Abstract
Adhesion of platelets to extracellular matrix via von Willebrand factor (vWF) and activation of platelets by thrombin are critical steps in hemostasis. Glycoprotein (GP) V is a component of the GPIb-V-IX complex, the platelet receptor for vWF. GPV is also cleaved by thrombin. Deficiency of GPIb or GPIX results in Bernard-Soulier syndrome (BSS), a bleeding disorder in which platelets are giant and have multiple functional defects. Whether GPV-deficiency might also cause BSS is unknown as are the roles of GPV in platelet-vWF interaction and thrombin signaling. We report that GPV-deficient mice developed normally, had no evidence of spontaneous bleeding, and had tail bleeding times that were not prolonged compared with wild-type mice. GPV-deficient platelets were normal in size and structure as assessed by flow cytometry and electron microscopy. GPV-deficient and wild-type platelets were indistinguishable in botrocetin-mediated platelet agglutination and in their ability to adhere to mouse vWF A1 domain. Platelet aggregation and ATP secretion in response to low and high concentrations of thrombin were not decreased in GPV-deficient platelets compared with wild-type. Our results show that (1) GPV is not necessary for GPIb expression and function in platelets and that GPV deficiency is not likely to be a cause of human BSS and (2) GPV is not necessary for robust thrombin signaling. Whether redundancy accounts for the lack of phenotype of GPV-deficiency or whether GPV serves subtle or as yet unprobed functions in platelets or other cells remains to be determined.
THE ABILITY OF PLATELETS to adhere to sites of vessel injury under conditions of flow is critical for hemostasis.1 The molecular basis for initial platelet adhesion after vessel disruption appears to involve the coordinate actions of 2 sets of adhesive receptors: the glycoprotein (GP) Ib-V-IX complex2,3 and the integrins α2β1 and αΙΙbβ3. The Ib-V-IX complex is composed of 4 related transmembrane GPs, ie, GPIbα, Ibβ, V, and IX, which are associated with each other in a stoichiometry of 2:2:1:2.4,5 Within this complex, Ibα and Ibβ are disulfide linked and noncovalently associated with IX.4,6 GPV is noncovalently associated with GPIb-IX,5 and surface expression of GPV is decreased in GPIb and GPIX deficiency states.7 Approximately 11,000 copies of the GPIb-IX complex are found on the surface of human platelets.4,5 This high density is consistent with its role in cell adhesion—under conditions of shear, the Ib-V-IX complex mediates reversible platelet binding to the subendothelial matrix of the vessel wall by binding to von Willebrand factor (vWF)8via sites on the Ibα chain.9 Subsequent platelet activation triggers integrin activation and irreversible binding to vWF by αΙΙbβ3.
Loss of GPIb-V-IX function causes Bernard-Soulier syndrome (BSS), a severe bleeding disorder.3,7,10 BSS is characterized by abnormal, giant circulating platelets with defective adhesion to vWF and reduced thrombin responsiveness.11 Classic BSS is associated with the loss of the entire Ib-V-IX complex.7Nonclassic presentations have also been reported in which the complex is present in normal amounts but defective in function.12,13 Mutations in the genes encoding both Ib12,13 and IX14 have been shown to cause BSS. GPV has been reported to enhance surface expression of Ib-IX in some heterologous expression systems,15,16 but not others.17 It is not known whether mutations in the gene encoding GPV will affect platelet expression of GPIb-IX and/or cause BSS.
Interestingly, GPV is a substrate for thrombin,18-21 a potent platelet activator. Thrombin binds to GPIb on human platelets,22 perhaps positioning itself to cleave an adjacent GPV molecule. These observations conjured the hypothesis that GPV might contribute to thrombin signaling.23,24 The observation that antibodies that inhibited thrombin cleavage of GPV failed to inhibit platelet activation by thrombin25suggested that GPV cleavage was not necessary for platelet activation by thrombin, and the identification of 3 distinct G protein-coupled receptors (GPCRs) for thrombin provided an alternative explanation of how platelets respond to thrombin.26-29 Nonetheless, the fact that thrombin cleavage site in GPV is conserved in the mouse, rat, and human proteins suggests that this sequence may be important for the structure or function of GPV,30 and available data do not formally exclude a contribution by GPV to thrombin signaling.
To address the role of GPV in vivo, we have generated GPV-deficient mice using gene targeting. GPV null mice developed normally and exhibited no spontaneous bleeding. GPV null platelets were normal in size and shape, responded normally to thrombin, and exhibited wild-type adhesion to mouse vWF A1 domain under shear. Thus, in the mouse, GPV is not necessary for surface expression or function of platelet Ib, for normal platelet cytoskeletal structure, or for normal responsiveness to thrombin. Our results suggest that loss of function mutations in the GPV gene are unlikely to be a cause of human BSS and support recent studies that suggest that platelet thrombin responses of shape change, aggregation, and granule secretion are mediated by protease-activated receptors (PARs).29 31
MATERIALS AND METHODS
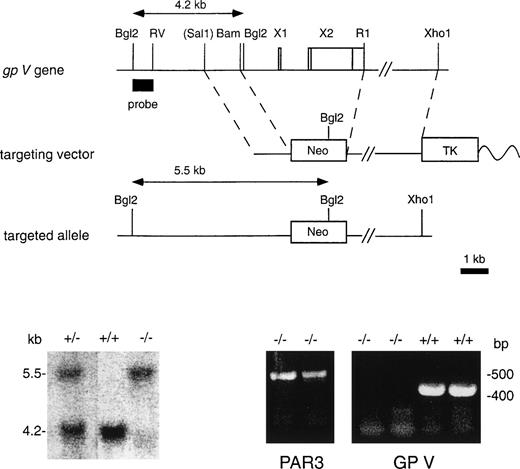
Targeted inactivation of the GpV gene.
Two P1 bacteriophage clones that contained the GpV gene were obtained by polymerase chain reaction (PCR) screen of a mouse genomic library (Genome Systems, St Louis, MO). A 1.3-kb SalI/BamHI fragment 5′ of exon 1 and a 7.0-kbEcoRI/Xho I fragment 3′ of exon 2 were cloned into the pNTK vector32 to create the targeting vector (Fig 1A). The 5′ Sal I site was contributed by the backbone vector (pAd10SacBII). A 0.6-kbBgl II/EcoRV fragment of the GpVgene 5′ of the short arm of homology was used as a probe to identify both the wild-type and targeted alleles (Fig 1A). RF8 ES cells33 (129/SvJae) were electroporated with the targeting construct, and clones resistant to G418 and FIAU were selected and screened by Southern blot. A highly chimeric male mouse derived fromGpV+/− ES cells was bred to C57Bl/6 females to generate approximately 30 F1 GpV+/− mice. All experiments reported here were performed using the F2 offspring of these mice.
Generation of GPV-deficient mice. (A) Gene-targeting strategy. A replacement vector69 was used to substitute a neomycin phosphotransferase expression cassette (Neo) for the entireGpV gene. The wavy line represents plasmid backbone; TK, HSV thymidine kinase expression cassette. X1 and X2 represent exons 1 and 2 of the GpV gene, with the coding region shown as a white box and the 5′ and 3′ untranslated regions shown as shaded boxes. (Sal1) indicates a Sal I restriction endonuclease site found in the P1 bacteriophage vector containing the GpV gene that was used to construct the targeting vector. (B) Southern blot analysis of Bgl2-digested genomic DNA from the tails of pups derived from GpV+/− matings using 5′ flanking probe (A). Targeting removed an endogenous Bgl2 site and introduced a new Bgl2 site. The 4.2- and 5.5-kb bands correspond to wild-type and targeted alleles, respectively. (C) RT-PCR analysis of GpV+/+ andGpV−/− mouse spleen total RNA using GpV (right panel) and Par3g (left panel) primers.
Generation of GPV-deficient mice. (A) Gene-targeting strategy. A replacement vector69 was used to substitute a neomycin phosphotransferase expression cassette (Neo) for the entireGpV gene. The wavy line represents plasmid backbone; TK, HSV thymidine kinase expression cassette. X1 and X2 represent exons 1 and 2 of the GpV gene, with the coding region shown as a white box and the 5′ and 3′ untranslated regions shown as shaded boxes. (Sal1) indicates a Sal I restriction endonuclease site found in the P1 bacteriophage vector containing the GpV gene that was used to construct the targeting vector. (B) Southern blot analysis of Bgl2-digested genomic DNA from the tails of pups derived from GpV+/− matings using 5′ flanking probe (A). Targeting removed an endogenous Bgl2 site and introduced a new Bgl2 site. The 4.2- and 5.5-kb bands correspond to wild-type and targeted alleles, respectively. (C) RT-PCR analysis of GpV+/+ andGpV−/− mouse spleen total RNA using GpV (right panel) and Par3g (left panel) primers.
Reverse transcription-PCR (RT-PCR) of mouse spleen.
Total RNA was obtained from individual mouse spleens using Trizol (GIBCO, Grand Island, NY). Two micrograms of total RNA was used as template to create first-strand cDNA using random hexamer primers and a commercially available kit per the manufacturer's instructions (Superscript; GIBCO). One microliter of the 20 μL reaction was used for PCR amplification of the GpV andPar3ggenes with 2 μmol/L primers as detailed below. ForGpV, the product size was 440 bp, the sense strand primer for PCR was 5′-TGC CTA CGA ACC TCA CAC ACA TC-3′, and the antisense primer for PCR was 5′-GCT TAA CTT GAG CCC CAA GCA G-3′. The conditions used were as follows: 94°C for 4 minutes and 72°C for 1 minute with the addition of Taq; then 94°C for 45 seconds, 60°C for 1 minute, and 72°C for 1 minute for 40 cycles; and then 72°C for 8 minutes. For Par3g, the product size was 511 bp, the sense strand primer for PCR was 5′-TCC TCA CTT GCA TGG GCA TC-3′, and the antisense primer for PCR was 5′-TCT AGG CAG CTA TTC AGG CTC CC-3′. The conditions used were as follows: 94°C for 4 minutes and 72°C for 1 minute with addition of Taq; then 94°C for 45 seconds, 58°C for 1 minute, and 72°C for 1 minute for 45 cycles; and then 72°C for 8 minutes.
Tail bleeding time.
The tail bleeding times of 45 progeny of GPV+/− matings 6 to 7 weeks of age were assayed using the technique of Dejana et al.34 The bleeding time was performed in a blinded fashion before tail cutting for genotyping by Southern blot.
Electron microscopy of murine platelets.
Platelets were fixed in 1.5% glutaraldehyde for 2 hours at 22°C in sodium cacodylate buffer, postfixed in 1% OsO4 in veronal-acetate buffer, stained with aqueous 1% uranyl acetate, dehydrated in ethyl alcohol, infiltrated with propylene oxide, and embedded in Epon (Ted Pella Inc, Redding, CA).
Flow cytometry.
For size analysis, washed platelets were fixed in 1% paraformaldehyde for 20 minutes at 4°C, washed 3 times with platelet buffer (20 mmol/L Tris-HCl, pH 7.4, 140 mmol/L NaCl, 2.5 mmol/L KCl, 1 mmol/L MgCl2, 1 mg/mL glucose, and 0.5% bovine serum albumin [BSA]) and analyzed by flow cytometry.
For measurement of GPIb-IX surface expression, washed platelets were fixed in 0.5% paraformaldehyde for 15 minutes at room temperature and then washed 3 times with phosphate-buffered saline (PBS). Platelets (5 × 107) were incubated in 0.25 mL Tyrode's buffer with BSA plus 1:1,000 (vol/vol) nonimmune rabbit serum or GPIb-IX immune serum (antiserum no. 3584; generously provided by Drs Sylvie Meyer and Beat Steiner, Hoffmann-LaRoche, Basel, Switzerland)35 for 1 hour at 4°C. Platelets were then washed once with PBS, incubated with fluorescein isothiocyanate (FITC)-goat antirabbit monoclonal antibody (Molecular Probes, Sunnyvale, CA) diluted 1:500, and analyzed by flow cytometry.36
Platelet aggregation and secretion.
Blood was collected into citrate buffer from the inferior vena cava of pentobarbital-anesthetized mice. Blood from 3 to 4GPV−/− mice or their wild-type littermates was pooled for each platelet study. Platelet-rich plasma (PRP) was prepared by centrifugation of whole blood at 200g for 7 minutes. EDTA (10 mmol/L) and prostaglandin E1(PGE1; 1 μmol/L) were then added and PRP was centrifuged at 500g for 10 minutes. Platelets were then washed in platelet buffer containing 1 mmol/L EDTA and 1 μmol/L PGE1, collected by centrifugation, resuspended to an OD500 of 1.0 (∼2.5 × 108 platelets/mL) in platelet buffer lacking EDTA and PGE1, and incubated on ice for 30 minutes before use. Aggregation and secretion were measured in a Chrono-Log lumiaggregometer (Havertown, PA). Three hundred microliters of platelet suspension was added to the aggregometer chamber. Luciferase (880 U/mL), luciferin (8 μg/mL), and CaCl2 (1 mmol/L) were then added, and aggregation was followed as change in light transmission with time after addition of agonist. Results were expressed as Δlight transmission, defined as the percentage increase in light transmission over that of the unactivated platelet suspension, with 100% representing light transmission of platelet buffer alone. Platelet ATP secretion was measured as luminescence generated by platelet-released ATP compared with that of an ATP standard. Studies using botrocetin were performed in PRP diluted using platelet-poor plasma (PPP; obtained by centrifugation of the remaining blood at 1,200g for 10 minutes) to an OD500 of 1.0, with 100% light transmission representing light transmission of PPP alone. Botrocetin was obtained as a kind gift from M. Berndt (Victoria, Australia).
Platelet adhesion to murine vWF: Construction of murine vWF-A1 domain expression vector.
A region corresponding to the human vWF-A1 (475-709) was cloned from mouse genomic DNA and used to produce recombinant protein (T. Diacovo, manuscript in preparation). The murine vWF-A1 domain was subcloned into the expression vector pQE9 (Qiagen, Valencia, CA) and expressed in Escherichia coli, and the protein was purified.37 Protein concentrations were determined using the BCA method (Pierce Chemical Co, Rockford, IL). Coomassie-blue staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels showed that greater than 99% of the protein in the prep was vWF-A1 domain.
Purified recombinant mouse vWF-A1 protein (diluted to 100 μg/mL with 10 mmol/L Tris, 150 mmol/L NaCl, pH 7.4) was loaded into microslides (rectangular glass tubes with a cross-section of 300 μm × 30 mm; H.P. Scientific, Inc, Concord, CA) by capillary action and stored overnight at 4°C. Coated microslides were subsequently rinsed and incubated with PBS containing 1% human serum albumin for 30 minutes at 37°C to block nonspecific interactions. Protein-coated microslides were secured to the stage of an inverted phase microscope (Diaphot–TMD; Nikon, Garden City, NJ), and plastic tubing was attached to each end. A uniform wall shear rate was generated by aspirating platelets through the microslide with a syringe pump (Harvard Apparatus, Holliston, MA). For attachment assays, platelets were purified by centrifugation of anticoagulated blood obtained from the retroorbital venous plexus of anesthetized mice. Platelets were washed twice in HEPES buffer (145 mmol/L NaCl, 10 mmol/L HEPES, 0.5 mmol/L Na2HPO4, 5 mmol/L KCl, 2 mmol/L MgCl2, 0.2% BSA, pH 7.4), resuspended at a concentration of 2 × 108/mL, and used within 2 hours. Platelet suspensions were drawn through microslides at shear rates of 50 or 600 s−1 for 5 minutes. The wall shear stress was calculated assuming a Newtonian fluid and a viscosity of 1.0 cP. Attached platelets were observed with phase contrast objectives and quantitated by analysis of videotape images. The number of platelets attached per unit area (0.67 mm2) was quantitated using 4 fields of view for each data point.
RESULTS
Generation of GPV-deficient mice.
The GpV gene was inactivated using a targeting vector that eliminated exons 1 and 2 by homologous recombination (Fig 1A). The targeted allele was detected by Southern blotting using the 5′ flanking probe shown and loss of GPV mRNA was confirmed by RT-PCR of total mouse spleen RNA (Fig 1B and C). Spleen is a hematopoietic organ in the mouse and contains megakaryocytes. GpV mRNA was detected in spleen RNA from wild-type mice but not knockout mice. Par3gmRNA, which is known to be expressed in mouse megakaryocytes,38 was detected in samples from both wild-type and knockout (Fig 1C).
GPV-deficient mice developed normally and exhibited normal hemostasis.
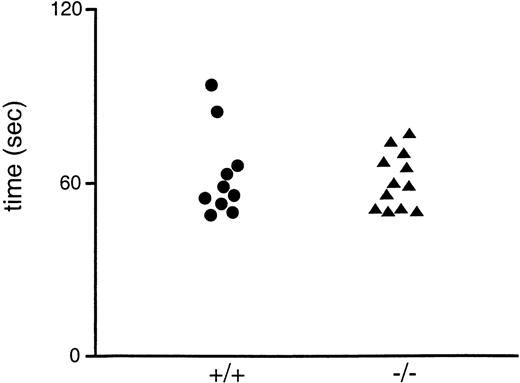
GpV+/− × GpV+/− matings producedGpV−/− offspring at the expected rate (eg, 31 +/+, 77 +/−, and 34 −/−); thus, there is no evidence for loss of GPV-deficient embryos during development. GPV-deficient mice were grossly normal at birth, grew like their wild-type littermates, and were fertile. They showed no evidence of spontaneous bleeding and had hematocrit levels and platelet counts indistinguishable from those of their wild-type littermates (data not shown). To test platelet function in vivo, tail bleeding times were determined on the progeny of heterozygous matings before genotyping (Fig2). GPV-deficient mice had bleeding times indistinguishable from those of their wild-type littermates, consistent with their lack of any overt bleeding.
Tail bleeding times of wild-type and GPV-deficient mice. The bleeding times of 6- to 7-week-old progeny of heterozygote matings were obtained in a blinded manner before genotyping. The results for mice subsequently identified as wild-type (+/+) and GPV-deficient (−/−) are shown.
Tail bleeding times of wild-type and GPV-deficient mice. The bleeding times of 6- to 7-week-old progeny of heterozygote matings were obtained in a blinded manner before genotyping. The results for mice subsequently identified as wild-type (+/+) and GPV-deficient (−/−) are shown.
GPV-deficient platelets were normal in size and structure.
The morphology of platelets from GPV-deficient and wild-type mice was examined by transmission electron microscopy. Electron micrographs of wild-type and GPV-deficient mouse platelets were indistinguishable. In particular, GPV-deficient platelets showed no giantism or cytoskeletal disruption typical of BSS (Fig 3). Granule counts of 300 platelets derived from 3 distinct platelet preparations of each genotype (100 each) were also equivalent: 300 wild-type platelets contained 1,200 granules and 300 GPV-deficient platelets contained 1,174 granules. On average, both wild-type and GPV-deficient platelets had 4.0 α-granules per thin section of platelet.
Transmission electron micrograph of mouse platelets. Two major granule types are present: the -granules, which are the predominant population (a), and the serotonin-containing dense granule (d) population. Some -granules contain small tubules cut in cross-section (A; arrow, and at higher magnification in [B], arrow) that is probably vWF.67 Others contain a regular fibrillar array, which is probably partially polymerized fibrinogen (C; arrowhead). Although the majority of granules are round, some have a tent-like shape (D) and a few are very large (E; denoted by asterisk). The platelets shown are GPV-deficient, but indistinguishable granules were also observed in the platelets of wild-type mice.
Transmission electron micrograph of mouse platelets. Two major granule types are present: the -granules, which are the predominant population (a), and the serotonin-containing dense granule (d) population. Some -granules contain small tubules cut in cross-section (A; arrow, and at higher magnification in [B], arrow) that is probably vWF.67 Others contain a regular fibrillar array, which is probably partially polymerized fibrinogen (C; arrowhead). Although the majority of granules are round, some have a tent-like shape (D) and a few are very large (E; denoted by asterisk). The platelets shown are GPV-deficient, but indistinguishable granules were also observed in the platelets of wild-type mice.
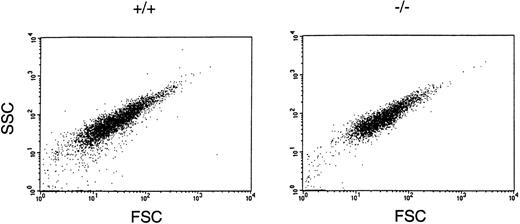
An independent analysis of the size of wild-type and GPV-deficient platelets was obtained by using flow cytometry to measure the forward and side scatter of light (Fig 4). GPV-deficient platelets exhibited the same range of forward and side scatter as that observed for wild-type platelets, confirming the microscopic observation that loss of GPV had no effect on platelet size or shape.
Assessment of platelet size and shape using flow cytometric analysis of wild-type (+/+) and GPV-deficient (−/−) platelets. Washed platelets were fixed and analyzed by flow cytometry for light forward and side scatter. Shown is the analysis of 10,000 platelets for each group.
Assessment of platelet size and shape using flow cytometric analysis of wild-type (+/+) and GPV-deficient (−/−) platelets. Washed platelets were fixed and analyzed by flow cytometry for light forward and side scatter. Shown is the analysis of 10,000 platelets for each group.
Adhesion to vWF was not impaired in GPV-deficient platelets.
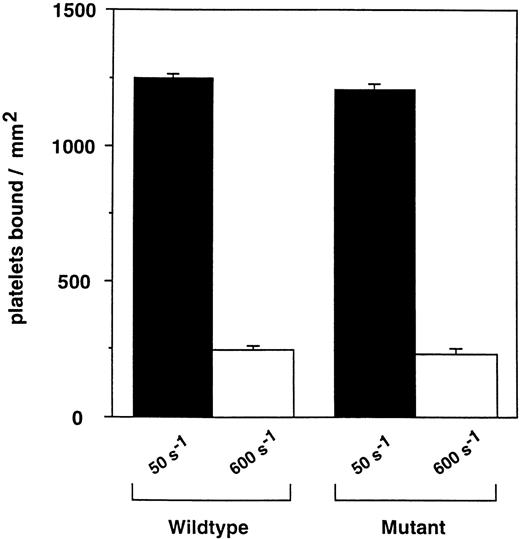
Platelet adhesion to vWF is dependent on GPIb function.8,39GPIb's interaction with vWF is species specific, and mouse GPIb will not bind human vWF.40 To measure the function of the mouse GPIb in wild-type and GPV-deficient mouse platelets, we therefore measured platelet adhesion to immobilized recombinant mouse vWF A1 domain. VWF-A1 domain contains unique sequences that provide a distinct binding site for GPIb.41,42 The importance of this domain with respect to vWF function is evident based on studies of type 2M vWD mutations. Patients with this genotype have mutations within the vWF-A1 domain that result in the impairment of hemostasis, but retain normal vWF multimer structure.43 44 Under conditions of both low and moderate shear, no difference in the steady-state number of adherent platelets was observed between GPV-null and wild-type platelets (Fig 5). Similarly, no difference was noted in the rate at which wild-type and GPV-deficient platelets rolled on the vWF-coated surface (data not shown). As observed for full-length vWF, the ability of recombinant vWF-A1 protein to support platelet adhesion in flow is species specific; murine, but not human, vWF-A1 bound murine platelets under flow conditions (T. Diacovo, manuscript in preparation), and no platelet adherence was noted when surfaces were not coated with vWF (not shown). Thus, GPV-deficient mouse platelets demonstrated no evidence for defective GPIb-vWF interaction in this functional assay.
Accumulation of platelets on immobilized monomeric murine vWF-A1 during flow. Washed murine platelets were infused through recombinant vWF-A1–coated microslides at a shear rate of 50 or 600 s−1. After 5 minutes of continuous flow, adherent platelets were counted in 4 different fields of view. Data are averaged from 2 experiments performed. Error bars represent the standard deviation.
Accumulation of platelets on immobilized monomeric murine vWF-A1 during flow. Washed murine platelets were infused through recombinant vWF-A1–coated microslides at a shear rate of 50 or 600 s−1. After 5 minutes of continuous flow, adherent platelets were counted in 4 different fields of view. Data are averaged from 2 experiments performed. Error bars represent the standard deviation.
Other measures suggest normal GPIb expression in GPV-deficient platelets.
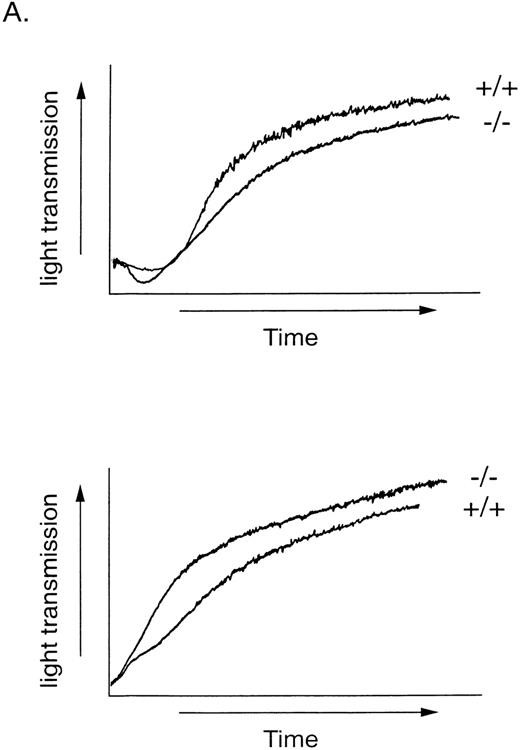
Surface expression of GPIb in wild-type and GPV-deficient platelets was also assessed by 2 other measures. First, botrocetin-mediated platelet agglutination was examined. Like ristocetin, the snake venom-derived botrocetin induces platelet agglutination in PRP by facilitating GPIb-vWF binding,45 but, unlike ristocetin, botrocetin is active on rodent platelets.46 47 Platelets were incubated with 1, 4, or 10 μg/mL botrocetin for 10 minutes with stirring, and agglutination was observed as an increase in light transmission. Representative tracings are shown in Fig 6. The percentages of maximal change in light transmission (0% equals PRP and 100% equals PPP) for wild-type versus GPV-deficient platelets were 5.7 ± 0.6 versus 6.0 ± 1.0 (mean ± SD; n = 3) at 1 μg/mL, 37 ± 6 versus 34 ± 2 at 4 μg/mL (n = 3), and 44 ± 4 versus 47 ± 4 at 10 μg/mL (n = 9), respectively. Thus, botrocetin-induced agglutination of wild-type and GPV-deficient platelets were indistinguishable, suggesting normal vWF binding of platelet GPIb in the absence of GPV.
Platelet agglutination by botrocetin. PRP were stirred and botrocetin was added at a final concentration of 10 (A), 4 (B), or 1 μg/mL (C). Agglutination was measured as the change in light transmission. +/+, wild-type platelets; −/−, GPV-deficient platelets. This experiment was replicated 3 times.
Platelet agglutination by botrocetin. PRP were stirred and botrocetin was added at a final concentration of 10 (A), 4 (B), or 1 μg/mL (C). Agglutination was measured as the change in light transmission. +/+, wild-type platelets; −/−, GPV-deficient platelets. This experiment was replicated 3 times.
Surface expression of GPIb-IX was also measured using a polyclonal antibody to Ib-IX and flow cytometry. No difference in antibody binding was detected in wild-type versus GPV-deficient platelets. For wild-type platelets, the values for nonimmune and immune antibody binding in average fluorescence units (mean ± SD; n = 3) were 10 ± 2 and 88 ± 35, respectively. The cognate values for GPV-deficient platelets were 8 ± 1 and 97 ± 5, respectively. These data suggest normal GPIb-IX expression in the absence of GPV, consistent with the functional data on platelet rolling on vWF-coated surfaces and botrocetin-induced agglutination.
Activation of platelets by thrombin and other agonists.
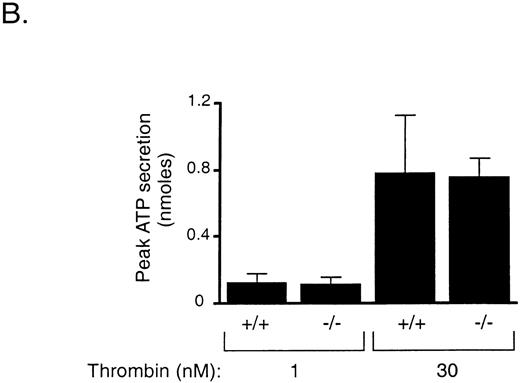
To determine if thrombin cleavage of GPV contributes to activation of platelets by thrombin, we measured shape change, aggregation, and ATP secretion by wild-type and GPV null platelets in response to low (0.5 and 1 nmol/L) and high (30 nmol/L) concentrations of thrombin. Unlike PAR3 null mouse platelets28 or PAR1-inhibited human platelets,29 GPV null platelets responded to 1 nmol/L thrombin like wild-type platelets (Fig 7A). The rate and extent of shape change and aggregation by wild-type and GPV-deficient platelets in response to 0.5 nmol/L thrombin, a concentration close to the threshold for aggregation under these conditions, were also indistinguishable (n = 5 for wild-type and n = 6 for GPV-deficient platelet preparations, data not shown). In addition, the time to half-maximal secretion, a sensitive measure of thrombin signaling in platelets,29,31 did not differ between wild-type and GPV-deficient platelets stimulated with 0.5, 1, or 30 nmol/L thrombin. The mean time to half-maximal secretion in seconds for wild-type and GPV-deficient platelets, respectively, was 53.33 (n = 3) and 54.5 (n = 4) at 0.5 nmol/L thrombin, 47.25 (n = 4) and 50.67 (n = 4) at 1.0 nmol/L thrombin, and 4.8 (n = 6) and 5.0 (n = 6) at 30 nmol/L thrombin. Even at 30 nmol/L thrombin, a concentration of thrombin demonstrated to efficiently cleave GPV,48 there was no detectable difference in the rate or extent of platelet aggregation or ATP secretion (Fig 7 and data not shown). GPV-deficient platelets also responded like wild-type to the thromboxane A2 analog U46619 (10 μmol/L) and to collagen (20 μg/mL, data not shown).
Platelet activation in response to thrombin. (A) Platelet aggregation. Washed wild-type (+/+) and GPV-deficient (−/−) platelets were stirred and exposed to 1 (top) or 30 nmol/L (bottom) thrombin at 0 seconds. Results are representative of 3 experiments. (B) Platelet secretion of ATP. Washed wild-type (+/+) and GPV-deficient (−/−) platelets were exposed to 1 or 30 nmol/L thrombin and the peak ATP secretion was measured by lumiaggregometry. Data represent the mean ± SD of 4 to 6 experiments.
Platelet activation in response to thrombin. (A) Platelet aggregation. Washed wild-type (+/+) and GPV-deficient (−/−) platelets were stirred and exposed to 1 (top) or 30 nmol/L (bottom) thrombin at 0 seconds. Results are representative of 3 experiments. (B) Platelet secretion of ATP. Washed wild-type (+/+) and GPV-deficient (−/−) platelets were exposed to 1 or 30 nmol/L thrombin and the peak ATP secretion was measured by lumiaggregometry. Data represent the mean ± SD of 4 to 6 experiments.
DISCUSSION
Since its identification as a platelet surface GP more than 20 years ago,18 GPV has been hypothesized to play 2 potential roles in hemostasis and thrombosis. It was proposed as a possible platelet thrombin receptor based on its proximity to the GPIb thrombin binding site and its susceptibility to thrombin cleavage.18,23 GPV was also postulated to be necessary for normal expression and function of GPIb based on GPV's association with Ib and IX on the platelet surface49 and its ability to enhance Ib expression in some studies.15,16 These ideas were concordant with the loss of GPV expression and vWF binding and decreased thrombin responses reported in BSS platelets7 50 and raised the important question of whether mutations in the GPV gene itself might cause BSS.
The cloning of GPV showed a type I membrane glycoprotein with 15 extracellular leucine-rich repeats (LRRs), a thrombin cleavage site just amino terminal to the transmembrane domain, and a short intracellular carboxyl tail.20,21 LRRs are also present in the extracellular domains of Ibα,51 Ibβ,52and IX.53 The functions of these presumed protein-protein interaction motifs in GPV and the other members of the Ib-V-IX complex are unknown. They may mediate interactions among Ib, V, and IX or bind to vWF or to as yet unidentified ligands. Especially intriguing is that the thrombin cleavage site of GPV is conserved in the mouse, rat, and human proteins,30 suggesting possible functional importance for this sequence and again raising the possibility that GPV might contribute to thrombin signaling.
To address the importance of GPV for Ib expression and function and for thrombin signaling, we generated GPV-deficient mice. These mice developed normally, were fertile, and had no overt bleeding phenotype. Their platelets showed normal secretion and aggregation responses to thrombin and U46619. They were normal in number and size, exhibiting none of the giant platelet morphology associated with human BSS.11 By contrast, mice lacking Ibα do have giant platelets and spontaneous hemorrhage,54 and vWF-deficient mice have a bleeding diathesis.55 The abnormal size and shape of platelets from patients with BSS were readily detected by flow cytometry56 and electron microscopy.57 These observations suggest that our results with GPV-deficient mice are unlikely to be due to insensitivity of the measurements used or to a species difference in the roles of the Ib-V-IX complex and vWF. Instead, our results show that loss of GPV is not sufficient to disrupt thrombin signaling or GPIb expression or function. The lack of a hemostatic defect and apparently normal adherence to vWF suggest that GPV is not necessary for vWF binding. This finding is consistent with recent studies comparing the function of Ib-IX and Ib-V-IX expressed heterologously.58 The normal number, size, shape, and cytoskeletal morphology of GPV-deficient platelets suggest that GPV is not required for normal thrombopoiesis or for formation of a normal platelet membrane cytoskeleton.59
The observation that disruption of the GpV gene in mouse had no deleterious effect on platelet thrombin responses is consistent with the observation that blocking thrombin cleavage of GPV with antibodies had no effect on the thrombin activation of human platelets25 and clearly demonstrates that GPV is not necessary for thrombin-triggered platelet secretion and aggregation. Indeed, available data suggest that these responses are mediated by G protein-coupled PARs.26,28,29,38,60,61 In human platelets, PAR1 and PAR4 can mediate thrombin signaling, and inhibition of both receptors virtually ablated responsiveness to thrombin.29In mouse platelets, PAR3 and PAR4 mediate thrombin signaling.28,38 Knockout of the G-protein α subunit Gq in mice ablated platelet aggregation and secretion to thrombin,62 which is also consistent with the notion that thrombin signaling is mediated, directly or indirectly, by GPCRs. Interestingly, thrombin-triggered shape change was intact in Gq-deficient platelets. However, shape change was inhibited by blockade of PAR1 and PAR4 in human platelets, and shape change was normal in GPV-deficient platelets. Thus, available data suggest that thrombin triggers platelet shape change through PARs via a G protein other than Gq, probably G12/13.63Indeed, when Gq-deficient platelets were exposed to the PAR4-activating peptide GYPGKF, they underwent rapid shape change but not aggregation or secretion (M.L.K. and S.R.C., unpublished observations). Thus, the lack of an effect of GPV-deficiency on thrombin signaling in platelets is consistent with the model that PARs are the major mediators of this process. Whether known PARs completely account for thrombin signaling in platelets will ultimately be tested in knockout mouse models.
As noted above, the observation that the knockout of Ibα results in a BSS-like phenotype suggests that the Ib-V-IX complex plays similar roles in mouse and human. Failure to observe thrombocytopenia, giant platelets, platelet cytoskeletal abnormalities, or bleeding in GPV-deficient mice suggests that loss of function mutations in the GPV gene is unlikely to be uncovered as a cause for BSS in humans.
As an aside, it is interesting to note that, although our transmission EM studies of mouse platelets did not show differences between wild-type and GPV-deficient mouse platelets, they did show interesting differences between mouse and human platelets. When compared with human platelets,64-67 the α-granules of mouse platelets displayed more variations in shape and content (Fig 3). These variations were present in both wild-type and GPV-deficient mouse platelets.
In summary, our results with GPV-deficient mice provide strong evidence that GPV is not necessary for GPIb function or thrombin signaling and is unlikely to be a cause of BSS. It is possible that GPV plays a subtle or redundant role in the function of the Ib-V-IX complex or in thrombin signaling. Alternatively, GPV may play a role in platelet function not probed in the present study. For example, the LRRs of GPV might bind an as yet unidentified ligand(s), or GPV shed from the cell surface upon cleavage by thrombin or another protease might signal to other cells. Lastly, GPV may play a role in cells other than platelets. Indeed, GPV expression has been reported in human endothelial cells.68 The GPV-deficient mouse provides a critical reagent for probing the role of GPV in endothelial cells and for testing other hypotheses regarding GPV's function as they are generated.
ACKNOWLEDGMENT
The authors thank Violetta Bigornia, Ivy Hsieh, and Martine Morales for their technical assistance and Michael Berndt for his kind gift of botrocetin.
Supported in part by National Institutes of Health (NIH) Grants No. HL44907 and HL59202 and by the Daiichi Research Center, University of California, San Francisco (S.R.C.). M.L.K. was supported by NIH Grant No. HL03731-01.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Shaun R. Coughlin, MD, PhD, CVRI, UCSF, 505 Parnassus Ave, San Francisco, CA 94143-0130; e-mail:shaun_coughlin@quickmail.ucsf.edu.

![Fig. 3. Transmission electron micrograph of mouse platelets. Two major granule types are present: the -granules, which are the predominant population (a), and the serotonin-containing dense granule (d) population. Some -granules contain small tubules cut in cross-section (A; arrow, and at higher magnification in [B], arrow) that is probably vWF.67 Others contain a regular fibrillar array, which is probably partially polymerized fibrinogen (C; arrowhead). Although the majority of granules are round, some have a tent-like shape (D) and a few are very large (E; denoted by asterisk). The platelets shown are GPV-deficient, but indistinguishable granules were also observed in the platelets of wild-type mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/12/10.1182_blood.v94.12.4112/5/m_blod42440003y.jpeg?Expires=1767716067&Signature=vNrxvNGyp4fv9o7fja~WBlLu1UNBTgdrm38fAGaoPzbG4psFgsIqCL0C8NWaig5uK575eeIdvvSgoDG2oN~YAa8noRocUifjRiTSkd181rmwBaWIvL~YF4QyUzcHki-wI35Q1OtElcLSdxxwQcZLEyT-t6BSfuFjtd09S7iX8BHR2lMzxRWNr4WwA0SHVyitrKW4Yi09SJJwUMyDqB~rZIjtta7sS9jnEyyIQxTUwu5qssewFQ5VPB1V3aHjVkpfqiItLJXV2u1sRHy10Hi8sddBLlfqnqU8dIhsc5VDpf8OiRidcBWinSl~fq6YNTW9WKknXtqKOFZcBY8EcNX~Jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal