We examined the effects of retinoids on the human mast cell development using a serum-deprived culture system. When 10-week cultured mast cells derived from CD34+ cord blood cells were used as target cells, both all-trans retinoic acid (ATRA) and 9-cis RA inhibited the progeny generation under stimulation with stem cell factor (SCF) in a dose-dependent manner (the number of progeny grown by SCF plus RA at 10−7 mol/L was one tenth of the value obtained by SCF alone). The early steps in mast cell development appear to be less sensitive to RA according to the single CD34+c-kit+ cord blood cell culture study. The optimal concentration of RAs also reduced the histamine concentration in the cultured mast cells (3.00 ± 0.47 pg per cell in SCF alone, 1.44 ± 0.18 pg per cell in SCF+ATRA, and 1.41 ± 0.10 pg per cell in SCF+9-cis RA). RT-PCR analyses showed the expression of RAR, RARβ, RXR, and RXRβ messenger ribonucleic acid (mRNA) in 10-week cultured mast cells. The addition of an RAR-selective agonist at 10−10 mol/L to 10−7 mol/L decreased the number of mast cells grown in SCF, whereas an RXR-selective agonist at up to 10−8 mol/L was inactive. Among RAR subtype selective retinoids used at 10−9 mol/L to 10−7 mol/L, only the RAR agonist was equivalent to ATRA at 10−7 mol/L in its ability to inhibit mast cell growth. Conversely, the addition of excess concentrations of a RAR antagonist profoundly counteracted the retinoid-mediated suppressive effects. These results suggest that RA inhibits SCF-dependent differentiation of human mast cell progenitors through a specific receptor.
Mast cells play a pivotal role as major effector cells in allergic disorders such as asthma, atopic dermatitis, and allergic rhinitis. Antigen-specific IgE-mediated degranulation of mast cells leads to the subsequent release of chemical mediators and multiple cytokines. Numerous investigators have developed antiallergic drugs to interfere with this process. Potent inhibitors of mast cell growth may provide a new strategy for prophylactic treatment of allergic disorders. In this regard, interferon gamma-1b is a candidate because this cytokine suppresses mast cell production.1
Retinoids are a group of natural and synthetic vitamin A analogues, and exert important effects on the growth and differentiation of various cell types, including hematopoietic progenitors.2-5 The action of retinoids is thought to be mediated by 2 types of nuclear retinoid receptors, the RARs and RXRs, which are members of the steroid/thyroid hormone receptor superfamily.6-8 Each class of the receptors comprises 3 subtypes designated α, β, and γ. All-transretinoic acid (ATRA) and9-cis RA are high affinity ligands for RARs, and 9-cis RA additionally binds RXRs.6-8 These receptors form RAR/RXR heterodimers and RXR/RXR homodimers, respectively, to function as ligand-activated transcription factors.
Stem cell factor (SCF) has been demonstrated to act as a major growth and differentiation factor for human mast cell lineage.9-13However, the purity of cultured mast cells grown by SCF alone has ranged from approximately 40% to 85%.9,11,12 We have recently reported the selective growth of a large number of mast cells from CD34+ human cord blood cells under stimulation with SCF in long-term serum-deprived cultures.14 In this study, we examined the effects of retinoids on the human mast cell development using this culture system.
Materials and methods
Cytokines, reagents, and antibodies
Human recombinant SCF was generously provided by Kirin Brewery Co Ltd (Takasaki, Japan). Human recombinant interleukin (IL)-4 was purchased from R & D Systems (Minneapolis, MN). Human recombinant IL-6 was a gift from Ajinomoto Co (Kawasaki, Japan).
ATRA was obtained from Sigma (St Louis, MO), and 9-cis-RA from Wako (Osaka, Japan). Ro 13-7410, Ro 25-7386, Ro 40-6055, Ro 19-0645, Ro 44-4753, and Ro 41-5253 were kindly provided by F. Hoffman-La Roche, Ltd (Basel, Switzerland). Ro 13-7410 has been shown to selectively bind and activate RARs but not RXRs.15 It does not discriminate among RARα, RARβ, and RARγ. Ro 25-7386 is indicated to be a specific RXR agonist and does not bind RARs.16 Three synthetic retinoids (Ro 40-6055, Ro 19-0645, and Ro 44-4753) are described to be selective for RARα, RARβ, and RARγ, respectively, when used at nanomolar concentrations.15,17,18 Ro 41-5253 acts as an RARα antagonist.16 18 All the retinoids were dissolved in ethanol at a concentration of 10−2 mol/L, and stored in light-protected vials at −80°C. Before their use in each experiment, they were further diluted with α-medium (Flow Laboratories, Rockville, MD) containing 1% deionized bovine serum albumin (BSA) (Sigma Chemical). All experiments were performed in subdued light, and the tubes containing retinoids were covered with aluminum foil. Preliminary experiments showed that controls containing an equivalent amount of ethanol were not different from medium controls in the development of the cultured mast cells under stimulation with SCF.
The polyclonal rabbit antihuman IL-6 antibody (Ab, 1.2 mg/mL)14 was a gift from Ajinomoto Co. One microgram of this Ab neutralized the activity of 3 ng IL-6, as determined with the use of the cell line, SKW6-CL4. A polyclonal sheep antihuman IL-4 Ab was purchased from Genzyme Co (Cambridge, MA). The Ab at 0.1 to 1 μg/mL neutralized the bioactivity of a 0.25 ng/mL solution of IL-4. The mouse monoclonal antibody (MoAb) against human granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from Oncogene Science Inc (Uniondale, NY). This azide-free antibody at 2 μg/mL reduced the growth of granulocyte-macrophage colonies supported by 10 ng/mL of GM-CSF to 37%.19 The neutralizing antihuman transforming growth factor (TGF)-β1 antibody was obtained from R & D Systems. The ND50 of the antibody was determined to be 0.2 to 0.6 μg/mL in the presence of 0.25 ng/mL of TGF-β1, using TGF-β responsive HT-2 cells.
For immunocytochemical staining, purified MoAbs for tryptase (MAB1222) and chymase (3D5) were purchased from Chemicon International Inc (Temecula, CA) and Biogenesis Inc (Sandown, NH), respectively.
For the flow cytometric analysis, the MoAbs for CD34 (HPCA-2, fluorescein isothiocyanate [FITC]) and c-kit (95C3, phycoerythrin [PE]) were purchased from Becton Dickinson Immunocytometry Systems (Mountain View, CA) and Immunotech SA (Marseilles, France), respectively.
Cell preparation
Cord blood samples were aspirated in heparinized plastic syringes from the umbilical vein at normal delivery. Fully informed consent was obtained from the mothers of all neonates before harvesting the specimens. Mononuclear cells (MNCs) were separated by density centrifugation over Ficoll-Paque (Pharmacia Fine Chemicals, Piscataway, NJ), washed twice, and suspended in Ca++- and Mg2+-free phosphate-buffered saline (PBS) containing 1 mmol/L EDTA-2Na and 2.5% fetal bovine serum (Hyclone, Logan, UT). After treatment with Silica (Immuno-Biological Laboratories, Fujioka, Japan) for 30 minutes at 37°C, CD34-positive cells were enriched using a Dynal CD34 Progenitor Cell Selection System (Dynal AS, Oslo, Norway). Briefly, 2 to 4 × 107 cells were mixed with the same number of polystyrene beads coated with an MoAb specific for CD34 (Dynabeads M-450 CD34), and incubated for 30 minutes at 4°C. Bead-rosetted cells were separated by a magnet. For the detachment of the beads from the cells, affinity-purified polyclonal antibodies against the Fab portion of anti-CD34 Ab (Detach-a-Bead CD34) were added, and incubation was carried out for 45 minutes at room temperature. The detached beads were removed by the magnet, and the cells were collected as CD34+ cells. More than 90% of the isolated cells were CD34-positive, as determined by FACScan flow cytometry (Becton Dickinson).
Suspension cultures
Serum-deprived liquid cultures were carried out in 24-well culture plates (#3047; Becton Dickinson) using a modification of the technique described previously.14,20 Ten thousand CD34+cells or 5000 CD34+c-kit+ cells were cultured in each well containing 2 mL of α-medium supplemented with 1% BSA, 300 μg/mL fully iron-saturated human transferrin (approximately 98% pure, Sigma), 16 μg/mL soybean lecithin (Sigma), 9.6 μg/mL cholesterol (Nakalai Chemicals, Tokyo, Japan), and 100 ng/mL SCF with or without different concentrations of ATRA. To examine the effects of retinoids, IL-6, and IL-4 on the SCF-dependent development of mast cells, we used the cultured cells grown in 10 ng/mL of SCF from CD34+ cord blood cells as target cells.14One × 104 or 5 × 104 10-week cells were incubated for 2 weeks in 24-well culture plates containing 100 ng/mL of SCF, different concentrations of retinoids, 50 ng/mL of IL-6 or 20 ng/mL of IL-4, alone or in combination. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. Half of the culture medium was replaced weekly with fresh medium containing the factor(s). The number of viable cells was determined by a trypan-blue exclusion test using a hemocytometer. We presented the actual counts of progeny in the results.
The DNA distribution was examined by flow cytometry after the cells had been stained with propidium iodide (PI), as described previously.21
Serum-deprived single-cell culture
Single-cell sorting was performed by 2-step sorting, as described previously.14 21-23 Cord blood MNCs (2 × 106) were stained with 20 μL of FITC-conjugated anti-CD34 MoAb and 20 μL of PE-conjugated anti-c-kit MoAb. After 2 washes, CD34+c-kit+ cord blood cells were collected in 5-mL tubes and were resorted into the individual wells of a 96-well U-bottomed tissue culture plate (#3077; Becton Dickinson) containing 100 μL of α-medium supplemented with 1% BSA, 300 μg/mL of fully iron-saturated human transferrin, 16 μg/mL of soybean lecithin, 9.6 μg/mL of cholesterol, 100 ng/mL of SCF, and different concentrations of ATRA, using the FACStarplus flow cytometer equipped with an automatic cell deposition unit (Becton Dickinson). Ninety-nine percent of the wells contained a single cell on the first day of culture. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. The number of cells in each well was serially counted until 4 weeks under direct microscopic visualization. Aggregates consisting of 30 or more cells were scored as colonies. Then, the colonies were individually lifted with a 3-μL Eppendorf micropipette, spread on glass slides using a Cytospin II (Shandon Southern, Sewickly, PA), and the constituent cells were stained with antitryptase MoAb.
Clonal cell cultures
The mast cell colony assay was carried out in 35-mm Lux suspension culture dishes (#171099; Nunc, Naperville, IL) using a modification of the technique described previously.24 The culture consisted of 10-week cultured cells (5000 cells/mL) grown in 10 ng/mL of SCF, α-medium, 0.9% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 1% BSA, 300 μg/mL of fully iron-saturated human transferrin, 16 μg/mL of soybean lecithin, 9.6 μg/mL of cholesterol, and 100 ng/mL of SCF with or without different concentrations of ATRA. Dishes were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. On day 14, aggregates consisting of 30 or more cells were scored as mast cell colonies, and those consisting of 10 to 29 cells as mast cell clusters. To confirm the in situ identification of mast cells, 60 individual colonies and clusters were lifted and stained with the antitryptase MoAb or mouse IgG1 using the alkaline phosphatase-antialkaline phosphatase (APAAP) technique. Almost all the constituent cells were positive for tryptase.
Immunocytochemical staining
The cultured cells were spread on glass slides using a Cytospin II. Reactions with mouse MoAbs against tryptase and chymase were detected using the APAAP method (Dako APAAP Kit System, Dako Corp, Carpinteria, CA), as described previously.25 The isotype mouse MoAb was also used as a control. Briefly, cytocentrifuged samples were fixed with Carnoy's fluid, washed with PBS, and preincubated with normal rabbit serum to saturate the Fc receptors on the cell surface. After being washed with PBS 3 times, the samples were reacted with a mouse MoAb for 30 minutes at room temperature in a humidified chamber. After 3 more washes with PBS, the samples were incubated with rabbit antimouse IgG antibody, washed 3 times, and successively reacted with the calf intestinal alkaline phosphatase-mouse monoclonal antialkaline phosphatase complex. Finally, alkaline phosphatase activity was detected with naphthol AS-MX phosphate, Fast Red TR, and levamisole to inhibit nonspecific alkaline phosphatase activity. The specimens were counterstained with hematoxylin.
The diameter of the mast cells was measured by calculating the average of 2 perpendicular diameters of tryptase+ cells on glass slides, using a microscope equipped with an ocular micrometer.
Reverse transcription-polymerase chain reaction
Reverse transcription-polymerase chain reaction (RT-PCR) was performed according to a modification of the procedure described previously.20 Total rubonucleic acid (RNA) was individually isolated from the cells, using Isogen (Wako). Next, 1 μg of RNA was reverse transcribed in 200 U of SuperScript II (Life Technologies, Gaithersburg, MD), 10−7 mol/L oligo dT primer (Takara Shuzo, Ohtsu, Japan), and 10 U RNase inhibitor (Boehringer Mannheim, Mannheim, Germany) in 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, and 3 mmol/L MgCl2. The prepared solution was incubated at 37°C for 1 hour. The PCR reaction was performed in a volume of 10 μL containing 1 μL of RT-PCR product, 1 μL of 10 × PCR buffer (100 mmol/L Tris-HCl, pH 8.3, 500 mmol/L KCl), 1.5 mmol/L MgCl2, 0.5 μmol/L of each primer, 1 μL of 0.5 mmol/L dNTP mix, and 0.5 U of Taq DNA polymerase (Takara Shuzo). The primers for amplification were designed on the basis of previous reports (RARγ,26 the other receptors27): 5′-CATTGAGACCCAGAGCAGC -3′ (nt 282-300) and 5′-CCGTCTCCGCATCATCCATC -3′ (nt 1062-1081) for RARα; 5′-CACTGGCTTGACCATCGCAGACC -3′ (nt 1047-1069) and 5′-GAGAGGTGGCATTGATCCAGG -3′ (nt 1507-1527) for RARβ; 5′-GGCCTGGGCCAGCCTGACCTC -3′ (nt 288-308) and 5′-CAGCCCCAGATCCAGCTGCACG-3′ (nt 803-824) for RARγ; 5′-CTCCTCAGGCAAGCACTATG-3′ (nt 498-517) and 5′-AGAGCTTAGCGAACCTTCCC-3′ (nt 1311-1330) for RXRα; 5′-TCAGGCAAACACTACGGGGT-3′ (nt 816-835) and 5′-GCATACACTTTCTCCCGCAG -3′ (nt 1566-1585) for RXRβ; 5′-CTCAGGAAAGCACTACGGGG-3′ (nt 465-484) and 5′-CAGGGTCATTTGTCGAGTTC-3′ (nt 804-823) for RXRγ; 5′-CTGGACTTCGAGCAAGAGAT-3′ (nt 702-721) and 5′-TCGTCATACGCCTGCTTGCT-3′ (nt 1132-1113) for β-actin. The samples were denatured at 95°C for 5 minutes, then subjected to 35 cycles at 95°C for 1 minute, at 54°C for 1 minute, and at 72°C for 1 minute, with a final 10 minutes of extension at 72°C in a Gene Amp PCR System 9600 (Perkin-Elmer Cetus, Norwalk, CT). PCR products (10 μL) were analyzed on a 1.5% agarose gel in TAE buffer (40 mmol/L Tris,40 mmol/L sodium acetate,1 mmol/L EDTA, pH 8.4) using a DNA ladder 100-base pair (bp) marker.
Assay of histamine, tryptase, and cytokine levels
Histamine concentrations in cell lysates obtained by the treatment of the cultured cells with 0.5% Nonidet P-40 and in supernatant were measured by the histamine radioimmunoassay (RIA) kit (Immunotech, SA). The detection limit was 0.05 ng/mL, and cross-reactions with t-methylhistamine or histidine were very low. Intra-assay CV and interassay CV were 7.6% to 8.4% and 8.2% to 11.5%, respectively. The tryptase concentrations in the cell lysates were measured with a fluoroenzymeimmunoassay (UniCAP Tryptase, Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden). The concentrations of GM-CSF, IL-4, IL-6, and TGF-β1 in the supernatant of the cultured cells were measured by an enzyme-linked immunosorbent assay (ELISA) (Amersham International, Buckinghamshire, UK). All assays were conducted in triplicate.
Statistical analysis
All experiments were carried out at least 3 times and were shown to be reproducible. Values are expressed as means ± SD. One-way analysis of variance, followed by post hoc contrasts with Bonferroni limitation, was used for more than 3 independent groups.
Results
Effects of all-trans retinoic acid or 9-cis retinoic acid on the growth and properties of cultured mast cells supported by stem cell factor
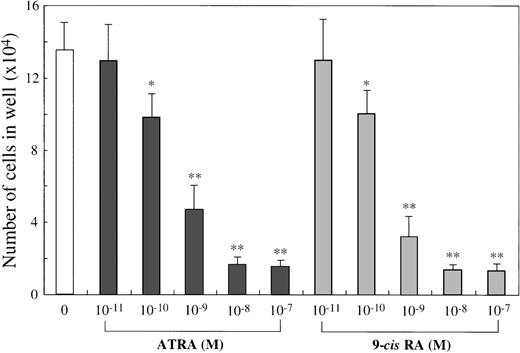
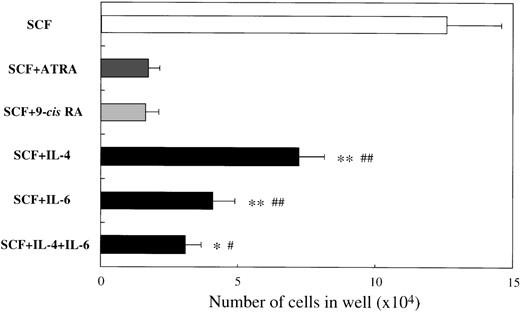
To examine the effects of ATRA or 9-cis RA on the human mast cell development, we first used 10-week cultured cells generated with 10 ng/mL of SCF from CD34+ cord blood cells as target cells. Immunocytochemical staining showed that almost all of these cultured cells were positive for tryptase, as described previously.14 One × 104 10-week cultured cells were incubated for 2 weeks in wells containing SCF at 100 ng/mL with or without ATRA or 9-cis RA at concentrations ranging from 10−11 mol/L to 10−7 mol/L. The results are shown in Figure1. The addition of either ATRA or9-cis RA to the culture with SCF gave rise to a dose-dependent decrease in the numbers of progeny. The maximal inhibition with both RAs was observed at a level of at least 10−8 mol/L. As shown in Figure2, the number of cultured mast cells grown in SCF+10−7 mol/L ATRA was similar to the value obtained by SCF+10−7 mol/L 9-cis RA. It is of particular interest that, at these optimal concentrations, the RAs inhibited the SCF-dependent mast cell generation to a greater extent than IL-4 or IL-6, alone or in combination.
Dose response to ATRA or 9-cis RA of mast cell growth supported by SCF.
The 1 × 104 10-week cultured mast cells were incubated in wells containing SCF at 100 ng/mL with either ATRA or 9-cis RA at 10−11 mol/L to 10−7 mol/L. After 2 weeks, the viable cells were enumerated. The results shown are from 1 representative experiment of 3. Similar results were obtained in the other 2 experiments. Significantly different from SCF alone (*P < .0005, **P < .0001).
Dose response to ATRA or 9-cis RA of mast cell growth supported by SCF.
The 1 × 104 10-week cultured mast cells were incubated in wells containing SCF at 100 ng/mL with either ATRA or 9-cis RA at 10−11 mol/L to 10−7 mol/L. After 2 weeks, the viable cells were enumerated. The results shown are from 1 representative experiment of 3. Similar results were obtained in the other 2 experiments. Significantly different from SCF alone (*P < .0005, **P < .0001).
Comparison of effects of RAs with those of IL-4, IL-6, or IL-4+IL-6 on mast cell growth supported by SCF.
The 1 × 104 10-week cultured mast cells were incubated in wells containing 100 ng/mL of SCF, ATRA or 9-cisRA at 10−7 mol/L, 20 ng/mL of IL-4, or 50 ng/mL of IL-6, alone or in combination. After 2 weeks, the viable cells were enumerated. Results shown are the mean ± SD of 3 experiments. Significantly different from ATRA (*P < .002, **P < .0001) and from 9-cis RA (#P < .001,##P < .0001).
Comparison of effects of RAs with those of IL-4, IL-6, or IL-4+IL-6 on mast cell growth supported by SCF.
The 1 × 104 10-week cultured mast cells were incubated in wells containing 100 ng/mL of SCF, ATRA or 9-cisRA at 10−7 mol/L, 20 ng/mL of IL-4, or 50 ng/mL of IL-6, alone or in combination. After 2 weeks, the viable cells were enumerated. Results shown are the mean ± SD of 3 experiments. Significantly different from ATRA (*P < .002, **P < .0001) and from 9-cis RA (#P < .001,##P < .0001).
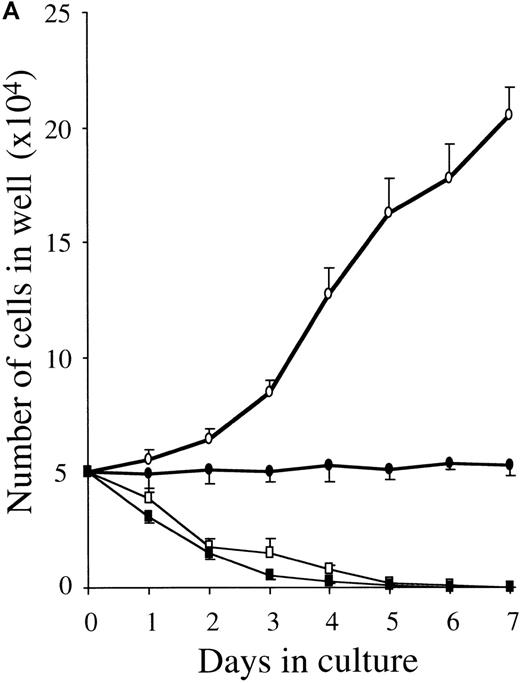
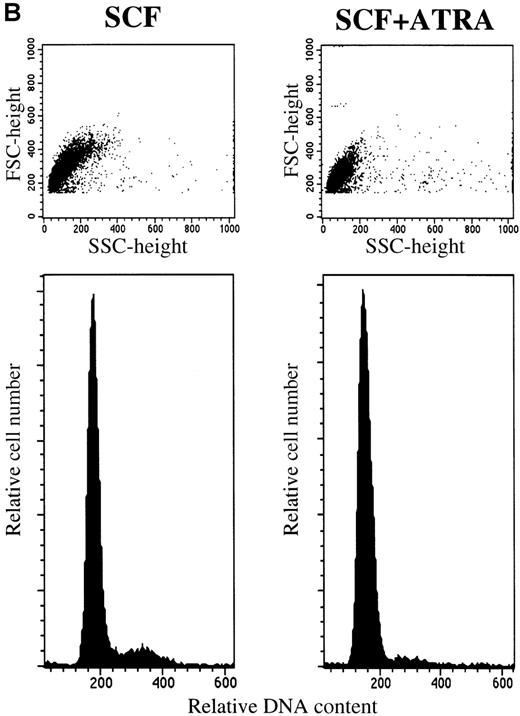
To clarify whether RA-induced inhibition represented a decrease in proliferation rate or a decreased survival, we examined the time course of the proliferative response and the cell cycle status of 10-week cultured mast cells exposed to SCF alone or SCF+ATRA. As shown in Figure 3, the total viable cell number was unchanged in the culture containing SCF+ATRA at 10−7mol/L. In the absence of SCF, the inhibitory effects of ATRA were either absent or present at low levels. The flow cytometric analysis revealed that the addition of ATRA to the culture with SCF caused a decrease in the percentage of S plus G2/M cells on day 2 (10.4% in SCF alone vs 3.0% in SCF+ATRA). However, there was no significant appearance of a sub-G1 peak of the cultured cells in the presence or absence of ATRA. Similar results were obtained on days 4 and 7.
ATRA reduces the proliferation rate of mast cells under stimulation with SCF.
The 5 × 104 10-week cultured mast cells were incubated in the presence or absence of SCF at 100 ng/mL for 1 week. ATRA was used at 10−7 mol/L. (A) The viable cells were enumerated every day. SCF alone, open circles; SCF+ATRA, closed circles; no factors, open squares; ATRA alone, closed squares. (B) The DNA distribution was examined by labeling of the cells with PI on day 2. FSC, forward light-scatter characteristics; SSC, side-scatter characteristics.
ATRA reduces the proliferation rate of mast cells under stimulation with SCF.
The 5 × 104 10-week cultured mast cells were incubated in the presence or absence of SCF at 100 ng/mL for 1 week. ATRA was used at 10−7 mol/L. (A) The viable cells were enumerated every day. SCF alone, open circles; SCF+ATRA, closed circles; no factors, open squares; ATRA alone, closed squares. (B) The DNA distribution was examined by labeling of the cells with PI on day 2. FSC, forward light-scatter characteristics; SSC, side-scatter characteristics.
Next, we examined whether ATRA and 9-cis RA influenced the size, intracellular histamine level, and chymase expression of the cultured mast cells grown in SCF. The results are presented in Table1. The RAs equivalently diminished the mean diameter and histamine content of the cultured mast cells, whereas IL-4 and IL-6 each increased these 2 parameters. The histamine level in the cultured cells grown in SCF plus ATRA or 9-cis RA was approximately 50% of that in the cultured cells grown in SCF alone. The histamine concentration in the supernatant of the cultured mast cells exposed to SCF+10−7 mol/L ATRA and in those exposed to SCF+10−7 mol/L 9-cis RA was one seventh and one sixth of the level obtained with SCF alone, respectively. At the initiation of culture, 4.0% ± 1.4% of the cells were positive for chymase. After 2 weeks, the percentage of chymase+ cells in the cultured mast cells increased in the presence of SCF+IL-4 or SCF+IL-6, compared with the level obtained with SCF alone, as described previously.14 On the other hand, neither ATRA nor 9-cis RA influenced the chymase expression. In addition, the content of tryptase was not markedly reduced by 10−7 mol/L ATRA (4.3 pg per cell in SCF alone vs 3.5 pg per cell in SCF+10−7 mol/L ATRA).
Effects of retinoids on properties of cultured mast cells supported by SCF
| Stimuli . | Cell size (μm) . | Histamine content (pg/cell) . | Chymase+ cells (%) . |
|---|---|---|---|
| SCF | 21.5 ± 3.6 | 3.00 ± 0.47 | 33.9 ± 7.5 |
| SCF + ATRA | 13.3 ± 3.3* | 1.44 ± 0.18† | 31.2 ± 8.4 |
| SCF + 9-cis RA | 13.0 ± 3.2* | 1.41 ± 0.10† | 32.8 ± 7.2 |
| SCF + Ro 13-7410 | 13.7 ± 3.6* | 1.47 ± 0.24† | 35.4 ± 5.1 |
| SCF + Ro 25-7386 | 20.1 ± 3.7 | 2.63 ± 0.77 | 34.7 ± 8.1 |
| SCF + IL-4 | 25.0 ± 3.3* | 6.24 ± 0.96* | 72.4 ± 9.1* |
| SCF + IL-6 | 29.7 ± 5.3* | 15.84 ± 2.17* | 82.5 ± 9.8* |
| Stimuli . | Cell size (μm) . | Histamine content (pg/cell) . | Chymase+ cells (%) . |
|---|---|---|---|
| SCF | 21.5 ± 3.6 | 3.00 ± 0.47 | 33.9 ± 7.5 |
| SCF + ATRA | 13.3 ± 3.3* | 1.44 ± 0.18† | 31.2 ± 8.4 |
| SCF + 9-cis RA | 13.0 ± 3.2* | 1.41 ± 0.10† | 32.8 ± 7.2 |
| SCF + Ro 13-7410 | 13.7 ± 3.6* | 1.47 ± 0.24† | 35.4 ± 5.1 |
| SCF + Ro 25-7386 | 20.1 ± 3.7 | 2.63 ± 0.77 | 34.7 ± 8.1 |
| SCF + IL-4 | 25.0 ± 3.3* | 6.24 ± 0.96* | 72.4 ± 9.1* |
| SCF + IL-6 | 29.7 ± 5.3* | 15.84 ± 2.17* | 82.5 ± 9.8* |
SCF = stem cell factor; ATRA = all-trans retinoic acid.
The 1 × 104 10-week cultured mast cells were incubated in culture wells containing SCF, retinoids, IL-6, or IL-4, alone or in combination. After 2 weeks, the cells were processed for immunocytochemical staining with an antitryptase or antichymase MoAb. The diameter of tryptase+ cells was measured, as described in “Materials and methods.” At the same time, intracellular histamine concentrations were determined by the radioimmunoassay. The results shown are the mean ± SD of 4 experiments. SCF, 100 ng/mL; ATRA, 9-cis RA, Ro 13-7410, Ro 25-7386, 10−7mol/L; IL-4, 20 ng/mL; IL-6, 50 ng/mL. Significantly different from SCF alone (*P < .0001,
P < .005).
Because GM-CSF, TGF-β1, IL-4, and IL-6 were reported to suppress the SCF-dependent mast cell growth by us or other investigators,14 28-31 it is possible that the effects of RAs on the mast cell development are mediated by these cytokines. The concentrations of all the factors in the supernatant of the 10-week-old mast cells after the 2-week incubation with SCF alone or SCF+10−7 mol/L ATRA were at levels below detectable limits. None of the neutralizing anti-GM-CSF Ab at 2 μg/mL, anti-TGF-β1 Ab at 100 μg/mL, anti-IL-4 Ab at 10 μg/mL, and anti-IL-6 Ab at 10 μg/mL affected the progeny generation under stimulation with 100 ng/mL of SCF or 100 ng/mL of SCF +10−7 mol/L ATRA (data not shown).
Expression of nuclear retinoid receptors in cultured mast cells grown in stem cell factor
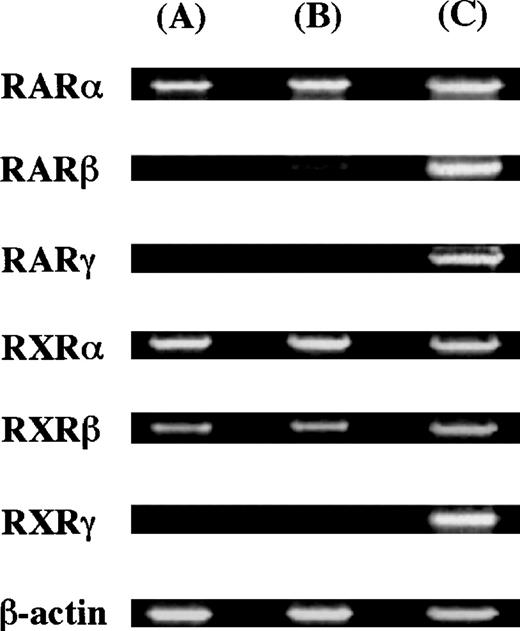
To elucidate whether ATRA and 9-cis RA exerted their action through the receptor(s), we examined the expression of messenger RNAs (mRNAs) for the retinoid receptor subtypes using RT-PCR analysis. Thirty-five cycles were used for amplification. As positive controls, we used the human pancreatic carcinoma cell line (Panc-1)32for the expression of RARγ, and the human myeloma cell line (RPMI 8226)27 for the expression of the other receptors. The molecular sizes of RT-PCR products obtained with the primers were compatible with the expected molecule size: 800 bp for RARα, 481 bp for RARβ, 537 bp for RARγ, 833 bp for RXRα, 770 bp for RXRβ, and 359 bp for RXRγ. The expression of RARα, RARβ, RXRα, and RXRβ was observed in 10-week cultured mast cells, as shown in Figure4.
Expression of nuclear retinoid receptors in cultured mast cells grown in SCF.
The cDNA of the nuclear retinoid receptors in cultured mast cells grown in SCF alone or SCF+ATRA at 10−7 mol/L was amplified by 35 cycles. As positive controls, Panc-1 cells were used for the expression of RARγ, and RPMI 8226 cells exposed to ATRA at 10−7 mol/L for 24 hours were used for the expression of the other receptors. (A) Mast cells grown in SCF at 100 ng/mL, (B) mast cells exposed to SCF at 100 ng/mL plus ATRA at 10−7 mol/L, and (C) positive controls.
Expression of nuclear retinoid receptors in cultured mast cells grown in SCF.
The cDNA of the nuclear retinoid receptors in cultured mast cells grown in SCF alone or SCF+ATRA at 10−7 mol/L was amplified by 35 cycles. As positive controls, Panc-1 cells were used for the expression of RARγ, and RPMI 8226 cells exposed to ATRA at 10−7 mol/L for 24 hours were used for the expression of the other receptors. (A) Mast cells grown in SCF at 100 ng/mL, (B) mast cells exposed to SCF at 100 ng/mL plus ATRA at 10−7 mol/L, and (C) positive controls.
Selective involvement of RAR in the regulation of stem cell factor–dependent mast cell development
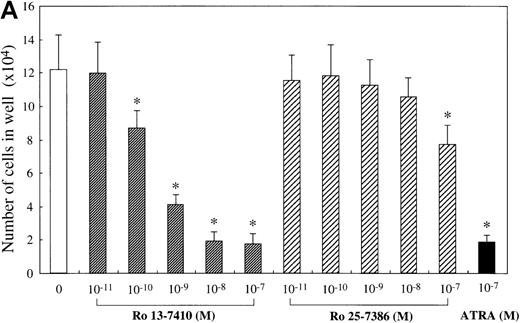
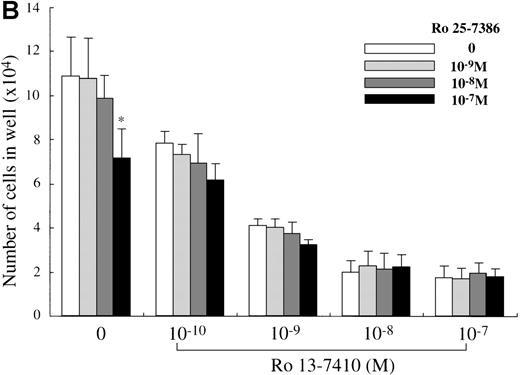
We attempted to determine which of the 2 retinoid responsive pathways is mainly involved in the regulation of the mast cell development, using 2 synthetic retinoids that selectively bind and activate RARs and RXRs, respectively. As shown in Figure5A, the addition of Ro 13-7410 (RAR agonist) at 10−10 mol/L to 10−7mol/L decreased the number of progeny grown by SCF, with a maximal inhibition of approximately 85% at a level of at least 10−8 mol/L. There was no difference in the number of the progeny between SCF+ Ro 13-7410 at 10−8 mol/L or 10−7 mol/L and SCF+ATRA at 10−7mol/L. On the other hand, Ro 25-7386 (RXR agonist) at 10−11 mol/L to 10−8 mol/L failed to inhibit the mast cell growth, though the number of mast cells grown by SCF was slightly reduced at 10−7 mol/L. The addition of Ro 25-7386 at 10−7 mol/L to the culture containing SCF plus Ro 13-7410 at concentrations of 10−10 mol/L to 10−7 mol/L did not further enhance the inhibition of the progeny production (Figure 5B). Both the mean diameter and the intracellular histamine content of the cultured mast cells were significantly attenuated by 10−7 mol/L Ro 13-7410, but not 10−7 mol/L Ro 25-7386 (Table 1). These results imply that the growth and properties of cultured mast cells are regulated through activation of the RAR-RXR response pathway rather than the RXR-RXR response pathway.
Effects of RAR- and RXR-selective retinoids on SCF-dependent mast cell growth.
(A) Dose response to RAR-selective retinoid (Ro 13-7410) or RXR-selective retinoid (Ro 25-7386) of mast cell growth supported by SCF. The 1 × 104 10-week cultured mast cells were incubated in wells containing SCF at 100 ng/mL with various concentrations of Ro 13-7410, Ro 25-7386, or ATRA. After 2 weeks, the viable cells were enumerated. Results shown are the mean ± SD of 3 experiments. Significantly different from SCF alone (*P < .0001). (B) No cooperative effects of Ro 13-7410 at concentrations of 10−10 mol/L to 10−7 mol/L and Ro 25-7386 at concentrations of 10−9 mol/L to 10−7 mol/L on the growth of mast cells supported by SCF. Significantly different from no Ro 25-7386 (*P < .0001).
Effects of RAR- and RXR-selective retinoids on SCF-dependent mast cell growth.
(A) Dose response to RAR-selective retinoid (Ro 13-7410) or RXR-selective retinoid (Ro 25-7386) of mast cell growth supported by SCF. The 1 × 104 10-week cultured mast cells were incubated in wells containing SCF at 100 ng/mL with various concentrations of Ro 13-7410, Ro 25-7386, or ATRA. After 2 weeks, the viable cells were enumerated. Results shown are the mean ± SD of 3 experiments. Significantly different from SCF alone (*P < .0001). (B) No cooperative effects of Ro 13-7410 at concentrations of 10−10 mol/L to 10−7 mol/L and Ro 25-7386 at concentrations of 10−9 mol/L to 10−7 mol/L on the growth of mast cells supported by SCF. Significantly different from no Ro 25-7386 (*P < .0001).
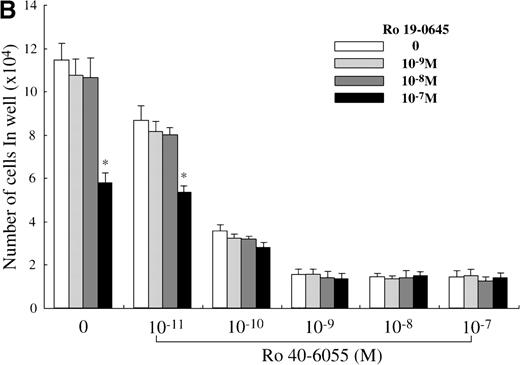
Next, we analyzed which of the endogenous RAR subtypes is involved in the RA-dependent regulation of mast cell generation, using Ro 40-6055 (RARα agonist), Ro 19-0645 (RARβ agonist), and Ro 44-4753 (RARγ agonist). As presented in Figure 6A, the addition of Ro 40-6055 at concentrations of at least 10−10 mol/L to the culture with SCF markedly decreased the number of progeny grown under stimulation with SCF. The inhibitory ability of Ro 40-6055 at a level of at least 10−9 mol/L was comparable to that of 10−7 mol/L ATRA. Neither Ro 19-0645 nor Ro 44-4753 suppressed the mast cell growth, except for 10−7mol/L Ro 19-0645. There were no cooperative effects between Ro 40-6055 at concentrations of 10−11 mol/L to 10−7 mol/L and Ro 19-0645 at a concentration of 10−7 mol/L in the suppression of the mast cell growth (Figure 6B).
Effects of RAR-, RARβ-, and RARγ-selective retinoids on SCF-dependent mast cell growth.
(A) Dose response to RARα-selective retinoid (Ro 40-6055), RARβ-selective retinoid (Ro 19-0645), or RARγ-selective retinoid (Ro 44-4753) of mast cell growth supported by SCF. One × 104 10-week cultured mast cells were incubated in wells containing SCF at 100 ng/mL with various concentrations of Ro 40-6055, Ro 19-0645, Ro 44-4753, or ATRA. After 2 weeks, the viable cells were enumerated. The results shown are from 1 representative experiment of 3. Similar results were obtained in the other 2 experiments. Significantly different from SCF alone (*P < .0001). (B) No cooperative effects of Ro 40-6055 at concentrations of 10−11 mol/L to 10−7 mol/L and Ro 19-0645 at concentrations of 10−9 mol/L to 10−7 mol/L on the growth of mast cells supported by SCF. Significantly different from no Ro 19-0645 (*P < .0001).
Effects of RAR-, RARβ-, and RARγ-selective retinoids on SCF-dependent mast cell growth.
(A) Dose response to RARα-selective retinoid (Ro 40-6055), RARβ-selective retinoid (Ro 19-0645), or RARγ-selective retinoid (Ro 44-4753) of mast cell growth supported by SCF. One × 104 10-week cultured mast cells were incubated in wells containing SCF at 100 ng/mL with various concentrations of Ro 40-6055, Ro 19-0645, Ro 44-4753, or ATRA. After 2 weeks, the viable cells were enumerated. The results shown are from 1 representative experiment of 3. Similar results were obtained in the other 2 experiments. Significantly different from SCF alone (*P < .0001). (B) No cooperative effects of Ro 40-6055 at concentrations of 10−11 mol/L to 10−7 mol/L and Ro 19-0645 at concentrations of 10−9 mol/L to 10−7 mol/L on the growth of mast cells supported by SCF. Significantly different from no Ro 19-0645 (*P < .0001).
To confirm the selective involvement of RARα, we examined whether Ro 41-5253 (RARα antagonist) counteracted the inhibition of the mast cell growth induced by ATRA, 9-cis RA, or Ro 40-6055. The results are presented in Figure 7. Ro 41-5253 (10−7 mol/L to 10−5 mol/L) showed no effects on the SCF-dependent generation of mast cells. The addition of 1 000- to 10 000-fold excess concentrations of Ro 41-5253 significantly increased the number of mast cells grown in the presence of SCF plus ATRA or 9-cis RA at a concentration of 10−9 mol/L or 10−8 mol/L. The suppression of the mast cell generation mediated by Ro 40-6055 at either 10−10 mol/L or 10−9 mol/L was also counteracted by the treatment with Ro 41-5253 at 10−5 mol/L (data not shown).
Effects of RAR-selective antagonist on mast cell growth under stimulation with SCF+ATRA or SCF+9-cis RA.
The effects of Ro 41-5253 (RARα antagonist) on the progeny generation from 1 × 104 10-week cultured mast cells in the presence of SCF plus ATRA or 9-cis RA were examined. Significantly different from no Ro 41-5253 (*P < .0005, **P < .0001).
Effects of RAR-selective antagonist on mast cell growth under stimulation with SCF+ATRA or SCF+9-cis RA.
The effects of Ro 41-5253 (RARα antagonist) on the progeny generation from 1 × 104 10-week cultured mast cells in the presence of SCF plus ATRA or 9-cis RA were examined. Significantly different from no Ro 41-5253 (*P < .0005, **P < .0001).
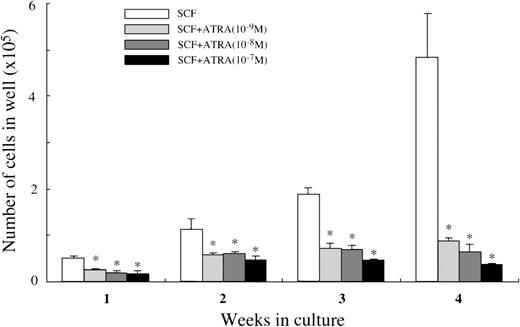
Effects of all-trans retinoic acid on the early phase of mast cell development
We then analyzed whether ATRA exerted its effects at the early phase of mast cell development. Ten thousand CD34+ cells were plated in wells containing 100 ng/mL of SCF with or without ATRA at 10−9 mol/L to 10−7 mol/L. As presented in Figure 8, all the concentrations of ATRA significantly decreased the cell production by CD34+ cells under stimulation with SCF. Almost all the 4-week cultured cells were positive for tryptase, both in the culture containing SCF alone and in the culture with SCF+10−7mol/L ATRA. Similar results were obtained by the addition of9-cis RA at 10−9 mol/L to 10−7 mol/L (data not shown). When 5000 CD34+c-kit+ cells sorted by a FACStarplus flow cytometer were used as target cells, the numbers of the viable cells were 5.4 ± 0.4 × 104 cells at 2 weeks, and 16.7 ± 1.3 × 104 cells at 4 weeks in 100 ng/mL of SCF alone; 3.1 ± 0.1 × 104 cells at 2 weeks, and 3.7 ± 0.5 × 104 cells at 4 weeks in 100 ng/mL of SCF+10−7 mol/L ATRA.
Effects of ATRA on mast cell growth by CD34+ cord blood cells.
CD34+ cord blood cells (1 × 104) were cultured with 100 ng/mL of SCF and/or ATRA at 10−9mol/L to 10−7 mol/L. The viable cells were enumerated until 4 weeks. The results shown are from 1 representative experiment of 3. Similar results were obtained in the other 2 experiments. Significantly different from SCF alone (*P < .0001).
Effects of ATRA on mast cell growth by CD34+ cord blood cells.
CD34+ cord blood cells (1 × 104) were cultured with 100 ng/mL of SCF and/or ATRA at 10−9mol/L to 10−7 mol/L. The viable cells were enumerated until 4 weeks. The results shown are from 1 representative experiment of 3. Similar results were obtained in the other 2 experiments. Significantly different from SCF alone (*P < .0001).
We attempted to elucidate the direct action of ATRA on the SCF-dependent generation of mast cells by hematopoietic progenitors, using single-cell culture studies. The results are presented in Table2. One sixth of the CD34+c-kit+ cells responded to SCF to form colonies at 4 weeks of culture, in which almost all constituent cells reacted with antitryptase MoAb. The addition of ATRA diminished the number of mast cell colonies grown by SCF at 4 weeks. However, the inhibitory effects of 10−9 mol/L to 10−8 mol/L ATRA were modest at the early stage of culture.
Effects of all-trans retinoic acid on mast cell colony growth by CD34+ c-kit+ cord blood cells
| Stimuli . | Number of Mast Cell Colonies . | |||
|---|---|---|---|---|
| 1 wk . | 2 wks . | 3 wks . | 4 wks . | |
| SCF | 7 | 13 | 17 | 17 |
| SCF + ATRA (10−9 mol/L) | 0 | 8 | 8 | 4 |
| SCF + ATRA (10−8 mol/L) | 0 | 9 | 7 | 3 |
| SCF + ATRA (10−7 mol/L) | 0 | 2 | 1 | 0 |
| Stimuli . | Number of Mast Cell Colonies . | |||
|---|---|---|---|---|
| 1 wk . | 2 wks . | 3 wks . | 4 wks . | |
| SCF | 7 | 13 | 17 | 17 |
| SCF + ATRA (10−9 mol/L) | 0 | 8 | 8 | 4 |
| SCF + ATRA (10−8 mol/L) | 0 | 9 | 7 | 3 |
| SCF + ATRA (10−7 mol/L) | 0 | 2 | 1 | 0 |
ATRA = all-trans retinoic acid; SCF = stem cell factor.
The single CD34+ c-kit+ cord blood cells were sorted into the individual wells of a 96-well culture plate containing 100 ng/mL of SCF with or without different concentrations of ATRA, as described in “Materials and methods.” The number of cells in each well was serially counted until 4 weeks. Similar results were obtained in the other 2 experiments.
Finally, we examined whether ATRA exerted an inhibitory effect at the mast cell-committed precursor level. The 10-week cultured cells were plated at 5000 cells per dish containing serum-deprived methylcellulose culture medium supplemented with SCF or SCF+ATRA, ranging from 10−10 mol/L to 10−7 mol/L. As shown in Figure 9, SCF alone supported the formation of 105.5 ± 20.0 mast cell colonies and 201.0 ± 35.9 clusters. The addition of ATRA at a level of at least 10−9 mol/L caused a significant reduction of the SCF-dependent growth of mast cell colonies and clusters.
Effects of ATRA on the early phase of mast cell development.
Five thousand 10-week cultured mast cells were plated per dish containing serum-deprived methylcellulose culture medium supplemented with SCF, SCF+ATRA, SCF+IL-4, or SCF+IL-6. After 2 weeks, aggregates consisting of 30 or more cells were scored as mast cell colonies, and those of 10 to 29 cells as mast cell clusters. SCF, 100 ng/mL; ATRA, 10−10 mol/L to 10−7 mol/L; IL-4, 20 ng/mL; IL-6, 50 ng/mL. Numbers of mast cell colonies (black bars) and mast cell clusters (gray bars) are shown. The results shown are from 1 representative experiment of 3. Similar results were obtained in the other 2 experiments. Significantly different from SCF alone (*P < .002, **P < .0001).
Effects of ATRA on the early phase of mast cell development.
Five thousand 10-week cultured mast cells were plated per dish containing serum-deprived methylcellulose culture medium supplemented with SCF, SCF+ATRA, SCF+IL-4, or SCF+IL-6. After 2 weeks, aggregates consisting of 30 or more cells were scored as mast cell colonies, and those of 10 to 29 cells as mast cell clusters. SCF, 100 ng/mL; ATRA, 10−10 mol/L to 10−7 mol/L; IL-4, 20 ng/mL; IL-6, 50 ng/mL. Numbers of mast cell colonies (black bars) and mast cell clusters (gray bars) are shown. The results shown are from 1 representative experiment of 3. Similar results were obtained in the other 2 experiments. Significantly different from SCF alone (*P < .002, **P < .0001).
Discussion
We previously reported that SCF alone could support the selective growth of mast cells from CD34+ human cord blood cells in serum-deprived cultures.14 When 10-week-old cultured mast cells were used as target cells, both ATRA and 9-cis RA suppressed the generation of progeny under stimulation with SCF in a dose-dependent manner. At the optimal concentration, ATRA was as potent an inhibitor as 9-cis RA.
RA-mediated inhibition may be based on a decrease in proliferation rate rather than an increase in apoptotic death. On the basis of the clonal cell culture, the inhibitory effects of RA appear to be exerted at the mast cell-committed precursor level. Furthermore, the addition of 10−9 mol/L to 10−7 mol/L ATRA decreased the SCF-dependent mast cell production by CD34+or CD34+c-kit+ cord blood cells. The single-cell culture study showed a direct action of ATRA on the growth of mast cell colonies by the CD34+c-kit+ cells. The inhibitory effects of 10−9 mol/L to 10−8 mol/L ATRA, however, were modest at the early phase of culture. These results suggest that RAs are able to inhibit the production of human mast cells under stimulation with SCF, whereas the early steps in mast cell development appear to be less sensitive to RA.
It has been demonstrated that GM-CSF, TGF-β1, IL-4, and IL-6 are potent inhibitors for the mast cell growth supported by SCF.14,28-31 The concentrations of the 4 cytokines in the supernatant of the cultured mast cells under stimulation with SCF alone or SCF+ATRA were at levels below detectable limits. Moreover, none of the neutralizing antibodies for the factors influenced the progeny generation in the presence of SCF alone or SCF+ATRA. Therefore, the effects of ATRA on the mast cell development are unlikely to be mediated by these cytokines. RT-PCR analysis showed that RARα, RARβ, RXRα, and RXRβ mRNA were expressed in 10-week cultured mast cells. The addition of Ro 13-7410 at 10−10 mol/L to 10−7 mol/L decreased the number of mast cells grown by SCF. The activity of the RAR selective agonist at a level of at least 10−8 mol/L was comparable to that of ATRA at 10−7 mol/L. On the other hand, all concentrations of Ro 25-7386, except for 10−7 mol/L, failed to inhibit the mast cell growth. The addition of the RXR selective agonist at 10−7 mol/L to the culture containing SCF plus Ro 13-7410 at concentrations of 10−10 mol/L to 10−7 mol/L did not further enhance the reduction of the progeny production. These results imply that the antiproliferative effects of RAs on the SCF-dependent mast cell growth are mainly induced through RAR/RXR heterodimers. Among 3 of the synthetic retinoids selective for RAR subtypes, when used at 10−9 mol/L to 10−7 mol/L, only the RARα agonist was equivalent to ATRA (10−7 mol/L) in the inhibition of the mast cell growth. Conversely, the addition of 1 000- to 10 000-fold excess concentrations of the RARα antagonist significantly counteracted the ATRA-, 9-cis RA-, and RARα agonist-mediated reduction of the number of mast cells grown under stimulation with SCF. Thus, RARα appears to be the major endogenous RAR subtype for RA-dependent regulation of mast cell production. In our previous study, the addition of IL-6 or IL-4 resulted in a substantial decrease in the number of cultured mast cells grown in SCF.14 Nevertheless, the cultured mast cells exposed to SCF+IL-6 or SCF+IL-4 for 2 weeks showed an apparent increment in the intracellular histamine level compared with those exposed to SCF alone. Additionally, the percentage of chymase+ cells was higher in the culture containing IL-6 or IL-4. This may be due to the enhancement of chymase expression mediated by these cytokines, because either IL-6 or IL-4 was required for the induction of chymase+ cells from 4-week cultured cells under stimulation with SCF (unpublished data). In this study, both ATRA and 9-cis RA, when used at 10−7 mol/L, inhibited the SCF-dependent mast cell generation to a greater extent than IL-4 or IL-6, alone or in combination. It is of particular interest that both of the RAs (10−7 mol/L) decreased the histamine concentration in the cultured mast cells by approximately 50%. In addition, the RAs did not influence the chymase expression. Therefore, RA appears to function as a negative regulator for both the multiplication and intracellular histamine level of human mast cells. Our results may provide a new strategy targeting the production process of mast cells for prophylactic treatment of allergic disorders.
Supported by grants-in aid nos. 11670753 and 09041178 from the Ministry of Education of Japan.
Reprints:Kenichi Koike, Department of Pediatrics, Shinshu University School of Medicine, 3-1-1, Asahi, Matsumoto, 390-8621, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal