Key Points
Genome-wide profiling reveals somatic alterations in NUP98-rearranged leukemia associated with fusion partners and phenotypes.
The dependency of NUP98-rearranged leukemia on menin can be affected by differentiation state and cooperating mutations.
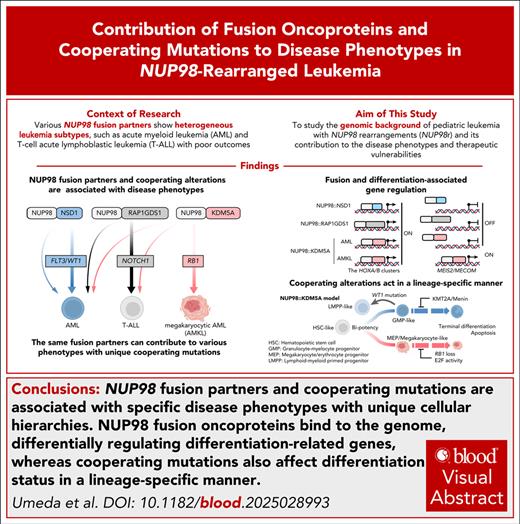
Visual Abstract
Leukemias with NUP98 rearrangements exhibit heterogeneous phenotypes such as acute myeloid leukemia, T-cell acute lymphoblastic leukemia (T-ALL), or myelodysplastic syndrome/neoplasms associated with fusion partners, whereas the mechanism responsible for this heterogeneity is poorly understood. Through genome-wide mutational and transcriptional analyses of 177 NUP98-rearranged leukemias, we show that cooperating alterations are associated with differentiation status even among leukemias sharing the same NUP98 fusions, such as NUP98::KDM5A acute megakaryocytic leukemia with RB1 loss or T-ALL with NOTCH1 mutations. CUT&RUN profiling of in vitro cord blood CD34+ cell (cbCD34) models of major NUP98 fusions revealed that NUP98-fusion oncoproteins (FOs) directly regulate differentiation-related genes contributing to the disease phenotypes, represented by NUP98::KDM5A binding to MEIS2 or GFI1B for megakaryocyte (MK) differentiation. In patient samples, NUP98-FO binding patterns are heterogeneous, potentially shaped by somatic mutations and differentiation status. Using cbCD34 models and CRISPR/Cas9 gene editing, we show that RB1 loss cooperates with NUP98::KDM5A by blocking terminal differentiation toward platelets and expanding MK-like cells, whereas WT1 frameshift mutations skew differentiation toward dormant lymphoid-myeloid primed progenitor cells and cycling granulocyte-monocyte progenitor cells, providing evidence for NUP98-rearranged leukemia phenotypes affected by cooperating alterations. NUP98::KDM5A cbCD34 models with RB1 or WT1 alterations have different sensitivities to menin inhibition, suggesting that cellular differentiation provides stage-specific menin dependencies and resistance mechanisms that can be leveraged for future treatment strategies for NUP98-rearranged leukemia.
Introduction
The prognosis of children with acute myeloid leukemia (AML) remains unsatisfactory, with high relapse rates.1-3 This is partly attributed to subtypes associated with poor treatment response, such as KMT2A rearrangements,4,5UBTF tandem duplications (UBTF-TD),6CBFA2T3::GLIS2,7,8 and NUP98 rearrangements (NUP98r).5,9,10 Understanding the genetic background and disease mechanisms of these subtypes is needed to develop efficient treatment strategies based on biology and molecular mechanisms.
NUP98r with various fusion partners are known to be associated with specific hematologic malignancies.11NUP98r accounts for ∼7% of pediatric AML, with NUP98::NSD1 predominantly found in adolescents with myelomonocytic AML, whereas NUP98::KDM5A is often found in acute megakaryocytic leukemia (AMKL) in young children.5,8,9 Other NUP98r fusion partners have also been reported in T-cell acute lymphoblastic leukemia (T-ALL),12 myelodysplastic syndrome/neoplasms,13 or mixed phenotype acute leukemia.14 Recent genomic studies focusing on NUP98r AML confirmed known associations of NUP98::NSD1 with FLT3 internal tandem duplications (ITDs), and WT1 mutations or NUP98::KDM5A with chromosome 13 loss involving RB1.8,10,15 However, genome-wide studies on various NUP98r leukemias are needed to better understand the association of genomic features with disease phenotypes.
Mechanistically, recent studies revealed that NUP98 fusion oncoproteins (FOs) bind to gene loci active in NUP98r AML, such as MEIS1, HOXA/B clusters, and CDK6.16-18 This binding is partly driven by intrinsically disordered regions of the N-terminal NUP98, which mediate transcriptional condensate formation,19,20 and DNA/chromatin binding domains of the C-terminal partners. This knowledge has mostly been derived from mouse or nonhematopoietic human models due to a lack of human NUP98r leukemia models. An understanding of molecular mechanisms, including NUP98-FO-binding profiles, in human hematopoietic cells is necessary for developing mechanism-based therapeutics for NUP98r leukemias.
To reveal phenotype-specific mutational profiles, we obtained and reanalyzed 175 publicly available AML and T-ALL data sets, along with genome-wide characterization of 10 new pediatric and young adult NUP98r patients using RNA sequencing (RNAseq) and whole genome/exome sequencing (WGS/WES). These data were corroborated by single-cell RNAseq (scRNAseq) and epigenetic profiling by CUT&RUN21 of samples with major NUP98 fusions, revealing NUP98-FO binding profiles associated with fusion partners and disease phenotypes. Functional characterization of recurrent gene alterations using cord blood CD34+ cell (cbCD34) models showed the NUP98 fusion- and phenotype-dependent impact on cell growth and transcriptional profiles associated with drug responses, which can be a basis for overcoming these refractory hematopoietic neoplasms.
Methods
Experimental details can be found in the supplemental Methods, available on the Blood website.
Patient cohort
Tumor samples from patients with acute leukemia were obtained with written informed consent using a protocol approved by the institutional review board in each institute. Samples for RNAseq (n = 10), WGS (n = 2), and WES (n = 2) were newly sequenced in this study. The rest of the data were obtained from previous publications (supplemental Tables 1-4)3,5,6,8,10,13,22-29 or public databases (see details in the supplemental Methods), establishing a cohort with 185 samples from 177 patients, including 8 patients with data at multiple time points (supplemental Table 1). RNAseq data were available for all samples (n = 185), and DNA data for 91 samples (WGS: 43, WES: 16, both: 32; supplemental Tables 2-9). Fusion genes were identified by CICERO30 and manual inspection of sequencing data, confirming NUP98 fusion transcripts in all cases.
cbCD34 modeling
cbCD34 were purchased from Lonza (2C-101) or the Carolinas Cord Blood Bank/Duke University. Cells were cultured in StemSpan SFEM II media (STEMCELL Technologies) supplemented with penicillin-streptomycin, l-glutamine, and recombinant human stem cell factor, FLT3 ligand, thrombopoietin, and interleukin-6 (all 50 ng/mL; PeproTech), StemRegenin1 (1 μmol/L), and UM171 (35 nmol/L, for Figure 4) or UM729 (10 nmol/L, for Figures 6 and 7; all STEMCELL Technologies). Cells were transduced with lentivirus expressing NUP98::KDM5A, NUP98::NSD1, NUP98::HOXA9, or empty vector controls, and transduced mCherry+ cells were enriched by flow sorting on day 7 of transduction. For the introduction of somatic alterations, cbCD34 models were transduced with lentivirus expressing Cas9 to establish cell lines with constitutive Cas9 expression. Transduction of guide RNA (gRNA)-expressing vectors (for WT1, RB1, and the AAVS locus) was performed at ∼day 40 to 60 from the transduction of NUP98-FOs.
Results
Transcriptional heterogeneity of pediatric NUP98r leukemia
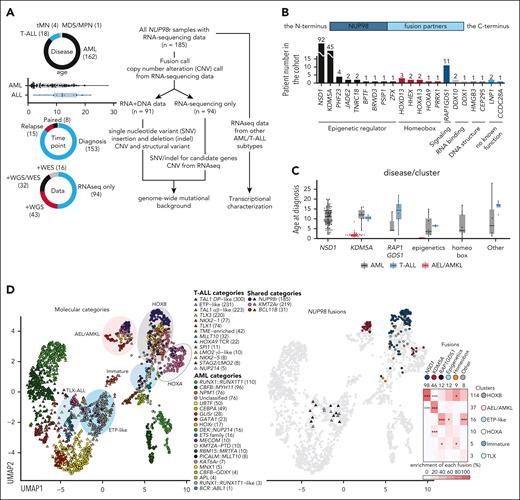
In our pediatric and young adult NUP98r leukemia cohort (median age: 8.6 years, range, 0.4-28 years), AML was the dominant disease (n = 162), followed by T-ALL (n = 18), therapy-related myeloid neoplasms (n = 4), and myelodysplastic syndrome/neoplasms (n = 1), reflecting heterogeneous NUP98r hematologic neoplasms (Figure 1A). Among fusion partners, NSD1 was the most frequent (n = 92, 51.7%), followed by KDM5A (n = 45, 25.3%; Figure 1B; supplemental Figure 1B; supplemental Table 8). RAP1GDS1 (n = 11, 6.2%) was the third most common partner, with 9 NUP98::RAP1GDS1 found in T-ALL cases. NUP98::KDM5A is enriched in infants, whereas NUP98::NSD1 and other partners were broadly found in adolescents and young adults (Figure 1C), consistent with its known occurrence in adult AML.9,15 Most of the other partners fall into the functional categories of epigenetic regulators (n = 12, 6.7%) or homeobox proteins (n = 9, 5.1%). Partners with epigenetic regulatory functions, including NSD1 and KDM5A, frequently have plant homeodomain fingers in the retained C-terminus, suggesting structural redundancy (supplemental Figure 1B-C). Functions of the remaining 6 partners were broad, ranging from RNA-binding (DDX1/10), maintenance of DNA structure (HMGB3 and CEP295), or without known function (LNP1 and CCDC28A), but all had domains with multiple α-helices with linker sequences resembling homeobox domains or plant homeodomains.
Heterogeneity of pediatric NUP98r leukemia. (A) Details of NUP98r leukemia samples (n = 185, left) and analytical pipelines (right). (B) Numbers and functional annotations of fusion partners in the study cohort. Colors indicate protein functional groups. (C) Age distribution related to fusion partners and disease types. Colors indicate disease types. (D) UMAP plots of the transcriptional cohort (n = 2321) colored according to leukemia subtypes (left), NUP98 fusion partners (middle), and enrichment of fusion partners in transcriptional clusters (right). The shapes of dots indicate disease types (circles, AML; triangles, ALL), and colors in the heat map indicate enrichment of fusions in each cluster. Asterisks indicate statistically significant adjusted P values from 2-sided Fisher’s exact tests and the Benjamini-Hochberg adjustment (∗P < .05; ∗∗∗P < .001; black: enriched, blue: exclusive). In panels A and C, lines of the box plots represent the 25% quantile, median, and 75% quantile, and the upper whisker represents the higher value of maxima or 1.5× interquartile range (IQR), and the lower whisker represents the lower value of minima or 1.5× IQR. Abbreviations in leukemia subtypes are found in supplemental Table 10. ETP, early T-cell precursor ALL; MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasms; tMN, therapy-related myeloid neoplasm; UMAP, uniform manifold approximation and projection.
Heterogeneity of pediatric NUP98r leukemia. (A) Details of NUP98r leukemia samples (n = 185, left) and analytical pipelines (right). (B) Numbers and functional annotations of fusion partners in the study cohort. Colors indicate protein functional groups. (C) Age distribution related to fusion partners and disease types. Colors indicate disease types. (D) UMAP plots of the transcriptional cohort (n = 2321) colored according to leukemia subtypes (left), NUP98 fusion partners (middle), and enrichment of fusion partners in transcriptional clusters (right). The shapes of dots indicate disease types (circles, AML; triangles, ALL), and colors in the heat map indicate enrichment of fusions in each cluster. Asterisks indicate statistically significant adjusted P values from 2-sided Fisher’s exact tests and the Benjamini-Hochberg adjustment (∗P < .05; ∗∗∗P < .001; black: enriched, blue: exclusive). In panels A and C, lines of the box plots represent the 25% quantile, median, and 75% quantile, and the upper whisker represents the higher value of maxima or 1.5× interquartile range (IQR), and the lower whisker represents the lower value of minima or 1.5× IQR. Abbreviations in leukemia subtypes are found in supplemental Table 10. ETP, early T-cell precursor ALL; MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasms; tMN, therapy-related myeloid neoplasm; UMAP, uniform manifold approximation and projection.
Appreciating the known heterogeneity of the disease phenotypes of NUP98r leukemia, we performed transcriptional analyses with other subtypes of AML5 (n = 816) and T-ALL29 (n = 1320) as a background cohort (supplemental Figure 2A; supplemental Table 10). Uniform manifold approximation and projection plots and unsupervised clustering revealed 10 clusters driven by driver alterations and differentiation status. NUP98r malignancies were distributed to 6 of these clusters (Figure 1D; supplemental Figure 2B-D). Most NUP98::NSD1 samples were clustered with AML, with NPM1 mutations or UBTF-TD showing high HOXA/B gene expression (the HOXB cluster, n = 114). By contrast, samples with NUP98::KDM5A were predominantly clustered with other acute erythroid leukemia (AEL)/AMKL subtypes (the AEL/AMKL cluster, n = 37), although rare cases clustered with subtypes with KMT2A-rearranged and KAT6A-rearranged AML with HOXA expression (the HOXA cluster, n = 10) or with mature ALL (the TLX cluster, n = 3). NUP98::RAP1GDS1 samples were mainly clustered with other early T-cell precursor (ETP)-like ALL (the ETP-like cluster, n = 16), whereas they were also found in the HOXB, TLX, or immature AML clusters (n = 5). Although HOXA/B gene expression is recognized as a hallmark of NUP98r leukemia, we observed that NUP98::NSD1 samples lack posterior HOXB (HOXB6-9) expression, and immature/T-ALL cases lack HOXB or both HOXA/B expression (supplemental Figure 2E). These data show that NUP98r leukemias are heterogeneous, and even the same fusion partners can contribute to variable phenotypes.
Mutational background of NUP98r leukemia associated with disease phenotypes
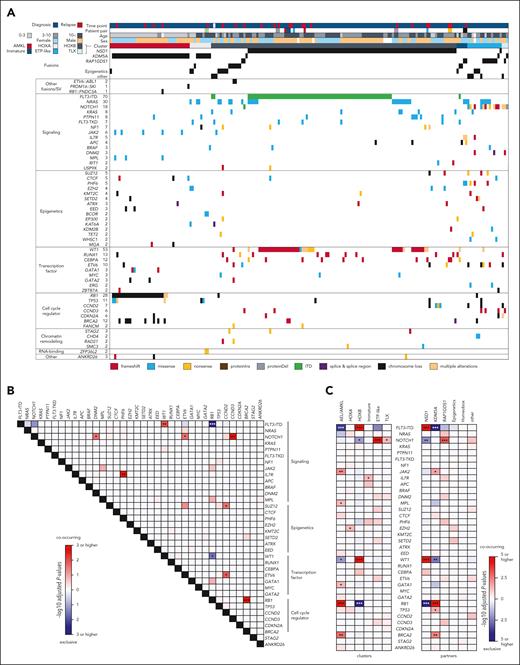
To assess genetic factors contributing to the transcriptional heterogeneity of NUP98r, we investigated the genomic landscape using DNA data and an RNAseq-based pipeline5,6 (Figure 1A), including single nucleotide variants, insertions and deletions (indel), ITD, copy number variations, and fusion genes other than NUP98 rearrangements (supplemental Tables 5-9). Among 53 recurrently altered genes, the most frequent alteration was FLT3-ITD (n = 70, 37.8%), followed by WT1 (n = 44, 23.8%), and NRAS mutations (n = 30, 16.2%; Figure 2A-C). Chromosome 13 deletions involving RB18,10 were found predominantly in NUP98::KDM5A cases (n = 23, 14.1%), with deleted regions extending in both 3ʹ and 5ʹ directions of RB1, often affecting the tumor suppressor BRCA2 (supplemental Figure 3A). We also identified RB1 loss in cases with only RNAseq by assessing for allelic imbalance of single-nucleotide polymorphisms within and around the RB1 locus; however, RB1 loss could still be underestimated as AMKL cases without detectable copy number variations also showed low RB1 RNA expression (supplemental Figure 3B-C). RB1 loss was associated with high expression of stem cell markers (GATA2, CD96, and BMI1) among NUP98::KDM5A AMKL samples, and gene set enrichment analysis (GSEA) showed enrichment of genes involving AMKL phenotypes (supplemental Figure 3D-F). GSEA also showed changes in genes related to cell growth and mitochondrial function, consistent with the known function of RB1/E2F31,32 (supplemental Figure 3F). Additionally, genome-wide profiling revealed JAK2 (n = 6, 3.2%), GATA1 (n = 3, 1.6%), and MPL (n = 3) mutations associated with the AEL/AMKL cluster (Figure 2C), all of which were frequently found in non-Down syndrome AMKL.8
Mutational background of NUP98r leukemia associated with disease phenotypes. (A) Genetic profiles of NUP98r samples in the cohort. Colors indicate patient annotations (top) and types of gene alterations (bottom). (B) Co-occurrence and mutual exclusivity among recurrent alterations (n ≥ 3). (C) Enrichment of somatic alterations in transcriptional clusters (left) and fusion partners (right). In panels B-C, colors indicate adjusted P values by 2-sided Fisher’s exact tests and the Benjamini-Hochberg adjustment (red: co-occurring, blue: mutually exclusive), and asterisks indicate statistically significant values (adjusted P values, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001). Annotations of genes in mutational heat maps depend on known general functions.
Mutational background of NUP98r leukemia associated with disease phenotypes. (A) Genetic profiles of NUP98r samples in the cohort. Colors indicate patient annotations (top) and types of gene alterations (bottom). (B) Co-occurrence and mutual exclusivity among recurrent alterations (n ≥ 3). (C) Enrichment of somatic alterations in transcriptional clusters (left) and fusion partners (right). In panels B-C, colors indicate adjusted P values by 2-sided Fisher’s exact tests and the Benjamini-Hochberg adjustment (red: co-occurring, blue: mutually exclusive), and asterisks indicate statistically significant values (adjusted P values, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001). Annotations of genes in mutational heat maps depend on known general functions.
FLT3-ITD and WT1 were highly co-occurring and enriched in NUP98::NSD1 AMLs (Figure 2A-C). Variant allele frequencies of WT1 and FLT3-ITD were generally high both at diagnosis and relapse (supplemental Figure 4A-C). FLT3-ITD and RAS-related mutations were less likely to co-occur, especially among NUP98::NSD1 samples, and variant allele frequencies of RAS mutations were variable (Figure 2B; supplemental Figure 4A-B), indicating RAS mutations existing in independent clones or subclonal to FLT3-ITD.33 Differentially expressed gene analysis showed high expression of immunity-related genes, such as MRC1 (CD206) or IL2RA (CD25), in FLT3-ITD+NUP98::NSD1 AML, consistent with previous findings in FLT3-ITD+ AML, where these markers are associated with inferior outcomes.34,35 GSEA confirmed known enrichment of inflammatory-related genes36 (supplemental Figure 4D-E). Samples with WT1 mutations had significantly high expression of genes such as DNTT (terminal deoxynucleotidyl transferase), a known marker for lymphoid-myeloid primed progenitors (LMPP),37,38 and gene sets related to mitochondria and translation.
Clinically, all 12 NUP98::RAP1GDS1 leukemias were either T-ALL or immature AML (FAB M0-1). Those with NOTCH1 mutations (n = 7, 58.3%) were predominantly found in the TLX and ETP-like clusters (6/7, 85.7%), and associated with T-cell–related expression profiles, whereas NRAS mutations were mainly found in the ETP and immature AML clusters (supplemental Figure 4F-H). Mutational profiling of samples in T-ALL/immature AML clusters showed NOTCH1 mutations enriched in the ALL clusters, including those in NUP98::KDM5A ALL, whereas IL7R mutations were enriched in immature AML clusters (Figure 2C). Thus, among immature NUP98r leukemias, NOTCH1 mutations likely contribute to T-ALL phenotypes.39 These data collectively show the association among fusion partners, cooperating mutations, and disease phenotypes of NUP98r malignancies.
Varieties of cellular hierarchies in NUP98r leukemia associated with fusions and cooperating alterations
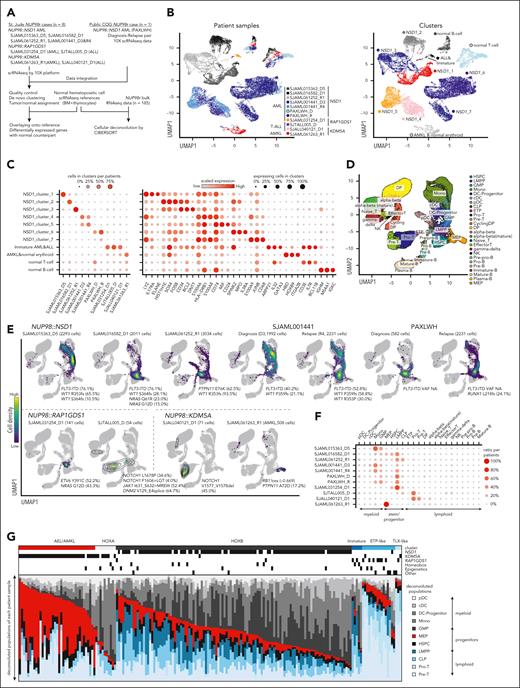
Recent research showed associations between differentiation status with outcomes or drug responses in AML.40-42 To investigate the differentiation status of NUP98r leukemia, we performed scRNAseq on 9 samples with representative fusions (NUP98::NSD1, ::KDM5A, and ::RAP1GDS1), and obtained publicly available data from 1 patient with diagnosis-relapse paired (D-R) samples of NUP98::NSD1 AML (PAXLWH)42 (Figure 3A; supplemental Figure 5A; supplemental Table 11). Uniform manifold approximation and projection plot showed distinct clusters for each sample, indicating unique expression profiles of each tumor, whereas T-cell or B-cell clusters consisted of cells from multiple patients, reflecting normal lymphocytes42,43 (Figure 3B-C; supplemental Figure 5B). To infer the differentiation stages corresponding to normal hematopoiesis, we projected scRNAseq data onto reference normal bone marrow and thymocyte scRNAseq data,43 which revealed unique distributions of tumor cells in each sample (Figure 3D-F; supplemental Figure 5C). Three NUP98::NSD1 samples (SJAML015363_D5, SJAML016582_D1, and SJAML061252_R1) showed various cellular hierarchies ranging from LMPP-like to monocyte-like cells (Figure 3E-F). SJAML001441 D-R pair (FLT3-ITD+/WT1+) had enriched monocyte and granulocyte-macrophage progenitor (GMP)-like cells at diagnosis, with increasing LMPP-like cells at relapse with an additional WT1 mutation. PAXLWH D-R pair (FLT3-ITD+) showed homogeneous LMPP-like cells at diagnosis, but increased GMPs or monocytic populations at relapse with an acquired RUNX1 mutation, suggesting different patterns of relapse of these NUP98::NSD1 cases. Leukemic cells from patients with NUP98::NSD1 showed aberrantly high HOXA/B gene expression along with low expression of marker genes (eg, S100A12 in monocytes, and ELANE in GMP) compared with normal counterparts (supplemental Figure 5D), confirming aberrant expression profiles. Deconvolution of bulk RNA samples using CIBERSORT44 revealed heterogeneous hierarchies among the entire cohort, and variable patterns among both D-R pairs and somatic mutations (Figure 3G; supplemental Figure 5E). Relapse samples commonly showed low monocyte and high LMPP signatures, indicating expansion of stem-like populations at relapse in a subset of NUP98::NSD1 AML, as reported in other subtypes.42
Varieties of cellular hierarchies in NUP98r leukemia. (A) Strategies for scRNAseq and deconvolution of bulk RNAseq data. (B) UMAP plots of patient samples colored by sample source (left) and transcriptional clusters (right). (C) Enrichment of cells in each cluster indicated by colors and sizes (left), and marker gene expression indicated by colors (averaged expression) and size (ratio of expressing cells: count >0 (right). (D) UMAP plot of reference bone marrow and thymocyte scRNAseq data, colored according to cell labels from the original reference data.43 (E) Distribution of patient sample scRNAseq on the reference data inferred by the MapQuery function in the Seurat package. Cells in normal hematopoietic cell clusters were excluded. Cells are colored according to the cell density on the UMAP plot. Cooperating mutations found in bulk samples are also shown. (F) Enrichment of cells with each cell label inferred by Seurat, indicated by colors and sizes. (G) Cellular component of bulk RNA samples (n = 185) inferred by CIBERSORT using a signature matrix derived from reference scRNAseq data. Bars are colored by cell populations in each sample. cDC, classic dendritic cell; CLP, common lymphoid progenitor; DP, CD4-CD8 double-positive T cell; GMP, granulocyte-monocyte progenitor; HSPC, hematopoietic stem and progenitor cell; NK, natural killer T cell; pDC, plasmacytoid dendritic cell; UMAP, uniform manifold approximation and projection.
Varieties of cellular hierarchies in NUP98r leukemia. (A) Strategies for scRNAseq and deconvolution of bulk RNAseq data. (B) UMAP plots of patient samples colored by sample source (left) and transcriptional clusters (right). (C) Enrichment of cells in each cluster indicated by colors and sizes (left), and marker gene expression indicated by colors (averaged expression) and size (ratio of expressing cells: count >0 (right). (D) UMAP plot of reference bone marrow and thymocyte scRNAseq data, colored according to cell labels from the original reference data.43 (E) Distribution of patient sample scRNAseq on the reference data inferred by the MapQuery function in the Seurat package. Cells in normal hematopoietic cell clusters were excluded. Cells are colored according to the cell density on the UMAP plot. Cooperating mutations found in bulk samples are also shown. (F) Enrichment of cells with each cell label inferred by Seurat, indicated by colors and sizes. (G) Cellular component of bulk RNA samples (n = 185) inferred by CIBERSORT using a signature matrix derived from reference scRNAseq data. Bars are colored by cell populations in each sample. cDC, classic dendritic cell; CLP, common lymphoid progenitor; DP, CD4-CD8 double-positive T cell; GMP, granulocyte-monocyte progenitor; HSPC, hematopoietic stem and progenitor cell; NK, natural killer T cell; pDC, plasmacytoid dendritic cell; UMAP, uniform manifold approximation and projection.
A NUP98::KDM5A AMKL case with RB1 loss contained a cluster corresponding to megakaryocyte (MK)/erythroid progenitors (MEPs) in the normal reference (Figure 3B,E-F), showing aberrant co-expression of HOXB genes and immune response-related IFI27 at the single cell level (supplemental Figure 6A-B). NOTCH1+NUP98::KDM5A ALL cells were enriched at pro- and pre-T-cell stages with aberrant expression of hemoglobin or HOXB genes (supplemental Figure 6A).
NOTCH1+NUP98::RAP1GDS1 cells were distributed broadly at the LMPP-ETP-pro-T stages, whereas NUP98::RAP1GDS1 AML cells were enriched in the LMPP cluster (Figure 3E-F; supplemental Figure 6C). Deconvoluted bulk NUP98::RAP1GDS1 samples in immature clusters showed LMPP-common lymphoid progenitor signatures, while those in the ALL clusters showed high pro-pre-T-cell signatures, suggesting that the differentiation block of NUP98::RAP1GDS1 occurs at various stages during T-cell development, likely affected by cooperating mutations39 (supplemental Figure 6C-E). Two NUP98::KDM5A ALL cases showed higher pre-T signals than NUP98::RAP1GDS1 cases (supplemental Figure 6F), indicating that NUP98::KDM5A could contribute to more mature ALL than NUP98::RAP1GDS1, both cooperating with NOTCH1 mutations.
cbCD34 models recapitulate the phenotypes of NUP98r leukemia
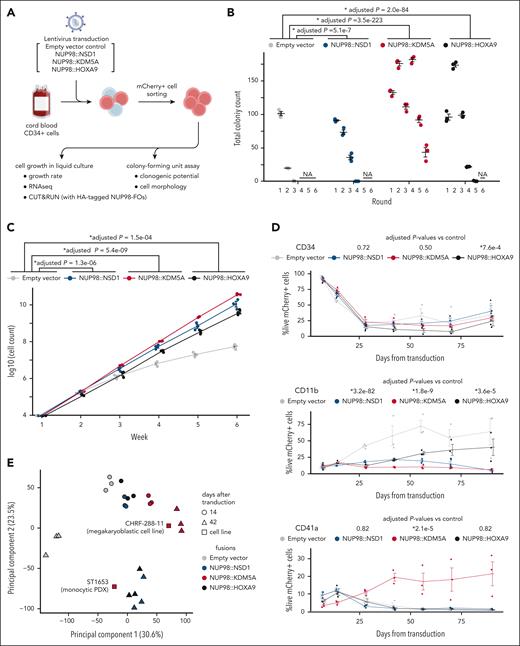
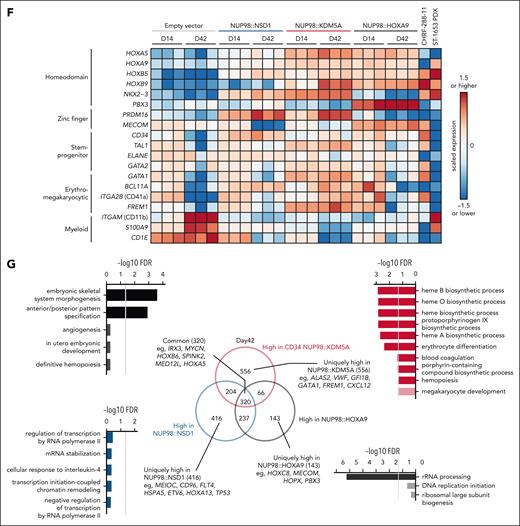
With limited human NUP98r leukemia models that recapitulate the disease phenotypes to study the underlying mechanisms,39,45-48 we tested the transforming potential of various NUP98 FOs by transducing cbCD34 cells with lentiviral particles encoding major NUP98-FOs (pediatrics-NUP98::NSD1 and NUP98:KDM5A, adult-NUP98::HOXA9). Expression of these NUP98-FOs increased clonogenic potential in methylcellulose and enhanced cell growth in liquid culture compared with an empty vector control (Figure 4A-C), despite a gradual decrease in CD34 expression. Flow cytometry analyses showed low CD11b expression in all conditions compared with the control, whereas only NUP98::KDM5A demonstrated consistent CD41a expression, indicating preferential differentiation toward MKs (Figure 4D; supplemental Figure 7A-B). RNAseq from liquid cultures at multiple time points revealed dynamic changes of transcriptional status unique to each NUP98-FO (Figure 4E-G; supplemental Figure 7C-E). Consistent with patient samples, NUP98-FO-transduced conditions showed high TAL1 and MYCN expression in addition to upregulation of HOXA/B genes, whereas control conditions showed reduced TAL1 and increased ITGAM (CD11b) expression, suggesting myeloid differentiation (Figure 4F). We identified 320 common genes with high expression in conditions with NUP98-FOs, which enriched homeobox genes (Figure 4G). Notably, we also identified uniquely high genes with each FO related to specific functions, such as GFI1B or GATA1 related to erythromegakaryocytic differentiation in cells with NUP98::KDM5A. Comparison with the patient cohort showed clustering of cbCD34 models with patient samples with similar fusions/phenotypes (supplemental Figure 7E). Two cell lines with NUP98::KDM5A, CHRF-288-1145 (megakaryocytic), and ST165349 (monocytic patient-derived xenograft), also showed unique patterns of expression (eg, ITGA2B and CD34 in CHRF-288-11, and ITGAM in ST1653), confirming the heterogeneity of leukemia with NUP98::KDM5A (Figure 4E-F; supplemental Figure 7E).
cbCD34 models recapitulate the phenotypes of NUP98r leukemia. (A) Experimental schema using cbCD34 models. (B) Colony-forming unit assays of cbCD34 models with empty control vectors or NUP98::NSD1, NUP98::KDM5A, or NUP98::HOXA9-expressing vectors. (C) Cell growth assays of cbCD34 models in liquid culture. (D) Flow cytometric analysis of cbCD34 models in liquid culture (top, CD34+; middle, CD11b+; bottom, CD41a+; population ratio, % in mCherry+ live cells). (E) Principal component analysis of RNAseq data from liquid culture. Colors indicate NUP98 fusions, and shapes indicate days after transduction. (F) Heat map showing expression of representative genes related to stemness or differentiation of hematopoietic cells. The colors of cells indicate expression levels normalized among samples, and genes are annotated on the left. (G) Comparison of differentially expressed genes in each cbCD34 model compared with empty vector controls at day 42. Venn diagram showing overlaps of highly expressed genes in each model (middle), and gene ontology term analyses of shared or specific differentially expressed genes are shown (left, right). Gray lines show −log10 FDR = 0.05. Data were obtained from 3 biological replicates (different lots of cord blood). In panels B-D, statistical tests were performed by a generalized linear mixed effect model with Poisson (B) and Gaussian (C-D) distributions, followed by comparison with empty vector control and the Benjamini-Hochberg adjustment, asterisks indicating adjusted P values, ∗P < .05. Error bars indicate mean ± standard error of the mean. FDR, false-discovery rate; PDX, patient-derived xenograft.
cbCD34 models recapitulate the phenotypes of NUP98r leukemia. (A) Experimental schema using cbCD34 models. (B) Colony-forming unit assays of cbCD34 models with empty control vectors or NUP98::NSD1, NUP98::KDM5A, or NUP98::HOXA9-expressing vectors. (C) Cell growth assays of cbCD34 models in liquid culture. (D) Flow cytometric analysis of cbCD34 models in liquid culture (top, CD34+; middle, CD11b+; bottom, CD41a+; population ratio, % in mCherry+ live cells). (E) Principal component analysis of RNAseq data from liquid culture. Colors indicate NUP98 fusions, and shapes indicate days after transduction. (F) Heat map showing expression of representative genes related to stemness or differentiation of hematopoietic cells. The colors of cells indicate expression levels normalized among samples, and genes are annotated on the left. (G) Comparison of differentially expressed genes in each cbCD34 model compared with empty vector controls at day 42. Venn diagram showing overlaps of highly expressed genes in each model (middle), and gene ontology term analyses of shared or specific differentially expressed genes are shown (left, right). Gray lines show −log10 FDR = 0.05. Data were obtained from 3 biological replicates (different lots of cord blood). In panels B-D, statistical tests were performed by a generalized linear mixed effect model with Poisson (B) and Gaussian (C-D) distributions, followed by comparison with empty vector control and the Benjamini-Hochberg adjustment, asterisks indicating adjusted P values, ∗P < .05. Error bars indicate mean ± standard error of the mean. FDR, false-discovery rate; PDX, patient-derived xenograft.
Differential gene regulation by NUP98-FOs
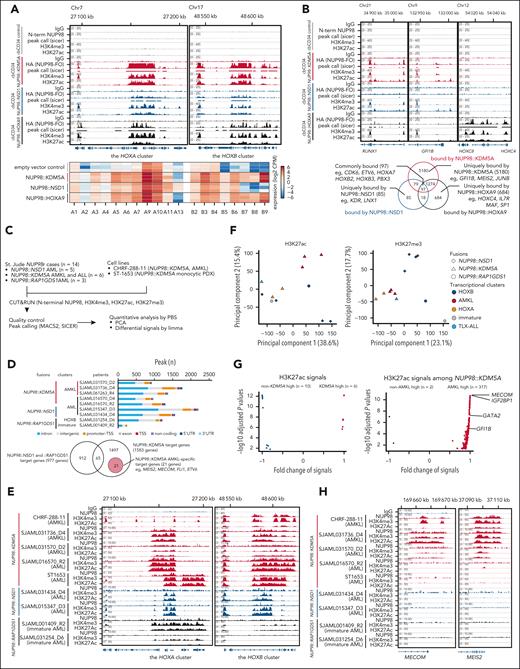
To further study how NUP98-FOs drive leukemogenesis, we profiled the genomic binding of N-terminal HA-tagged NUP98-FOs (supplemental Figure 8A), using anti-HA antibody in cbCD34 models along with histone modifications (H3K4me3 and H3K27ac) and menin using CUT&RUN21 (Figure 5A-B; supplemental Figure 8B-D). FO-binding peaks were enriched in the promoter and intronic regions of protein-coding genes with high reproducibility among biological replicates (supplemental Figure 8C). All three FOs showed binding to the HOXA-B clusters as previously reported using different models,16-19 whereas the binding patterns along the anterior-posterior axes were unique to each fusion, corresponding to RNA expression, active histone modifications, and menin binding (Figure 5A; supplemental Figure 8D). Cells with empty vector controls showed only weak H3K4me3 and H3K27ac signals on the HOXA/B clusters, consistent with the observed low RNA expression (Figure 5A). Comparisons of protein-coding genes with FO-binding peaks showed 97 common target genes for all 3 NUP98-FOs, including genes involved in leukemogenesis and hematopoiesis (eg, RUNX1, CDK6), whereas each FO also showed specific targets and binding motifs (Figure 5B; supplemental Figure 8B). Notably, NUP98::KDM5A uniquely showed peaks at ETS motifs and on GFI1B and MEIS2, which are MK/erythroid differentiation regulators,50-53 indicating that direct regulation of differentiation-related genes could drive phenotypes of NUP98::KDM5A AMKL.
Differential gene regulation by NUP98-FOs. (A) Integrative Genome Viewer (IGV) tracks of the HOXA-B clusters from CUT&RUN using HA, H3K4me3, and H3K27ac antibodies in HA-tagged NUP98r cbCD34 models (top: empty vector control, gray; HA-NUP98::KDM5A, red; HA-NUP98::NSD1, blue; HA-NUP98::HOXA9, black), and heat map showing expression levels of HOXA-B genes (bottom). N-terminal NUP98 antibody was applied for the empty vector control. (B) IGV tracks of differentiation-related gene loci (top: RUNX1, GFI1B, and the HOXC cluster) and Venn diagram showing overlap of protein-coding genes with annotated peaks (bottom: false-discovery rate <0.00001). (C) CUT&RUN strategy from primary patient samples or NUP98::KDM5A cell lines (CHRF-288-11 and ST1653). (D) Counts of peaks from the N-terminus NUP98 antibody in primary samples (top) and overlaps of target genes among non-NUP98::KDM5A and NUP98::KDM5A (bottom). NUP98::KDM5A AMKL-specific 21 target genes are highlighted. Colors indicate peak annotations. (E) IGV tracks of the HOXA-B cluster from CUT&RUN using N-terminal NUP98, H3K4me3, and H3K27ac antibodies in primary leukemia samples and NUP98::KDM5A cell lines. (F) Principal component analysis of genome-wide PBS (probability of being signals) scores of H3K27ac (left) and H3K27me3 (right) from primary samples. Colors indicate expression clusters, and shapes indicate fusion partners. (G) Differential signal analysis using H3K27ac PBS scores between NUP98::KDM5A and other (NUP98::NSD1 and NUP98::RAP1GDS1) samples (left) and NUP98::KDM5A AMKL and non-AMKL (right) calculated by limma, followed by the Benjamini-Hochberg adjustment. Only regions with significant enrichment (adjusted P < .05) are shown. (H) IGV tracks of the MECOM and MEIS2 gene loci from CUT&RUN using N-terminal NUP98, H3K4me3, and H3K27ac antibodies in primary leukemia samples and NUP98::KDM5A cell lines. IgG, immunoglobulin G.
Differential gene regulation by NUP98-FOs. (A) Integrative Genome Viewer (IGV) tracks of the HOXA-B clusters from CUT&RUN using HA, H3K4me3, and H3K27ac antibodies in HA-tagged NUP98r cbCD34 models (top: empty vector control, gray; HA-NUP98::KDM5A, red; HA-NUP98::NSD1, blue; HA-NUP98::HOXA9, black), and heat map showing expression levels of HOXA-B genes (bottom). N-terminal NUP98 antibody was applied for the empty vector control. (B) IGV tracks of differentiation-related gene loci (top: RUNX1, GFI1B, and the HOXC cluster) and Venn diagram showing overlap of protein-coding genes with annotated peaks (bottom: false-discovery rate <0.00001). (C) CUT&RUN strategy from primary patient samples or NUP98::KDM5A cell lines (CHRF-288-11 and ST1653). (D) Counts of peaks from the N-terminus NUP98 antibody in primary samples (top) and overlaps of target genes among non-NUP98::KDM5A and NUP98::KDM5A (bottom). NUP98::KDM5A AMKL-specific 21 target genes are highlighted. Colors indicate peak annotations. (E) IGV tracks of the HOXA-B cluster from CUT&RUN using N-terminal NUP98, H3K4me3, and H3K27ac antibodies in primary leukemia samples and NUP98::KDM5A cell lines. (F) Principal component analysis of genome-wide PBS (probability of being signals) scores of H3K27ac (left) and H3K27me3 (right) from primary samples. Colors indicate expression clusters, and shapes indicate fusion partners. (G) Differential signal analysis using H3K27ac PBS scores between NUP98::KDM5A and other (NUP98::NSD1 and NUP98::RAP1GDS1) samples (left) and NUP98::KDM5A AMKL and non-AMKL (right) calculated by limma, followed by the Benjamini-Hochberg adjustment. Only regions with significant enrichment (adjusted P < .05) are shown. (H) IGV tracks of the MECOM and MEIS2 gene loci from CUT&RUN using N-terminal NUP98, H3K4me3, and H3K27ac antibodies in primary leukemia samples and NUP98::KDM5A cell lines. IgG, immunoglobulin G.
We next sought to profile endogenous NUP98-FO binding without epitope tags in patient samples or cell lines. We tested N-terminus NUP98 (N-NUP98) antibodies in cbCD34, which showed similar binding patterns albeit with weaker signals yielding fewer peaks than HA-antibody, possibly underestimating weak FO-binding (supplemental Figure 9A). CHRF-288-11 and ST1653 showed NUP98 signals at genes highly expressed in these cell lines, such as HOXA/B genes and MEIS1, with menin colocalization (supplemental Figure 9B). However, weak or low signals were observed in an empty vector control from cbCD34 models or RUNX1::RUNX1T1 AML (lacking HOX gene expression) or UBTF-TD AML (resembling NUP98::NSD1 AML expression6; Figure 5A; supplemental Figure 9B). We then applied CUT&RUN using antibodies for N-NUP98 and histone modifications (H3K4me3, H3K27ac, H3K27me3) for 14 NUP98r samples from patients covering NUP98::NSD1, NUP98::KDM5A, and NUP98::RAP1GDS1 (Figure 5C; supplemental Figure 9C; supplemental Table 12). N-NUP98 antibodies revealed target genes for each FO, including HOXA/B genes with heterogeneous patterns even within the same NUP98-FO groups (Figure 5D-E). Also, N-NUP98 antibodies showed NUP98::KDM5A-specific targets (1518 genes), whereas 21 genes, including MEIS2 or MECOM, were NUP98::KDM5A AMKL-specific. Genes with H3K4me3 peaks showed a broad overlap among samples, whereas 61 genes, including IGF2BP3 associated with MK development,54 were unique to NUP98::KDM5A AMKL (supplemental Figure 9D). For H3K27ac and H3K27me3 signals with broad peaks that could span multiple genes, we applied the PBS55 (probability of being a signal) approach to quantify the genome-wide signals. This analysis revealed that genome-wide H3K27ac status correlated with disease phenotypes, whereas H3K27me3 status was associated with fusion partners (Figure 5F). Comparisons of NUP98::KDM5A with the other fusions (NUP98::NSD1 and NUP98::RAP1GDS1) showed limited regions with differential H3K27ac signals (Figure 5G). In contrast, a comparison between NUP98::KDM5A AML and AMKL showed enrichment of MECOM, IGF2BP1, or GATA2 regions in NUP98::KDM5A AMKL, indicating phenotype-specific epigenetic status (Figure 5G-H; supplemental Figure 9E). These data suggest that both fusion partners and cellular differentiation status can affect FO-binding patterns to establish the heterogeneous epigenetic and expression profiles in NUP98r leukemia.
Functional characterization of recurrent somatic alterations in NUP98r leukemia
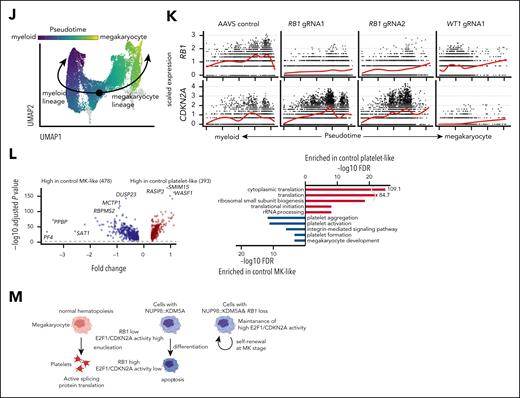
Given the differential NUP98-FO binding and mutation patterns related to disease phenotypes, we investigated the impact of co-occurring alterations on NUP98r cbCD34 models. We first established cbCD34 models with constitutive Cas9 expression, followed by lentiviral transduction of gRNA designed to mimic WT1 frameshift mutations or RB1 loss (Figure 6A; supplemental Figure 10A-B). In NUP98::KDM5A cbCD34 models, WT1 gRNAs enhanced cell growth compared with AAVS-targeting controls, whereas out-of-frame indels in RB1 were enriched at later time points, indicating growth advantages of cells with RB1 loss (Figure 6B-C). Morphologically, AAVS-controls showed cells with various degrees of myeloid and erythrocyte/MK differentiation, whereas RB1-gRNAs yielded larger cells with features of maturing MKs, and WT1-gRNAs enriched homogeneously small blast-like cells (Figure 6B). Flow cytometric analysis showed that RB1-gRNAs enriched CD34+CD41a+ cells, whereas WT1-gRNAs depleted the CD41a+ cells with increased CD34+CD41a– cells (Figure 6D; supplemental Figure 10C). NUP98::NSD1 cbCD34 models showed growth advantages with WT1-gRNAs, whereas RB1-gRNAs did not show either growth advantage or consistent enrichment of out-of-frame indels, suggesting that RB1 loss specifically synergizes with NUP98::KDM5A (supplemental Figure 10D-E). RNAseq revealed global transcriptional changes in RB1- or WT1-gRNA conditions (Figure 6E). RB1-gRNAs led to upregulation of CD34 and CDKN2A, which is downstream of RB1/E2F and forms a feedback loop,56,57 and changes in immunity genes compared with AAVS-controls (Figure 6F; supplemental Figure 11A). WT1-gRNAs also increased CD34 expression, along with increased major histocompatibility complex class II and decreased MK/platelet-related gene expression (Figure 6G).
Functional characterization of recurrent somatic alterations in NUP98r leukemia. (A) Experimental schema of induction of cooperating alterations (RB1, WT1) in cbCD34/Cas9 models. (B) Cell growth assays of cbCD34/Cas9 NUP98::KDM5A with gRNAs targeting the AAVS, RB1, or WT1 loci (left), and cytospin of cells on day 35 (right; scale bars, 50 μm). (C) Rates of indel (insertions and deletions) at days 4 and 39 in each condition. Bars represent fractions of indel rates in all target sequence reads, and dots represent the out-of-frame indel ratio among total indels. (D) Flow gating (left), CD34+ CD41a+ positivity (middle), and CD34+ CD41a– (right) among mCherry+ GFP+ mAmetrine+ live cells. (E) Principal component analysis of RNAseq of gRNA-transduced NUP98::KDM5A cbCD34 models at day 35. (F) Differentially expressed genes analysis between AAVS controls and RB1-gRNA conditions (left), and gene ontology term analysis of differentially expressed genes (right). Colors indicate differentially expressed genes and gene ontology terms (red: high in RB1-gRNA conditions, blue: low in RB1-gRNA conditions). (G) Differentially expressed genes analysis between AAVS controls and WT1-gRNA conditions as shown in panel F. (H) UMAP plots of scRNAseq data from gRNA-transduced NUP98::KDM5A cbCD34 models at day 35, showing marker gene expression (left), annotated clusters (middle), and cell distributions among conditions (right). Colors in plots indicate relative expression levels, clusters, and cell density, respectively. (I) Enrichment of cells with each cluster indicated by colors and sizes. (J) Pseudotime along myeloid (HSC → GMP → monocytes) and platelet (HSC → MEP → MK → platelet) trajectories. Colors represent pseudotime scores of each single cell inferred by Slingshot. (K) RB1 (top) and CDKN2A (bottom) expression along the pseudotime axis in each condition, with red curves showing average expressions. (L) Differentially expressed genes analysis between the platelet-like and MK-like clusters in the AAVS-control condition (left) and gene ontology term analysis (right) of genes high in the platelet-like cluster (red) and the MK-like cluster (blue). (M) Schematics illustrating platelet differentiation in normal hematopoiesis and NUP98::KDM5A models (created in BioRender. Umeda, M. (2025) https://BioRender.com/kreq8kl). Assay data were obtained in technical triplicates from an established NUP98::KDM5A/Cas9 line and independent experiments. One data point in panel C was not obtained due to technical errors. RNAseq data were obtained from 6 independent experiments. In panels B-D, statistical tests were performed by linear mixed effect model (B) or 2-sided Student t test by comparing day 4 and day 39 (C) or gRNA conditions and AAVS controls (D), and limma (F-G), followed by the Benjamini-Hochberg adjustment when applicable. Differentially expressed genes in scRNAseq (L) were identified using the FindMarker function in the Seurat package with default settings, which calculates adjusted P values with limma implementation of the Wilcoxon rank-sum test followed by Bonferroni correction. Asterisks indicating P values or adjusted P values <.05. Error bars indicate mean ± standard error of the mean. HSC, hematopoietic stem cell; UMAP, uniform manifold approximation and projection.
Functional characterization of recurrent somatic alterations in NUP98r leukemia. (A) Experimental schema of induction of cooperating alterations (RB1, WT1) in cbCD34/Cas9 models. (B) Cell growth assays of cbCD34/Cas9 NUP98::KDM5A with gRNAs targeting the AAVS, RB1, or WT1 loci (left), and cytospin of cells on day 35 (right; scale bars, 50 μm). (C) Rates of indel (insertions and deletions) at days 4 and 39 in each condition. Bars represent fractions of indel rates in all target sequence reads, and dots represent the out-of-frame indel ratio among total indels. (D) Flow gating (left), CD34+ CD41a+ positivity (middle), and CD34+ CD41a– (right) among mCherry+ GFP+ mAmetrine+ live cells. (E) Principal component analysis of RNAseq of gRNA-transduced NUP98::KDM5A cbCD34 models at day 35. (F) Differentially expressed genes analysis between AAVS controls and RB1-gRNA conditions (left), and gene ontology term analysis of differentially expressed genes (right). Colors indicate differentially expressed genes and gene ontology terms (red: high in RB1-gRNA conditions, blue: low in RB1-gRNA conditions). (G) Differentially expressed genes analysis between AAVS controls and WT1-gRNA conditions as shown in panel F. (H) UMAP plots of scRNAseq data from gRNA-transduced NUP98::KDM5A cbCD34 models at day 35, showing marker gene expression (left), annotated clusters (middle), and cell distributions among conditions (right). Colors in plots indicate relative expression levels, clusters, and cell density, respectively. (I) Enrichment of cells with each cluster indicated by colors and sizes. (J) Pseudotime along myeloid (HSC → GMP → monocytes) and platelet (HSC → MEP → MK → platelet) trajectories. Colors represent pseudotime scores of each single cell inferred by Slingshot. (K) RB1 (top) and CDKN2A (bottom) expression along the pseudotime axis in each condition, with red curves showing average expressions. (L) Differentially expressed genes analysis between the platelet-like and MK-like clusters in the AAVS-control condition (left) and gene ontology term analysis (right) of genes high in the platelet-like cluster (red) and the MK-like cluster (blue). (M) Schematics illustrating platelet differentiation in normal hematopoiesis and NUP98::KDM5A models (created in BioRender. Umeda, M. (2025) https://BioRender.com/kreq8kl). Assay data were obtained in technical triplicates from an established NUP98::KDM5A/Cas9 line and independent experiments. One data point in panel C was not obtained due to technical errors. RNAseq data were obtained from 6 independent experiments. In panels B-D, statistical tests were performed by linear mixed effect model (B) or 2-sided Student t test by comparing day 4 and day 39 (C) or gRNA conditions and AAVS controls (D), and limma (F-G), followed by the Benjamini-Hochberg adjustment when applicable. Differentially expressed genes in scRNAseq (L) were identified using the FindMarker function in the Seurat package with default settings, which calculates adjusted P values with limma implementation of the Wilcoxon rank-sum test followed by Bonferroni correction. Asterisks indicating P values or adjusted P values <.05. Error bars indicate mean ± standard error of the mean. HSC, hematopoietic stem cell; UMAP, uniform manifold approximation and projection.
scRNAseq from these conditions collectively showed differentiation trajectories resembling normal hematopoiesis (Figure 6H-I; supplemental Figure 11B). A control condition showed broad distributions with enrichment at the platelet-like populations adjacent to the MEP-like or MK-like clusters. By contrast, RB1-gRNAs led to enrichment at the MEP or MK-like clusters and lacked platelet-like populations, and WT1-gRNA resulted in a bias toward the GMP- and LMPP-like populations rarely found in control and RB1-gRNA conditions. Pseudotime analyses showed that RB1 was upregulated through platelet differentiation and highest in the platelet-like cluster, whereas CDKN2A expression was high in the MEP or MK clusters but low or undetectable in the platelet cluster (Figure 6J-K; supplemental Figure 11C). We also observed negative correlations with RB1 and CDKN2A among NUP98::KDM5A patient samples (supplemental Figure 11D). The platelet-like cluster had higher expression of translation-related genes than the MK-like cluster (Figure 6L), recapitulating changes in translation in normal platelet production from MK or proplatelets.58 These data suggest that RB1 loss inhibits terminal platelet differentiation in the NUP98::KDM5A model, leading to the accumulation of cells at the MK-like or MEP-like stages, resembling the megakaryocytic differentiation block seen in E2F transgenic mice59 (Figure 6M). The LMPP population enriched in the WT1-gRNA condition was characterized by high major histocompatibility complex class II gene and low cell-division gene expression, and cell-cycle inference indicated enrichment of cells at the G1 stage in the LMPP cluster and S/G2M in the GMP cluster (supplemental Figure 11E-G), suggesting a unique hierarchy of proliferating GMP-like cells and dormant LMPP-like cells with WT1-gRNA-induced mutations.
Cooperating alterations and differentiation status affect sensitivity to menin inhibition
NUP98r leukemia has been reported to be dependent on KMT2A/menin complex,18 and menin inhibitors are currently being tested in clinical trials enrolling patients with AML, including those with NUP98r.60 Mouse models showed that menin inhibition led to myeloid differentiation in NPM1-mutant and NUP98r leukemia models,18,61-63 although whether menin is required for megakaryocytic lineages or AMKL is currently unclear. We tested a menin inhibitor (revumenib) in NUP98::KDM5A CD34 models with cooperating alterations to assess the sensitivity and impact on cellular populations (Figure 7A). Two-week treatment with revumenib (1 μM) decreased cell growth in AAVS conditions (14.1% ± 4.6%; mean ± standard error) compared with dimethyl sulfoxide, whereas RB1- or WT1-gRNA conditions were less sensitive (RB1-gRNA: 64.3% ± 10.3%, WT1-gRNA: 56.8% ± 4.0%; Figure 7B). Flow cytometric analysis showed significant increases in the CD11b+ differentiated myeloid population in AAVS-control and WT1-gRNA conditions, whereas RB1-gRNA conditions increased the CD34+CD41a+ population (Figure 7C), suggesting adaptive changes of cellular hierarchies. RNAseq on day 7 of treatment showed increased expression of genes previously reported to be downregulated in NUP98r models in all conditions, whereas CDKN2 family,64 inflammation, and erythroid/MK-related genes were enriched in AAVS conditions compared with RB1-gRNA conditions, suggesting differentiation induced by menin inhibition (Figure 7D). Menin inhibition in WT1-gRNA showed immunity-related changes as in AAVS controls, along with activation of monocyte/lymphocyte genes (eg, CD48, MAFB; supplemental Figure 11H), suggesting partial differentiation from dominant GMP or LMPP clusters. We also tested revumenib on ST1653 (monocytic) and CHRF-288-11 (megakaryocytic), which showed that ST1653 was sensitive to menin inhibition but CHRF-288-11 was not, further confirming the association of differentiation status with menin-dependency (Figure 7E). These collective data indicate that cellular differentiation and cooperating mutations can both influence menin dependency of NUP98r leukemias (Figure 7F).
Cooperating alterations and differentiation status affect sensitivity to menin inhibition. (A) Experimental schema showing revumenib treatment. (B) Relative cell growth of cbCD34 NUP98::KDM5A models with gRNA treated with revumenib (0.1-1 μM) compared with dimethyl sulfoxide controls. (C) Flow gating (top), CD34+CD41a+ population (left), CD34–CD41a+ population (middle), and CD34–CD11B+ populations (right) among mCherry+ GFP+ mAmetrine+ live population at day 15. (D) GSEA analyses between dimethyl sulfoxide and revumenib-treated conditions, colors showing NES (top-left), comparison of expression changes between AAVS and RB1-gRNA1 conditions (right), and gene ontology term analysis of changes enriched (difference of fold changes >1) in AAVS conditions (bottom-left). (E) Relative cell growth of unedited cbCD34 NUP98::KDM5A (control, black), CHRF-288-11 (red), and ST1653 patient-derived xenograft (blue) treated with revumenib (0.1-1 μM) compared with dimethyl sulfoxide controls. (F) Schematics illustrating a cellular hierarchy of NUP98::KDM5A models created in BioRender. Umeda, M. (2025) https://BioRender.com/kreq8kl. Data were obtained from 3 technical replicates using gRNA-transduced cells in Figure 6. One data point at day 15 was excluded for technical errors. Statistical tests were performed by a generalized linear mixed effect model with Gaussian distribution, followed by the Benjamini-Hochberg adjustment (B,E) or Student t test by comparing gRNA conditions with AAVS controls (C). Asterisks indicate P values or adjusted P values; ∗P < .05. Error bars indicate mean ± standard error of the mean. DMSO, dimethyl sulfoxide; NES, normalized enrichment score.
Cooperating alterations and differentiation status affect sensitivity to menin inhibition. (A) Experimental schema showing revumenib treatment. (B) Relative cell growth of cbCD34 NUP98::KDM5A models with gRNA treated with revumenib (0.1-1 μM) compared with dimethyl sulfoxide controls. (C) Flow gating (top), CD34+CD41a+ population (left), CD34–CD41a+ population (middle), and CD34–CD11B+ populations (right) among mCherry+ GFP+ mAmetrine+ live population at day 15. (D) GSEA analyses between dimethyl sulfoxide and revumenib-treated conditions, colors showing NES (top-left), comparison of expression changes between AAVS and RB1-gRNA1 conditions (right), and gene ontology term analysis of changes enriched (difference of fold changes >1) in AAVS conditions (bottom-left). (E) Relative cell growth of unedited cbCD34 NUP98::KDM5A (control, black), CHRF-288-11 (red), and ST1653 patient-derived xenograft (blue) treated with revumenib (0.1-1 μM) compared with dimethyl sulfoxide controls. (F) Schematics illustrating a cellular hierarchy of NUP98::KDM5A models created in BioRender. Umeda, M. (2025) https://BioRender.com/kreq8kl. Data were obtained from 3 technical replicates using gRNA-transduced cells in Figure 6. One data point at day 15 was excluded for technical errors. Statistical tests were performed by a generalized linear mixed effect model with Gaussian distribution, followed by the Benjamini-Hochberg adjustment (B,E) or Student t test by comparing gRNA conditions with AAVS controls (C). Asterisks indicate P values or adjusted P values; ∗P < .05. Error bars indicate mean ± standard error of the mean. DMSO, dimethyl sulfoxide; NES, normalized enrichment score.
Discussion
Biology-informed treatment of subtypes of pediatric leukemia is paving the way for improved clinical outcomes;3,65 however, the biology driving the heterogeneous phenotypes of NUP98r leukemia has not yet been fully characterized. Using genome-wide mutational and transcriptional analyses of a large collective cohort of 185 patients with NUP98r leukemia, we expand on the known association of NUP98r with WT1, FLT3-ITD, and RB1 loss to highlight JAK2, GATA1, and MPL alterations associated with AMKL and NOTCH1 alterations with T-ALL phenotypes. Profiling with scRNAseq and deconvolution of bulk RNAseq data additionally shows heterogeneous cellular hierarchies even with the same fusion partners, suggesting that both fusion partners and cooperating mutations can contribute to the expression profiles and cellular hierarchies.40,42
Despite broadly shared binding to the HOXA/B clusters, NUP98 FO-binding patterns are also unique to each fusion partner, whereas profiling of patient samples and cell lines showed that the binding patterns are not static and could be dynamic according to the differentiation status, represented by AMKL-specific NUP98::KDM5A-binding to MEIS2 or MECOM. These data highlight the importance of using human hematopoietic cells to profile NUP98 FO-binding and epigenetic status to determine their contribution to leukemogenesis. We also found that RB1 deletion and WT1 frameshift mutations could render cells with growth advantages and alter the cellular hierarchies of NUP98r in vitro models in a fusion partner-specific manner. Previously, transplant models of cbCD34 NUP98::KDM5A cells showed heterogeneous disease phenotypes,48 and whether these cooperating mutations can affect phenotypes in vivo is to be investigated. Although acquired mutations in the MEN1 gene have been clinically observed as mechanisms of resistance to menin inhibitors in other AML subtypes,66,67 our in vitro models also suggest that differentiation-specific menin dependency can be a different resistance mechanism for NUP98r leukemia. It will be critical to investigate clinical outcomes of NUP98r AML treated with menin inhibitors according to fusion partners, cooperating mutations, cellular differentiation, and expression profiles to identify patients who will not benefit from menin inhibition, and treat them with alternative approaches.
Mechanistically, RB1 is a repressor of E2F1 proteins, which in turn activate the expression of cell cycle-related genes, including CDKN2 families.56,57 E2F1 has been shown to inhibit normal platelet differentiation, leading to the accumulation of abnormal MKs in E2F1 transgenic mice,59 whereas Cdkn2a-deficient mice showed enhanced platelet production.68 NUP98-FO binding data from cbCD34 models suggest that preferential differentiation toward the megakaryocytic lineage is NUP98::KDM5A-intrinsic, whereas RB1 loss could lead to the accumulation of megakaryocytic cells and ultimately development of AMKL via high E2F activity and CDKN2 family expression. Another consideration is the cell-of-origin of fetal hematopoiesis, which has been shown to affect the AMKL phenotype of CBFA2T3(ETO2)-GLIS2 leukemia.69 Similarly, myelogenic phenotypes of NUP98::NSD1, which are common in adolescents and adults, may be supported preferentially in the adult hematopoietic niche.70 Future studies using mice at the developmental stages or transplant models could dissect the contribution of cell-of-origin (fetal or adult definitive hematopoiesis) or microenvironments (fetal liver or bone marrow) to the NUP98r leukemogenesis, and provide mechanistic insights needed for better treatment for these refractory diseases.
Acknowledgments
The authors thank all the patients and their families at St. Jude Children’s Research Hospital (SJCRH) for their contribution to the biological specimens used in this study. The authors also thank the Biorepository, the Flow Cytometry and Cell Sorting Core, the Center for Applied Bioinformatics, the Center for Advanced Genome Engineering, and the Hartwell Center for Biotechnology at SJCRH for their essential services, which are supported by the National Cancer Institute (NCI), National Institutes of Health (NIH) (P30 CA021765, Cancer Center Support Grant).
This work was funded by the American Lebanese Syrian Associated Charities of SJCRH, and grants from the NCI, NIH (U54 CA243124, Fusion Oncoproteins in Childhood Cancers [FusOnC2] Consortium [C.G.M. and J.M.K.], R35CA197695 [C.G.M.], R01 CA276079 and CA285272 [J.M.K.], and F32 CA261011 and K99 CA283256 [N.L.M.]). J.M.K. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund, and is a previous recipient of the V Foundation Scholar Award (Pediatric). D.D.G. received financial support under the National Recovery and Resilience Plan, Mission 4, Component 2, Investment 1.1, Call for tender No. 1409 published on 14.9.2022 by the Italian Ministry of University and Research (MUR), funded by the European Union-NextGenerationEU, Project Title “Experimental modeling to identify molecular mechanisms and therapeutic vulnerabilities in NUP98-rearranged leukemia,” CUP:J53D23017510001 Grant Assignment Decree No. 1384 adopted on 1 September 2023 by the MUR. C.M. was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) 5×1000 called “Metastatic disease: the key unmet need in oncology” to MYNERVA project, #21267 (Myeloid Neoplasms Research Venture AIRC). R.M. was supported by AIRC (AIRC IG 26039). J.M.B. is a Fellow of The Jane Coffin Childs Fund for Medical Research. F.L. is a recipient of “European Union–Next Generation EU, Mission 4, Component 2, CUP B93D21010860004, National Center for Gene Therapy and Drugs Based on RNA Technology,” “Hub Life Science–Terapia Avanzata PNC-E3-2022-23683269–CUP E83C22006230001 from the Italian Ministry of Health-Piano Nazionale Complementare Ecosistema Innovativo della Salute-cod PNC-E.3.,” and Ministero dell’Università e della Ricerca grants PRIN 2020 and PRIN 2022.
The content does not necessarily represent the official views of the NIH, and is solely the responsibility of the authors.
Authorship
Contribution: J.M.K. and C.G.M. conceptualized the study; J.M.K., M.P.W., G.S., J.M., T.W., P.P., I.I., and C.G.M. collected data; J.M.K., M.U., M.P.W., G.S., J.M., and T.W. curated data; M.U. and J.M. conducted formal analyses and performed statistical tests; M.U., R.H., N.L.M., J.M.B., T.W., M.E.T., B.A., and A.K. performed experiments; M.U. made figures; P.P., C.M., D.D.G., F.L., R.M., S.N.B., M.P., S.M.P.-M., S.P., J.R., H.I., K.P.P., M.J.W., I.I., and C.G.M. provided resources; M.U. and J.M.K. wrote the original draft; and all the coauthors reviewed the draft and were involved in the editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffery M. Klco, St. Jude Children’s Research Hospital, MS 342, D4047B, 262 Danny Thomas Pl, Memphis, TN 38105; email: jeffery.klco@stjude.org.
References
Author notes
The sequencing data from patient samples newly generated in this study (RNA sequencing [RNAseq], n = 10; whole genome sequencing, n = 2; whole exome sequencing, n = 2; single-cell RNAseq [scRNAseq], n = 9; CUT&RUN, n = 64) and undeposited data from previous studies (RNAseq, n = 3) have been deposited in the European Genome-Phenome Archive (EGA), which is hosted by the European Bioinformatics Institute (accession number EGAS00001005760).
Output files from Cell Ranger for scRNAseq data of 9 samples from 8 patients with NUP98r and 4 samples from cord blood CD34+ cell (cbCD34) models in this study were deposited in the Gene Expression Omnibus (GEO) database (accession number GSE287716). Bulk RNAseq data (log2CPM count data) from cbCD34 models were deposited in the GEO database (accession number GSE287297). Processed CUT&RUN data (bigwig files and binding peaks) from primary patient samples and CD34 models were deposited in the GEO database (accession number GSE287298).
Patient samples were deidentified and replaced with unique research identifiers (IDs), which are maintained using an honest broker, in accordance with institutional protocols and ethical guidelines. This unique research ID cannot be linked to patient identification outside of the research team.
Other data generated in this study are available in supplemental Tables or on request to the corresponding author, Jeffery M. Klco (jeffery.klco@stjude.org). Custom code was not used in this study.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal