Recent evidence indicates that human hematopoietic stem cell properties can be found among cells lacking CD34 and lineage commitment markers (CD34−Lin−). A major barrier in the further characterization of human CD34− stem cells is the inability to detect this population using in vitro assays because these cells only demonstrate hematopoietic activity in vivo. Using cell surface markers AC133 and CD7, subfractions were isolated within CD34−CD38−Lin− and CD34+CD38−Lin− cells derived from human cord blood. Although the majority of CD34−CD38−Lin− cells lack AC133 and express CD7, an extremely rare population of AC133+CD7− cells was identified at a frequency of 0.2%. Surprisingly, these AC133+CD7− cells were highly enriched for progenitor activity at a frequency equivalent to purified fractions of CD34+ stem cells, and they were the only subset among the CD34−CD38−Lin− population capable of giving rise to CD34+ cells in defined liquid cultures. Human cells were detected in the bone marrow of non-obese/severe combined immunodeficiency (NOD/SCID) mice 8 weeks after transplantation of ex vivo–cultured AC133+CD7− cells isolated from the CD34−CD38−Lin− population, whereas 400-fold greater numbers of the AC133−CD7− subset had no engraftment ability. These studies provide novel insights into the hierarchical relationship of the human stem cell compartment by identifying a rare population of primitive human CD34− cells that are detectable after transplantation in vivo, enriched for in vitro clonogenic capacity, and capable of differentiation into CD34+ cells.
The human hematopoietic system is sustained by rare pluripotent stem cells that are capable of extensive proliferation and differentiation.1 Utility of human stem cells ranges from gene therapy for the correction of genetic disorders to ex vivo expansion and transplantation for the purposes of hematological recovery and long-term engraftment in patients undergoing aggressive cancer therapy.2-4 Evidence in mice, monkeys, and humans indicates that the most primitive blood stem cells reside in a phenotypically and functionally heterogeneous population of cells having “stem cell” attributes.5-8 Therefore the apex of the hematopoietic hierarchy appears to comprise an array of primitive cells that form the stem cell compartment. The bulk of experimental studies to support this notion comes from purification of various primitive subsets using cell surface markers or DNA stains.9-12 Subpopulations derived from hematopoietic sources can be isolated and subjected to in vitro and in vivo assays that are capable of measuring stem cell function.12 13 The most critical marker used for the isolation of human blood stem cells is the sialomucin CD34, and accordingly, all experimental and clinical protocols involving gene transfer, stem cell transplantation, and expansion are designed for CD34+subsets.
Previous studies in the murine system indicate that some stem cells capable of long-term repopulation do not express detectable levels of cell surface CD34.10 A study by Osawa et al12demonstrated that single murine CD34−Lin− cells could be transplanted into lethally irradiated mice and sustain long-term multilineage engraftment, but they were devoid of short-term repopulation ability. Recently primitive populations of cells with DNA dye efflux properties (“SP cells”) have been isolated from monkey and human hematopoietic tissue.14 These cells do not express cell surface CD34. While monkey-derived SP cells were shown to possess primitive hematopoietic capacity using in vitro assays, human CD34−Lin− cells were unable to demonstrate hematopoietic activity in vitro,14and it remained unclear whether the human CD34−CD38−Lin−cells had any primitive hematopoietic function. Using in vivo transplantation assays, the groups of Zanjani15 and Dick16 demonstrated that a population of CD34−Lin− cells was capable of engrafting non-obese/severe combined immunodeficiency (NOD/SCID) mice and fetal sheep. These studies provided the first and only evidence of hematopoietic activity from CD34−Lin− cells derived from human tissue. From these earlier studies, it has been shown that the CD34−Lin− population is made up of a large number of cells found in human cord blood (CB) and bone marrow (BM). CD34−Lin− stem cells have been hypothesized to be the most primitive blood cells identified to date, and it is surprising that this population comprises a 3- to 4-fold greater number of cells than all other CD34+subfractions combined. However, the frequency of repopulating cells within the CD34−Lin− fraction was shown to be far lower than that found in the CD34+subfraction.16 17 Therefore, the biological function of CD34− stem cells more closely resembles the rare frequency expected of such a primitive cell and may suggest the absence of a sufficiently purified population of human stem cells devoid of CD34.
Using cell surface markers CD7 and AC133,18-20 we identified biologically distinct subsets from human CD34−CD38−Lin−and CD34+CD38−Lin−populations. A unique subset expressing AC133 and lacking CD7 was found at a frequency of 0.2% within the CD34−CD38−Lin−fraction, which contained all of the progenitor capacity previously thought to be deficient in human CD34−stem cells.14 16 Furthermore, using defined in vitro culture conditions, these AC133+CD34−CD38−Lin−cells were capable of acquiring CD34 and possessed a clonogenic progenitor capacity equivalent to primitive CD34+ cells. Human cells were detected in the BM of NOD/SCID mice after transplantation of ex vivo–cultured AC133+CD34−CD38−Lin−cells, which suggests that this population contains primitive repopulating cells. Identification of these cells demonstrates a previously uncharacterized heterogeneity within the human CD34−Lin− population and provides insights into the relationship of CD34− cells to other cells in the human stem cell compartment.
Materials and methods
Human cells
Samples of human CB were obtained from placental and umbilical tissues and diluted 1:3 in Iscove's modified Dulbecco's medium (IMDM) or α–modified Eagle medium (α-MEM) (Gibco Life Technologies, Grand Island, NY). The mononuclear cells were collected by centrifugation (Ficoll-Hypaque, Pharmacia Biotech, Uppsala, Sweden).
Cell purification
AC133 and CD7 subsets were isolated and analyzed from CD34−CD38−Lin−and CD34+CD38−Lin−cells using standard protocols.16 CB cells were first enriched for lineage-depleted (Lin−) cells by negative selection using a cocktail of lineage antibodies and a device similar to that described by the manufacturers (StemSep; Stem Cell Technologies Inc, Vancouver, BC, Canada). These cell fractions were then stained with antihuman CD38 allophycocyanin (APC), antihuman CD34 peridinin chlorophyll protein (Per-CP), antihuman CD7 fluorescein isothiocyanate (FITC) (Becton Dickinson Immunocytometry Systems, San Jose, CA), and anti-hu AC133 phycoerythrin (PE) (Miltenyi Biotech, Bergisch Gladbach, Germany) and then analyzed and sorted on a fluorescence-activated cell sorter (FACS) (FACS Vantage SE, Becton Dickinson). Sorting gates used are indicated in Figure1. Data acquisition and analysis were then performed (Cell Quest software, Becton Dickinson).
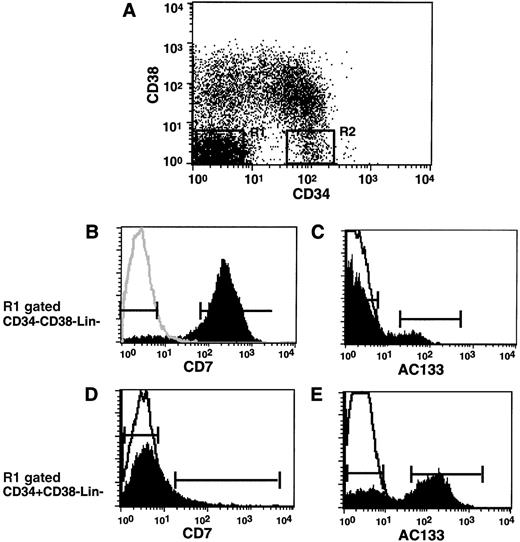
Phenotypic analysis of cell surface AC133 and CD7 on primitive subpopulations of CD34−CD38−Lin− and CD34+CD38−Lin−populations of human CB cells. Representative analysis of cell surface CD34 and CD38 by multiparameter flow cytometry of human CB cells depleted for cells expressing lineage commitment markers (Lin−). (A) Subpopulations of CD34−CD38−Lin−and CD34+CD38−Lin−cells were gated R1 and R2, respectively. Histograms showing the expression of CD7 and AC133 on gated subfractions of (B, C) CD34−CD38−Lin−cells and (D, E) CD34+CD38−Lin− cells. Due to the low number of AC133 cells detected in the CD34−CD38−Lin−fraction, cells shown in (C) are also gated CD7−using (B) markers. (A to E) Markers indicate sorting gates used for the isolation of specified subfractions. Analysis is representative of 4 independent CB samples. A single-line histogram indicates isotype overlay.
Phenotypic analysis of cell surface AC133 and CD7 on primitive subpopulations of CD34−CD38−Lin− and CD34+CD38−Lin−populations of human CB cells. Representative analysis of cell surface CD34 and CD38 by multiparameter flow cytometry of human CB cells depleted for cells expressing lineage commitment markers (Lin−). (A) Subpopulations of CD34−CD38−Lin−and CD34+CD38−Lin−cells were gated R1 and R2, respectively. Histograms showing the expression of CD7 and AC133 on gated subfractions of (B, C) CD34−CD38−Lin−cells and (D, E) CD34+CD38−Lin− cells. Due to the low number of AC133 cells detected in the CD34−CD38−Lin−fraction, cells shown in (C) are also gated CD7−using (B) markers. (A to E) Markers indicate sorting gates used for the isolation of specified subfractions. Analysis is representative of 4 independent CB samples. A single-line histogram indicates isotype overlay.
Clonogenic progenitor assays
Human clonogenic progenitor assays were performed by plating various sorted cell populations at concentrations ranging from 1 × 102 cells to upward of 1 × 103 cells into a methylcellulose cocktail (MethoCult H4434, Stem Cell Technologies) containing 50 ng/mL recombinant human (rH) stem cell factor (SCF), 10 ng/mL rH granulocyte-macrophage–colony-stimulating factor (GM-CSF), 10 ng/mL rH interleukin-3 (IL-3), and 3 units/mL rH erythropoietin. The cocktail was then incubated at 37°C with 5% carbon dioxide (CO2) in a humidified atmosphere. Differential colony counts were scored after 10-14 days by morphological characteristics using an inverted microscope.
Liquid suspension cultures
Sorted cells were incubated in serum-free conditioned BSA, insulin, transferrin (BIT) media (Stem Cell Technologies) that was previously shown to maintain primitive human populations.21Briefly, the serum-free BIT media was supplemented with 10−4 mol/L β-mercaptoethanol (2 mmol/L), L-glutamine, and human growth factors. The growth factor cocktail was used at final concentrations of 300 ng/mL of SCF (Amgen Inc, Thousand Oaks, CA) and Flt-3 (R & D Systems, Minneapolis, MN); 50 ng/mL G-CSF (R&D Systems); and 10 ng/mL of IL-3 and IL-6 (R & D Systems). Cells were cultured in flat- bottomed suspension wells of 96-well plates (Falcon, San Jose, CA) and incubated the indicated times at 37°C and 5% CO2 with 50 μL fresh media. The growth factor cocktail was added to each well every other day.
Transplantation of purified cells into NOD/SCID mice
Cells were transplanted by tail vein injection into sublethally irradiated NOD/LtSz-scid/scid (NOD/SCID) mice (350 rads137 cesium) according to standard protocols.16 The mice were sacrificed 6-8 weeks after transplantation in accordance to local animal welfare protocols, and BM cells were collected from femurs, tibiae, and iliac crests.
Analysis of human cell engraftment
High molecular weight DNA was extracted from BM cells of transplanted mice and digested with EcoR1 restriction enzyme (MBI Fermentus, Flamborough, Ontario, Canada). The percentage of human cells was determined by probing Southern blot analyses with a human chromosome 17–specific α-satellite probe as previously described.16 The level of human cell engraftment was determined by analysis of Southern blots (PhosphoImager; Molecular Dynamics, Sunnyvale, CA) and quantified by using software (Image-Quant; Molecular Dynamic, Sunnyvale, CA) to compare the characteristic 2.7-kilobase (2.7-kb) band with human:mouse DNA mixture controls. This was accomplished by using a lower limit of detection of 0.05% human DNA, which provided a linear signal response. In cases where the level of human cells was less than or equal to 0.05%, polymerase chain reaction (PCR) for the human-specific geneCART-1 was used as shown previously.22 Briefly,CART-1 primers 5′-AAGGATACCACAATAAGCTGC-3′ and 5′-GGTTTGTGGAGACTGGCAC-3′ were used to amplify (Perkin-Elmer 9700; Perkin Elmer, Norwalk, CT) a 156–base pair (156-bp) product from the untranslated region of the humanCART-1 gene at 96°C for 2 minutes followed by 35 cycles at 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 15 seconds at 1 mmol/L magnesium dichloride (MgCl2). In addition, the BM of transplanted mice was analyzed by staining with the human pan-leukocyte marker CD45 to detect the presence of human hematopoietic cells by flow cytometric analysis using a FACS (FACSCalibur, Becton Dickinson) as described below.
Flow cytometric analysis of murine BM
To prepare cells for flow cytometry, contaminating red cells were lysed with a 0.8% ammonium chloride solution, and the remaining cells were washed in phosphate-buffered saline (PBS) containing 5% fetal calf serum (FCS). Approximately 106 cells were resuspended in 1 mL PBS and 5% FCS, washed, and then incubated with monoclonal antibodies (mAbs) at a concentration of 5 μg/mL for 30 minutes at 4°C. The mAb combinations used are indicated in Figure 5B. (CD45 was conjugated to PerCP; CD20 and CD33 were conjugated to FITC; CD38, CD15, and CD19 were conjugated to PE; CD34 was conjugated to APC.) Cells were then washed 3 times in PBS plus 5% FCS and analyzed (FACSCalibur or FACS Vantage SE, Becton Dickinson). For each mouse analyzed, an aliquot of cells was also stained with mouse IgG1 conjugated to FITC, PE, PerCP, and APC as an isotype control.
Results
Phenotypic identification and isolation of subfractions comprising CD34−CD38−Lin− and CD34+CD38−Lin− populations
Mononuclear cells from human CB were enriched for cells that do not express lineage-specific antigens (Lin−) by magnetic depletion of lineage-committed cells stained with a cocktail of mAbs directed against a variety of lymphoid, myeloid, and erythroid antigens.17 Figure 1A shows the analysis of CD34 and CD38 cell surface antigens expressed on a representative Lin− population measured by flow cytometry. Subpopulations of interest were gated R1 and R2 for the analysis of CD34−CD38−Lin−and CD34+CD38−Lin−cells, respectively. Approximately 96% of the CD34−CD38−Lin−population (gated R1), previously shown to contain no progenitor cell activity in vitro but capable of modest repopulation in NOD/SCID mice, was found to express cell surface CD7 (Figure 1B, Table1). Although most of the CD34−CD38−Lin−cells did not express AC133, a very rare population of cells within the CD34−CD38−Lin−fraction that did not express CD7 were found to be AC133+(Figure 1C).
Frequency of subpopulations within CD34−CD38−Lin− and CD34+CD38−Lin− cells based on cell surface AC133 and CD7 expression
| Subpopulation Phenotype . | CD34−CD38−Lin− (%) . | CD34+CD38−Lin− (%) . |
|---|---|---|
| CD7− | 4.2 ± 3.8 | 92 ± 8.1 |
| CD7+ | 96 ± 4.1 | 8.7 ± 3.8 |
| AC133− | 98.8 ± 0.9 | 11.2 ± 7.1 |
| AC133+ | 0.2 ± 0.2 | 88.4 ± 12.4 |
| Subpopulation Phenotype . | CD34−CD38−Lin− (%) . | CD34+CD38−Lin− (%) . |
|---|---|---|
| CD7− | 4.2 ± 3.8 | 92 ± 8.1 |
| CD7+ | 96 ± 4.1 | 8.7 ± 3.8 |
| AC133− | 98.8 ± 0.9 | 11.2 ± 7.1 |
| AC133+ | 0.2 ± 0.2 | 88.4 ± 12.4 |
CD34+CD38−Lin− cells have been shown to be highly enriched for colony-forming cells (CFCs); contain SCID repopulating cells (SRCs), which are capable of multilineage repopulation in NOD/SCID mice; and comprise only 5%-8% of the total CD34+ population.21 23 A low frequency of CD34+CD38−Lin− cells (gated R2) were found to express CD7 on the cell surface (Figure 1D, Table 1), whereas analysis of AC133 expression demonstrated 2 distinct populations of AC133+ and AC133− cells (Figure 1E). AC133+ and AC133− cells represent previously unidentified subfractions within the highly purified population of CD34−CD38−Lin−and CD34+CD38−Lin−cells that have yet to be characterized in functional assays. A summary of the relative content and frequency of both AC133 and CD7 subpopulations within both CD34−CD38−Lin−and CD34+CD38−Lin−cells are shown in Table 1. AC133 and CD7 are therefore capable of distinguishing previously unknown phenotypic heterogeneity within human subpopulations of both CD34−CD38−Lin−and CD34+CD38−Lin−cells.
Clonogenic progenitor capacity of CD34−CD38−Lin− and CD34+CD38−Lin− subpopulations
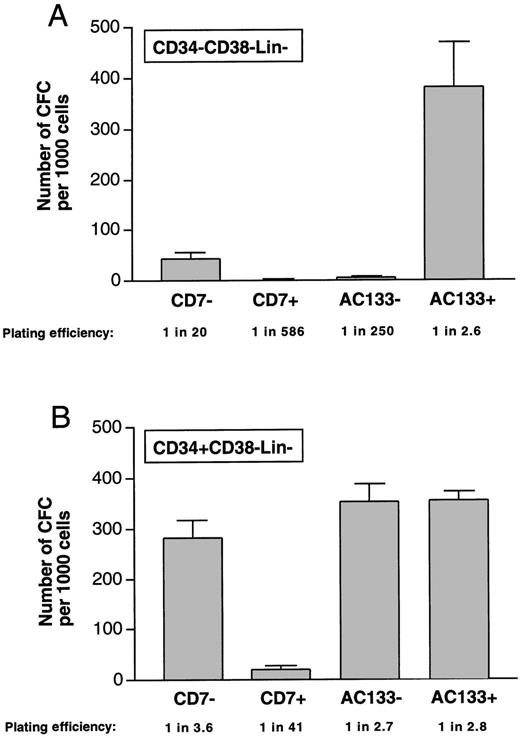
Hematopoietic assays that detect CFCs were used as a measure of primitive progenitor cell function. Novel subpopulations identified within both CD34−CD38−Lin−and CD34+CD38−Lin−fractions were isolated based on the absence or presence of detectable cell surface CD7 or AC133 expression using sorting gates as indicated in Figure 1. Sorted populations were reanalyzed to assess purity, which was found to be greater than 98% (data not shown). The CD7+ and AC133− subfractions isolated from CD34−CD38−Lin−cells had little to no detectable progenitor capacity (Figure2A), which was similar to previous studies using the CD34−CD38−Lin−fraction purified from human sources of hematopoietic tissue.14 16 It is important to note that more than 99% of the cells within the CD34−CD38−Lin−fraction are AC133−CD7+ (Table 1). CD34−CD38−Lin−cells that lack CD7 cell surface expression were capable of producing all types of CFCs at frequencies ranging from 40-60 colonies per 1000 cells (Figure 2A, Table 2). The frequency of colony types from all subpopulations assayed for progenitor content in Figure 2A and B are shown in Table 2. The population of even rarer AC133+ cells within the CD34−CD38−Lin−fraction (Table 1) could be isolated for functional analysis, but only 30-800 cells could be obtained from as much as 75 mL of whole cord blood.
Clonogenic progenitor cell capacity of subfractions isolated from CD34−CD38−Lin− and CD34+CD38−Lin−populations. CFC capacity was assessed in subfractions isolated from (A) CD34−CD38−Lin−cells and (B) CD34V+CD38−Lin− cells based on the absence or presence of CD7 and AC133 and represented as the number of CFCs per 1000 purified cells. Sorting gates used for isolation of subfractions are indicated in Figure 1 (A to E). Values are the mean and the SEM of determinations from up to 4 separate CB samples. Plating efficiencies were calculated by dividing the number of cells plated by the number of CFCs detected.
Clonogenic progenitor cell capacity of subfractions isolated from CD34−CD38−Lin− and CD34+CD38−Lin−populations. CFC capacity was assessed in subfractions isolated from (A) CD34−CD38−Lin−cells and (B) CD34V+CD38−Lin− cells based on the absence or presence of CD7 and AC133 and represented as the number of CFCs per 1000 purified cells. Sorting gates used for isolation of subfractions are indicated in Figure 1 (A to E). Values are the mean and the SEM of determinations from up to 4 separate CB samples. Plating efficiencies were calculated by dividing the number of cells plated by the number of CFCs detected.
Percentage of clonogenic progenitors within subpopulations of CD34−CD38−Lin− and CD34+CD38−Lin− cells
| Subpopulation Phenotype . | CFU-M, % . | CFU-G, % . | CFU-GM, % . | BFU-E, % . | CFU-GEMM, % . |
|---|---|---|---|---|---|
| 34−38−7− | 4.3 ± 1.7 | 25 ± 7.5 | 0.5 ± 0.5 | 65 ± 9.5 | 5.2 ± 0.9 |
| 34−38−7+ | 1.3 ± 1.3 | 25.5 ± 17.5 | 0 | 73 ± 16 | 0 |
| 34+38−7− | 12.3 ± 2.9 | 43.5 ± 10.4 | 3.3 ± 1.8 | 36.8 ± 9.5 | 4 ± 2 |
| 34+38−7+ | 20.1 ± 13.5 | 53.6 ± 15.6 | 0 | 20.4 ± 11.9 | 5.6 ± 3.2 |
| 34−38−AC133− | 1.4 ± 1.4 | 45.6 ± 37.4 | 0 | 52.1 ± 35.5 | 0.7 ± 0.7 |
| 34−38−AC133+ | 2.9 ± 1.5 | 36.2 ± 6.5 | 1.2 ± 1.2 | 54.3 ± 8.3 | 5.2 ± 3.6 |
| 34+38−AC133− | 2.6 ± 1.6 | 31.5 ± 5.3 | 0.4 ± 0.4 | 61.4 ± 8.3* | 3.8 ± 1.1 |
| 34+38−AC133+ | 11.7 ± 0.5 | 66 ± 5.7 | 2.5 ± 2.0 | 18.4 ± 3.0 | 1.7 ± 1.3 |
| Subpopulation Phenotype . | CFU-M, % . | CFU-G, % . | CFU-GM, % . | BFU-E, % . | CFU-GEMM, % . |
|---|---|---|---|---|---|
| 34−38−7− | 4.3 ± 1.7 | 25 ± 7.5 | 0.5 ± 0.5 | 65 ± 9.5 | 5.2 ± 0.9 |
| 34−38−7+ | 1.3 ± 1.3 | 25.5 ± 17.5 | 0 | 73 ± 16 | 0 |
| 34+38−7− | 12.3 ± 2.9 | 43.5 ± 10.4 | 3.3 ± 1.8 | 36.8 ± 9.5 | 4 ± 2 |
| 34+38−7+ | 20.1 ± 13.5 | 53.6 ± 15.6 | 0 | 20.4 ± 11.9 | 5.6 ± 3.2 |
| 34−38−AC133− | 1.4 ± 1.4 | 45.6 ± 37.4 | 0 | 52.1 ± 35.5 | 0.7 ± 0.7 |
| 34−38−AC133+ | 2.9 ± 1.5 | 36.2 ± 6.5 | 1.2 ± 1.2 | 54.3 ± 8.3 | 5.2 ± 3.6 |
| 34+38−AC133− | 2.6 ± 1.6 | 31.5 ± 5.3 | 0.4 ± 0.4 | 61.4 ± 8.3* | 3.8 ± 1.1 |
| 34+38−AC133+ | 11.7 ± 0.5 | 66 ± 5.7 | 2.5 ± 2.0 | 18.4 ± 3.0 | 1.7 ± 1.3 |
CFU-M indicates CFU-macrophage; CFU-G, CFU-granulocyte; CFU-GM, CFU-granulocyte, macrophage; BFU-E, burst forming unit–erythroid; and CFU-GEMM, CFU-granulocytic, erythroid, macrophage, megakaryocytic. The percentage of each colony type is calculated as the mean ± SEM (standard error of the mean) (n = 2-4).
Progenitor content was measured by enumerating the colony forming units (CFUs) from equal numbers of phenotypically distinct subpopulations isolated from lineage-depleted samples of human CB. The number of each type of CFU was determined from plating conditions outlined in “Materials and methods” and identified according to standard protocols.
Despite the low frequency of AC133+CD34−CD38−Lin−cells, this subset demonstrated CFC capacity equal to that of CD34+CD38−Lin− cells (Figure 2A and B). As many as 400 CFCs were detectable per 1000 AC133+CD34−CD38−Lin−cells, thereby providing evidence for the identification of a rare cell population that is functionally distinct from the remaining CD34−CD38−Lin−cells. Previous studies indicate that isolation of CD34+CD38−Lin− cells allow for the enrichment of CFCs to a frequency of 1 in 4.17,21 All subpopulations within the CD34+CD38−Lin−population demonstrated CFC capacity of multiple myeloid and erythroid lineages, although cells expressing CD7 showed a lower frequency of progenitors (Figure 2B, Table 2). CD34+CD38−Lin− cells lacking CD7 expression were able to generate progenitors at levels previously demonstrated by the CD34+CD38−Lin−fraction.21 Isolated subpopulations of either AC133− or AC133+ within the CD34+CD38−Lin− fraction (Figure 1E) were found to have equivalent CFC capacity (Figure 2B). There was no significant difference in the type of CFCs produced between these subpopulations, with one exception: in erythroid progenitors, AC133 allows for discrimination of this lineage in the AC133 subset (Table 2). With the exception of the CD7+subfraction, all other subsets of the CD34+CD38−Lin−population had similar CFC capacity. Consistent with recent studies, all subsets within the CD34−CD38−Lin−population displayed poor progenitor capacity in vitro, with the exception of the CD34−CD38−Lin−cells, which express AC133. AC133+CD34−CD38−Lin−cells therefore represent a unique population of human CD34− cells, which are enriched for primitive hematopoietic activity.
Developmental capacity of subsets isolated from the CD34−CD38−Lin− population
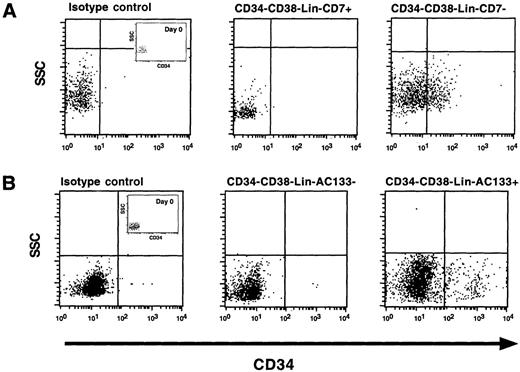
We further characterized the developmental capacity of AC133 and CD7 subsets isolated from the CD34−CD38−Lin−fraction. Using defined in vitro culture conditions previously shown to support CD34+CD38−Lin−cells,21 we cultured subsets of AC133+ and AC133− cells and CD7+ and CD7− cells for 3 days in these serum-free conditions. Cell surface phenotype and side scatter properties of subpopulations prior to culture are indicated as Day 0 (inset with isotype control, Figure 3). Responsiveness to serum-free culture conditions was also assessed by visual inspection and defined by changes in cell number or side scatter properties. CD7+cells, which represent the majority of this fraction, remained unresponsive and showed no signs of proliferation (Figure 3A). In contrast, the CD7− subfraction was capable of both acquiring CD34 and demonstrating mild proliferation in response to in vitro culture (Figure 3A). Similar to CD7+ cells, AC133− cells derived from the CD34−CD38−Lin−population did not respond to in vitro culture and were not capable of acquiring CD34 surface expression (Figure 3B). However, AC133+CD34−CD38−Lin−cells possessed the capacity to acquire cell-surface CD34 in vitro (Figure 3B) and demonstrated a proliferative response by increasing the total cell number by 3- to 4-fold after 3 days (data not shown). These experiments suggest that the developmental capacity of AC133+CD34−CD38−Lin−cells is biologically unique within the CD34−CD38−Lin−population and contains the primitive precursors capable of differentiation into CD34+ cells in vitro.
Analysis of CD34 expression of purified subpopulations after in vitro culture for 3 days. A representative experiment (n = 5) of CD34 cell surface expression performed on subpopulations of cells isolated from the CD34−CD38−Lin−population based on the absence or presence of (A) CD7 or (B) AC133 cell surface expression. The entire contents of individual wells was collected after 3 days (2000-10 000 cells), washed, stained with mAbs against CD34, and analyzed using flow cytometric analysis. Cell surface phenotypes of subpopulations prior to culture are indicated as Day 0 (A, B) within isotype control dot plots.
Analysis of CD34 expression of purified subpopulations after in vitro culture for 3 days. A representative experiment (n = 5) of CD34 cell surface expression performed on subpopulations of cells isolated from the CD34−CD38−Lin−population based on the absence or presence of (A) CD7 or (B) AC133 cell surface expression. The entire contents of individual wells was collected after 3 days (2000-10 000 cells), washed, stained with mAbs against CD34, and analyzed using flow cytometric analysis. Cell surface phenotypes of subpopulations prior to culture are indicated as Day 0 (A, B) within isotype control dot plots.
Transplantation of AC133+ and AC133−cells from both CD34−CD38−Lin− and CD34+CD38−Lin− subsets into NOD/SCID mice
Primitive cells (SRCs) capable of repopulating NOD/SCID mice have previously been shown to be enriched in the highly purified population of CD34−CD38−Lin−and CD34+CD38−Lin−cells from both human CB and BM, termed CD34−SRCs and CD34+SRCs, respectively.17,21,23 Other stem cell–associated markers, such as Thy-1, human leukocyte antigen-DR (HLA-DR), and c-kit, have been used for the selection of subpopulations to further characterize and isolate primitive cells within these fractions. But the markers are unable to identify functionally distinct subsets within these fractions.8,24 25 AC133+ and AC133− cells were isolated from the CD34−CD38−Lin−population and cultured in defined serum-free media previously shown to enhance repopulating capacity in NOD/SCID mice. The level of human cell engraftment was determined by analysis of DNA extracted from the BM cells of recipient mice 8 weeks after intravenous transplantation of cell fractions using PCR analysis for the human-specific gene sequenceCART-1.
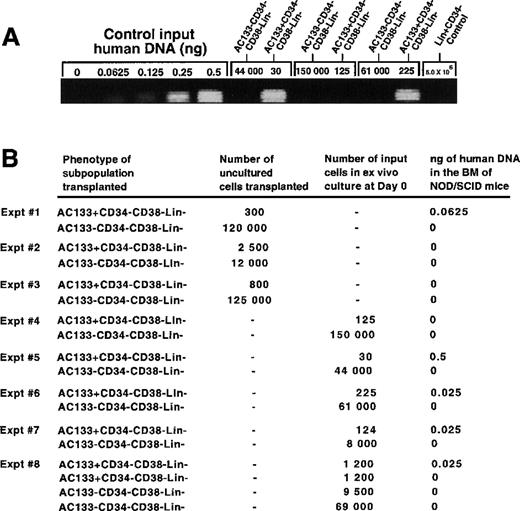
Figure 4A shows the results of PCR using human-mouse mixtures of DNA as controls. Indicated amounts of human DNA were added to PCR reactions containing 200 ng of genomic DNA extracted from the BM of nontransplanted NOD/SCID mice. This technique allowed for a linear signal at lower levels of detection compared with Southern blot analysis used previously, which detected 0.05% human cells, thereby extending the lower limit of detection for human cells (Figure 4A). As noted previously (Table 1), the proportion of AC133+ cells within the CD34−CD38−Lin−fraction is relatively low, and a range of only 30-800 cells could be isolated from a single CB sample. Accordingly, in some cases individual CB samples were pooled to obtain a greater number of cells, and the average number of attainable cells can be estimated by multiplying the range of AC133+CD34−CD38−Lin−cells that can be isolated from a single CB sample (30-800 cells) by the total number of samples pooled. Individual and pooled human CB samples of 50-200 mL were used to purify AC133+ and AC133− subsets from the CD34−CD38−Lin−population, and the total cells attainable were either transplanted immediately after isolation (experiments 1 to 3, Figure 4B) or cultured in vitro for 3 days (experiments 4 to 8, Figure 4B) prior to intravenous transplantation into NOD/SCID mice.
Transplantation into NOD/SCID mice of ex vivo cultured subsets of AC133+and AC133− cells isolated from the CD34−CD38−Lin−population. (A) Human-mouse DNA mixtures were used for PCR amplification of CART-1 human-specific gene sequence. A total of 200 ng of genomic DNA from BM cells of nontransplanted control NOD/SCID mice was mixed with equal volumes of serially diluted (0, 0.0625, 0.125, 0.25, and 0.5 ng) human genomic DNA. There were no detectable PCR products in the absence of human DNA, indicating the specificity of the PCR reaction to human sequence. However, increased amounts of human DNA allowed for the detection of a linearly increasing signal. PCR reactions of the human-mouse DNA mixture were compared to 200 ng DNA extracted from NOD/SCID mice transplanted with ex vivo–cultured cell populations as indicated. (B) Summary of the level of human cell engraftment detectable in NOD/SCID mice transplanted with AC133+ and AC133− subfractions isolated from the CD34−CD38−Lin−populations in 8 independent experiments. Experiments 1 to 3 represent transplantation of NOD/SCID with uncultured de novo isolated subfractions, whereas experiments 4-8 represent results from mice transplanted with subfractions cultured for 3 days in serum-free media. Experiments 2, 3, and 8 represent transplantation of cells isolated from 3 pooled CB samples.
Transplantation into NOD/SCID mice of ex vivo cultured subsets of AC133+and AC133− cells isolated from the CD34−CD38−Lin−population. (A) Human-mouse DNA mixtures were used for PCR amplification of CART-1 human-specific gene sequence. A total of 200 ng of genomic DNA from BM cells of nontransplanted control NOD/SCID mice was mixed with equal volumes of serially diluted (0, 0.0625, 0.125, 0.25, and 0.5 ng) human genomic DNA. There were no detectable PCR products in the absence of human DNA, indicating the specificity of the PCR reaction to human sequence. However, increased amounts of human DNA allowed for the detection of a linearly increasing signal. PCR reactions of the human-mouse DNA mixture were compared to 200 ng DNA extracted from NOD/SCID mice transplanted with ex vivo–cultured cell populations as indicated. (B) Summary of the level of human cell engraftment detectable in NOD/SCID mice transplanted with AC133+ and AC133− subfractions isolated from the CD34−CD38−Lin−populations in 8 independent experiments. Experiments 1 to 3 represent transplantation of NOD/SCID with uncultured de novo isolated subfractions, whereas experiments 4-8 represent results from mice transplanted with subfractions cultured for 3 days in serum-free media. Experiments 2, 3, and 8 represent transplantation of cells isolated from 3 pooled CB samples.
In 3 experiments, de novo isolation of AC133+ or AC133− subsets demonstrated that only 1 out of 3 NOD/SCID recipient mice contained human cells when transplanted with the AC133+ subfraction, whereas there were no human cells detected in animals transplanted with the AC133−cells (Figure 4B). However, after ex vivo culture, as few as 30 AC133+CD34−CD38−Lin−human cells were detected in the BM of 4 out of 6 NOD/SCID mice, whereas 44 000 and 150 000 AC133−CD34−CD38−Lin−transplanted cells were not detectable (Figure 4A and B). A representative analysis of 3 experiments is shown adjacent to human-mouse mixtures (Figure 4A) for mice transplanted with cultured AC133− and AC133+ subfractions. Transplantation of 5 × 106CD34−Lin+ control carrier cells was not detectable in the BM of recipient mice (Figure 4A). Based on an average of 80 × 106 cells comprising the total murine BM and molecular weight of human genomic DNA per cell, it can be estimated that our level of detection represents an average of 15 000 human cells in the BM of transplanted mice. This estimate suggests that AC133+CD34−CD38−Lin−cells are capable of a significant level of cellular expansion and survival over an 8-week period in vivo as compared with 400 times the number of transplanted AC133−CD34−CD38−Lin−cells that were not detectable. Taken together, these experiments demonstrate that rare AC133+ cells possess in vivo capacity which is distinct from the bulk population of CD34−CD38−Lin−cells.
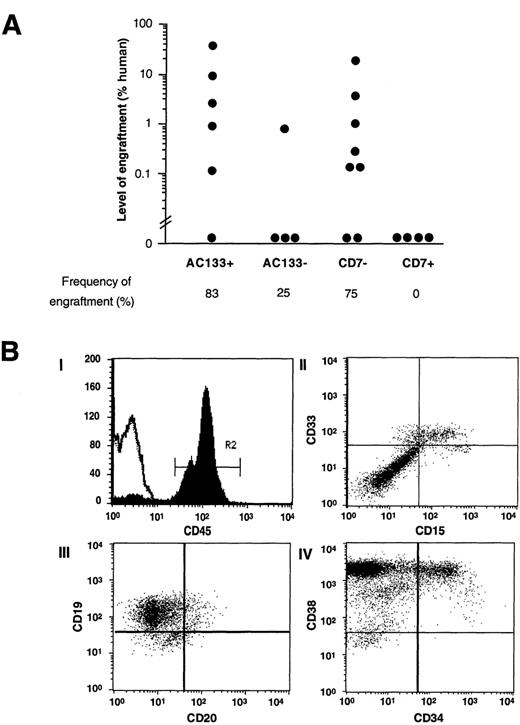
For comparison, AC133+ and AC133− cells and CD7+ and CD7− cells were isolated from the CD34+CD38−Lin−population and transplanted into NOD/SCID mice to evaluate their repopulating capacity. Figure 5A summarizes the levels of human cell engraftment and frequency of SRC detection within the indicated subfractions from 4 independent CB samples. Engraftment was detected after transplantation with as few as 900 CD34+CD38−Lin−cells expressing AC133. With the exception of 1 animal transplanted with the highest dose of AC133−CD34+CD38−Lin−cells (20 000 cells), this novel population was devoid of SRC activity. In addition, the CD7− subpopulation contained SRCs, whereas the CD7+ population did not contain repopulating cells (Figure 5A). Because all AC133+ cells are CD7− in this fraction, the AC133+CD7− cells represent a unique population within the CD34+CD38−Lin− fraction that is enriched for primitive repopulating cells.
Analysis of SRC capacity in subpopulations of CD34+CD38−Lin− cells based on the expression or absence of cell surface CD7 and AC133. (A) Summary of the level of human engraftment in NOD/SCID mice transplanted with AC133+ and AC133− subfractions and CD7+ and CD7− subfractions isolated from the CD34+CD38−Lin−population of human CB. Eight weeks after transplantation, the presence of human cells in the BM of 22 mice was assessed by extraction and hybridization of DNA using either Southern blot analysis with a human chromosome 17–specific α-satellite probe or multiparameter flow cytometric analysis with the human-specific pan-leukocyte marker CD45. Each symbol represents a single NOD/SCID recipient. (B) Multilineage differentiation of human AC133+ + CD34+ + CD38−Lin−cells in NOD/SCID mice. Bone marrow from a representative engrafted mouse transplanted with 10 000 AC133+ + CD34+ + CD38−Lin−CB cells was stained with various human-specific mAbs and analyzed by flow cytometry. (I) Histogram of CD45 (pan-leukocyte marker) expression indicates that 65% of the cells present in the murine BM were human. Analysis of lineage markers was done on cells within gate R2 (CD45+). Histogram overlay (single line) represents isotype control for nonspecific IgG staining. Expressions of (II) myeloid marker CD33 and mature myeloid marker CD15, (III) pan-B cell markers CD19 and CD20, and (IV) CD38 and immature hematopoietic marker CD34 are shown. Multilineage engraftment shown here was similar to that found in mice transplanted with CD7−CD34+CD38−Lin−cells (data not shown).
Analysis of SRC capacity in subpopulations of CD34+CD38−Lin− cells based on the expression or absence of cell surface CD7 and AC133. (A) Summary of the level of human engraftment in NOD/SCID mice transplanted with AC133+ and AC133− subfractions and CD7+ and CD7− subfractions isolated from the CD34+CD38−Lin−population of human CB. Eight weeks after transplantation, the presence of human cells in the BM of 22 mice was assessed by extraction and hybridization of DNA using either Southern blot analysis with a human chromosome 17–specific α-satellite probe or multiparameter flow cytometric analysis with the human-specific pan-leukocyte marker CD45. Each symbol represents a single NOD/SCID recipient. (B) Multilineage differentiation of human AC133+ + CD34+ + CD38−Lin−cells in NOD/SCID mice. Bone marrow from a representative engrafted mouse transplanted with 10 000 AC133+ + CD34+ + CD38−Lin−CB cells was stained with various human-specific mAbs and analyzed by flow cytometry. (I) Histogram of CD45 (pan-leukocyte marker) expression indicates that 65% of the cells present in the murine BM were human. Analysis of lineage markers was done on cells within gate R2 (CD45+). Histogram overlay (single line) represents isotype control for nonspecific IgG staining. Expressions of (II) myeloid marker CD33 and mature myeloid marker CD15, (III) pan-B cell markers CD19 and CD20, and (IV) CD38 and immature hematopoietic marker CD34 are shown. Multilineage engraftment shown here was similar to that found in mice transplanted with CD7−CD34+CD38−Lin−cells (data not shown).
The differentiative and proliferative capacity of highly purified SRCs from CD34+CD38−Lin−cells selected for AC133 expression was assessed by flow cytometric analysis of engrafted NOD/SCID mice 8 weeks after transplantation. A representative analysis of a NOD/SCID mouse transplanted with 10 000 AC133+CD34+CD38−Lin−cells is shown in Figure 5B. The BM of this mouse contained 65% CD45+ human cells (Figure 5B, panel I). CD45 is a human-specific pan-leukocyte marker. Human CD45+ cells within gated region R2 were analyzed for multiple cell surface markers to assess pluripotency of repopulating cells. Figure 5B (panel II) demonstrates the presence of mature cells (CD15+) among myeloid progeny (CD33+). As shown previously, B-lymphoid cells are dominantly represented in the murine BM, as detected by staining for CD19 and CD20 (Figure 5B, panel III).17 In addition to differentiated human cells, a large proportion of CD34+ cells was detected (Figure 5B, panel IV), which provides evidence that immature cells are produced and maintained in the murine BM in mice transplanted with this population. Similar compositions of human hematopoietic engraftment were seen in animals transplanted with CD7− cells within the CD34+CD38−Lin− fraction (data not shown). Taken together these results indicate that CD34−SRCs and CD34+SRCs share the same AC133+CD7− phenotype. AC133 therefore may provide a common cell surface marker to isolate both CD34− and CD34+ human stem cells.
Discussion
The relationship of primitive cells within the human hematopoietic hierarchy has been difficult to clarify due to the heterogeneity of the stem cell compartment. This heterogeneity creates a major barrier in the isolation of discrete populations among putative stem cells for comparative analysis in functional assays. Using differences in the cell surface expression of CD7 and AC133, we identified subpopulations among both CD34−CD38−Lin−and CD34+CD38−Lin−cells that have distinct biological functions. Using in vitro and in vivo assays, our results demonstrate that cell populations lacking CD7 but expressing AC133 (AC133+CD7−) possessed primitive hematopoietic activity unique to remaining CD34−38−Lin− or CD34+CD38−Lin−populations. An extremely rare subset of AC133+CD34−CD38−Lin−cells was identified at a frequency of 0.2% of the total CD34−CD38−Lin−cells from human CB. This was the only subset among the CD34−CD38−Lin−cells that possessed progenitor activity equivalent to that of CD34+CD38−Lin−cells, acquired cell surface expression of CD34, and was detected in the BM of NOD/SCID mice 8 weeks after intravenous transplantation. Taken together, we suggest that this previously unidentified population contains primitive precursors of CD34+ cells and represents the most highly purified fraction of primitive human CD34− cells to date.
Until recently, characterization of primitive cell populations in the human hematopoietic system was limited to those populations expressing cell surface CD34.16 The isolation of highly purified cell populations that do not express markers associated with lineage commitment, termed lineage negative (Lin−), has allowed for the comparison and investigation of populations that are Lin− but do not express CD34 (CD34−Lin−).16 Using in vitro assays, human CD34−Lin− cells have been postulated to have little to no progenitor capacity due to their extreme immature nature and/or lack of appropriate growth factors to support these novel cell populations.14,16 The ability of cells within this large population to repopulate recipient animals, however, demonstrated that this population was capable of hematopoietic stem cell activity.16 We identified a rare population of cells within the CD34−CD38−Lin−population that expresses AC133 and possesses in vitro progenitor capacity equivalent to the most highly purified CD34+ human stem cell fractions. The identification of these cells indicates that primitive subsets of CD34−Lin−cells are capable of being detected in vitro and respond to similar cytokines as CD34+Lin− cells. Furthermore, this same population is capable of producing CD34+ cells in serum-free liquid cultures.
Similar to previous studies using CD34−CD38−Lin−cells, attempts to demonstrate repopulation capacity in NOD/SCID mice from de novo isolated AC133+CD34−CD38−Lin−or AC133−CD34−CD38−Lin−cells have been infrequent. In the case of the AC133+CD34−CD38−Lin−subfraction, this may be attributed to the fact that the number of cells available for transplantation is lower than the number of cells required to successfully demonstrate repopulation using the NOD/SCID assay or the number of cells required for ex vivo stimulation prior to transplantation, as shown previously.16 Ex vivo culture of the AC133+ subset from CD34−CD38−Lin−cells allowed for a higher frequency of detection of human cells in mice transplanted with the AC133+ population, which indicates that CD34−SRCs require prestimulation. However, the levels of human chimerism in the BM of recipient mice from CD34−SRCs are low, even after ex vivo culture. Based on the results of this study and previous work, it is clear that detection of CD34−SRCs in the NOD/SCID mouse is inferior to the engraftment capacity of CD34+SRCs. An appropriate facilitating population of cells, which differs from accessory populations required for low numbers of CD34+repopulating cells to engraft NOD/SCID mice, may be required for these distinct CD34−cells.26 The NOD/SCID assay therefore requires further development to be useful for the future characterization of human CD34− stem cells and to fully elucidate the role of this cell in clinical stem cell transplants.
Previous studies have suggested that CD7 is a marker for lymphoid cells and may also serve as a primitive stem cell marker because many acute myeloid leukemic blasts coexpress CD7 and CD34.27,28 Our results indicate that CD34+CD38−Lin− cells expressing CD7 had limited progenitor capacity within the myeloid lineage, yet they failed to demonstrate repopulation capacity in NOD/SCID mice. This was consistent with CD7−subfractions found in the CD34−CD38−Lin−populations, and we propose that the bulk of these CD7+cells within the CD34−CD38−Lin−population are fully mature and fail to be removed during lineage depletion. AC133+ and AC133− cells within the CD34+CD38−Lin−population demonstrated no detectable difference in the number or type of progenitors. This is in contrast to previous studies in which AC133+ demonstrated a higher frequency of total and multipotent progenitors when compared with AC133−cells.29CD34+CD38−Lin− cells represent only 5%-8% of the total CD34+ cells. These discrepancies may be due to the fact that both AC133+ and AC133− cells were isolated from a more primitive and homogenous population in this study and that they were derived from human CB as opposed to adult BM. The AC133+CD34+CD38−Lin−population almost exclusively contains CD34+SRCs, which is similar to previous studies demonstrating that the AC133+CD34+ population from BM is enriched for repopulating cells.29 30 All AC133+ cells within the CD34+CD38−Lin− fraction are CD7−, and our data indicates that human CD34+SRCs are found exclusively in a more highly purified population of AC133+CD7−CD34+CD38−Lin−cells. This represents a significant refinement in the purification of candidate human CD34+ stem cells.
Similar populations of AC133+CD34− cells have been detected in human leukemias.31,32 Acute myeloid leukemia (AML) patients present with both CD34+ and AC133+CD34− blasts, and as a result, these investigators31 hypothesized that the presence of AC133+CD34− blasts may represent a developmental transition in the hierarchy of hematopoiesis to CD34+ blasts. AML has been shown to arise from a stem cell population,33 and it is equally possible that some leukemias occur from a transformation event in primitive AC133+CD34− cells which leads to a clonal expansion of leukemic stem cells. A proportion of these transformed AC133+CD34− cells may have the capacity to differentiate into CD34+ cells that lead to the CD34+ leukemic clone. In this study, our identification of a normal primitive population of AC133+CD34− cells, which is capable of producing CD34+ cells, supports this hypothesis. Therefore it will be critical to isolate AC133+CD34− cells from AML patients to determine whether they have undergone transformation using chromosomal/molecular diagnostic markers. Alternatively, AC133+CD34− cells derived from AML patients may have normal hematopoietic function and would then serve as an ideal candidate for purging strategies during autologous transplantation.
Preliminary data from our laboratory indicates that AC133+CD34−Lin− cells are present in adult BM (data not shown). Thus, identification of a population of cells within the CD34−CD38−Lin−compartment that can be selected and quantitated using a positive marker may permit the diagnostic evaluation of human CD34− stem cells in various clinical procedures. These include both autologous and allogenic BM transplantation and the ability to evaluate stem cell mobilization using this phenotype. In addition, the AC133+CD34−CD38−Lin−phenotype will assist in developing methods for gene transfer into this population by first optimizing transduction efficiencies in the clonogenic progeny of these cells using in vitro assays. AC133 is expressed on both repopulating CD34+ cells and primitive CD34− subpopulations, and AC133 may, therefore, provide a more appropriate method to enrich stem cells than CD34 selection alone, thus preventing the discard of CD34− subsets that could be critical in human hematopoietic engraftment.
Acknowledgments
We thank Amgen Inc, Thousand Oaks, CA, for cytokines and the staff of the labor and delivery departments of St Joseph's Hospital and London Health Sciences, London, Ontario, Canada, and especially Marlene Watson and Jan Popma for providing cord blood specimens. In addition, we would like to thank Dr C. Awaraji for his technical support and Dr D. Kelvin for critically reviewing this manuscript.
Supported by a grant from the Cancer Research Society Inc, Quebec, Canada; a grant (#MT-15063) from the Medical Research Council of Canada, Ontario, Canada; and a scholarship award (#MSH-35681) to M.B. from the Medical Research Council of Canada, Ontario, Canada.
L. G. and B. M. contributed equally to this work.
Submitted June 28, 1999; accepted January 4, 2000.
Reprints:Mickie Bhatia, The John P. Robarts Research Institute, 100 Perth Drive, London, Ontario, N6A 5K8 Canada; e-mail: mbhatia@rri.on.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal