Issue Archive
Table of Contents
BLOOD COMMENTARIES
SPECIAL REPORT
Perspectives on drug development in chronic myelomonocytic leukemia: changing the paradigm
Clinical Trials & Observations
Therapy for chronic myelomonocytic leukemia (CMML) has made little progress during the last 15 years, mainly because standard therapies have been adopted from treatment of myelodysplasia and acute myeloid leukemia. In a Special Report, Hunter et al describe the challenges of successful drug development for CMML. They emphasize the need for disease-specific trials focused on CMML, prioritizing and maximizing enrollment of all patients with CMML in clinical trials, and collaboration among academic medical centers, industry, patient advocacy groups, and regulatory bodies to promote development of novel therapies.
CLINICAL TRIALS AND OBSERVATIONS
Asciminib monotherapy as frontline treatment of chronic-phase chronic myeloid leukemia: results from the ASCEND study
Clinical Trials & Observations
Yeung and colleagues report results of the ASCEND study from the Australasian Leukaemia and Lymphoma Group, which assessed the efficacy of asciminib, a novel allosteric BCR::ABL1 inhibitor, as first-line therapy for chronic-phase chronic myeloid leukemia (CP-CML). Early (at 3 months) and major (at 12 months) molecular responses were seen in 93% and 79% of patients, respectively, and therapy was well tolerated. This study supports the efficacy of asciminib for frontline therapy of CP-CML.
LYMPHOID NEOPLASIA
NG2 is a target gene of MLL-AF4 and underlies glucocorticoid resistance in MLLr B-ALL by regulating NR3C1 expression
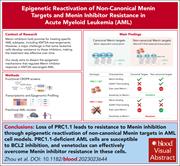
Two papers in this week’s issue explore divergent epigenetic mechanisms of drug resistance in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) carrying rearrangements of MLL (KMT2A). Glucocorticoids (GCs) are a backbone of therapy for B-cell ALL, but B-ALL harboring rearrangements of the MLL gene (MLLr B-ALL), a poor-prognosis variant seen in infants, is notably GC resistant. Lopez-Millan et al elucidate the mechanism of GC resistance in MLLr B-ALL. Having previously demonstrated that neuron-glial antigen 2 (NG2) is highly expressed in MLLr B-ALL, they demonstrate that NG2 is an epigenetic regulator of the GC receptor through FLT3, identifying FLT3 inhibitors as potential agents to restore GC resistance in these patients. AML with MLL (KMT2A) rearrangements has a poor prognosis but responds to menin inhibitors, which disrupt the menin-MLL interaction; however, resistance to menin inhibitors remains an issue. Zhou and colleagues used chromatin-focused CRISPR screens to identify a novel resistance mechanism independent of menin mutations related to loss of polycomb repressive complex 1.1 (PRC1.1) subunits, allowing reactivation of noncanonical MLL targets. They show that cells with loss of PCR1.1 subunits are sensitive to BCL2 inhibition and that venetoclax can overcome resistance to menin inhibition.
MYELOID NEOPLASIA
Epigenetic regulation of noncanonical menin targets modulates menin inhibitor response in acute myeloid leukemia
Two papers in this week’s issue explore divergent epigenetic mechanisms of drug resistance in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) carrying rearrangements of MLL (KMT2A). Glucocorticoids (GCs) are a backbone of therapy for B-cell ALL, but B-ALL harboring rearrangements of the MLL gene (MLLr B-ALL), a poor-prognosis variant seen in infants, is notably GC resistant. Lopez-Millan et al elucidate the mechanism of GC resistance in MLLr B-ALL. Having previously demonstrated that neuron-glial antigen 2 (NG2) is highly expressed in MLLr B-ALL, they demonstrate that NG2 is an epigenetic regulator of the GC receptor through FLT3, identifying FLT3 inhibitors as potential agents to restore GC resistance in these patients. AML with MLL (KMT2A) rearrangements has a poor prognosis but responds to menin inhibitors, which disrupt the menin-MLL interaction; however, resistance to menin inhibitors remains an issue. Zhou and colleagues used chromatin-focused CRISPR screens to identify a novel resistance mechanism independent of menin mutations related to loss of polycomb repressive complex 1.1 (PRC1.1) subunits, allowing reactivation of noncanonical MLL targets. They show that cells with loss of PCR1.1 subunits are sensitive to BCL2 inhibition and that venetoclax can overcome resistance to menin inhibition.
Risk prediction for clonal cytopenia: multicenter real-world evidence
Clinical Trials & Observations
Clonal cytopenia of undetermined significance (CCUS) is defined by an absence of bone marrow evidence of myeloid neoplasm (MN) but has a risk of future progression to MN. Xie et al analyzed 357 patients with CCUS to develop a clonal cytopenia risk score (CCRS) predicting cumulative incidence of MN. Splicing mutations, platelet counts of <100 × 109/L, and the presence of 2 or more mutations factor into a simple score that identifies low-risk (6.4%), intermediate-risk (14.1%), and high-risk (37.2%) groups for progression. The CCRS was validated in an independent CCUS cohort and should inform future CCUS management and clinical trials.
TRANSFUSION MEDICINE
Genotyped RhD+ red cells for D-positive patients with sickle cell disease with conventional RHD and unexpected anti-D
Brief Report
Patients with sickle cell disease (SCD) who are Rh positive (RhD+) can unexpectedly develop anti-D antibodies, likely a reflection of variant Rh expression on donor cells. After such an episode, these patients are usually transfused strictly with Rh-negative blood, which places a strain on the blood supply given the high rate of transfusions in patients with SCD. Chou et al demonstrate that these patients can be transfused with D-positive blood if they are confirmed to have RhD by genotyping and have RHD-genotyped matched units from Black donors.
VASCULAR BIOLOGY
Less-deformable erythrocyte subpopulations biomechanically induce endothelial inflammation in sickle cell disease
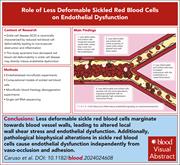
Sickle cell disease (SCD) is associated with decreased red blood cell (RBC) deformability, leading to microvascular obstruction and inflammation. Caruso and colleagues investigated the contribution of RBCs with impaired deformability to vasculopathy. Their results suggest that rigid RBCs are abnormally marginated in small vessels, causing localized increased shear stress, increased inflammatory gene expression by endothelial cells, and inflammation-associated vascular dysfunction.
LETTER TO BLOOD
Real-world impact of differences in the WHO and ICC classifications of non-Hodgkin lymphoma: a LEO cohort study analysis
BLOOD WORK
ERRATA
-
Cover Image
Cover Image
![issue cover]()
A Wright-Giemsa stain of OCI-AML2 KMT2A-rearranged acute myeloid leukemia cells with PRC1.1 subunit BCOR knockout, demonstrating that the cells remain undifferentiated despite menin inhibitor treatment. See the article by Zhou et al on page 2018.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals