], 86-97%), and major molecular response by 12 months achieved in 79%; (95% CI, 70-87%), respectively. Cumulative incidence of molecular response 4.5 was 53% by 24 months. One patient had 2 cerebrovascular events; no other arterial occlusive events were reported. Asciminib as frontline CP-CML therapy leads to high rates of molecular response with excellent tolerance and a low rate of discontinuation for toxicity. This trial was registered at https://www.anzctr.org.au/ as #ACTRN12620000851965.
Introduction
Small-molecule adenosine triphosphate (ATP)-competitive inhibitors of BCR::ABL1 were first introduced into clinical practice in chronic-phase chronic myeloid leukemia (CP-CML) 2 decades ago, beginning with imatinib, the first generation tyrosine kinase inhibitor (TKI).1 With widespread clinical adoption of TKI treatment, the majority of patients with CP-CML now enjoy survival similar to their age-matched peers.2,3 However, a substantial minority of patients continue to experience treatment failure, either because of intolerance of currently available drugs, treatment resistance, or a combination of both. These events contribute to treatment discontinuations and adverse outcomes. More potent drugs were developed in response to observations of treatment resistance to imatinib. Examples include the second generation drugs nilotinib, dasatinib, and bosutinib, as well as the third generation drug, ponatinib.4-7
Asciminib (formerly ABL001) is a small-molecule allosteric inhibitor of BCR::ABL1, binding to a myristoyl pocket in ABL1. This pocket is usually occupied by the myristoylated N-terminal of ABL1, an element of negative autoregulation on kinase activity that is lost with the truncation of N-terminus residuals when the BCR::ABL1 fusion protein is formed. Asciminib binding mimics this interaction, restoring the autoinhibitory activity.8,9 This agent was initially developed for patients with resistance or intolerance to other TKIs, postulating that it may be useful in patients with resistance mediated by mutations in the ATP-binding pocket, a commonly encountered mechanism of therapy failure. The majority of mutations that confer resistance to ATP-competitive inhibitors were predicted to be susceptible to asciminib, based on in vitro data.8 Conversely, myristoyl pocket mutations artificially generated to confer asciminib resistance were shown to have in vitro sensitivity to ATP-pocket–binding TKIs.8 In addition, the specificity of asciminib for ABL proteins and lack of activity against other kinases may, in theory, reduce the frequency and severity of toxicities that are common to ATP-pocket–binding TKIs as a class effect.10
A phase 1 (X2101) study that enrolled patients with CP-CML with either resistance and/or intolerance to earlier line therapies established the safety of asciminib at various doses. This study also noted early efficacy signals.11 Subsequently, in the randomized ASCEMBL study, asciminib monotherapy proved to be superior to bosutinib in patients resistant to, or intolerant of, at least 2 other TKIs.12 Based on these results, asciminib is now registered in many jurisdictions internationally for patients with CP-CML who experience treatment failure to ≥2 of therapy.
Given its activity in ATP-competitive TKI-refractory disease and its favorable tolerability profile, the Australasian Leukemia and Lymphoma Group (ALLG) conducted the ALLG CML13 Asciminib Evaluation in Newly Diagnosed CML (ASCEND) study to evaluate the safety and efficacy of asciminib for frontline treatment of CP-CML. The study design included the addition of an ATP-pocket–binding TKI for patients with treatment failure, based on the theoretical synergy between asciminib and ATP-pocket–binding TKIs. In addition to BCR::ABL1 inhibition at 2 distinct sites, in vitro modeling also suggest asciminib binding may stabilize BCR::ABL1 conformation, and enhance effectiveness of ATP-competitive TKIs when concomitantly administered.13
Methods
The ASCEND study is a prospective single-arm phase 2 study, sponsored by the ALLG, registered through the Australian and New Zealand Clinical Trials Registry (ACTRN12620000851965), and conducted with approval from relevant human research ethics committees in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Patients were eligible if they were aged ≥18 years, had an Eastern Cooperative Oncology Group performance status score of 0 to 2, and had CP-CML (defined as per European LeukemiaNet 2013)14 diagnosed in the preceding 6 months with BCR::ABL1 transcript of e13a2, e14a2, or e1a2. All patients were TKI treatment naïve, although short-term cytoreduction with hydroxyurea, anagrelide, or leukapheresis was permitted before study entry. Patients with elevated liver enzymes (bilirubin of >1.5 × upper limit of normal [ULN], aspartate aminotransferase/alanine aminotransferase of >3 × ULN), creatinine (>1.5 × ULN), and pancreatic enzymes (>1.5 × ULN amylase/lipase) were excluded, as were patients with comorbid conditions requiring acute management, including active infection; uncontrolled cardiovascular conditions including arrhythmias, angina, or congestive cardiac failure; and uncontrolled diabetes. Patients with well-managed chronic illnesses that did not require intensive monitoring, frequent adjustments of concomitant medications, or impending interventions were eligible to participate. This included patients on antihypertensives, lipid lowering medications, oral hypoglycemics, and insulin for diabetes. Patient with preexisting arterial-occlusive disease or cancer not requiring immediate treatment were not excluded.
All patients started treatment with asciminib monotherapy at 40 mg twice a day, and were thereafter assessed according to modified 2020 European LeukemiaNet targets.15BCR::ABL1 quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed monthly for the first 3 months, then every 3 months thereafter. BCR::ABL1 qRT-PCR at months 1, 2, 3, 6, 12, 18, 24, 36, 48, and 60 were performed at a central laboratory (SA Pathology, Adelaide), with other time points performed at local laboratories.16 All molecular targets and results are expressed on the standardized International Scale.17 Central laboratory BCR::ABL1 qRT-PCR confirmation, and mutation testing (via Sanger sequencing) of the kinase and myristoyl domain, was performed for failure to meet molecular targets, for loss of molecular response 4 (MR4, BCR::ABL1 ≤ 0.01%) or major molecular response (MMR, BCR::ABL1 ≤ 0.1%), or for blastic transformation. Additional testing occurred at discretion of the physician for greater than twofold rise, or a lack of an expected decline, in BCR::ABL1. Patients with optimal responses (BCR::ABL1 of ≤10%, ≤1%, ≤0.1%, and ≤0.01% at 3, 6, 12, and 18 months, respectively) continued on asciminib monotherapy at 40 mg twice daily. Based on contemporaneous pharmacokinetic data, a protocol amendment 18 months after study commencement changed patients to 80 mg daily after 12 months of therapy, rather than 40 mg twice daily, for ease of administration, providing that the patient has achieved MMR.18,19 Patients with treatment failure (BCR::ABL1 of >10% at 3 or 6 months, and >1% at 12 or 18 months) continued asciminib and added either imatinib (400 mg once a day, with asciminib 60 mg once a day), dasatinib (100 mg once a day, with asciminib 80 mg once a day), or nilotinib (300 mg twice daily, with asciminib 40 mg twice daily), according to physician decision, individualized to the patient’s comorbidities. Patients in the warning category (>1%-10% at 6 months, >0.1%-1% at 12 months, and >0.01%-1% at 18 months) were given the option of doubling the asciminib dose to 80 mg twice daily at physician discretion.
The coprimary end points of the study were the proportion of patients achieving (1) BCR::ABL1 of ≤10% at 3 months (early molecular response [EMR]); and (2) BCR::ABL1 of ≤0.1% by 12 months (MMR). These were chosen for their demonstrated correlation with long term event-free survival and treatment-free remission eligibility (statistical considerations are included in supplemental Material, available on the Blood website). Achievement of deep molecular reponse (MR), such as MR4 (BCR::ABL1 of ≤0.01%) and MR4.5 (BCR::ABL1 of ≤0.0032%), and survival analyses were key secondary end points. Overall survival (OS), progression-free survival (PFS), and event-free survival (EFS) were analyzed using Kaplan-Meier survival methods. Death from any cause was an event for OS; events for PFS additionally included progression beyond chronic phase; whereas EFS included also loss of response as well as asciminib discontinuation for any reason. Achievement of MR at prespecified landmark time points was calculated as a frequency, as well as a cumulative incidence accounting for competing risks, which included discontinuation from study treatment for any reason. Confidence intervals (CIs) for the coprimary end points were reported with modifications for α-spending as a result of per-protocol interim analyses, using a repeated CI methodology.20
Results
The ASCEND study enrolled 101 patients through 14 Australasian sites between 2020 and 2022. Patient demographics and disease characteristic at study entry are summarized in Table 1. The median age of this cohort was 57 years, with a male preponderance (61%), and a relatively high proportion of patients with low EUTOS long-term survival (ELTS) score (72%). These characteristics are consistent with a cohort of newly diagnosed patients with CP-CML in a high-income country. One patient had persistent elevated lipase at baseline (despite normal lipase at screening) and withdrew from study without starting therapy.
Baseline demographics
| Total patients | 101 |
| Median age at diagnosis, y (range) | 57 (19-88) |
| Gender, female, n (%) | 39 (38.6%) |
| Race, n (%) | |
| White | 79 (78.2%) |
| East or South-Central Asian | 12 (11.9%) |
| Other | 10 (9.9%) |
| ELTS risk, n (%) | |
| Low | 73 (72.3%) |
| Intermediate | 22 (21.8%) |
| High | 5 (5.0%) |
| NA | 1 (1.0%) |
| Therapies before asciminib commencement, n (%) | |
| Prior hydroxyurea | 50 (49.5%) |
| Prior leukapheresis | 2 (2.0%) |
| Total patients | 101 |
| Median age at diagnosis, y (range) | 57 (19-88) |
| Gender, female, n (%) | 39 (38.6%) |
| Race, n (%) | |
| White | 79 (78.2%) |
| East or South-Central Asian | 12 (11.9%) |
| Other | 10 (9.9%) |
| ELTS risk, n (%) | |
| Low | 73 (72.3%) |
| Intermediate | 22 (21.8%) |
| High | 5 (5.0%) |
| NA | 1 (1.0%) |
| Therapies before asciminib commencement, n (%) | |
| Prior hydroxyurea | 50 (49.5%) |
| Prior leukapheresis | 2 (2.0%) |
ELTS, EUTOS long-term survival score; NA, not available.
The median follow-up was 21 months (range, 0-36), with 82 patients continuing to receive study treatment at the census date of 8 February 2024. The first coprimary end point, EMR, was achieved by 94 of 101 patients (93%; 96% CI, 86-97). Early MMR and MR4 responses were notably achieved by 48% and 14%, respectively, after 3 months of therapy. Of the 6 remaining patients who did not achieve EMR, 1 patient missed their BCR::ABL1 assessment at 3 months, 3 were already off-study, and 2 had BCR::ABL1 of >10%. Reasons for being off study at 3 months were (1 each) withdrawal of consent (with unresolved grade 2 headache and anorexia), loss to follow-up, and lipase elevation. There were 2 patients with BCR::ABL1 of >10% at 3 months. One patient with BCR::ABL1 of 24% had dose interruptions because of thrombocytopenia, which limited capacity for the addition of an ATP-competitive TKI. The other patient had BCR::ABL1 of 210%, without an identified cause for treatment failure, such as significant asciminib dose interruptions, nor BCR::ABL1 mutations. This latter patient received dasatinib in combination with asciminib but withdrew from study because of toxicity (persistent headaches), and subsequently had resistance to 3 other TKIs after study withdrawal.
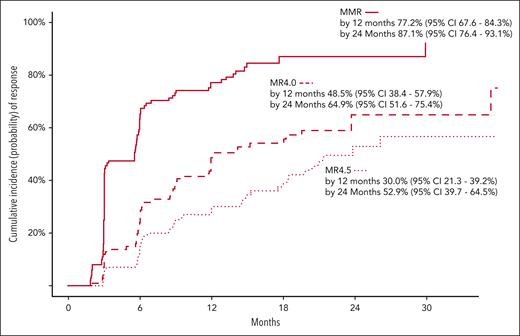
The second coprimary end point, MMR by 12 months, was achieved by 80 of 101 patients (79.2%; 95% CI, 69.7-86.8). Figure 1 summarizes the cumulative incidence of MRs over time. The cumulative incidence of MMR was 77.2% (95% CI, 67.6-84.3); rising to 87.1% by 24 months (95% CI, 76.4-93.1). The cumulative incidence of MR4 was 48.5% (95% CI, 38.4-57.9), rising to 64.9% (95% CI, 51.6-75.4) by 24 months. The cumulative incidence of MR4.5 was 30.0% (95% CI, 21.3-39.2), rising to 52.9% (95% CI, 39.7-64.5) by 24 months (Figure 1). Of the 80 patients who achieved MMR before 12 months, 75 remained on asciminib monotherapy and in MMR at 12 months.
Cumulative incidences of MMR, MR4.0, and MR4.5, performed with competing risk regression. Competing risks events are defined as any event that would result in permanent asciminib discontinuation on study. The cumulative incidence of MMR was 77.2% (95% CI, 67.6-84.3) and 87.1% (95% CI, 76.4-93.1) by 12 and 24 months, respectively. The cumulative incidence of MR4 was 48.5% (95% CI, 38.4-57.9) and 64.9% (95% CI, 51.6-75.4) by 12 and 24 months, respectively. The cumulative incidence of MR4.5 was 30.0% (95% CI, 21.3-39.2) and 52.9% (95% CI, 39.7-64.5) by 12 and 24 months, respectively.
Cumulative incidences of MMR, MR4.0, and MR4.5, performed with competing risk regression. Competing risks events are defined as any event that would result in permanent asciminib discontinuation on study. The cumulative incidence of MMR was 77.2% (95% CI, 67.6-84.3) and 87.1% (95% CI, 76.4-93.1) by 12 and 24 months, respectively. The cumulative incidence of MR4 was 48.5% (95% CI, 38.4-57.9) and 64.9% (95% CI, 51.6-75.4) by 12 and 24 months, respectively. The cumulative incidence of MR4.5 was 30.0% (95% CI, 21.3-39.2) and 52.9% (95% CI, 39.7-64.5) by 12 and 24 months, respectively.
No deaths had been reported on study. Figure 2 depicts the PFS and EFS. The PFS was 99% (95% CI, 96.8-100) at 24 months (Figure 2A). The EFS was 90% (95% CI, 82.1-94.4) and 85% (95% CI 75.7-91.4) at 12 and 24 months, respectively (Figure 2B). Four patients received allogeneic stem cell transplantation after discontinuation: the patient with lymphoid blast crisis, the 2 patients who discontinued because of prolonged treatment-emergent cytopenia, and 1 of 2 patients with EMR failure.
PFS and EFS. Deaths from any cause and transformation to accelerated/blastic phase disease were considered events for PFS calculations. For EFS, events included deaths, progression beyond CP-CML, loss of response, and trial discontinuation for any reason. Two-year PFS (A) and EFS (B) were 98.9% (95% CI, 96.8%-100%) and 85.3% (95% CI, 75.7%-91.4%), respectively.
PFS and EFS. Deaths from any cause and transformation to accelerated/blastic phase disease were considered events for PFS calculations. For EFS, events included deaths, progression beyond CP-CML, loss of response, and trial discontinuation for any reason. Two-year PFS (A) and EFS (B) were 98.9% (95% CI, 96.8%-100%) and 85.3% (95% CI, 75.7%-91.4%), respectively.
Eighteen patients (17%) prematurely discontinued asciminib therapy (Table 2). Six patients discontinued for treatment-emergent adverse events (TEAE), 7 for resistance, and 5 for loss of follow-up/withdrawal of consent. The 6 TEAEs that caused withdrawal were lipase/amylase elevations in 3 patients, clinical pancreatitis in 1, and prolonged cytopenia in 2 patients.
Patient disposition after median 21 months of follow-up
| Patient disposition . | n . |
|---|---|
| Ongoing asciminib | 82 |
| 80 mg once a day/40 mg twice daily | 75 |
| <80 mg once a day/40 mg twice daily | 6 |
| In combination with dasatinib | 1 |
| Registered but never started treatment | 1 |
| Discontinued asciminib | 18 |
| Adverse events | 6 |
| Failure to achieve EMR with intolerance for combination therapy3 | 2 |
| Loss of response | 4 |
| Progression to AP/BC | 1 |
| Lost to follow-up/withdrawn consent | 5 |
| Patient disposition . | n . |
|---|---|
| Ongoing asciminib | 82 |
| 80 mg once a day/40 mg twice daily | 75 |
| <80 mg once a day/40 mg twice daily | 6 |
| In combination with dasatinib | 1 |
| Registered but never started treatment | 1 |
| Discontinued asciminib | 18 |
| Adverse events | 6 |
| Failure to achieve EMR with intolerance for combination therapy3 | 2 |
| Loss of response | 4 |
| Progression to AP/BC | 1 |
| Lost to follow-up/withdrawn consent | 5 |
AP, accelerated phase; BC, blast crisis.
The 7 patients who discontinued asciminib because of treatment resistance included the 2 patients with failure to achieve EMR, 4 patients who lost response, and 1 patient who progressed to lymphoid blast crisis (BC). The 4 patients who lost response are as follows: 1 patient achieved MR4.5 after 3 months of therapy, then lost MR4.5 with T315I and M244V mutations (70% and 30%, respectively), at 13 months; 1 patient achieved MMR at 3 months, then lost MMR at 6 months with an A337T mutation (90%); 1 patient lost MR2 with a V506L mutation (100%); and 1 patients lost MMR without an ABL1 mutation (mutation analysis results are detailed in supplemental Table 1.) The only transformation event reported on our trial occurred in a patient who achieved a BCR::ABL1 nadir of 0.37%, who progressed to lymphoid blast crisis at 6 months with mutations A337T, A337V, and P465S (at 40%, 10%, and 10%, respectively).
The most frequent TEAEs are represented in Table 3. The majority manifested within the first 3 months of treatment. Hematological TEAEs only contributed to treatment discontinuation in 3 patients (2 with cytopenia as the primary cause of study withdrawal, and 1 failing to achieve EMR as the primary cause, with thrombocytopenia limiting the ability to tolerate combination treatment). The majority of nonhematological toxicities were mild (grade 1/2), and resolved without leading to treatment discontinuation. The most common biochemical abnormality we saw was increased lipase, seen in 21 (21%) patients, with a grade 3/4 frequency of 10%. A subset of these patients were also reported as having elevations in amylase. This rate is similar to previously reported studies. supplemental Table 3 lists protocol guidance on management on lipase and amylase elevations. Dose reductions occurred in 14 patients, including a cessation of asciminib in 10 patients. Permanent asciminib discontinuation was necessary in 4 patients: 3 with persistent amylase/lipase elevation upon asciminib rechallenge, and 1 patient who had abdominal pain and radiological evidence of pancreatitis in the context of asciminib dose escalation to 80 mg twice daily for suboptimal response. Of the 21 patients, 13 resumed asciminib dose at either 80 mg once a day or above, whereas 4 patients continue asciminib on study with a dose of either 20 or 40 mg once a day.
TEAEs, regardless of relationship to study drug
| Adverse event . | Any grade (%) . | Grade III/IV (%) . |
|---|---|---|
| Hematological TEAEs | ||
| Anemia | 8% | 2% |
| Neutropenia | 7% | 6% |
| Thrombocytopenia | 15% | 5% |
| Nonhematological TEAE, frequency of >10% | ||
| Infection | 30% | 3%‡ |
| Fatigue | 26% | |
| Upper respiratory infection | 24% | |
| Increased lipase | 21% | 10% |
| Skin disorders, including rash | 20% | |
| Headache | 19% | |
| Abdominal pain | 18% | 2% |
| Nausea | 17% | |
| Diarrhea | 17% | |
| Musculoskeletal disorder | 16% | |
| Other | 16% | |
| Gastrointestinal | 13% | 1% |
| Back pain | 11% | 1% |
| Arthralgia | 10% | |
| Selected nonhematological TEAEs, frequency of <10% | ||
| Hypertension∗ | 22% | 3% |
| Increased amylase | 7% | 2% |
| Increased alkaline phosphatase | 5% | |
| Increased AST | 6% | 1% |
| Increased ALT | 4% | 1% |
| Increased bilirubin | 3% | |
| Stroke† | 1% | 1% |
| Adverse event . | Any grade (%) . | Grade III/IV (%) . |
|---|---|---|
| Hematological TEAEs | ||
| Anemia | 8% | 2% |
| Neutropenia | 7% | 6% |
| Thrombocytopenia | 15% | 5% |
| Nonhematological TEAE, frequency of >10% | ||
| Infection | 30% | 3%‡ |
| Fatigue | 26% | |
| Upper respiratory infection | 24% | |
| Increased lipase | 21% | 10% |
| Skin disorders, including rash | 20% | |
| Headache | 19% | |
| Abdominal pain | 18% | 2% |
| Nausea | 17% | |
| Diarrhea | 17% | |
| Musculoskeletal disorder | 16% | |
| Other | 16% | |
| Gastrointestinal | 13% | 1% |
| Back pain | 11% | 1% |
| Arthralgia | 10% | |
| Selected nonhematological TEAEs, frequency of <10% | ||
| Hypertension∗ | 22% | 3% |
| Increased amylase | 7% | 2% |
| Increased alkaline phosphatase | 5% | |
| Increased AST | 6% | 1% |
| Increased ALT | 4% | 1% |
| Increased bilirubin | 3% | |
| Stroke† | 1% | 1% |
Listed are hematological events, nonhematological events that occurred at >10% frequency, as well as selected events of interest at <10% frequency.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, common terminology criteria for adverse events; TEAE, treatment-emergent adverse events; TIA, transient ischemic attack.
Treatment-emergent hypertension in this table is defined as any 2 consecutive measurements meeting the CTCAE criteria, without a prior history of hypertension at study entry. The relevant parameters are: SBP of ≥140 to 159 mmHg and/or DBP of ≥90 to 99 mmHg for all grades, and SBP of ≥160 mmHg and/or DBP of ≥100 mmHg for grade ≥3. Refer to text for further information.
A 73-year-old woman with preexisting type 2 diabetes, hypertension, and high body mass index had a lacunar infarct 20 months into treatment presenting as a TIA, with a second TIA affecting the same territory 28 months into treatment. There was complete neurological recovery after each episode. No other treatment emergent arterial occlusive event was reported.
The 3 grade 3 infective episodes were hospitalizations in 3 patients for pneumonia, skin infection from cat scratch, and septic arthritis.
In the ASCEND trial, hypertension was reported as a clinically significant adverse event in 5% of patients, with a grade 3/4 event of 3%. Given that hypertension was 1 of the more commonly reported TEAEs in other asciminib studies, we reviewed blood pressure measurements across 1068 time points. Thirty-nine patients had 2 consecutive measurements for which systolic blood pressure (SBP) was >140 mmHg, and/or diastolic blood pressure (DBP) of >100 mmHg; 22 (22%) of whom did not have a history of hypertension at study entry. This included a subset of 10 patients who had 2 consecutive measurements of SBP of ≥160 mmHg and/or DBP of ≥100 mmHg, 3 (3%) of whom had no previous history of hypertension, meeting the criteria for grade 3 treatment emergent hypertension. Only 1 patient, with multiple risk factors, has had an arterial occlusive event, equating to an arterial occlusive event rate of 0.59 episodes per 100 patient-years of cumulative asciminib exposure. (Table 3). The dose density during the first 3 months was excellent, with 86% of patients taking ≥90% of protocol assigned doses (supplemental Figure 1).
We assessed the impact of clinical risk factors on MR. Given the low numbers of patients in each risk group, high and intermediate ELTS patients were grouped together in MR analyses. There was no statistically significant difference in the achievement of MMR, MR4, nor MR4.5 when patients were stratified by ELTS (supplemental Figure 2A-C). Cytogenetics at diagnosis was also assessed. Eighty-three patients (82%) had the Philadelphia chromosome as the sole cytogenetic abnormality at diagnosis. Ten patients had variant translocations and 5 patients had additional chromosomal abnormalities (supplemental Table 2). These lesions made no significant clinical impact on subsequent achievement of MR.
Of 20 patients who started asciminib but did not achieve MMR in the first 12 months, 8 patients discontinued therapy before this assessment. One patient had a BCR::ABL1 of 39% at 12 months, nonadherence was suspected and the patient withdrew consent to participate soon after. The remaining 11 patients had BCR::ABL1 of between 0.14% and 1.1% at 12 months. Five of these patients who failed to achieve MMR at 12 months had ≥6 months of additional follow-up. Two patients (with BCR::ABL1 of 0.16% and 0.13% at 12 months) remained on asciminib 80 mg once a day and subsequently achieved MMR. Three patients doubled their dose to 80 mg twice daily, 1 of whom has subsequently achieved MMR (Figure 3A). The patient with the BCR::ABL1 of 1.1% at 12 months is receiving combination dasatinib/asciminib therapy, with further molecular assessments awaited. There were 23 patients who did not achieve MR4 by 18 months, of whom 11 had ≥6 months additional follow-up. Seven of these 11 patients doubled their asciminib dose to 80 mg twice daily, with a median BCR::ABL1 drop of 0.22 log in the subsequent 6 months with subsequent assessments, compared with a median drop of 0.08 log in the 4 patients who remained on asciminib 80 mg once a day (Figure 3B-C).
BCR::ABL1 kinetics for patients with suboptimal response at 12 and 18 months. There were 9 assessable patients who did not achieve MMR by 12 months, of whom 5 had ≥6 months additional follow-up. (A, dashed lines) Two patients (with BCR::ABL1 of 0.16% and 0.13% at 12 months) remained on asciminib 80 mg once a day (QD) and subsequently achieved MMR. (A) Three patients doubled their dose to asciminib 80 mg twice daily (BID), of whom 1 subsequently achieved MMR. There were 23 assessable patients who did not achieve MR4 by 18 months, of whom 11 had ≥6 months additional follow-up. (B, dashed lines) Four patients who remained on asciminib 80 mg once a day had a median fall of 0.08 log in the BCR::ABL1 qRT-PCR in the subsequent 6 months. (C) The 7 patients who doubled their asciminib dose to 80 mg twice daily had a median BCR::ABL1 drop of 0.22 log in the subsequent 6 months.
BCR::ABL1 kinetics for patients with suboptimal response at 12 and 18 months. There were 9 assessable patients who did not achieve MMR by 12 months, of whom 5 had ≥6 months additional follow-up. (A, dashed lines) Two patients (with BCR::ABL1 of 0.16% and 0.13% at 12 months) remained on asciminib 80 mg once a day (QD) and subsequently achieved MMR. (A) Three patients doubled their dose to asciminib 80 mg twice daily (BID), of whom 1 subsequently achieved MMR. There were 23 assessable patients who did not achieve MR4 by 18 months, of whom 11 had ≥6 months additional follow-up. (B, dashed lines) Four patients who remained on asciminib 80 mg once a day had a median fall of 0.08 log in the BCR::ABL1 qRT-PCR in the subsequent 6 months. (C) The 7 patients who doubled their asciminib dose to 80 mg twice daily had a median BCR::ABL1 drop of 0.22 log in the subsequent 6 months.
Discussion
In the phase 1 X2101 and the phase 3 ASCEMBL studies of patients with CP-CML with resistance or intolerance to prior lines of therapies, asciminib demonstrated a relatively favorable safety profile, and encouraging MRs. These data suggested a potential role for asciminib as frontline treatment in CP-CML, and led to the ASCEND study, 1 of the first prospective studies to test the safety and efficacy of asciminib in newly diagnosed CP-CML.
The high achievement of EMRs associated with asciminib in the ASCEND study is encouraging, because EMR has long been used as a surrogate end point, given its association with PFS, MMR, MR4.5, and successful achievement of TFR.21-23 With the obvious caveats in cross-trial comparisons, the EMR rate in the ASCEND study of 93% appears favorable, given that, historically, the observed EMR rate is 64% to 71% for imatinib, and 84% to 91% for second generation TKIs such as nilotinib, bosutinib, radotinib, and dasatinib.24-27 Although ponatinib led to an EMR rate of 94% in the EPIC study, an excess of arterial occlusive events related to ponatinib use led to the early discontinuation of the trial.28 Early achievement of deep MRs also appears high in the ASCEND study, with MMR and MR4 rates of 48% and 14%, respectively, after 3 months of treatment. This accords with the other coprimary end point, MMR at 12 months, achieved by 79%.
These high MR rates likely reflect both the intrinsic potency of asciminib to inhibit BCR::ABL1, and its favorable tolerability, resulting in a high rate of treatment adherence and a low rate of withdrawal because of adverse events. Elevated pancreatic enzymes is 1 of the more commonly encountered adverse events associated with TKI treatment. In our study, the frequency of lipase elevations were similar to that reported in other studies, although only 4 patients had to discontinue asciminib because of this reason, with clinical symptoms in 1 patient. Management guidelines within the protocol to address lipase elevation, as well as prior clinical experience with asciminib in clinical studies in the intolerance/resistance setting, may have minimized the discontinuation rate. Another frequently encountered TEAE is hypertension. We observed a discrepancy between the rate of hypertension reported as a TEAE vs the proportion of patients with sustained, elevated SBP or DBP when numerical values were analyzed. We suspect that many investigators only reported instances of hypertension that they have assessed as clinically significant, potentially leading to an underreporting bias, although significant sequalae were rare. Arterial occlusive events are another concern with TKI treatment, and events have been reported in previous trials in which asciminib was used after ATP-competitive TKIs. With a median follow-up of 21 months, an arterial occlusive event was only observed in 1 patient with a high cardiovascular risk profile; longer term follow-up and reporting of this study will help clarify whether asciminib alone is a predisposing factor for arterial occlusive events.
Preclinical models of asciminib treatment anticipated myristoyl mutations as an important mechanism of treatment resistance.8 Some of the predicted mutations emerged in the relatively heavily pretreated populations in the phase 1 X2101 and ASCEMBL studies. Such mutational patterns were confirmed in the ASCEND trial, in which myristoyl mutations, A337T/V, V506L, as well as T315I and M244V in the ATP binding pocket, were observed in 4 patients in the context of asciminib resistance. Some of these mutations occurred in patients who had already achieved MMR or MR4.5, traditionally regarded as milestones associated with minimal risk of subsequent resistance and disease progression events. This pattern of secondary resistance may give rise to concerns regarding the durability of MRs achieved with asciminib; again, prolonged follow-up in a larger cohort of patients will be needed to address this. In contrast to the experience with asciminib therapy in heavily pretreated patients,12 emergence of conventional TKI resistance mutations was uncommon in the ASCEND trial. Patients who lose their response may have intrinsic disease biology that predisposes them to resistance. The challenge will be early identification of these patients using prognostic markers at diagnosis or early in treatment to evaluate whether treatment intensification or combination therapy may prevent secondary resistance.
A secondary objective of the ASCEND trial was to test the safety and efficacy of treatment intensification for patients who failed to achieve optimal treatment response. Patients meeting the criteria for treatment failure had protocol mandated addition of an ATP-pocket–binding TKI to asciminib. Given the high rate of molecular target achievement in the ASCEND trial, we were unable to assess the efficacy of combination therapy in this setting, because only 3 patients met protocol-defined criteria for combination treatment. This includes the 2 patients who experienced EMR failure, and neither could tolerate combination treatment. An additional patient is currently receiving combination therapy after failure to achieve BCR::ABL1 of ≤1% at 12 months. For MRs between optimal and warning categories, patients with BCR::ABL1 of ≤1% at 12 months but who have not achieved MMR at 12 months, or MR4 at 18 months, we speculated that an increased dose of asciminib to 80 mg twice daily may be of benefit. Although MRs did appear to improve over time in many of these patients, it is unclear whether these improvements are attributable to protocol-driven intervention, or whether MRs would have deepened to this extent with continuing treatment over time even without intervention. Further data from this and other studies are needed to definitively answer this question.
The German CML XI study (ClinicalTrials.gov identifier: NCT03906292) in which patients were assigned asciminib combined with either nilotinib, dasatinib, or imatinib in the frontline setting has maturing data that may provide further information regarding the safety, tolerability, and efficacy of combination therapy. Similarly, we cannot definitively comment on the clinical utility of asciminib dose escalation to 80 mg twice daily for patients who are neither in the optimal nor the failure category, given the relatively short follow-up for this group of patients. However, the preliminary observations suggest that patients not achieving MR4 may benefit from dose escalation.
We also recognize that a phase 2 design is unlikely to provide definitive data to determine the advantages and disadvantages of asciminib as frontline treatment compared with standard-of-care therapies. Nonetheless, the ASCEND trial does provide clinically relevant data to support a potential role of asciminib monotherapy for newly diagnosed patients with CP-CML. Our data will inform future clinical development of asciminib, which appears to be a very promising therapy for newly diagnosed CP-CML. Outcomes from other ongoing studies, including the German CML XI and the phase 3 ASC4FIRST study, in which patients are randomized to asciminib or an investigator-selected TKI, will add further evidence to define the role of asciminib in this setting.29
Acknowledgments
The authors thank Jodi Braley and her colleagues for performing molecular diagnostics, and Stephanie Arbon for logistics assistance. The authors also thank the coordinators at each of the sites for their assistance in this study. The authors are grateful to the patients who participated in the study, and their families for supporting them.
This study was sponsored by the ALLG, with support from Novartis Pharmaceuticals Corp. D.T.Y. is a South Australian Cancer Council Beat Cancer Clinical investigator.
Authorship
Contribution: D.T.Y. and T.P.H. designed the study; S.B. supervised molecular studies and critically reviewed the manuscript; J.R. performed biostatistics and critically reviewed the manuscript; M. Walia coordinated data collection; D.T.Y., J.R., and M. Walia analysed the data; D.T.Y., A.P.G., and T.P.H. drafted the manuscript; D.Y., N.S., A.S.M.Y., L.C., J.S., N.V., I.C., D.M.R., A.D., M. Wright, R.H., C.F., R.F., and S.L. provided study patients, critically reviewed and edited the manuscript; and P.B. and C.G. served on the study management committee, and critically reviewed and edited the manuscript.
Conflict-of-interest disclosure: S.B. received research support from Novartis and Cepheid; and received honoraria from Novartis, Cepheid, and Qiagen. L.C. served on the advisory board for Otsuka and Novartis; reports consultancy for Keros Therapeutics; and received honoraria from Otsuka, Novartis, and Keros Therapeutics. C.F. received honorarium from, and has served on advisory boards for Novartis. T.P.H. received honoraria from, or has served on the advisory board of Pfizer, Novartis, Terns, Enliven, and Takeda; and received research funding from Novartis and Bristol Myers Squibb. D.M.R. has served on the advisory board of Novartis, Keros, Menarini, Takeda, and Avance CRO; has received honoraria from Novartis; and reports research funding from Novartis, Keros, MSD, Morphosys, Protagonist, Incyte, and Sumitomo. J.R. hold stocks in Novartis AG. J.S. served on advisory boards of Bristol Myers Squibb, Pfizer, Astellas, and Otsuka; received research funding from Bristol Myers Squibb and Astex; and received speakers honoraria from Mundipharma and Novartis. N.V. has received advisory board fees and/or travel sponsorship from Sandoz and Novartis. A.S.M.Y. received research funding from Novartis, Bristol Myers Squibb, and Celgene; received honoraria from Novartis and Bristol Myers Squibb; and is a member of the advisory board of Novartis. D.T.Y. received honoraria from, or has served on the advisory board of Pfizer, Amgen, Ascentage, Novartis, and Takeda; and received research funding from Novartis and Bristol Myers Squibb. The remaining authors declare no competing financial interests.
A complete list of the members of the Australasian Leukaemia and Lymphoma Group appears in “Appendix.”
Correspondence: Timothy Hughes, Precision Cancer Medicine Theme, South Australian Health and Medical Research Institute, North Terrace, Adelaide, SA 5000, Australia; email: tim.hughes@sahmri.com.
Appendix
The members of the Australasian Leukaemia and Lymphoma Group are Peter Browett, Lynette Chee, Ilona Cunningham, Alwyn D’Souza, Robin Filshie, Cecily Forsyth, Andrew P. Grigg, Carolyn Grove, Rosemary Harrup, Timothy P. Hughes, Steven Lane, David M. Ross, Jake Shortt, Naranie Shanmuganathan, Nicholas Viiala, Mannu Walia, Matthew Wright, Paul Yeh, David T. Yeung, and Agnes S.M. Yong.
References
Author notes
Interim results from this trial were presented as abstract 79 at the American Society of Hematology annual scientific meeting, New Orleans, LA, 12 December 2022; and as abstract 865 at the American Society of Hematology annual scientific meeting, San Diego, CA, 11 December 2023.
Data are available on request from the corresponding author, Timothy Hughes (tim.hughes@sahmri.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal