Key Points
Ven can be safely used in combination with 7+3 chemotherapy for remission induction in patients with newly diagnosed AML.
Ven plus 7+3 chemotherapy led to high rates of MRD-negative complete remission.
Visual Abstract
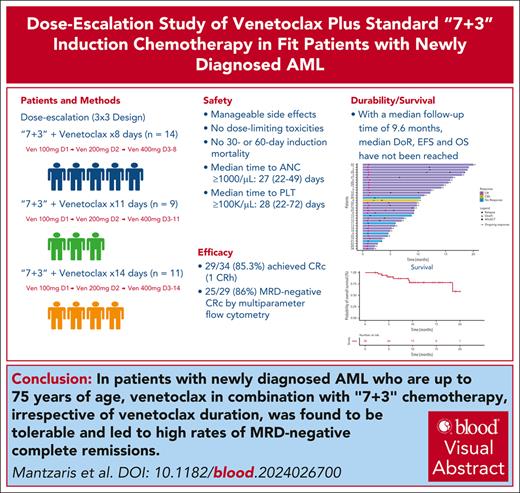
Venetoclax (Ven), when combined with intensive chemotherapy, shows promise for untreated acute myeloid leukemia (AML), but its integration with the 7+3 regimen remains underexplored. In a phase 1b study, we assessed the safety and efficacy of Ven with daunorubicin and cytarabine in patients with newly diagnosed AML. A total of 34 patients (median age, 59 years; 62% non-White) received Ven at escalating durations (8, 11, or 14 days). Adverse events included febrile neutropenia (100%), sepsis (29%), and enterocolitis (23.5%), but there were no induction deaths. The median recovery times for neutrophils (>1.0 × 103/μL) and platelets (>100 × 103/μL) were less than 30 days. Composite complete remission was achieved in 85.3% of patients, and 86.2% were negative for measurable residual disease (MRD). Responses spanned all European Leukemia Net 2022 risk categories. With a median follow-up of 9.6 (2-20) months, the median duration of response, event-free survival, and overall survival were not reached. Ven (400 mg), when combined with 7+3 chemotherapy, was safe and effective in achieving MRD-negative remissions across all durations. Ven dose optimization is being explored in the expansion phase of this trial. Future multicenter studies should confirm our findings. This trial was registered at clinicaltrials.gov as #NCT05342584.
Introduction
The addition of venetoclax (Ven) to intensive chemotherapy (IC) regimens, such as fludarabine, cytarabine, and granulocyte colony-stimulating factor (FLAG)/idarubicin or cladribine, idarubicine, and cytarabine (CLIA), has recently been shown to be safe and highly effective.1,2 However, data on its combination with the widely used 7+3 regimen remain limited to a study from Zhejiang University.3 Although various Ven durations have been explored,4,5 the optimal duration with IC is unknown. In this study, we report the initial safety and preliminary efficacy of Ven at escalating durations when combined with daunorubicin (Dauno) and cytarabine (AraC), followed by Ven plus intermediate-dose cytarabine (IDAC) in patients with newly diagnosed AML.
Study design
Adult patients with newly diagnosed AML, aged 18 to 75 years, who were deemed fit for IC were eligible. Hydroxyurea and/or AraC cytoreduction (0.5-1 g/m2 per dose, up to 2 doses) was permitted to achieve a white blood cell count of ≤25 × 10³/μL (eligibility details available in the protocol). All investigations were approved by the Einstein Institutional Review Committee and conducted in accordance with the Declaration of Helsinki.
A cytogenetic evaluation was performed using standard metaphase karyotype analysis. For genomic profiling at enrollment, a 29- or 63-gene next-generation sequencing platform (Cairo Diagnostics and NeoGenomics Laboratories, respectively) was employed. Measurable residual disease (MRD) was assessed using multiparameter flow cytometry at Hematologics Inc using a different-from-normal approach (limit of detection [LoD], 0.02%).6 Reverse transcription quantitative polymerase chain reaction was performed for NPM1-mutated cases (transcripts A, B, and D) and core binding factor AML (CBFB-MYH11 types A, D, and E, and RUNX1-RUNX1T1 fusions) with an LoD of 1 × 10−5 (ARUP Laboratories).7 The FLT3–internal tandem duplication (ITD) MRD assay (Invivoscribe, Inc) was used when available in FLT3 ITD-mutated cases (LoD: 5 × 10−5).8
In a 3+3 design, Ven at escalating durations (8, 11, and 14 days, including a 3-day ramp-up to 400 mg) was combined with 100 mg/m2 AraC and 60 mg/m2 Dauno in 2 age groups (≤60 and >60 years) (supplemental Figure 1, available on the Blood website). In the same schema, 90 mg/m2 Dauno (Dauno90) was administered in patients ≤60 years but was closed in April 2023 after randomized data showed no added benefit. Three patients received Dauno90 plus 8-day Ven before closure. Backfilling was permitted, but those patients were not eligible for dose-limiting toxicity (DLT) evaluations.
Responding patients proceeded to age-adjusted IDAC consolidation (AraC: 1.5 g/m2 for ≤60 years, 1 g/m2 for >60 years, every 12 hours on days 1, 3, 5) with Ven (200 mg for 7 days) for up to 4 cycles. After 2 hematologic DLTs (hDLTs), IDAC alone was used in 3 patients >60 years. Eligible patients were offered allogeneic transplantation off-protocol during the first remission at the treating physician’s discretion.
Concurrent use of azoles with Ven was discouraged but allowed when needed for the management of active or previous fungal infections with appropriate Ven dose adjustments per the US Food and Drug Administration label. All patients received micafungin during Ven induction, followed by triazole prophylaxis for the remainder of severe neutropenia. Antiviral, antibacterial, and antifungal prophylaxis was provided throughout neutropenia in all therapy courses.
All patients received IV hydration and oral allopurinol for tumor lysis syndrome prophylaxis. During induction, granulocyte colony-stimulating factor was administered after interim marrow assessment or during infections at the treating physician’s discretion. During consolidation, all patients received pegfilgrastim following Ven/IDAC completion.
Adverse events (AEs) were assessed according to Common Terminology Criteria for Adverse Events version 5.
Response evaluation
All patients underwent marrow aspiration/biopsy to evaluate the response on days 14 to 21 and 28 to 42 of the induction course and on days 28 to 42 of each consolidation cycle. The clinical activity of 7+3 with Ven was assessed based on the 2022 ELN recommendations.9
DLTs were defined during induction and the first consolidation cycle as either (1) grade ≥3 nonhematologic toxicity at least possibly attributed to Ven within the first 28 days or (2) grade ≥3 neutropenia (<1.0 × 103/μL) or thrombocytopenia (<50 × 103/μL) that persists beyond day 42 without residual AML.
Statistical methods
The primary objective was to evaluate the safety of the combination and to determine the optimal Ven duration. Secondary objectives included the following efficacy end points: composite complete remission (CRc; including CR, CR with partial hematologic recovery, CR with incomplete count recovery), MRD-negative CRc, overall survival (OS; time from treatment initiation to death), event-free survival (EFS; time from treatment initiation to no response, relapse, or death), and duration of response (DoR; time from CRc to relapse or death). Response date was based on bone marrow sampling with a 7-day window used to align biopsy and blood count recovery.
Response rates were estimated using proportions with exact 95% confidence intervals. Trends across dose cohorts were analyzed using the Cochran-Armitage Trend test. The Kaplan-Meier method was used to estimate OS, EFS, and DoR distributions, and the log-rank test was used to compared survival rates by ELN 2022 risk and TP53 mutation status. The hematologic recovery cumulative incidence was also estimated using the Kaplan-Meier method, and a random-effects proportional hazards model was used to evaluate cycle effects on recovery.
Results and discussion
Between 6 June 2022 and 15 February 2024, 34 of 35 screened patients with AML completed induction (Table 1; supplemental Figure 2). Of those, 20 patients were aged ≤60 years and 14 were >60 years (supplemental Tables 1 and 2). The median age was 59 (27-71) years, and most patients (n = 21, 62%) were non-White.
Baseline characteristics of the overall patient population
| Characteristics . | 8-day Ven (n = 14) . | 11-day Ven (n = 9) . | 14-day Ven (n = 11) . | All cohorts (N = 34) . |
|---|---|---|---|---|
| Age, median (range), y | 54 (27-69) | 57 (43-71) | 59 (42-71) | 59 (27-71) |
| Sex (male), n (%) | 8 (57) | 6 (67) | 5 (45) | 19 (56) |
| Race, n (%) | ||||
| White | 7 (50) | 3 (33.3) | 3 (27.3) | 13 (38.2) |
| Non-White | 7 (50) | 6 (66.7) | 8 (72.7) | 21 (61.8) |
| WBC at diagnosis, median (range), ×103/μL | 20.6 (1.6-223.6) | 3.4 (1.4-343.6) | 15.2 (1.2-241.7) | 16.4 (1.2-343.6) |
| WBC on day 1, median (range), ×103/μL | 4.6 (1.3-22.3) | 4.7 (1.7-24.4) | 2 (0.7-24.1) | 4 (0.7-24.4) |
| Secondary AML, n (%) | 3 (21) | 1 (11) | — | 4 (11.7) |
| Cytogenetic risk, n (%) | ||||
| Favorable | 2 (14) | 2 (22) | 1 (9) | 5 (14.7) |
| Intermediate | 7 (50) | 6 (67) | 7 (64) | 20 (58.8) |
| Adverse | 5 (36) | 1 (11) | 3 (27) | 9 (26.5) |
| ELN 2022 risk, n (%) | ||||
| Favorable | 5 (36) | 4 (44) | 4 (36) | 13 (38.2) |
| Intermediate | 1 (7) | 2 (22) | 3 (27) | 6 (17.7) |
| Adverse | 8 (57) | 3 (33) | 4 (36) | 15 (44.1) |
| Molecular subtypes | ||||
| NPM1 | 4 (28) | 3 (33) | 6 (54) | 13 (38.2) |
| FLT3 ITD | 2 (14) | 2 (22) | 3 (27) | 7 (20.6) |
| TP53 | 3 (21) | 1 (11) | 1 (9) | 5 (14.7) |
| Characteristics . | 8-day Ven (n = 14) . | 11-day Ven (n = 9) . | 14-day Ven (n = 11) . | All cohorts (N = 34) . |
|---|---|---|---|---|
| Age, median (range), y | 54 (27-69) | 57 (43-71) | 59 (42-71) | 59 (27-71) |
| Sex (male), n (%) | 8 (57) | 6 (67) | 5 (45) | 19 (56) |
| Race, n (%) | ||||
| White | 7 (50) | 3 (33.3) | 3 (27.3) | 13 (38.2) |
| Non-White | 7 (50) | 6 (66.7) | 8 (72.7) | 21 (61.8) |
| WBC at diagnosis, median (range), ×103/μL | 20.6 (1.6-223.6) | 3.4 (1.4-343.6) | 15.2 (1.2-241.7) | 16.4 (1.2-343.6) |
| WBC on day 1, median (range), ×103/μL | 4.6 (1.3-22.3) | 4.7 (1.7-24.4) | 2 (0.7-24.1) | 4 (0.7-24.4) |
| Secondary AML, n (%) | 3 (21) | 1 (11) | — | 4 (11.7) |
| Cytogenetic risk, n (%) | ||||
| Favorable | 2 (14) | 2 (22) | 1 (9) | 5 (14.7) |
| Intermediate | 7 (50) | 6 (67) | 7 (64) | 20 (58.8) |
| Adverse | 5 (36) | 1 (11) | 3 (27) | 9 (26.5) |
| ELN 2022 risk, n (%) | ||||
| Favorable | 5 (36) | 4 (44) | 4 (36) | 13 (38.2) |
| Intermediate | 1 (7) | 2 (22) | 3 (27) | 6 (17.7) |
| Adverse | 8 (57) | 3 (33) | 4 (36) | 15 (44.1) |
| Molecular subtypes | ||||
| NPM1 | 4 (28) | 3 (33) | 6 (54) | 13 (38.2) |
| FLT3 ITD | 2 (14) | 2 (22) | 3 (27) | 7 (20.6) |
| TP53 | 3 (21) | 1 (11) | 1 (9) | 5 (14.7) |
WBC, white blood cells.
The addition of 8 to 14 days of Ven to 7+3 chemotherapy was acceptably well tolerated in both younger and older patients. The 30- and 60-day mortality rate was 0%. No protocol-defined DLTs occurred at any Ven duration, including in the 3-patient Dauno90 + 8-day Ven cohort. AEs for Ven plus the 7+3 regimen aligned with the safety profile of the 7+3 regimen alone (Table 2; supplemental Tables 3 and 4),10,11 and no tumor lysis syndrome was observed. Increasing the Ven duration did not seem to increase toxicity. Neutropenic enterocolitis (NEC; febrile neutropenia + abdominal pain + bowel wall thickening on computed tomography) occurred in 23.5% of patients (n = 8/34), which is potentially higher than the historic rates for the 7+3 regimen.10-13 However, the small sample size, wide NEC incidence reported in the literature,12,14 and its low incidence in recent IC plus Ven studies1-3,15 make comparisons difficult. There was no association between NEC and age, Ven duration, or previous cytoreduction (supplemental Table 5). Although Ven may potentiate chemotherapy toxicity to intestinal mucosa, it is reassuring that NEC resolved in all cases with conservative management and no fatalities occurred.
Most common treatment emergent adverse effects in all patients
| TEAEs ≥5% . | 8-day Ven (n = 14) . | 11-day Ven (n = 9) . | 14-day Ven (n = 11) . | All cohorts (N = 34) . | ||||
|---|---|---|---|---|---|---|---|---|
| Any grade, n (%) . | Grade ≥3, n (%) . | Any grade, n (%) . | Grade ≥3, n (%) . | Any grade, n (%) . | Grade ≥3, n (%) . | Any grade, n (%) . | Grade ≥3, n (%) . | |
| WBC/ANC decreased | 14 (100) | 14 (100) | 9 (100) | 9 (100) | 11 (100) | 11 (100) | 34 (100) | 34 (100) |
| Anemia | 14 (100) | 14 (100) | 9 (100) | 9 (100) | 11 (100) | 11 (100) | 34 (100) | 34 (100) |
| PLT decreased | 14 (100) | 14 (100) | 9 (100) | 9 (100) | 11 (100) | 11 (100) | 34 (100) | 34 (100) |
| Neutropenic fever | 14 (100) | 14 (100) | 9 (100) | 9 (100) | 11 (100) | 11 (100) | 34 (100) | 34 (100) |
| NEC | 3 (21) | 3 (21) | 1 (11) | 1 (11) | 4 (36) | 4 (36) | 8 (23.5) | 8 (23.5) |
| Sepsis/bacteremia | 5 (36) | 5 (36) | — | — | 5 (45.5) | 5 (45.5) | 10 (29) | 10 (29) |
| Lung infection | 2 (14) | 2 (14) | 1 (11) | 1 (11) | 3 (27) | 3 (27) | 6 (18) | 6 (18) |
| Soft tissue infection | 2 (14) | 2 (14) | — | — | — | — | 2 (6) | 2 (6) |
| Skin infection | 1 (7) | 1 (7) | 1 (11) | — | — | — | 2 (6) | 1 (3) |
| Infectious colitis | 1 (7) | — | — | — | 2 (18) | 2 (18) | 3 (9) | 2 (6) |
| Joint infection | — | — | — | — | 1 (9) | 1 (9) | 1 (3) | 1 (3) |
| Fungemia | — | — | — | — | 1 (9) | 1 (9) | 1 (3) | 1 (3) |
| Nausea | 7 (50) | — | 3 (33) | — | 6 (54.5) | — | 16 (47) | — |
| Vomiting | 3 (21) | — | — | — | 5 (45.5) | — | 8 (23.5) | — |
| Diarrhea | 6 (43) | — | 6 (67) | — | 3 (27) | — | 15 (44) | — |
| Mucositis oral | 1 (7) | 1 (7) | 4 (44) | 1 (11) | 1 (9) | 1 (9) | 6 (18) | 3 (9) |
| Alk Phos increased | 11 (79) | — | 7 (78) | 1 (11) | 9 (82) | 3 (27) | 27 (79) | 4 (12) |
| ALT increased | 7 (50) | — | 3 (33) | 1 (11) | 7 (64) | 2 (18) | 17 (50) | 3 (9) |
| AST Increased | 10 (71) | 1 (7) | 7 (78) | — | 9 (82) | 1 (9) | 26 (76.5) | 2 (6) |
| Hypokalemia | 10 (71) | 5 (36) | 6 (67) | 1 (11) | 9 (82) | 3 (27) | 25 (73.5) | 9 (26.5) |
| Hyponatremia | 6 (43) | 1 (7) | 2 (22) | — | 6 (54.5) | — | 14 (41) | 1 (3) |
| TEAEs ≥5% . | 8-day Ven (n = 14) . | 11-day Ven (n = 9) . | 14-day Ven (n = 11) . | All cohorts (N = 34) . | ||||
|---|---|---|---|---|---|---|---|---|
| Any grade, n (%) . | Grade ≥3, n (%) . | Any grade, n (%) . | Grade ≥3, n (%) . | Any grade, n (%) . | Grade ≥3, n (%) . | Any grade, n (%) . | Grade ≥3, n (%) . | |
| WBC/ANC decreased | 14 (100) | 14 (100) | 9 (100) | 9 (100) | 11 (100) | 11 (100) | 34 (100) | 34 (100) |
| Anemia | 14 (100) | 14 (100) | 9 (100) | 9 (100) | 11 (100) | 11 (100) | 34 (100) | 34 (100) |
| PLT decreased | 14 (100) | 14 (100) | 9 (100) | 9 (100) | 11 (100) | 11 (100) | 34 (100) | 34 (100) |
| Neutropenic fever | 14 (100) | 14 (100) | 9 (100) | 9 (100) | 11 (100) | 11 (100) | 34 (100) | 34 (100) |
| NEC | 3 (21) | 3 (21) | 1 (11) | 1 (11) | 4 (36) | 4 (36) | 8 (23.5) | 8 (23.5) |
| Sepsis/bacteremia | 5 (36) | 5 (36) | — | — | 5 (45.5) | 5 (45.5) | 10 (29) | 10 (29) |
| Lung infection | 2 (14) | 2 (14) | 1 (11) | 1 (11) | 3 (27) | 3 (27) | 6 (18) | 6 (18) |
| Soft tissue infection | 2 (14) | 2 (14) | — | — | — | — | 2 (6) | 2 (6) |
| Skin infection | 1 (7) | 1 (7) | 1 (11) | — | — | — | 2 (6) | 1 (3) |
| Infectious colitis | 1 (7) | — | — | — | 2 (18) | 2 (18) | 3 (9) | 2 (6) |
| Joint infection | — | — | — | — | 1 (9) | 1 (9) | 1 (3) | 1 (3) |
| Fungemia | — | — | — | — | 1 (9) | 1 (9) | 1 (3) | 1 (3) |
| Nausea | 7 (50) | — | 3 (33) | — | 6 (54.5) | — | 16 (47) | — |
| Vomiting | 3 (21) | — | — | — | 5 (45.5) | — | 8 (23.5) | — |
| Diarrhea | 6 (43) | — | 6 (67) | — | 3 (27) | — | 15 (44) | — |
| Mucositis oral | 1 (7) | 1 (7) | 4 (44) | 1 (11) | 1 (9) | 1 (9) | 6 (18) | 3 (9) |
| Alk Phos increased | 11 (79) | — | 7 (78) | 1 (11) | 9 (82) | 3 (27) | 27 (79) | 4 (12) |
| ALT increased | 7 (50) | — | 3 (33) | 1 (11) | 7 (64) | 2 (18) | 17 (50) | 3 (9) |
| AST Increased | 10 (71) | 1 (7) | 7 (78) | — | 9 (82) | 1 (9) | 26 (76.5) | 2 (6) |
| Hypokalemia | 10 (71) | 5 (36) | 6 (67) | 1 (11) | 9 (82) | 3 (27) | 25 (73.5) | 9 (26.5) |
| Hyponatremia | 6 (43) | 1 (7) | 2 (22) | — | 6 (54.5) | — | 14 (41) | 1 (3) |
Alk Phos, alkaline phosphatase; ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; TEAE, treatment emergent adverse effects; WBC, white blood cells.
After the interim marrow assessment (day 21), 14 patients received filgrastim support for a median of 2 days (range, 1-7). Delayed hematologic recovery occurred in only 1 patient (AML, myelodysplasia related) who responded to therapy (Dauno60 + Ven8d). For all responders, the median time to absolute neutrophil count of ≥1.0 × 103/μL and platelet (PLT) count of ≥100 × 103/μL was 27 (22-49) and 28 (22-72) days, respectively (supplemental Figure 3). The median hematologic recovery time was <30 days across both age groups and across all 3 Ven cohorts and was consistent with previous reports for the 7+3 regimen alone (supplemental Table 6; supplemental Figure 3).13,16
Ven plus 7+3 chemotherapy yielded high MRD-negative response rates. CRc was achieved in 85.3% of patients (28 CR and 1 CR with partial hematologic recovery) after single induction, and 86% achieved multiparameter flow cytometry–MRD negativity (Table 3). Despite the small sample size and enrichment of the 8-day Ven cohorts with poor-risk genotypes, response rates remained consistently high across the cohorts. High MRD-negative CR rates were observed across the ELN22 risk categories and molecular AML subgroups, except in TP53-mutated disease (supplemental Table 6; supplemental Figure 4). Notably, MRD-negative rates exceeded the historic 7+3 regimen outcomes.17,18
Disease response by Ven duration and for all patients
| Response . | 8-day Ven (n = 14), n (%) . | 11-day Ven (n = 9), n (%) . | 14-day Ven (n = 11), n (%) . | All Cohorts (N = 34), n (%) . |
|---|---|---|---|---|
| No response | 3 (21) | 1 (11) | 1 (9) | 5 (15) |
| CR | 10 (71) | 8 (89) | 10 (91) | 28 (82) |
| CRh | 1 (7) | — | — | 1 (3) |
| CRcMRD– | 9/11 (82) | 7/8 (87.5) | 9/10 (90) | 25/29 (86) |
| Allogeneic transplant in CR1 | 3 (22) | 3 (33) | 4 (36) | 10 (29) |
| Response . | 8-day Ven (n = 14), n (%) . | 11-day Ven (n = 9), n (%) . | 14-day Ven (n = 11), n (%) . | All Cohorts (N = 34), n (%) . |
|---|---|---|---|---|
| No response | 3 (21) | 1 (11) | 1 (9) | 5 (15) |
| CR | 10 (71) | 8 (89) | 10 (91) | 28 (82) |
| CRh | 1 (7) | — | — | 1 (3) |
| CRcMRD– | 9/11 (82) | 7/8 (87.5) | 9/10 (90) | 25/29 (86) |
| Allogeneic transplant in CR1 | 3 (22) | 3 (33) | 4 (36) | 10 (29) |
CR, complete remission; CRh, complete remission with partial hematologic recovery; CRcMRD–, measurable residual disease–negative composite CR by multiparameter flow cytometry (Hematologics Inc); CR1, first complete remission.
Ven addition to IDAC during consolidation was tolerated differently by age group, likely reflecting age-related marrow reserve differences. Of the 29 responders, 23 entered consolidation; 20 received at least 1 IDAC + Ven cycle (supplemental Figure 2), whereas 3 patients >60 years received IDAC alone after 2 hDLTs (delayed PLT recovery) occurred among the first 7 older patients. Only 1 of 13 patients ≤60 years experienced an hDLT (delayed absolute neutrophil count and PLT recovery). The median neutrophil recovery (≥1000/μL) was consistent at 19 to 20 days across the cycles, but PLT recovery was significantly delayed with subsequent cycles (P = .001; supplemental Table 7). Infectious complications were the most common nonhematologic AEs (supplemental Table 8), but no infection-related deaths occurred.
The durability and survival trends are promising. With a median follow-up of 9.6 months (range, 2-20), the median DoR, EFS, and OS remain unreached (supplemental Figure 5). At data cutoff, 29% (n = 10) underwent transplantation, 93% (n = 27/29) of responders were alive, and 76% (n = 22/29) maintained continuous MRD-negative CR (supplemental Figure 6; supplemental Table 9). The median EFS and OS for ELN22 adverse-risk patients were 4.1 (1.4-11.6) and 18.5 (9.2 to not reached) months, largely reflecting poor, short-lived responses in TP53-mutated cases (supplemental Figures 7 and 8).
The generalizability of our findings is limited by the single-center study design and tertiary care setting. However, our results, combined with recent reports on other Ven + IC combinations,1-3,5 highlight the deep-remission potential of such regimens, particularly Ven plus 7+3, in newly diagnosed AML.
The Bronx, NY, population served by the Montefiore Medical System provided a unique opportunity to test this intervention in an ethnically and racially diverse patient population that is often underrepresented in clinical trials. The inclusion of older but fit patients with AML in our study, coupled with zero induction mortality and high response rates, supports the role of the Ven plus 7+3 induction strategy for carefully selected patients in this age group that is increasingly prioritized for lower-intensity therapies.
Finally, the high MRD-negative CR rates across most genotypes underscore the potential for a genotype-agnostic induction strategy, reserving targeted approaches for consolidation. TP53-mutated AML remains an exception, necessitating exploration of noncytotoxic therapies.
The optimal Ven duration when combined with the 7+3 regimen remains under investigation in our study’s expansion phase in which a 1:1 randomized design is being used to compare 8 with 14 days of Ven. The benefit of Ven addition to the 7+3 regimen is currently being examined in randomized studies.
Acknowledgments
This study was supported with departmental funds from the Department of Oncology, Montefiore Einstein Comprehensive Cancer Center and in part by a gift from Izzy Englander.
Authorship
Contribution: I.M., M.G., and E.J.F. were responsible for the conception and design of the study; I.M., M.G., A.M., A.D., K.F., B.T., L. Shah, S.C., M.K., and E.J.F. were responsible for trial management; I.M., M.G., E.J.F., A.S., N.S., K.G., N.S.K., L. Shapiro, R.A.S., M.K., D.L.C., A.V., M.U., and N.C. were responsible for recruitment and the treatment of patients; I.M., A.M., and A.D. had access to the raw data; I.M., A.M., A.D., J.A.V., K.F., B.T., S.C., and M.U. were responsible for data collection; C.Z. and M.K. were responsible for statistical analysis; I.M. wrote the first draft of the manuscript; and all the authors interpreted the data and reviewed and approved the manuscript.
Conflict-of-interest disclosure: I.M. reports serving on the board of directors or on advisory committees for SYNDAX Pharmaceuticals. A.S. reports receiving research funding from Kymera; serving as a consultant for Gilead, Rigel, Kymera, and Janssen; and receiving honoraria from NACE and PeerView. M.K. reports serving as a consultant for AbbVie, AstraZeneca, Boehringer, F. Hoffman-LaRoche, Genentech, Gilead, Janssen, Legend Biotech, MEI Pharma, Redona, Sanofi Aventis, Sellas, Menarini Group, Stemline, and Vincerx; serving on the board of directors or on advisory committees for AbbVie, Auxenion GmbH, Bakx Therapeutics, Dark Blue Therapeutics, F. Hoffmann-LaRoche, Genentech, Gilead, Immune Oncology, Menarini Group, and Vincerx; receiving research funding from AbbVie, Allogene, AstraZeneca, Genentech, Gilead, ImmunoGen, Janssen, MEI Pharma, Pfizer, Precision Biosciences, Rafael Pharmaceutical, Sanofi Aventis, Stemline, and Menarini Group; and holding stock options in Reata Pharmaceuticals. E.J.F. reports serving as a consultant for Stelexis. A.V. reports receiving research funding from Bristol Myers Squibb, Janssen, Curis, Prelude, and Ryvu and Company; serving as a scientific advisor for Stelexis Therapeutics, Calico, Acceleron Pharma, Aurigene, and Celgene; and owning equity in BioConvergent Health, Throws Exception, and Stelexis Therapeutics. K.G. reports receiving research funding from ADC Therapeutics and iOnctura. The remaining authors declare no competing financial interests.
Correspondence: Ioannis Mantzaris, Department of Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th St, Bronx, NY 10467; email: imantzar@montefiore.org.
References
Author notes
Original data are available on request from the corresponding author, Ioannis Mantzaris (imantzar@montefiore.org). Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 5 years after the publication date at www.ashpublications.org. The study protocol is included as a data supplement that is available with the online version of this article.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal