Issue Archive
Table of Contents
BLOOD COMMENTARIES
PERSPECTIVE
CAR T cells in autoimmunity: game changer or stepping stone?
Clinical Trials & Observations
Chimeric antigen receptor (CAR) T-cell therapy has transformed the treatment of CD19- and BCMA–positive hematologic malignancies, and emerging evidence highlights its potential in autoimmune diseases. This Perspective from Mougiakakos and coauthors explores the early successes and future prospects of B-cell–targeting CAR T-cell therapy in autoimmune disease, offering a balanced overview of current data and anticipated developments, including potential alternatives such as bispecific antibodies.
BLOOD SPOTLIGHT
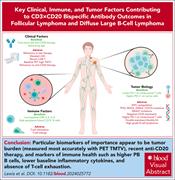
Emerging biomarkers for CD3×CD20 bispecific antibodies in lymphoma
In recent years, several novel CD3×CD20 bispecific immunotherapies have received accelerated approval for the treatment of follicular lymphoma and diffuse large B-cell lymphoma. In this Blood Spotlight, Lewis and colleagues review the challenges in patient selection and discuss current prognostic and potentially predictive biomarkers to help guide therapy.
CLINICAL TRIALS AND OBSERVATIONS
Glucarpidase for treatment of high-dose methotrexate toxicity
Clinical Trials & Observations
With therapeutic drug monitoring to guide leucovorin rescue, high-dose methotrexate (MTX) can be safely administered; however, a subset of patients may still develop life-threatening toxicities. Gupta et al report significant improvements in the recovery from renal failure and other less frequent MTX-related toxicities with the use of glucarpidase, a recombinant, bacterial-derived enzyme that cleaves the glutamate residue from folate analogues in patients receiving high-dose MTX who develop acute kidney injury. The authors’ study supports the use of glucarpidase in cases of delayed MTX elimination and acute renal failure following high-dose MTX therapy, although more precise threshold criteria for its use are still needed.
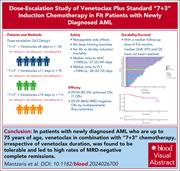
Venetoclax plus daunorubicin and cytarabine for newly diagnosed acute myeloid leukemia: results of a phase 1b study
Clinical Trials & Observations
Brief Report
When combined with a hypomethylating agent, venetoclax has transformed the treatment landscape for newly diagnosed acute myeloid leukemia in older patients or those with significant comorbidities, and many studies are now exploring its role as part of initial therapy for fit patients. Mantzaris et al report on results from a phase 1b study evaluating venetoclax in combination with standard 7+3 induction chemotherapy, demonstrating that venetoclax can be safely administered alongside cytarabine and daunorubicin. An extension study and other ongoing trials will provide further efficacy data to help guide frontline treatment decisions.
LYMPHOID NEOPLASIA
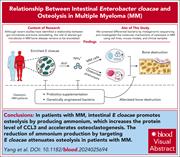
Targeting Enterobacter cloacae attenuates osteolysis by reducing ammonium in multiple myeloma
Skeletal complications of multiple myeloma (MM) are common and contribute significantly to patient morbidity. Yang et al demonstrate that Enterobacter cloacae is significantly enriched in the intestines of patients with osteolytic lesions. In preclinical models, E cloacae promoted osteolysis by increasing circulating ammonium levels, which can be reversed by targeting E cloacae, thereby identifying a potential therapeutic avenue for preventing MM-associated bone disease.
Prognostic and therapeutic implications of measurable residual disease levels during remission induction of childhood ALL
Clinical Trials & Observations
In pediatric acute lymphoblastic leukemia (ALL), measurable residual disease (MRD) monitoring is a standard tool for identifying patients at higher risk of relapse who may benefit from more intensive therapy. In a large cohort of 7640 patients treated under the Chinese Children Cancer Group ALL 2015 protocol, Zhang et al demonstrated that treatment intensification for patients with B-cell ALL with MRD ≥1% on day 19 of induction significantly improved event-free survival, and patients who were MRD-negative at both day 19 and day 46 exhibited particularly favorable outcomes. These findings affirm the utility of MRD testing as a critical tool for risk stratification and treatment guidance in pediatric ALL.
MYELOID NEOPLASIA
PSTK inhibition activates cGAS-STING, precipitating ferroptotic cell death in leukemic stem cells
Differentiation arrest and reliance on oxidative metabolism are shared features across genetically diverse acute myeloid leukemias (AMLs). In preclinical models, He et al identified that inhibition of phosphoseryl-transfer RNA kinase (PSTK), a key enzyme in selenoprotein synthesis, induced ferroptotic cell death and promoted differentiation in AML cells via a cGAS-STING–dependent mechanism and synergized with chemotherapy and venetoclax, while sparing normal hematopoietic cells. These findings support a strategy of timed metabolic intervention to enhance standard therapies and target residual leukemic cells.
RED CELLS, IRON, AND ERYTHROPOIESIS
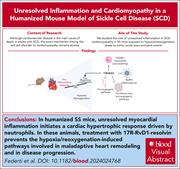
17(R)-Resolvin D1 protects against sickle cell–related inflammatory cardiomyopathy in humanized mice
Cardiovascular disease is a leading cause of morbidity and mortality in adults with sickle cell disease (SCD), yet the mechanisms underlying SCD-associated cardiomyopathy remain unclear. Using a humanized mouse model of SCD, Federti et al demonstrated that cardiomyocyte dysfunction is driven by unresolved inflammation due to hypoxia/reoxygenation stress. Treatment with 17(R)-resolvin D1 effectively mitigates this dysfunction, supporting development of novel anti-inflammatory therapies aimed at reducing cardiovascular disease in patients with SCD.
THROMBOSIS AND HEMOSTASIS
Functional assessment of genetic variants in thrombomodulin detected in patients with bleeding and thrombosis
Thrombomodulin (TM) is a transmembrane glycoprotein primarily expressed on the surface of endothelial cells that inhibits the procoagulant activity of thrombin while significantly enhancing the activation of protein C, a key anticoagulant enzyme, as well as thrombin activatable fibrinolysis inhibitor. These critical functions suggest that both quantitative and qualitative alterations in TM may contribute to bleeding or thrombotic disorders. Van Laer et al expressed and characterized 8 TM gene variants of uncertain significance identified in patients with venous thromboembolism (VTE) or bleeding. Two variants, L433P and C175S, demonstrated defective anticoagulant activity, supporting their potential pathogenic role in VTE.
TRANSPLANTATION
Common hereditary variants of the APOE gene and posttransplant outcome in acute myeloid leukemia
Clinical Trials & Observations
Apolipoprotein E (APOE) is a glycoprotein involved in metabolism, immune regulation, and neurodegenerative disease. In a retrospective analysis of 2 modern cohorts undergoing allogeneic hematopoietic cell transplantation for acute myeloid leukemia, Ronnacker et al report that patients carrying at least 1 APOE2 allele have an increased risk of posttransplant mortality, driven by higher rates of severe chronic graft-versus-host disease (GVHD) and nonrelapse death, while transplantation of APOE2-positive allografts is associated with elevated incidence of both acute and chronic GVHD and reduced overall survival. Further validation in a prospective study is essential to confirm these results and elucidate the biological mechanisms linking APOE variants to posttransplant outcomes.
LETTER TO BLOOD
Non-V600E BRAF mutations and treatment for hairy cell leukemia
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
A 3-dimensional model was used to visualize the location of the thrombomodulin leucine433, a thrombosis-risk variant predicted to disrupt the thrombin-thrombomodulin interaction. See the article by Van Laer et al on page 1929.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals







Methotrexate and glucarpidase: the emperor has new clothes
Clinical Trials & Observations