Abstract
To quantify osseous breakdown in multiple myeloma (MM), monoclonal gammopathy of undetermined significance (MGUS), and benign osteoporosis, we measured urinary levels of pyridinium cross-links of collagen in 50 patients with newly diagnosed and untreated MM, 40 patients with MGUS, 40 untreated patients with osteoporotic vertebral fractures, and 64 healthy adults. Ion-paired, reverse-phase high-performance liquid chromatography (HPLC) was used to measure total urinary excretion of pyridinoline (h-PYD) and deoxypyridinoline (h-DPD). Urinary excretion of free immunoreactive deoxypyridinoline (i-DPD) was determined with an enzyme immunoassay. MM patients had significantly (P < .0001) higher levels of h-PYD, h-DPD, and i-DPD than the healthy adults, patients with MGUS, or patients with osteoporosis. The MGUS and osteoporosis groups presented with elevated (P < .05) levels of urinary pyridinium cross-links when compared with healthy controls. In 20 MM patients who subsequently received chemotherapy, the percent changes in i-DPD did not correlate with the changes in the monoclonal protein. In one of three patients experiencing a transition of initial MGUS into stage I MM, i-DPD increased above the upper limit of the normal range. In 13 patients with stable MGUS, i-DPD remained normal in repeated measurements. Based on the upper limits of the normal range, the sensitivity of urinary pyridinium cross-links in stage I and II MM was low (<50%), but it was between 78% (h-DPD) and 93% (i-DPD) in stage III MM. Specificity in patients with MGUS was between 87% (h-PYD) and 97% (h-DPD). In conclusion, determining the urinary excretion of pyridinium cross-links seems to be a promising noninvasive and thus easily repeatable method for evaluating the actual degree of osseous breakdown. Although measurement of pyridinium cross-link levels is not useful in discriminating patients with MGUS from early-stage myeloma patients, determination of i-DPD levels may contribute importantly to clinical guidance, since increased i-DPD levels seem to identify patients who are particularly likely to benefit from osteoclast-inhibiting drugs such as bisphosphonates. The fact that in a number of patients paraprotein concentrations and i-DPD levels did not change in parallel but instead diverged strongly after chemotherapy might explain the observation that bone lesions sometimes progress even in patients who achieve complete remission.
NEOPLASTIC BONE involvement is the major source of morbidity in multiple myeloma (MM), leading both to diffuse osteopenia (ie, osteoporosis-like changes) and to circumscribed osteolytic lesions. Like other tumor cells, myeloma cells do not directly erode the bone surface, but exert a paracrine stimulation of the host's osteoclasts to resorb bone.1 The osteoclast-activating cytokines that are probably involved in myeloma bone disease are lymphotoxin, interleukin-1, interleukin-6, and macrophage colony-stimulating factor.1,2 Although plain skeletal radiographs, computed tomographic scans, and magnetic resonance images provide a more or less precise picture of the extent of myeloma bone disease, none of them offer information on the actual activity of the bone resorbing process. Especially in cases presenting either with the transition of a monoclonal gammopathy of undetermined significance (MGUS) into a low-stage MM or in asymptomatic MM patients (primarily asymptomatic or in plateau phase following chemotherapy), knowing the intensity of osseous breakdown could be important for precise evaluation of the clinical situation: It could assist in finding the optimal time point for starting or resuming treatment with cytostatic drugs, corticosteroids, or bisphosphonates.3
Bataille et al4 used bone histomorphometry to quantify bone turnover in patients with plasma cell dyscrasias. This approach provides an accurate assessment of osteoblastic and osteoclastic activity, but is tedious and requires special experience and equipment.4 Because of the invasiveness of the method, it cannot be used for multiple evaluations repeated at short intervals during the course of the disease. Moreover, quantitative bone histology reveals increased bone resorption rates only in biopsies from areas invaded by MM cells.5
An alternative and more practical way of assessing bone metabolism is to determine biochemical markers. The pyridinium cross-links of collagen have been established as specific urinary markers of bone resorption.6,7 Pyridinoline and deoxypyridinoline are mature, covalent cross-links of collagen and contribute to the stability of the extracellular matrix of bone. In healthy adults, about 40% of the urinary cross-links are excreted in free form; the remaining 60% are bound to fragments of collagen peptides.8 The standard method for determining urinary pyridinium cross-links is ion-paired high-performance liquid chromatography (HPLC), which may be used to measure both total and free cross-link levels in urine.6,8 Recently, an enzyme immunoassay highly specific for free urinary deoxypyridinoline has been developed, and results obtained with the immunoassay are reported to correlate well with HPLC measurements of total deoxypyridinoline.9,10
In the present investigation, we used both techniques of measuring urinary pyridinium cross-links to quantify the degree of bone resorption in patients with MM, MGUS, and benign osteoporosis. Further, we analyzed the effect on bone resorption of a chemotherapy-induced decrease in tumor mass, and examined the diagnostic validity of urinary pyridinium cross-links in differentiating patients with MGUS and with benign osteoporosis from those with low-stage myeloma.
SUBJECTS AND METHODS
The study was performed as a cross-sectional investigation comparing biochemical markers of bone resorption in normal controls versus patients with MM, MGUS, and osteoporosis. To evaluate urinary markers of bone resorption under conditions as similar as possible to routine practice, we conducted the investigation without dietary restrictions.
Selection of patients with MM and MGUS. Fifty consecutive patients with overt MM and 40 patients with MGUS participated in the investigation (Table 1). Five patients included in the MGUS group actually fulfilled diagnostic criteria for smoldering myeloma: like patients with MGUS, they did not have anemia, renal failure, or osteolytic bone lesions; on the other hand, they presented with a higher plasma cell content (>10% and <30% of nucleated cells) in the bone marrow. All patients with plasma cell disorders were characterized by a monoclonal protein detected by serum or urine electrophoresis and by a serum creatinine concentration of 350 μmol/L or less. The median concentration of the serum monoclonal protein was 29.7 g/L (range, 8.60 to 91.87) in myeloma patients and 14.9 g/L (range, 5.70 to 38.30) in MGUS patients. All patients underwent examination of the skull, spine, and pelvis by plain x-ray. In case of painful regions outside the axial skeleton, screening for neoplastic bone involvement was extended to those areas. Bone biopsies were performed with the Yamshidi needle after blood and urine had been collected to determine biochemical markers of bone metabolism.
Characteristics of the Diagnostic Groups
| Characteristic . | Normal Controls . | MM . | MGUS . | Osteoporosis . |
|---|---|---|---|---|
| No. of subjects | 64 | 50 | 40 | 40 |
| Sex (male/female) | 35/29 | 23/27 | 15/25 | 16/24 |
| Age | ||||
| Median | 65.5 | 67 | 66 | 70 |
| Range | 39-84 | 28-87 | 40-86 | 51-83 |
| Bone disease* (n) | ||||
| None | 64 | 8 | 28 | 0 |
| Osteoporosis | 0 | 11 | 12 | 40 |
| Osteolysis | 0 | 14 | 0 | 0 |
| Osteoporosis and osteolysis | 0 | 17 | 0 | 0 |
| Vertebral fractures | 0 | 24 | 7 | 40 |
| Plasma cell content of the bone marrow (%)† | ||||
| Median | ND | 50 | 5 | ND |
| Range | 5-85 | 0-20 | ||
| Serum creatinine (μmol/L) | ||||
| Median | 88.4 | 88.4 | 79.5 | 88.4 |
| Range | 61.8-150.2 | 36.2-344.7 | 61.8-132.6 | 44.2-150.2 |
| Serum calcium (mmol/L) | ||||
| Median | 2.42 | 2.34 | 2.34 | 2.42 |
| Range | 2.17-2.59 | 1.99-3.52 | 1.93-2.60 | 2.27-2.56 |
| Serum β2-microglobulin (mg/L) | ||||
| Median | 1.50 | 4.25 | 1.95 | 1.60 |
| Range | 1.00-3.30 | 1.40-16.30 | 0.90-6.30 | 1.00-3.90 |
| Characteristic . | Normal Controls . | MM . | MGUS . | Osteoporosis . |
|---|---|---|---|---|
| No. of subjects | 64 | 50 | 40 | 40 |
| Sex (male/female) | 35/29 | 23/27 | 15/25 | 16/24 |
| Age | ||||
| Median | 65.5 | 67 | 66 | 70 |
| Range | 39-84 | 28-87 | 40-86 | 51-83 |
| Bone disease* (n) | ||||
| None | 64 | 8 | 28 | 0 |
| Osteoporosis | 0 | 11 | 12 | 40 |
| Osteolysis | 0 | 14 | 0 | 0 |
| Osteoporosis and osteolysis | 0 | 17 | 0 | 0 |
| Vertebral fractures | 0 | 24 | 7 | 40 |
| Plasma cell content of the bone marrow (%)† | ||||
| Median | ND | 50 | 5 | ND |
| Range | 5-85 | 0-20 | ||
| Serum creatinine (μmol/L) | ||||
| Median | 88.4 | 88.4 | 79.5 | 88.4 |
| Range | 61.8-150.2 | 36.2-344.7 | 61.8-132.6 | 44.2-150.2 |
| Serum calcium (mmol/L) | ||||
| Median | 2.42 | 2.34 | 2.34 | 2.42 |
| Range | 2.17-2.59 | 1.99-3.52 | 1.93-2.60 | 2.27-2.56 |
| Serum β2-microglobulin (mg/L) | ||||
| Median | 1.50 | 4.25 | 1.95 | 1.60 |
| Range | 1.00-3.30 | 1.40-16.30 | 0.90-6.30 | 1.00-3.90 |
Data refer to the evaluation of bone disease by plain radiographs.
Expressed as the percentage of nucleated cells.
The differential diagnosis between MM and MGUS was based on the criteria of Salmon and Cassady,11 and the staging of MM, on the system of Durie and Salmon.12 In patients with MM, 11 had stage I disease, 12 stage II disease, and 27 stage III disease. Eight MM patients had serum creatinine levels of 176.8 μmol/L or greater (substage B). With the exception of one patient who refused cytostatic treatment and three cases with indolent stage I MM, all MM patients received chemotherapy with vincristine, melphalan, cytoxan, and prednisolone (VMCP protocol). Response to chemotherapy was evaluated according to Southwest Oncology Group criteria.11
In the MGUS group, 12 patients (seven females and five males; median age, 70 years; range, 56 to 86) presented with radiologic evidence of osteoporotic bone loss13 (Table 1). Vertebral fractures were demonstrable in six females from this group, as well as a 55-year-old male whose radiographs showed no signs of osteoporotic bone loss: three patients had wedge-shaped vertebrae, two had concave deformities of the superior or inferior surfaces of vertebral bodies, one had a combination of both deformities, and one had a singular flattened vertebral body. On radiographs, the vertebral deformities in female patients appeared typical for benign osteoporosis, although it cannot be excluded that such deformities are an early sign of malignant bone disease. In the male patient, the change in the shape of the vertebral body was explained as the result of an old trauma. In none of the MGUS patients with osteoporosis (with or without vertebral fractures) was hemoglobin less than 130 g/L, serum creatinine greater than 135 μmol/L, or the percentage of plasma cells greater than 15% in the bone marrow. During follow-up study, one male patient with osteoporotic features on baseline radiographs experienced a transition of MGUS into stage I MM (patient no. A).
All plasma cell dyscrasias were studied at the time of primary diagnosis. Patients with MM had not been pretreated with chemotherapy, corticoids, or radiotherapy. Because bisphosphonate treatment results in a rapid decrease of urinary pyridinium cross-links, no patient previously treated with bisphosphonates was included in the study.14
Selection of patients with osteoporosis. The osteoporotic group consisted of 40 consecutive patients with newly diagnosed, untreated osteoporosis (Table 1). These patients had at least one typical vertebral fracture (wedge, compression, or biconcave) and a bone mineral density (determined at Ward's triangle by dual x-ray absorptiometry) greater than 2.5 SD below the age- and sex-matched mean. None had a history of neoplastic disease. Moreover, there was no paraprotein detectable on serum electrophoresis and no other symptoms (eg, anemia or renal failure) indicating plasma cell dyscrasia or neoplastic diseases.
Selection of healthy controls. The normal controls were 64 normocalcemic adults with a bone mineral density within 2 SD of the sex- and age-matched mean. None of them presented with vertebral fractures (as assessed by lateral x-ray of the spine), nor did any have a history of metabolic, neoplastic, or inflammatory disease. None of the controls were taking any medication known to affect bone metabolism, including hormone-replacement therapy, osteotropic vitamins, or calcium supplements.
Written informed consent was obtained from both the healthy controls and the cancer patients before collection of blood or urinary samples. The study was approved by the local ethics committee.
Laboratory parameters. Total urinary pyridinoline and deoxypyridinoline were determined by HPLC.7 In brief, 250 μL urine was hydrolyzed with an equal volume of 12-mol/L HCl at 107°C for 16 hours to convert all urinary cross-links to the peptide-free form. Following partition chromatography on CF1 cellulose, pyridinium cross-links were separated by ion-paired HPLC, and concentrations were determined by fluorometry of the eluant peaks. Standards were derived from sheep bone and were a generous gift from Dr Simon P. Robins (Aberdeen, Scotland). The overall reproducibility of the assay, including the cellulose formation step, was between 8% and 12%.
Laboratory Findings
| Parameter . | Normal Controls (n = 64) . | MM (n = 50) . | MGUS (n = 40) . | Osteoporosis (n = 40) . | Significance . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | MM v MGUS . | MM v Osteo . | MGUS v Osteo . |
| h-PYD (μmol/mol) | |||||||
| Median | 22.9 | 60.5‡ | 28.2† | 28.2* | P < .0001 | P < .0001 | NS |
| Range | 11.0-65.4 | 23.4-773.7 | 11.7-63.6 | 11.4-78.00 | |||
| h-DPD (μmol/mol) | |||||||
| Median | 5.5 | 18.0‡ | 6.45† | 7.8* | P < .0001 | P < .0001 | NS |
| Range | 2.4-15.5 | 5.1-313.9 | 3.4-21.8 | 2.70-21.50 | |||
| i-DPD (μmol/mol) | |||||||
| Median | 4.1 | 10.6‡ | 5.1* | 6.8† | P < .0001 | P < .0001 | NS |
| Range | 1.8-11.4 | 4.4-170.7 | 0.56-8.66 | 2.30-18.7 | |||
| uCa (mol/mol) | |||||||
| Median | 0.34 | 0.45* | 0.35 | 0.42 | NS | NS | NS |
| Range | 0.01-1.29 | 0.01-23.38 | 0.04-3.11 | 0.06-1.30 | |||
| Parameter . | Normal Controls (n = 64) . | MM (n = 50) . | MGUS (n = 40) . | Osteoporosis (n = 40) . | Significance . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | . | . | MM v MGUS . | MM v Osteo . | MGUS v Osteo . |
| h-PYD (μmol/mol) | |||||||
| Median | 22.9 | 60.5‡ | 28.2† | 28.2* | P < .0001 | P < .0001 | NS |
| Range | 11.0-65.4 | 23.4-773.7 | 11.7-63.6 | 11.4-78.00 | |||
| h-DPD (μmol/mol) | |||||||
| Median | 5.5 | 18.0‡ | 6.45† | 7.8* | P < .0001 | P < .0001 | NS |
| Range | 2.4-15.5 | 5.1-313.9 | 3.4-21.8 | 2.70-21.50 | |||
| i-DPD (μmol/mol) | |||||||
| Median | 4.1 | 10.6‡ | 5.1* | 6.8† | P < .0001 | P < .0001 | NS |
| Range | 1.8-11.4 | 4.4-170.7 | 0.56-8.66 | 2.30-18.7 | |||
| uCa (mol/mol) | |||||||
| Median | 0.34 | 0.45* | 0.35 | 0.42 | NS | NS | NS |
| Range | 0.01-1.29 | 0.01-23.38 | 0.04-3.11 | 0.06-1.30 | |||
P < .05, † P < .005, ‡ P < .0001: v normal adults.
Free urinary deoxypyridinoline was also determined with a competitive monoclonal enzyme immunoassay (Pyrilinks-D; Metra Biosystems, Mountain View, CA). The characteristics of this assay have been recently reported by Robins et al.9 The monoclonal antibody has less than 2.5% cross-reactivity with free pyridinoline and 10% cross-reactivity with cross-linked peptides. In our laboratory, intraassay and interassay variations were less than 10% and 15%, respectively.
Urinary concentrations are expressed relative to urinary creatinine levels throughout. In the present report, h-PYD and h-DPD refer to the urinary pyridinoline to creatinine ratio (μmol/mol) and the urinary deoxypyridinoline to creatinine ratio (μmol/mol) measured with HPLC. i-DPD represents the deoxypyridinoline to creatinine ratio (μmol/mol) determined with the immunoassay. In previous reports, h-PYD and h-DPD determined in spot urine correlated highly with the 24-hour excretion of these cross-links.15 Urinary calcium excretion is represented by the calcium to creatinine ratio ([uCa] mol/mol). In our laboratory, the upper limit of the 95% confidence interval determined in our population of normal adults is 50 μmol/mol for h-PYD, 15 μmol/mol for h-DPD, 8 μmol/mol for i-DPD, and 0.5 mol/mol for uCa.16
Statistical procedures. Data are expressed as the median and range. The significance of differences in continuous data was evaluated by nonparametric methods (analysis of variance on ranks and Wilcoxon test for paired samples). The strength of association between variables was assessed by Spearman's rank correlation procedure. The percentage reduction in monoclonal protein and in i-DPD was calculated by dividing the follow-up values by the corresponding baseline values. Calculation of sensitivity (as the ratio of the true positives to the sum of true positives and false negatives) was based on laboratory values measured in patients with stage I and II MM and with stage III MM; calculation of specificity (as the ratio of the true negatives to the sum of true negatives and false positives) was based on results obtained in patients with MGUS and patients with osteoporosis. All P values are two-sided. P less than .05 was considered to indicate significance.
RESULTS
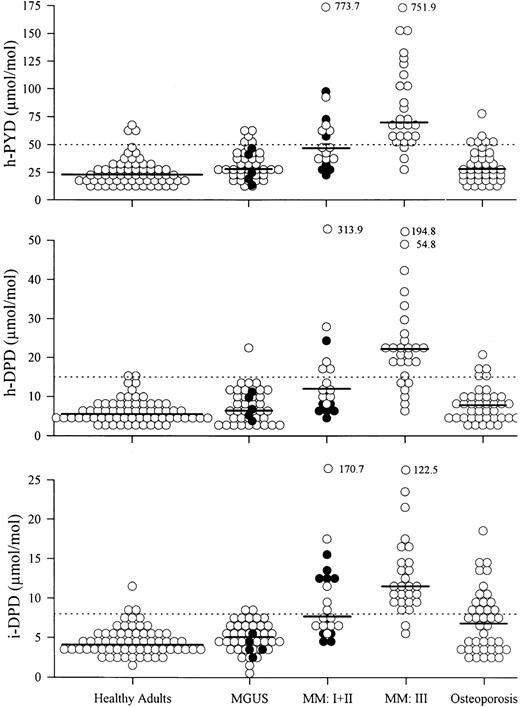
Comparison of healthy controls and patients with MM, MGUS, or osteoporosis. Patients with MM, MGUS, and osteoporosis showed higher median levels of h-PYD, h-DPD, and i-DPD than normal controls (Table 2). Only patients with MM had elevated uCa compared with healthy adults. MM patients showed significantly higher levels of h-PYD, h-DPD, and i-DPD than patients with MGUS or osteoporosis (Table 2 and Fig 1). Among the three groups of patients, there was no statistically significant difference in the levels of uCa. Comparison of the MGUS group and the osteoporosis group yielded no differences in the amount of urinary pyridinium cross-links excreted.
Urinary excretion of pyridinium cross-links in healthy adults and patients with MGUS, MM, and osteoporosis. Patients with smoldering myeloma (•) are included in the MGUS section. MM I + II, MM patients with stage I (•) and stage II (○) disease; MM III, MM patients with stage III disease. (——) Median; (⋅⋅⋅⋅⋅) upper limit of normal range.
Urinary excretion of pyridinium cross-links in healthy adults and patients with MGUS, MM, and osteoporosis. Patients with smoldering myeloma (•) are included in the MGUS section. MM I + II, MM patients with stage I (•) and stage II (○) disease; MM III, MM patients with stage III disease. (——) Median; (⋅⋅⋅⋅⋅) upper limit of normal range.
Effect of the Extent of Bone Disease on Biochemical Markers of Bone Resorption
| Parameter . | MGUS (n = 40) . | Normocalcemic Myeloma . | Hypercalcemic Myeloma . | ||
|---|---|---|---|---|---|
| . | . | Without Evidence of Bone Disease (n = 8) . | Osteoporosis-Like Bone Involvement . | Overt Lytic Bone Lesions (± osteoporosis) . | (n = 8)3-150 . |
| . | . | . | (n = 10) . | (n = 24) . | . |
| Serum calcium (mmol/L) | |||||
| Median | 2.34 | 2.40 | 2.31 | 2.27 | 2.99‡ρ |
| Range | 1.93-2.60 | 2.12-2.45 | 2.04-2.49 | 1.99-2.61 | 2.71-3.52 |
| h-PYD (μmol/mol) | |||||
| Median | 28.2 | 47.93-151 | 50.6‡ | 64.4‡ | 69.7‡ |
| Range | 11.7-63.6 | 23.4-73.0 | 36.1-751.9 | 25.8-773.7 | 48.3-127.8 |
| h-DPD (μmol/mol) | |||||
| Median | 6.45 | 13.43-151 | 11.2‡ | 21.0‡ | 21.8‡ |
| Range | 3.4-21.8 | 6.5-24.7 | 6.3-194.8 | 5.1-313.9 | 12.8-36.4 |
| i-DPD (μmol/mol) | |||||
| Median | 5.1 | 6.93-151 | 10.7‡ | 11.4‡ | 9.9‡ |
| Range | 0.56-8.66 | 4.4-17.7 | 6.7-122.5 | 4.6-170.7 | 8.6-14.7 |
| uCa (mol/mol) | |||||
| Median | 0.35 | 0.32 | 0.44 | 0.43 | 1.1‡ρ |
| Range | 0.04-3.11 | 0.01-0.95 | 0.05-18.44 | 0.08-23.38 | 0.23-2.51 |
| Parameter . | MGUS (n = 40) . | Normocalcemic Myeloma . | Hypercalcemic Myeloma . | ||
|---|---|---|---|---|---|
| . | . | Without Evidence of Bone Disease (n = 8) . | Osteoporosis-Like Bone Involvement . | Overt Lytic Bone Lesions (± osteoporosis) . | (n = 8)3-150 . |
| . | . | . | (n = 10) . | (n = 24) . | . |
| Serum calcium (mmol/L) | |||||
| Median | 2.34 | 2.40 | 2.31 | 2.27 | 2.99‡ρ |
| Range | 1.93-2.60 | 2.12-2.45 | 2.04-2.49 | 1.99-2.61 | 2.71-3.52 |
| h-PYD (μmol/mol) | |||||
| Median | 28.2 | 47.93-151 | 50.6‡ | 64.4‡ | 69.7‡ |
| Range | 11.7-63.6 | 23.4-73.0 | 36.1-751.9 | 25.8-773.7 | 48.3-127.8 |
| h-DPD (μmol/mol) | |||||
| Median | 6.45 | 13.43-151 | 11.2‡ | 21.0‡ | 21.8‡ |
| Range | 3.4-21.8 | 6.5-24.7 | 6.3-194.8 | 5.1-313.9 | 12.8-36.4 |
| i-DPD (μmol/mol) | |||||
| Median | 5.1 | 6.93-151 | 10.7‡ | 11.4‡ | 9.9‡ |
| Range | 0.56-8.66 | 4.4-17.7 | 6.7-122.5 | 4.6-170.7 | 8.6-14.7 |
| uCa (mol/mol) | |||||
| Median | 0.35 | 0.32 | 0.44 | 0.43 | 1.1‡ρ |
| Range | 0.04-3.11 | 0.01-0.95 | 0.05-18.44 | 0.08-23.38 | 0.23-2.51 |
Seven patients had lytic bone lesions and 1 patient had osteoporosis-like bone disease.
P < .025, ‡ P < .0001: v MGUS.
ρ P < .05 v 3 subgroups of normocalcemic myeloma patients.
The levels of h-PYD, h-DPD, i-DPD, and uCa determined in the urine of patients with smoldering myeloma did not differ from those measured in patients fulfilling the diagnostic criteria for MGUS. In five patients with smoldering myeloma (included in the combined MGUS + smoldering myeloma group), the median level of h-PYD was 33.8 μmol/mol (range, 14.6 to 43.6), h-DPD 7.6 μmol/mol (range, 3.9 to 11.6), i-DPD 4.1 (range, 2.7 to 7.9), and uCa 0.3 (range, 0.12 to 0.41).
Effect of the extent of bone disease on biochemical parameters of bone metabolism. MM patients were subdivided into four subgroups (Table 3) according to the extent of bone disease on plain radiographs and the serum calcium level (< or ≥2.7 mmol/L; normocalcemic patients without evidence of bone involvement, normocalcemic patients with an osteoporosis-like appearance of myeloma bone disease, normocalcemic patients with overt lytic bone disease (with or without osteoporosis), and hypercalcemic patients. A comparison of urinary pyridinium cross-links among the four subgroups revealed no differences (Table 3). Each subgroup presented with significantly higher median levels of h-PYD, h-DPD, and i-DPD than patients with MGUS (Table 3). Hypercalcemic MM patients had a significantly higher uCa than patients with MGUS or normocalcemic MM subgroups.
Correlation of laboratory parameters. When data obtained from healthy adults and the three groups of patients were pooled, the closest relationship was observed for h-DPD and h-PYD (r = .89, P < .0001). i-DPD also correlated highly (r = .77, P < .0001) with h-PYD and with h-DPD. We did not find any correlations between urinary pyridinium cross-links and either uCa or serum levels of creatinine and calcium.
In MM patients, serum concentrations of β2-microglobulin correlated (P < .0001) with plasma cell density in the bone marrow (r = .58) and with the serum level of the monoclonal protein (r = .52). No such correlations were found between levels of urinary pyridinium cross-links and either serum β2-microglobulin levels, monoclonal protein concentrations, or bone marrow plasma cell density.
Follow-up study of patients with MGUS and with MM. The median observation time in patients with MGUS was 26 months (range, 3 to 34). Within this period, 29 of 32 patients originally included in this group remained stable. Urine samples were tested repeatedly for i-DPD in 13 MGUS patients with clinically stable disease (median interval between baseline and follow-up test, 25 months; range, 8 to 32) and in three cases with progressive disease. None of the stable MGUS patients showed an increase of i-DPD above the upper limit of the normal range (median initial i-DPD, 4.4 μmol/mol; range, 0.56 to 7.59; median follow-up i-DPD, 5.1 μmol/mol; range, 3.0 to 7.1). In one of three MGUS patients who progressed to stage I MM, a marked increase in i-DPD levels was seen (Table 4). One patient with stage II MM refused cytostatic treatment. Subsequent disease progression in this patient was associated with an increase in i-DPD.
Serial Determinations of i-DPD in Patients With Progressive Disease
| Patient No. . | Initial State of Disease . | Baseline . | Baseline Monoclonal Protein . | Baseline Percentage of Plasma Cells . | Evolved State of Disease . | Follow-up i-DPD . | Follow-up Monoclonal Protein . | Follow-up Percentage of Plasma Cells . | Time Between Baseline and Follow-up Examination . |
|---|---|---|---|---|---|---|---|---|---|
| . | . | i-DPD . | (g/L) . | . | . | (μmol/mol) . | (g/L) . | . | (mo) . |
| . | . | (μmol/mol) . | . | . | . | . | . | . | . |
| A | MGUS | 6.1 | 19.55 | 5% | Stage I MM | 4.0 | 24.33 | 30% | 19 |
| B | MGUS | 4.9 | 27.95 | 5% | Stage I MM4-151 | 4.1 | 35.44 | 20% | 20 |
| C | MGUS | 5.36 | 28.88 | 5% | Stage I MM4-151 | 12.0 | 38.88 | ND | 31 |
| D4-150 | Stage II MM | 7.6 | 45.12 | 70% | Stage III MM‡ | 11.96 | 63.50 | ND | 7 |
| Patient No. . | Initial State of Disease . | Baseline . | Baseline Monoclonal Protein . | Baseline Percentage of Plasma Cells . | Evolved State of Disease . | Follow-up i-DPD . | Follow-up Monoclonal Protein . | Follow-up Percentage of Plasma Cells . | Time Between Baseline and Follow-up Examination . |
|---|---|---|---|---|---|---|---|---|---|
| . | . | i-DPD . | (g/L) . | . | . | (μmol/mol) . | (g/L) . | . | (mo) . |
| . | . | (μmol/mol) . | . | . | . | . | . | . | . |
| A | MGUS | 6.1 | 19.55 | 5% | Stage I MM | 4.0 | 24.33 | 30% | 19 |
| B | MGUS | 4.9 | 27.95 | 5% | Stage I MM4-151 | 4.1 | 35.44 | 20% | 20 |
| C | MGUS | 5.36 | 28.88 | 5% | Stage I MM4-151 | 12.0 | 38.88 | ND | 31 |
| D4-150 | Stage II MM | 7.6 | 45.12 | 70% | Stage III MM‡ | 11.96 | 63.50 | ND | 7 |
Because of the high sensitivity of i-DPD in identifying patients with MM and the close correlations between h-PYD, h-DPD, and i-DPD, only i-DPD levels were determined in follow-up urine samples.
Patient refused cytostatic treatment.
The criterion for the transition of MGUS into stage I MM was a newly diagnosed solitary osteolysis on plain x-ray examination.
The criterion for the transition of stage II MM into III MM was the first radiographic evidence of multiple lytic bone lesions.
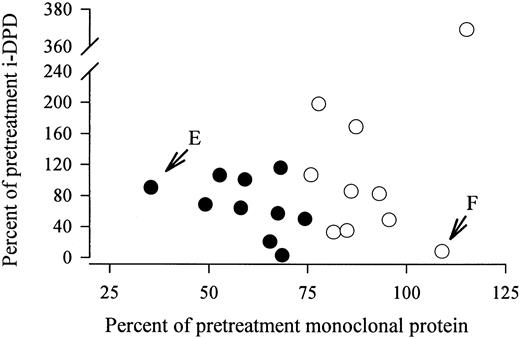
In 20 MM patients receiving chemotherapy, i-DPD excretion was repeatedly determined. None of the patients were treated with bisphosphonates (Table 5). Patients were stratified according to changes in the monoclonal protein: 10 patients improved on chemotherapy and showed a decline in the monoclonal protein of greater than 25%; in the other 10 patients, the monoclonal protein remained stable or increased. A significant decrease in median i-DPD levels was observed in patients responding to chemotherapy, whereas median i-DPD did not change in nonresponders (Table 5). The percent change in the monoclonal protein did not correlate with the percent change in i-DPD (Fig 2). In two patients, an almost complete uncoupling of the course of the monoclonal protein and of i-DPD was observed (Fig 2). Patient no. E showed a decrease in the monoclonal component to 35% of the pretreatment concentration after 10 months of chemotherapy. His i-DPD level remained nearly unchanged (91% of the pretreatment value). Patient no. F presented with an increase in the monoclonal protein to 110% of the initial value (after 5 months of treatment), but she showed a concomitant decrease of i-DPD to 8.3% of the baseline level.
Serial Determinations of i-DPD in Myeloma Patients Receiving Chemotherapy
| Patient Stratification5-150 . | No. of Patients . | Baseline i-DPD . | Follow-up i-DPD . | Time Between Baseline and Follow-up Measurement (mo) . | Significance of Changes in i-DPD . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | (μmol/mol) . | (μmol/mol) . | Median . | Range . | . | ||
| . | . | Median . | Range . | Median . | Range . | . | . | . |
| >25% decrease in the monoclonal protein | 105-151 | 10.9 | 5.7-170.7 | 6.5 | 2.38-21 | 5 | 1-10 | P = .0371 |
| ≤25% decrease or increase in the monoclonal protein | 10 | 8.9 | 6.67-122.5 | 9.8 | 3.0-59.3 | 6 | 1-17 | NS |
| Patient Stratification5-150 . | No. of Patients . | Baseline i-DPD . | Follow-up i-DPD . | Time Between Baseline and Follow-up Measurement (mo) . | Significance of Changes in i-DPD . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | (μmol/mol) . | (μmol/mol) . | Median . | Range . | . | ||
| . | . | Median . | Range . | Median . | Range . | . | . | . |
| >25% decrease in the monoclonal protein | 105-151 | 10.9 | 5.7-170.7 | 6.5 | 2.38-21 | 5 | 1-10 | P = .0371 |
| ≤25% decrease or increase in the monoclonal protein | 10 | 8.9 | 6.67-122.5 | 9.8 | 3.0-59.3 | 6 | 1-17 | NS |
Patients were stratified according to the level of the monoclonal protein at the time of i-DPD measurement.
None of the patients achieved complete remission (monoclonal protein undetectable by immunofixation).
No correlation between the percent change in the monoclonal protein and in i-DPD in 20 MM patients receiving chemotherapy. (•) Patients who improved on chemotherapy, with a decrease in the monoclonal protein of ≥25% of the pretreatment level. (E) Patient no. E, who showed a decrease in the monoclonal component to 35% of the pretreatment concentration after 10 months of chemotherapy. i-DPD remained nearly unchanged (91% of the pretreatment value). (F) Patient no. F, who presented with an increase in the monoclonal protein to 110% of the initial value (after 5 months of treatment) but also showed a concomitant decrease in i-DPD to 8.3% of the baseline level.
No correlation between the percent change in the monoclonal protein and in i-DPD in 20 MM patients receiving chemotherapy. (•) Patients who improved on chemotherapy, with a decrease in the monoclonal protein of ≥25% of the pretreatment level. (E) Patient no. E, who showed a decrease in the monoclonal component to 35% of the pretreatment concentration after 10 months of chemotherapy. i-DPD remained nearly unchanged (91% of the pretreatment value). (F) Patient no. F, who presented with an increase in the monoclonal protein to 110% of the initial value (after 5 months of treatment) but also showed a concomitant decrease in i-DPD to 8.3% of the baseline level.
Diagnostic accuracy. The calculation of sensitivity and specificity was based on cutoff levels referring to the upper limits of the normal range for the individual bone resorption markers (Table 6). Sensitivity of the urinary pyridinium cross-links in stage I and II MM was 41% for h-PYD, 46% for h-DPD, and 48% for i-DPD. For stage III MM, the proportions were between 78% (h-DPD) and 93% (i-DPD). Specificity in patients with MGUS was 87% for h-PYD, 97% for h-DPD, and 95% for i-DPD. In patients with osteoporosis, only 15% had levels of h-PYD and h-DPD above the upper limit of normal (specificity, 85%), whereas 38% of these patients presented with elevated i-DPD levels (specificity, 62%).
Differentiation of Patients With MM From Patients With MGUS or Osteoporosis
| Parameter . | MM . | MGUS (n = 40) . | Osteoporosis (n = 40) . | ||
|---|---|---|---|---|---|
| . | Stage I and II (n = 23) . | Stage III (n = 27) . | All Stages (n = 50) . | . | . |
| h-PYD | |||||
| ≤50 μmol/mol | 59% | 12% | 33% | 87%6-151 | 85%6-151 |
| >50 μmol/mol | 41%6-150 | 88%6-150 | 67%6-150 | 13% | 15% |
| h-DPD | |||||
| ≤15 μmol/mol | 64% | 22% | 40% | 97%6-151 | 85%6-151 |
| >15 μmol/mol | 46%6-150 | 78%6-150 | 60%6-150 | 3% | 15% |
| i-DPD | |||||
| ≤8 μmol/mol | 52% | 7% | 28% | 95%6-151 | 62%6-151 |
| >8 μmol/mol | 48%6-150 | 93%6-150 | 72%6-150 | 5% | 38% |
| Parameter . | MM . | MGUS (n = 40) . | Osteoporosis (n = 40) . | ||
|---|---|---|---|---|---|
| . | Stage I and II (n = 23) . | Stage III (n = 27) . | All Stages (n = 50) . | . | . |
| h-PYD | |||||
| ≤50 μmol/mol | 59% | 12% | 33% | 87%6-151 | 85%6-151 |
| >50 μmol/mol | 41%6-150 | 88%6-150 | 67%6-150 | 13% | 15% |
| h-DPD | |||||
| ≤15 μmol/mol | 64% | 22% | 40% | 97%6-151 | 85%6-151 |
| >15 μmol/mol | 46%6-150 | 78%6-150 | 60%6-150 | 3% | 15% |
| i-DPD | |||||
| ≤8 μmol/mol | 52% | 7% | 28% | 95%6-151 | 62%6-151 |
| >8 μmol/mol | 48%6-150 | 93%6-150 | 72%6-150 | 5% | 38% |
Cutoff levels for the individual markers refer to the upper limit of their 95% confidence interval determined in the collective of healthy controls.
Sensitivity.
Specificity.
DISCUSSION
The present investigation focused on a noninvasive method of measuring bone resorption in plasma cell dyscrasias. MM patients were found to have significantly higher bone resorption rates (as reflected by the amount of pyridinium cross-links of collagen excreted in the urine) than healthy adults, patients with MGUS, and patients with vertebral osteoporosis (Table 2 and Fig 1). Interestingly, the MGUS group was also characterized by higher median levels of h-PYD, h-DPD, and i-DPD than sex- and age-matched healthy controls. This difference persisted even when 12 MGUS patients with radiographic evidence of osteoporotic bone loss were excluded from the analysis (data not shown). As reported previously,7 bone resorption rates in patients with smoldering myeloma did not differ from those in patients fulfilling strict diagnostic criteria for MGUS (Fig 1). Overall, the degree of bone resorption in the combined MGUS–smoldering myeloma group was comparable to that observed in patients with vertebral fractures due to osteoporosis. The enhancement of osteoclast activity and the subsequent increase in biochemical markers of bone resorption appear to represent an early sign of malignant cell behavior.4
In the myeloma group, the extent of bone disease as determined from plain radiographs did not correlate with the amount of pyridinium cross-links excreted in the urine. Patients with overt lytic bone disease did not have higher urinary levels of h-PYD, h-DPD, and i-DPD than patients with osteoporosis-like bone changes or patients without evidence of bone involvement (Table 3). This finding may partially be due to the low sensitivity of plain radiographs in identifying osteolysis.17 On the other hand, it reflects the importance of unbalanced bone remodeling in the development of lytic lesions: as reported by Bataille et al,18 patients presenting with lytic lesions at diagnosis of MM are not characterized by a higher bone resorption rate (compared with MM patients without osteolysis) but by the relatively low activity of bone-forming cells, which cannot compensate for bone loss. With progression of the disease, bone formation is increasingly inhibited. This process leads to the development of lytic lesions also in patients who present initially with osteoporosis-like bone changes or with normal-appearing skeletal radiographs. Similar to what we have already shown in solid tumors,16 hypercalcemic MM patients did not have a higher median bone resorption rate (as reflected by levels of urinary pyridinium cross-links) than normocalcemic MM patients. Thus, besides bone resorption, another factor, ie, decreased renal calcium excretion, must be responsible for the development of hypercalcemia in MM patients. In contrast to solid tumors, myeloma cells do not secrete parathyroid hormone–related protein, a substance that has been shown to enhance calcium resorption in the kidney.19 In MM, the nephrotoxic effects of paraproteins probably lead to the inability of the kidney to excrete calcium in the same amount as it is released into the extracellular fluid by the increased bone resorption.20 The hypercalcinuria in the hypercalcemic MM patients appears to be secondary to the elevation of serum calcium16: with increasing concentrations of serum calcium, the absolute amount of calcium excreted in the urine also increases, while being too low to compensate for calcium input.21 The observation that urinary calcium excretion is more a function of the serum calcium level (and of circulating parathyroid hormone) and less of bone resorption explains why uCa did not differ significantly among patients with MM, MGUS, or benign osteoporosis (Table 2).
The production of osteoclast-activating factors and therefore bone-resorbing capacity vary considerably among tumor cell clones of individual MM patients: in some patients with low tumor mass, bone resorption is extensive, whereas in other patients with high tumor load, only minor bone involvement is detectable.4 Our investigation contributes further to the concept of a rather loose correlation between tumor burden and intensity of bone degradation: Following chemotherapy, diverging changes in the monoclonal protein (which is considered to reflect tumor burden in MM) and in the urinary bone resorption parameter i-DPD were seen. Although patients who presented with greater than a 25% decrease in the monoclonal component showed a significant decrease in median i-DPD (Table 5), the percent change of i-DPD levels in individual patients did not correlate with the decline in the corresponding monoclonal components (Fig 2). In patients unresponsive to chemotherapy, there was similarly no relationship between the percent change in the two parameters. Thus, chemotherapy seems to affect myeloma cell clones in different ways, in some patients leading to a concomitant but not parallel reduction in tumor load and bone-resorbing activity, and in others, probably because of the selection of subclones with a high rate of synthesis of osteoclast-activating factors, to a reduction in tumor mass in combination with a continued high level of bone resorption (patient no. E, Fig 2). In patients with unchanged monoclonal protein but markedly decreased i-DPD (patient no. F, Fig 2), the divergency in the course of the two parameters could be due to a direct inhibitory effect of the glucocorticoids (included in the chemotherapy regimen) on the release of osteoclast-stimulating cytokines by tumor cells or their microenvironment.22 The uncoupling of tumor cell reduction from the inhibition of bone degradation might explain the clinical observation that myeloma bone lesions rarely heal and sometimes even progress in patients who have achieved significant tumor cell reduction, which qualifies them as complete responders.23,24 In the case of persistently elevated urinary pyridinium cross-links despite a successful reduction in tumor burden, the addition of osteoclast-inhibiting compounds, eg, bisphosphonates, to the therapeutic regimen appears reasonable and has already been demonstrated to reduce the skeletal complications of MM.25 With regard to the costs of bisphosphonate treatment, future investigations should evaluate whether bisphosphonate therapy could be restricted to the group of patients whose bone resorption is not normalized after induction chemotherapy.
The overall sensitivity of urinary pyridinium cross-links in patients with MM was between 60% and 72% (Table 6). Subdividing our MM patients according to tumor stage showed that the sensitivity of h-PYD, h-DPD, and i-DPD was uniformly low in patients with stage I and II MM (Table 6). The limited usefulness of these bone resorption markers in identifying patients with low-stage MM was underlined by the fact that only one of three patients in whom initial MGUS transformed into a stage I MM showed an increase in i-DPD (Table 5). The specificity of the three urinary bone resorption markers in patients with MGUS was between 87% and 97%. Thus, depending on the marker used, only 3% to 13% of MGUS patients had elevated levels of urinary pyridinium cross-links (Table 6). In distinguishing MM patients from patients with overt osteoporotic bone loss, measuring total urinary pyridinium cross-link levels by the HPLC method proved superior to the enzyme immunoassay. In contrast to h-PYD and h-DPD, which were highly specific (85%) for excluding patients with osteoporosis, i-DPD levels were elevated in 38% of cases with vertebral osteoporosis. When the mutual relationship of specificity and sensitivity is considered, the higher proportion of osteoporotic patients with increased i-DPD levels indicates that i-DPD was a more sensitive marker for bone loss due to benign osteoporosis than were h-PYD and h-DPD.
In conclusion, determining the urinary excretion of pyridinium cross-links seems to be a promising noninvasive and thus easily repeatable method for evaluating the actual degree of osseous breakdown. Although measurement of pyridinium cross-link levels is not useful in discriminating patients with MGUS from early-stage myeloma patients, determination of i-DPD levels may contribute importantly to clinical guidance, since increased i-DPD levels seem to identify patients who are particularly likely to benefit from osteoclast-inhibiting drugs such as bisphosphonates. The fact that in a number of patients paraprotein concentrations and i-DPD levels did not change in parallel but instead diverged strongly after chemotherapy may explain the observation that bone lesions sometimes progress even in patients who achieve complete remission.
ACKNOWLEDGMENT
We thank A. Millendorfer and Dr G. Nirnberger (Bioconsult) for statistical advice.
Supported by a grant from Österreichisches Forum gegen Krebs.
Address reprint requests to Martin Pecherstorfer, MD, First Department of Medicine and Medical Oncology, Wilhelminenspital, Montleartstrasse 37, A-1171 Vienna, Austria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal