Loss of long-term hematopoietic stem cell function in vitro is associated with cell cycle progression. To determine whether cytokine-induced proliferation also limits the rate of short-term engraftment and potential clinical utility of ex vivo expanded hematopoietic cells, murine Sca-1+c-kit+Lin− cells were cultured in interleukin-6 (IL-6), IL-11, granulocyte colony-stimulating factor (G-CSF), stem cell factor, flk-2 ligand, and thrombopoietin for 7 days. Cells amplified 2000-fold were then stained with Hoechst 33342, separated into G0/G1 (72% ± 3%) or S/G2/M (27% ± 3%) fractions by flow sorting, and injected into lethally irradiated mice. Although long-term (more than 6 months) engraftment of lymphoid and myeloid lineages was greater in primary and secondary recipients of expanded cells residing in G0/G1 at the time of transplantation, there were no noted differences in the short-term (less than 6 weeks) recovery kinetics of circulating blood cells. When hematopoietic cells were expanded in cultures containing the tetrapeptide stem cell inhibitor N-Acetyl-Ser-Asp-Lys-Pro (AcSDKP) to reduce progenitor cycling prior to transplantation, again there were no differences observed in short-term reconstitution by inhibited or uninhibited cells. Interestingly, AcSDKP significantly accelerated engraftment by expanded hematopoietic cells when administered in vivo at the time of transplantation. Leukocytes recovered to 20% of normal levels approximately 1 week faster, and thrombocytopenia was largely abrogated in AcSDKP-treated versus untreated mice. Therefore, while AcSDKP can accelerate the engraftment of ex vivo expanded hematopoietic progenitors, which suggests a relatively simple approach to improve their clinical utility, its effects appear unrelated to cell cycle arrest.
Cancer patients receiving high-dose chemotherapy and/or radiotherapy followed by hematopoietic stem cell (HSC) transplantation typically endure between 10 days and 5 weeks of profound neutropenia and thrombocytopenia, depending on the number and source of HSCs infused.1-4 During this time, infections and clotting disorders can be controlled by the administration of antibiotics, hematopoietic growth factors, and platelet transfusions, but none of these treatments completely abrogate the depression in blood cell counts or usefully hasten hematopoietic recovery. In recent years, several clinical studies have examined the possibility that HSCs stimulated with recombinant cytokines in vitro may generate partially differentiated progeny able to mediate more rapid hematopoietic rescue.5-10 Although moderately efficacious in mice,11-13 this cell-based therapy has not yet been proven to be consistently beneficial in humans and underscores our currently limited understanding of the role of different classes of hematopoietic cells in early engraftment and potential changes in their transplantation potential during ex vivo culture. For example, although expanded cells can accelerate hematological recovery by 1-2 weeks when transplanted into lethally irradiated mice, engraftment is slower than predicted from the large number of in vitro colony-forming cells (CFCs) and spleen colony-forming units (CFU-S) infused.12,14 One possible explanation for this discrepancy is provided by our recent demonstration that CFCs generated in vitro acquire a homing defect that reduces by 10-fold their ability to localize to hematopoietic organs after intravenous infusion.15 It will be critical to resolve this issue before further clinical testing of expanded hematopoietic cells because their functional potency may be severely compromised even if large numbers of primitive cells can be generated in culture.
Several recent studies indicate that the entry of cultured HSCs into active cell cycle dramatically reduces their homing ability and subsequent long-term repopulating potential. For example, a 2-hour preincubation of murine bone marrow (BM) cells with either interleukin-3 (IL-3) or IL-3 plus IL-12 and stem cell factor (SCF) substantially decreases the seeding of all subsets of cobblestone area–forming cells (CAFCs) to both the BM and spleen.16 A similar decline in the seeding efficiency of murine CFU-S isolated from regenerating marrow after hydroxyurea treatment was noted 20 years ago.17 Human CD34+–mobilized peripheral blood cells that traverse from G0 to G1 over a 36-hour incubation with IL-3, SCF, and flk-2 ligand also lose their capacity to regenerate hematopoiesis in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice.18 Of critical importance to the successful clinical application of cultured hematopoietic cells, these effects on engraftment are not irreversible. Rather, they constitute a plastic feature associated with the cyclic oscillation of expanding stem/progenitor cells between proliferation and quiescence.19 It is not known whether similar deleterious effects of cell cycling also extend to cells that mediate early hematopoietic reconstitution. If so, the seeding and early engraftment kinetics of vigorously proliferating stem and progenitor cells generated in culture might be improved by transiently inhibiting their cycling immediately prior to transplantation.
In the present study, we tested this hypothesis using N-Acetyl-Ser-Asp-Lys-Pro (AcSDKP) as a candidate stem cell inhibitor that may mediate such effects on short-term engraftment. This tetrapeptide, which is naturally present in serum, can protect mice against lethal doses of cytosine arabinoside (Ara-C) by preventing the entry of CFU-S into S phase.20 In Ara-C–treated patients, administration of AcSDKP also decreases the depth of granulocyte and platelet nadirs.21 Recently, Suzuki et al22 showed that AcSDKP increased the survival and engraftment of lethally irradiated mice transplanted with a normally limiting dose of unfractionated BM cells. This effect was mediated by an increased localization of CFU-S and granulocyte-macrophage colony-forming units (CFU-GM) to the marrow as early as 1 day after transplantation,22suggesting an effect on the homing of primitive hematopoietic cells.
Therefore, we stimulated highly enriched Sca-1+c-kit+Lin− murine HSCs in serum-free suspension cultures optimized for maximal generation of clonogenic progeny. Ex vivo expanded CFCs (almost entirely in S phase) were then inhibited by in vitro exposure to AcSDKP. When we compared the rate of short-term hematopoietic reconstitution in mice that were transplanted with arrested or unmanipulated expanded cells, no beneficial effects of cell cycle down-modulation were noted. This lack of effect of cell cycle activation on short-term engraftment potential was supported by additional experiments in which ex vivo expanded cells in G0/G1 or S/G2/M were physically separated before transplantation by cell sorting. Both fractions exhibited identical short-term engraftment kinetics, but only expanded cells in G0/G1 retained high levels of long-term multilineage repopulating ability. Interestingly, and in marked contrast to the inability of AcSDKP to improve engraftment of in vitro–treated cells, in vivo administration of the tetrapeptide significantly accelerated leukocyte and platelet recovery in lethally irradiated mice transplanted with ex vivo expanded cells. Our data indicate that the potential clinical utility of expanded hematopoietic cells for mediating rapid hematological recovery after myeloablative cancer therapy can be improved by the relatively simple treatment of recipients with a bioactive peptide. Although the mechanism of AcSDKP action is currently undefined, it appears unrelated to its ability to inhibit cell cycle entry.
Materials and methods
Animals
Marrow donors were 5- to 8-week-old C57BL/6 (B6) mice (Ptprca [Ly-5.2]). Age-matched B6 or B6.SJL (Ptprcb[Ly-5.1]) mice were used as recipients as indicated. Mice were purchased via the National Cancer Institute Animal Program from Charles River Laboratories (Frederick, MD) and maintained under specific pathogen-free conditions in the animal facility of the Chandler Medical Center (University of Kentucky, Lexington, KY).
Enrichment of hematopoietic stem cells
Sca-1+c-kit+Lin− cells were isolated from the BM of B6 mice injected 1 day earlier with 150 mg/kg 5-fluorouracil (5-FU; Roche Laboratories, Nutley, NJ) as previously described15 using a dual-laser fluorescence-activated cell sorter (FACSVantage; Becton Dickinson Immunocytometry Systems, San Jose, CA). Reanalysis of enriched cells immediately after sorting indicated a purity typically greater than 90%.
Competitive repopulation assays
The frequency of competitive long-term repopulating units (CRU) among freshly isolated Sca-1+c-kit+Lin− marrow cells was determined by limiting-dilution analysis in vivo (3, 10, 30, or 100 cells/mouse) as previously described.15 16 A level of at least 5% donor-derived (Ly-5.2+) peripheral blood cells detectable among B (B220+) and T (Thy-1+) lymphoid compartments and myeloid (Mac-1/Gr-1+) cells 10 weeks after transplantation of Ly-5.1 recipients was defined as the threshold for positive engraftment for maximum likelihood analysis.
Ex vivo expansion of sorted stem cells
Sorted Sca-1+c-kit+Lin−cells were cultured (2 × 103/mL) in serum-free medium (StemPro-34; Life Technologies, Gaithersburg, MD) containing 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mmol/L L-glutamine, and 0.05 mmol/L 2-mercaptoethanol (2-ME). The medium was supplemented with the following recombinant cytokines: 10 ng/mL murine IL-6 (Peprotech, Rocky Hill, NJ) and human G-CSF (Amgen, Thousand Oaks, CA); 50 ng/mL murine IL-11; and 100 ng/mL each of murine SCF, murine thrombopoietin (TPO) (R&D Systems, Minneapolis, MN), and human flk-2 ligand (FLK) (Peprotech). Cultures were incubated undisturbed at 37°C and harvested after 7 days. In some experiments AcSDKP (purchased initially from Sigma [St Louis, MO] and later generously provided by Biomeasure [Milford, MA]) was added daily to duplicate cultures to a final concentration of 10−8 to 10−18 mol/L (with 100-fold serial dilutions between) beginning on day 1.
CFC assays
Unseparated, sorted, or ex vivo expanded BM cells were assayed for CFCs as previously described.12 The proportion of progenitors in S phase was determined by incubating 2 aliquots of cells for 1 hour at 37°C in Dulbecco's modified Eagle's medium (DMEM) containing 2% fetal bovine serum (FBS) with or without 200 μg/mL hydroxyurea (HU)(Sigma). Cells were then washed and plated at 104 or 2 × 103 cells/dish, respectively. Colonies were counted 12 days later, and the percent of CFCs in S phase (%S) was calculated using the following formula:
where x = colony counts after exposure to HU, and y = colony counts after incubation without HU.
Cell cycle fractionation by FACS
Ex vivo expanded BM cells were separated into fractions in G0/G1 or S/G2/M phases of the cell cycle as follows. Cells were resuspended at approximately 5 × 106/mL in Hank's balanced salt solution (HBSS) containing 10% FBS, 1 mg/mL glucose, 20 mmol/L HEPES (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid), and 5 μg/mL Hoechst 33342 (HO) (Molecular Probes, Eugene, OR) and incubated in the dark at 37°C for 30 minutes. Cells were then washed twice in the same buffer without HO dye and analyzed on a FACSVantage flow cytometer equipped with an Enterprise II laser providing the 351-nm excitation wavelength for HO. HO fluorescence was detected with a 424/44-nm bandpass filter, and sort windows were established using linear gates. Cells were kept on ice during sorting and protected from light. In preliminary experiments, 50 μmol/L Verapamil (Sigma) was included in the medium during HO staining and sorting to prevent contamination of the G0/G1fraction by cycling cells that have effluxed the dye. No differences were noted in the cloning efficiency or engraftment of the sorted fractions with or without Verapamil; so it was not routinely included in this method. Finally, cells were assayed before and after HO staining to verify that these procedures were not toxic to progenitors able to generate colonies of mature myeloid and erythroid cells in semisolid medium.
Measurement of short-term hematopoietic reconstitution kinetics and long-term repopulating ability
Just prior to transplantation, B6.SJL mice were exposed to 9 Gy total body γ-irradiation administered in 2 doses of 4.5 Gy approximately 3 hours apart. Ablated animals were injected intravenously with either 103 freshly sorted Sca-1+c-kit+Lin− BM cells, the entire product of 103 sorted cells obtained after ex vivo expansion as described, or 106 expanded BM cells that had been separated into G0/G1 or S/G2/M populations by FACS. When AcSDKP was administered, it was mixed with the cells to be injected immediately prior to transplantation, and each animal received 10 or 100 μg. After transplantation, mice were bled from the retro-orbital sinus on days 6, 9, 12, 15, 18, 25, 32, 42, 56, and 120. Until day 25, only half of the mice in each cohort were analyzed alternately each time so that no individual animal was bled more frequently than every 7 days. Circulating leukocyte, erythrocyte, and platelet counts were measured by analysis of 40 μL blood using a System 9118+Hematology Series Cell Counter (BioChem ImmunoSystems, Allentown, PA). At selected times, blood samples were also stained with a donor-specific anti–Ly-5.2+ monoclonal antibody (mAb) (clone ALI4A2) conjugated with fluorescein isothiocyanate (FITC) and phycoerythrin-conjugated (PE-conjugated) mAbs specific for B lymphocytes (anti-CD45R/B220; clone RA3-6B2); T lymphocytes (anti–Thy-1.2; clone 30H12); or granulocytes (anti-Ly6G/Gr-1; clone RB6-8C5) and macrophages (anti-CD11b/Mac-1; clone M1/70). Multilineage progeny of transplanted stem and/or progenitor cells were quantitated using a FACScan instrument (Becton Dickinson). After 120 days, BM cells were assayed for CFCs and injected into lethally irradiated B6.SJL mice (0.5 femurs/mouse) for secondary repopulation. Secondary mice were analyzed 10 weeks later for donor-derived peripheral blood leukocytes and BM CFCs as described above.
Statistical analysis
The statistical significance of differences between means was assessed using the 2-tailed Student t test and assuming unequal variances.
Results
Delayed hematopoietic reconstitution by enriched Sca-1+c-kit+Lin− stem cells
In previous studies, we established the efficacy of using large numbers of ex vivo expanded murine hematopoietic cells as a cellular therapy to support the normally delayed engraftment observed after transplantation of highly enriched HSCs.12 Toward optimization of this preclinical model of stem cell expansion and to further define the parameters that influence the engraftment of cultured hematopoietic cells, we initiated the present study with minor modifications to our earlier method. First, we used a different stem cell sorting procedure to isolate Sca-1+c-kit+Lin− cells (instead of Thy-1loSca-1+H-2Khicells used previously12) that are highly enriched in competitive repopulating units. Limiting-dilution assays established that Sca-1+c-kit+Lin− cells contain 1 CRU per 15 cells (95% confidence limits, 1 per 12 to 1 per 23 cells)16 (data not shown). More mature progenitors such as CFCs (approximately 60 per 103 cells) and CFU-S (approximately 3 per 103 cells on day 12 and 0 per 103 cells on day 8) are largely depleted.15
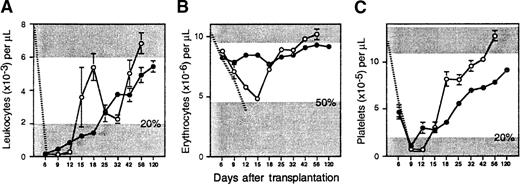
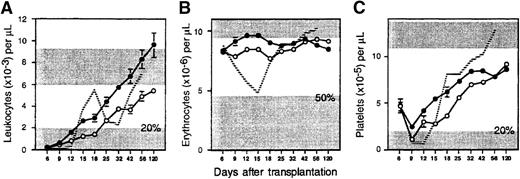
To determine if Sca-1+c-kit+Lin− cells exhibit delayed hematopoietic reconstitution kinetics, as we have demonstrated previously with other enriched stem cell populations,12 103 sorted cells (containing at least 67 CRU) were transplanted into lethally irradiated Ly-5 congenic hosts. Circulating blood cells were counted once or twice per week from 6 days to 4 months after transplantation to measure early hematopoietic reconstitution kinetics. Transplanted mice required approximately 13 days to recover 20% normal levels of leukocytes and platelets (Figure1). Leukocyte recovery was biphasic, characterized by a transient fall in counts to a nadir just above 20% of normal on day 32, but reached normal levels by day 56. Erythrocytes declined to a nadir just above 50% of normal values on day 15, but they also recovered slowly thereafter. Notably, normal blood counts were not achieved for any compartment until after approximately 7 weeks.
Hematopoietic reconstitution kinetics of enriched stem cells and their ex vivo expanded progeny.
Lethally irradiated B6.SJL mice were injected with 103 Ly-5 congenic Sca-1+c-kit+Lin+ BM cells (open circles; n = 7 mice from 3 experiments), or their entire expanded progeny (approximately 2 × 106 cells) generated after 7 days of culture in IL-6, IL-11, G-CSF, SCF, FLK, and TPO (closed circles; n = 37 mice from 5 experiments). Shown are the mean ± SEM (standard error of the mean) number of (A) peripheral blood leukocytes, (B) erythrocytes, and (C) platelets counted on the indicated days after transplantation. The ranges of blood counts in normal B6.SJL mice are defined by the upper shaded areas; the upper bounds for leukocytes (9.2 × 103/μL) and erythrocytes (11.4 × 106/μL) are off scale. The radiation-induced decline of circulating cells in controls that did not receive a transplant is denoted, until their deaths, by the hatched lines. The lower shaded areas indicate minimal engraftment thresholds for leukocytes, 2 × 103/μL (20% of normal); erythrocytes, 4.7 × 106/μL (50% of normal); and platelets, 2 × 105/μL (20% of normal). Standard errors not shown are too small for the scale used.
Hematopoietic reconstitution kinetics of enriched stem cells and their ex vivo expanded progeny.
Lethally irradiated B6.SJL mice were injected with 103 Ly-5 congenic Sca-1+c-kit+Lin+ BM cells (open circles; n = 7 mice from 3 experiments), or their entire expanded progeny (approximately 2 × 106 cells) generated after 7 days of culture in IL-6, IL-11, G-CSF, SCF, FLK, and TPO (closed circles; n = 37 mice from 5 experiments). Shown are the mean ± SEM (standard error of the mean) number of (A) peripheral blood leukocytes, (B) erythrocytes, and (C) platelets counted on the indicated days after transplantation. The ranges of blood counts in normal B6.SJL mice are defined by the upper shaded areas; the upper bounds for leukocytes (9.2 × 103/μL) and erythrocytes (11.4 × 106/μL) are off scale. The radiation-induced decline of circulating cells in controls that did not receive a transplant is denoted, until their deaths, by the hatched lines. The lower shaded areas indicate minimal engraftment thresholds for leukocytes, 2 × 103/μL (20% of normal); erythrocytes, 4.7 × 106/μL (50% of normal); and platelets, 2 × 105/μL (20% of normal). Standard errors not shown are too small for the scale used.
Four months after transplantation, virtually all of the circulating B (98%) and T (77%) lymphocytes and myeloid cells (91%) in these mice were derived from donor stem cells (Table1). Femoral cellularity and CFC content had also recovered slightly above normal. When BM from reconstituted animals was transplanted into secondary lethally irradiated B6.SJL mice, high levels of engraftment were again achieved in both lymphoid (approximately 50%) and myeloid (66%) compartments after 10 weeks, and marrow progenitors were regenerated to 90% of normal numbers. Thus, Sca-1+c-kit+Lin− cells represent very primitive HSCs with multipotent, albeit delayed, repopulating ability.
Multilineage hematopoietic reconstitution potential of enriched HSCs and their ex vivo expanded progeny
| Engrafted Compartment . | Sca-1+c-kit+Lin− . | Expanded Product . | Expanded Product + AcSDKP . | |||
|---|---|---|---|---|---|---|
| 1° Recipients (n = 7) . | 2° Recipients (n = 7) . | 1° Recipients (n = 25) . | 2° Recipients (n = 19) . | 1° Recipients (n = 20) . | 2° Recipients (n = 7) . | |
| Peripheral blood, % donor: | ||||||
| Total leukocytes | 91 ± 2 | 75 ± 4 | 85 ± 3 | 54 ± 6 | 93 ± 1 | 44 ± 3 |
| B cells | 98 ± 1 | 50 ± 9 | 93 ± 2 | 66 ± 6 | 98 ± 1 | 53 ± 4 |
| T cells | 77 ± 4 | 51 ± 4 | 72 ± 3 | 48 ± 5 | 81 ± 3 | 37 ± 3 |
| Myeloid cells | 91 ± 3 | 66 ± 7 | 76 ± 4 | 40 ± 7 | 87 ± 3 | 29 ± 6 |
| Marrow, % of normal: | ||||||
| Cells/femur | 115 ± 14 | 42 ± 11 | 70 ± 7 | 47 ± 5 | 65 ± 7 | 39 ± 5 |
| CFCs/femur | 122 ± 11 | 90 ± 15 | 230 ± 23 | 99 ± 13 | 186 ± 25 | 84 ± 11 |
| Engrafted Compartment . | Sca-1+c-kit+Lin− . | Expanded Product . | Expanded Product + AcSDKP . | |||
|---|---|---|---|---|---|---|
| 1° Recipients (n = 7) . | 2° Recipients (n = 7) . | 1° Recipients (n = 25) . | 2° Recipients (n = 19) . | 1° Recipients (n = 20) . | 2° Recipients (n = 7) . | |
| Peripheral blood, % donor: | ||||||
| Total leukocytes | 91 ± 2 | 75 ± 4 | 85 ± 3 | 54 ± 6 | 93 ± 1 | 44 ± 3 |
| B cells | 98 ± 1 | 50 ± 9 | 93 ± 2 | 66 ± 6 | 98 ± 1 | 53 ± 4 |
| T cells | 77 ± 4 | 51 ± 4 | 72 ± 3 | 48 ± 5 | 81 ± 3 | 37 ± 3 |
| Myeloid cells | 91 ± 3 | 66 ± 7 | 76 ± 4 | 40 ± 7 | 87 ± 3 | 29 ± 6 |
| Marrow, % of normal: | ||||||
| Cells/femur | 115 ± 14 | 42 ± 11 | 70 ± 7 | 47 ± 5 | 65 ± 7 | 39 ± 5 |
| CFCs/femur | 122 ± 11 | 90 ± 15 | 230 ± 23 | 99 ± 13 | 186 ± 25 | 84 ± 11 |
Lethally irradiated Ly-5.1 mice were transplanted with 103 Sca-1+c-kit+Lin−BM cells (Ly-5.2+) or their entire expanded progeny (approximately 2 × 106 cells) generated after 1 week of serum-free culture in IL-6, IL-11, G-CSF, SCF, FLK, and TPO. Some recipients of expanded cells were simultaneously injected with 10 or 100 μg AcSDKP. Primary mice were analyzed after 4 months. Secondary recipients, receiving a transplant of primary BM cells (0.5 femur/mouse), were analyzed 10 weeks later. Data shown are the mean ± SEM percentage of Ly-5.2+ cells detected in each blood cell compartment and the percentage of normal femoral cellularity and CFC content compared to 4 age-matched B6.SJL mice.
Ex vivo expansion of enriched hematopoietic stem cells generates progeny with more rapid engraftment kinetics
To promote the generation from enriched stem cells of hematopoietic cells with potentially more rapid engraftment kinetics, Sca-1+c-kit+Lin−cells were cultured in StemPro-34 serum-free medium containing a more extensive cocktail of cytokines (IL-6, IL-11, G-CSF, SCF, FLK, and TPO) than used in our previous studies.12 This combination was selected on the basis of preliminary experiments in which 10 different cytokine combinations, added to 5 different commercially available serum-free media, were compared for their ability to support a maximal generation of total nucleated cells and CFCs from enriched HSCs (data not shown). After 7 days, the entire expanded progeny of 103Sca-1+c-kit+Lin− cells (approximately 2 × 106 cells containing 91 600 ± 11 600 CFCs) were transplanted into 37 lethally irradiated mice (5 experiments). Compared to freshly isolated HSCs, posttransplant anemia was now completely eliminated, and the duration of thrombocytopenia was reduced by approximately 4 days (Figure 1). Leukocyte counts were also moderately elevated prior to day 12. However, the transient burst in leukocyte production which peaked on day 18 after transplantation of fresh stem cells was lost (presumably due to differentiation of myeloid progenitors in vitro), so the overall time to sustained leukocyte recovery was extended by approximately 1 week.
Four months after transplantation, 85% of leukocytes were derived from expanded cells and comprised the majority of B (93%) and T (72%) lymphocytes and granulocytes and/or monocytes (76%) (Table 1). The levels of B (P < .05) and myeloid (P < .005) engraftment were significantly lower than observed with uncultured Sca-1+c-kit+Lin− cells, indicating that expanded cells were somewhat compromised in long-term hematopoietic potential. Marrow cellularity recovered to only 70% of normal, but each femur contained 2.3-fold more CFCs than were present in either normal animals or recipients of uncultured HSCs. Marrow from recipients of expanded cells also retained the ability to regenerate multilineage progeny and CFCs 10 weeks after retransplantation into secondary hosts, but total leukocyte and myeloid engraftment was again impaired (P < .007 and P < .02, respectively) compared with marrow from recipients of fresh Sca-1+c-kit+Lin− cells (Table1). Thus, expansion of enriched stem cells under these optimized conditions rendered progeny able to mediate moderately earlier hematopoietic reconstitution, albeit with some reduction in long-term potential.
Cell cycle activation compromises the long-term, but not short-term, hematopoietic reconstitution potential of ex vivo expanded progenitors
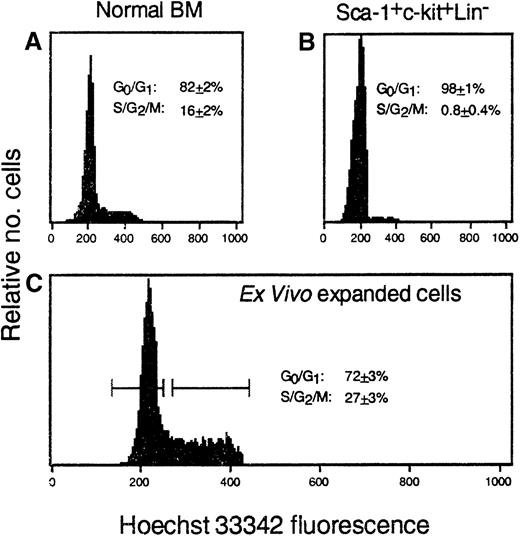
Although ex vivo expanded hematopoietic cells engrafted moderately faster than freshly isolated HSCs in these and our earlier studies, reconstitution was still slower than expected considering their high progenitor content. Several recent studies indicate that cycling stem cells exhibit diminished long-term repopulating potential.18,19 23 To examine the possibility that cytokine-induced proliferation of hematopoietic cells may compromise the short-term repopulating ability and potential clinical utility of ex vivo expanded cells, we initially measured the number of expanded CFCs in S phase. In normal BM, 53% ± 1% of clonogenic progenitors were killed by exposure to HU (5 experiments). In striking contrast, 90% ± 1% of CFCs generated from Sca-1+c-kit+Lin− cells after 7 days of culture in IL-6, IL-11, G-CSF, SCF, FLK, and TPO were killed by HU (15 experiments). To compare the functional properties of expanded hematopoietic cells that were either proliferating or not proliferating at the time of transplantation, cultured cells were stained with HO dye and separated into G0/G1 and S/G2/M fractions. In normal BM, approximately 82% and 16% of all nucleated cells (n = 9) were in G0/G1 and S/G2/M, respectively (Figure 2). As expected, virtually all Sca-1+c-kit+Lin− cells (98%) isolated from day 1 post–5-FU BM were in G0/G1. S/G2/M cells increased significantly during culture and represented almost one-third of all expanded cells (Figure 2). Note that because HO staining measures the cycling (DNA content) of all nucleated cells in these cultures, many of which are nearing terminal differentiation (entering G0/G1) and only approximately 5% of which are detectable as CFCs, this measurement is not directly comparable to the functional assessment of CFC cycling (90%) by HU exposure. Nevertheless, both types of experiments provide evidence of dramatic cycle activation of ex vivo expanded hematopoietic cells.
Ex vivo expanded hematopoietic cells are actively cycling.
The proportion of nucleated cells in G0/G1 or S/G2/M phases of the cell cycle was determined by HO staining of (A) normal murine BM, (B) Sca-1+c-kit+Lin+ cells isolated from day 1 post–5-FU BM, or (C) these cells' progeny generated after ex vivo expansion. Numbers in each panel represent the mean ± SEM of 3-9 measurements per population. The horizontal bars (C) represent the gates used for sorting expanded cells into G0/G1 or S/G2/M fractions prior to transplantation.
Ex vivo expanded hematopoietic cells are actively cycling.
The proportion of nucleated cells in G0/G1 or S/G2/M phases of the cell cycle was determined by HO staining of (A) normal murine BM, (B) Sca-1+c-kit+Lin+ cells isolated from day 1 post–5-FU BM, or (C) these cells' progeny generated after ex vivo expansion. Numbers in each panel represent the mean ± SEM of 3-9 measurements per population. The horizontal bars (C) represent the gates used for sorting expanded cells into G0/G1 or S/G2/M fractions prior to transplantation.
Lethally irradiated Ly-5.1 mice were transplanted with 106(Ly-5.2+) G0/G1 or S/G2/M expanded cells, and the rate of short-term hematopoietic reconstitution was measured by temporal analysis of circulating blood counts, as described above. Figure3 shows that these fractions did not differ significantly from each other with respect to their short-term engraftment kinetics. However, as demonstrated previously by others in different culture systems, proliferating and nonproliferating expanded cells did differ significantly in their contribution to lympho-hematopoiesis at later times. Four months after transplantation, both populations contributed measurably to B, T, and myeloid cells, but recipients of S/G2/M cells exhibited significantly lower engraftment that was particularly pronounced in the myeloid lineage (Table 2). Similar defects in repopulating ability were noted in secondary animals transplanted 10 weeks previously with primary marrow regenerated from S/G2/M cells. These data demonstrate that the deleterious effects of cycle activation on hematopoietic cell function after ex vivo expansion are limited only to their long-term repopulating ability. The short-term hematopoietic reconstitution potential of cycling expanded cells is not reduced.
Lack of cell cycle effects on short-term reconstitution by ex vivo expanded hematopoietic cells.
Lethally irradiated B6.SJL mice were injected with either 106 G0/G1 expanded cells (open squares; n = 23 mice from 4 experiments); 106S/G2/M expanded cells (closed squares; n = 10 mice from 4 experiments); or 2 × 106 unfractionated expanded cells that had been subjected to cell cycle arrest by in vitro exposure to AcSDKP (open circles; n = 13 mice from 2 experiments). Shown are the mean ± SEM number of (A) peripheral blood leukocytes, (B) erythrocytes, and (C) platelets counted on the indicated days after transplantation. Details of normal counts and thresholds for engraftment, as denoted by the shaded areas, are described in the Figure 1 legend.
Lack of cell cycle effects on short-term reconstitution by ex vivo expanded hematopoietic cells.
Lethally irradiated B6.SJL mice were injected with either 106 G0/G1 expanded cells (open squares; n = 23 mice from 4 experiments); 106S/G2/M expanded cells (closed squares; n = 10 mice from 4 experiments); or 2 × 106 unfractionated expanded cells that had been subjected to cell cycle arrest by in vitro exposure to AcSDKP (open circles; n = 13 mice from 2 experiments). Shown are the mean ± SEM number of (A) peripheral blood leukocytes, (B) erythrocytes, and (C) platelets counted on the indicated days after transplantation. Details of normal counts and thresholds for engraftment, as denoted by the shaded areas, are described in the Figure 1 legend.
Reduced long-term hematopoietic reconstitution potential of expanded cells that are cycling at transplantation
| . | G0/G1 . | S/G2/M . | P Value . |
|---|---|---|---|
| 1° Recipients, n: | 15 | 6 | |
| Total leukocytes | 77 ± 5 | 39 ± 14 | .04 |
| B cells | 87 ± 5 | 48 ± 15 | .05 |
| T cells | 62 ± 4 | 29 ± 11 | .03 |
| Myeloid cells | 65 ± 7 | 22 ± 11 | .007 |
| 2° Recipients, n: | 9 | 5 | |
| Total leukocytes | 52 ± 8 | 15 ± 12 | .04 |
| B cells | 66 ± 8 | 21 ± 16 | .05 |
| T cells | 39 ± 5 | 14 ± 10 | .06 |
| Myeloid cells | 40 ± 12 | 22 ± 17 | .04 |
| . | G0/G1 . | S/G2/M . | P Value . |
|---|---|---|---|
| 1° Recipients, n: | 15 | 6 | |
| Total leukocytes | 77 ± 5 | 39 ± 14 | .04 |
| B cells | 87 ± 5 | 48 ± 15 | .05 |
| T cells | 62 ± 4 | 29 ± 11 | .03 |
| Myeloid cells | 65 ± 7 | 22 ± 11 | .007 |
| 2° Recipients, n: | 9 | 5 | |
| Total leukocytes | 52 ± 8 | 15 ± 12 | .04 |
| B cells | 66 ± 8 | 21 ± 16 | .05 |
| T cells | 39 ± 5 | 14 ± 10 | .06 |
| Myeloid cells | 40 ± 12 | 22 ± 17 | .04 |
The expanded progeny of Sca-1+c-kit+Lin− BM cells (Ly-5.2+) were labeled with HO dye, sorted into G0/G1 or S/G2/M fractions, and injected into lethally irradiated Ly-5.1 mice (106cells/mouse). The data shown are the mean ± SEM percentage of Ly-5.2+ blood cells measured 4 months later (pooled data from 3 experiments). Primary BM cells were then transplanted into secondary mice (0.5 femur/mouse), which were analyzed after 10 weeks.P values represent comparisons of engraftment by G0/G1 and S/G2/M cells.
AcSDKP accelerates the engraftment of ex vivo expanded hematopoietic cells through a mechanism unrelated to cell cycle inhibition
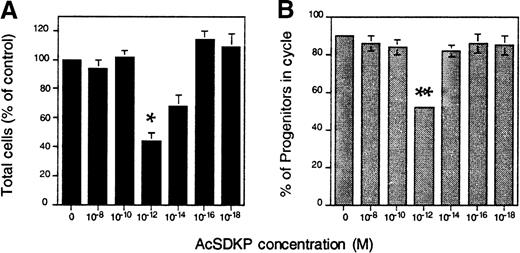
To further examine the effects of cell cycling on the short-term repopulating ability of ex vivo expanded hematopoietic cells, we used the tetrapeptide AcSDKP to inhibit the cells' proliferation in culture. AcSDKP was selected for these experiments because of its ability to block proliferation of murine and human CFCs in vitro24,25 and its demonstrated ability to enhance the engraftment of normally limiting numbers of whole mouse BM cells in irradiated hosts.22Sca-1+c-kit+Lin− cells were expanded in IL-6, IL-11, G-CSF, SCF, FLK, and TPO, as described above. Beginning 1 day after culture initiation, AcSDKP was added daily to duplicate cultures to a final concentration of 10−18to 10−8 mol/L. Nucleated cells were counted after a total of 7 days, and the proportion of expanded CFCs in S phase was measured after exposure to HU. Because each of the 3 experiments performed yielded slightly different overall expansion, results were pooled and are expressed as a fraction of control values (Figure4). Maximal inhibition of CFC cycling was observed with 10−12 mol/L AcSDKP, resulting in a reduction from 90% in S phase, as observed in control cultures (without AcSDKP), to approximately 50% (P = .03). Net cell expansion was also reduced by approximately 40% (P = .009) compared with controls (Figure 4). As reported previously by others,25 the effective dose range was narrow, and this inhibitory effect was lost above or below 10−12mol/L.
AcSDKP inhibits the cycling of hematopoietic progenitors generated in vitro.
Sca-1+c-kit+Lin− cells were cultured (2 × 103/mL) in serum-free medium containing IL-6, IL-11, G-CSF, SCF, FLK, and TPO. Except for control cultures, AcSDKP was added daily to duplicate wells on days 1-6. Shown are (A) the mean ± SEM number of nucleated cells counted after 7 days and (B) the proportion of cycling CFCs in each culture assayed by plating cells in methylcellulose-based medium before or after exposure to HU, as described in the “Materials and methods.” The reduction in total nucleated cell production and CFC cycling in cultures containing 10−12 mol/L AcSDKP was statistically significant. (*indicates P < .01, and **indicatesP < .03.) The data given are pooled from 3 experiments.
AcSDKP inhibits the cycling of hematopoietic progenitors generated in vitro.
Sca-1+c-kit+Lin− cells were cultured (2 × 103/mL) in serum-free medium containing IL-6, IL-11, G-CSF, SCF, FLK, and TPO. Except for control cultures, AcSDKP was added daily to duplicate wells on days 1-6. Shown are (A) the mean ± SEM number of nucleated cells counted after 7 days and (B) the proportion of cycling CFCs in each culture assayed by plating cells in methylcellulose-based medium before or after exposure to HU, as described in the “Materials and methods.” The reduction in total nucleated cell production and CFC cycling in cultures containing 10−12 mol/L AcSDKP was statistically significant. (*indicates P < .01, and **indicatesP < .03.) The data given are pooled from 3 experiments.
To determine whether expanded cells that had been subjected to cycle inhibition exhibited any differences in their early hematopoietic reconstitution kinetics, 2 × 106cells (ie, the average number of cells generated from 103Sca-1+c-kit+Lin− cells in cultures without AcSDKP) were transplanted into individual lethally irradiated mice. The rate of leukocyte recovery in 13 mice (2 experiments) was essentially identical to that of mice transplanted with an equal number of hematopoietic cells expanded without AcSDKP (compare Figures 1A and 3A). Thus, cell cycle inhibition was certainly of no benefit and, in fact, appeared to be moderately deleterious to erythrocyte and platelet recovery (compare Figure 3B with Figure 1B, and Figure 3C with Figure 1C). These results were not unexpected given the lack of effect of cell cycle status on short-term engraftment, as determined from the HO sorting experiments above.
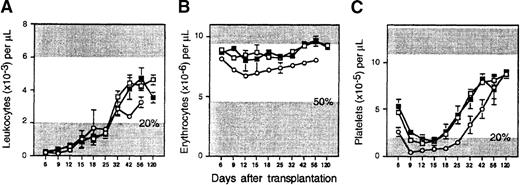
Suzuki et al22 demonstrated an improved rate of survival and recovery of circulating blood cells in mice injected simultaneously with normal BM cells and AcSDKP. This in vivo treatment was thus evaluated in a final attempt to hasten reconstitution by purified HSCs or their ex vivo expanded progeny. In 10 myeloablated animals injected with 103 sorted cells together with 10 μg AcSDKP (3 experiments), we did not observe any improvement in leukocyte, erythrocyte, or platelet regeneration kinetics; the results, identical to Sca-1+c-kit+Lin− cells in Figure 1, are not shown. An additional 20 mice (2 experiments) each received the entire expanded progeny of 103Sca-1+c-kit+Lin− cells (approximately 2 × 106 cells) and either 10 or 100 μg AcSDKP. The recovery of circulating blood cells was monitored as above and is compared in Figure 5 to the rate of hematopoietic reconstitution observed with fresh HSCs or expanded cells injected without the peptide. The 10 and 100 μg AcSDKP groups gave identical results and were thus pooled. Engraftment was clearly enhanced in mice that were treated with AcSDKP. Leukocyte and erythrocyte counts were significantly higher (P < .005) in treated versus untreated recipients of expanded cells on every day they were measured, from 9 days to 4 months after transplantation. As a result, white blood cells recovered permanently to 20% of normal counts within approximately 13 days. This recovery was approximately 9 days faster than in mice receiving a transplant of expanded cells without AcSDKP and contemporaneously with uncultured stem cells, but with the added benefit of elevated and sustained counts prior to reaching this threshold (Figure 5A). Indeed, when last analyzed on day 120, leukocyte counts in mice injected with expanded cells and AcSDKP were almost 2-fold higher than leukocyte counts in mice that received a transplant of expanded cells alone. Platelets also never declined below 20% of normal at any time after transplantation in mice injected with ex vivo expanded cells and AcSDKP. The improvement over untreated controls was significant on every time between day 9 and day 42 (P < .005) (Figure 5C).
In vivo treatment of transplant recipients with AcSDKP promotes the engraftment of ex vivo expanded hematopoietic cells.
Lethally irradiated mice were injected with the expanded progeny of Sca-1+c-kit+Lin− BM cells administered simultaneously with a single dose of 10 or 100 μg/mouse of AcSDKP. Recovery kinetics of (A) peripheral blood leukocytes, (B) erythrocytes, and (C) platelets were measured as described in Figure 1and are depicted by the closed circles (mean ± SEM values for 20 mice from 2 experiments). Hematopoietic reconstitution by freshly isolated Sca-1+c-kit+Lin− cells (hatched lines) or their expanded progeny transplanted without AcSDKP (open circles) is duplicated from Figure 1 for comparison (note scale change in panel A). Details of normal counts and thresholds for engraftment, as denoted by the shaded areas, are described in the Figure 1 legend.
In vivo treatment of transplant recipients with AcSDKP promotes the engraftment of ex vivo expanded hematopoietic cells.
Lethally irradiated mice were injected with the expanded progeny of Sca-1+c-kit+Lin− BM cells administered simultaneously with a single dose of 10 or 100 μg/mouse of AcSDKP. Recovery kinetics of (A) peripheral blood leukocytes, (B) erythrocytes, and (C) platelets were measured as described in Figure 1and are depicted by the closed circles (mean ± SEM values for 20 mice from 2 experiments). Hematopoietic reconstitution by freshly isolated Sca-1+c-kit+Lin− cells (hatched lines) or their expanded progeny transplanted without AcSDKP (open circles) is duplicated from Figure 1 for comparison (note scale change in panel A). Details of normal counts and thresholds for engraftment, as denoted by the shaded areas, are described in the Figure 1 legend.
AcSDKP also improved the ultimate level of engraftment by ex vivo expanded hematopoietic cells (Table 1). Four months after transplantation, these animals exhibited significantly higher levels of Ly-5.2+ leukocytes in peripheral blood than recipients of cultured cells not treated with the peptide (93% vs 85%;P < .03). Compared to the latter, a greater proportion of circulating B lymphocytes (98% vs 93%;P < .03) and T lymphocytes (81% vs 72%;P < .04) were also derived from cultured cells, but the slight improvement in myeloid engraftment (87% vs 76%) did not quite reach statistical significance (P = .06). As in recipients of expanded cells alone, BM CFCs recovered to higher levels in animals transplanted with expanded cells plus AcSDKP than in animals transplanted with uncultured HSCs (Table 1). Stem cells with secondary transplantation potential were also regenerated in the BM of these mice. Although the level of secondary engraftment in all lineages was slightly reduced compared with BM from recipients of expanded cells alone, this difference was not statistically significant (P = .09 to .24). Overall, these data support the feasibility of enhancing the clinical utility of cultured hematopoietic cells using AcSDKP. The mechanism of this action is yet to be defined, but it appears unrelated to the activity of AcSDKP as a cell cycle inhibitor.
Discussion
The ability of many cytokines to stimulate the proliferation and differentiation of primitive HSCs into progenitors of multiple lineages has been exploited in various in vitro systems to generate grafts of tailored composition, with the hope that such cells will support rapid hematopoietic rescue when transplanted into myeloablated patients. Unfortunately, despite significant advances in identifying culture conditions that support impressive amplification of progenitor cell numbers and, to a lesser degree, stem cells,26-29 the comparative lack of efficacy of ex vivo expanded human hematopoietic cells in numerous clinical trials has been disappointing.5,7,10 Nevertheless, the success of such cell therapies in mice provides reason for optimism that this strategy is sound11-13 and validates a preclinical model for detailed study of the effect of in vitro manipulation on transplantable hematopoietic cells.
Using such animal models, several recent reports have focused attention on the role of the cell cycle in regulating engraftment potential.18,19 Notably, analysis of the long-term repopulating ability of murine BM cells cultured in IL-3, IL-6, IL-11, and SCF have revealed marked cyclic fluctuations in stem cell activity over 24-48 hours, with nadirs in engraftment potential occurring in late S and early G2 phases of the cell cycle.19Taken together with the empirical finding that the most rapid hematopoietic reconstitution observed clinically is rendered by cytokine-mobilized peripheral blood cells1 in which stem/progenitor cells are largely quiescent,30 these data suggest a basis for experimental manipulation of engraftment kinetics by inducing cell cycle arrest in hematopoietic cells prior to their infusion.
In the present study we examined the hypothesis that cycle activation of short-term repopulating cells generated in vitro might compromise their ability to rapidly alleviate posttransplant cytopenia in a manner analogous to the loss of long-term function observed with stem cell proliferation. Two approaches were used to compare the short-term hematopoietic reconstitution potential of actively cycling versus quiescent expanded cells. In the first, the progeny of highly enriched Sca-1+c-kit+Lin− stem cells generated after 1 week of serum-free culture in IL-6, IL-11, G-CSF, SCF, FLK, and TPO were separated into G0/G1 and S/G2/M fractions by FACS. During the first several weeks after transplantation of equal numbers of cells from these populations, circulating blood cells regenerated at a similar rate. In contrast and as confirmation of the efficiency of cell separation in these experiments, expanded cells residing in G0/G1at the time of transplantation gave rise to significantly higher levels of donor lymphoid and myeloid cells after a cumulative 6.5 months in primary and secondary recipients. This latter finding is consistent with numerous published studies indicating a deleterious effect of cycle activation on long-term stem cell function.18,19 23
Next we inhibited the cycling of progenitor cells during ex vivo expansion by simultaneous exposure to the tetrapeptide AcSDKP. Although we were able to achieve an approximately 50% reduction in the number of CFCs in S phase, expanded progenitors that had been subjected to cycle inhibition did not exhibit any improvement in short-term reconstitution kinetics. Wiesmann et al31obtained similar results with long-term repopulating RhloThy-1.1loSca-1+c-kit+Lin−BM cells cultured for 48 hours in SCF, IL-6, and FLK and then subjected to cycle arrest by exposure to SCF and transforming growth factor-β1 (TGF-β1) for an additional 24 hours. The percent of cells in S/G2/M decreased 2-fold after TGF-β1 exposure (cycling was not measured functionally), but no significant effects on peripheral white blood cell recovery were observed between 2 and 12 weeks after transplantation. Taken together with our present findings, these data suggest that, in contrast to more primitive stem cells that maintain long-term hematopoiesis in vivo, cell cycle activation does not compromise the functional activity of hematopoietic cells necessary for early reconstitution. Despite the correlation between quiescence and rapid reconstitution potential as exemplified by mobilized peripheral blood progenitors,32 cell cycle down-modulation thus does not appear to be an effective strategy to hasten engraftment by cultured hematopoietic cells.
These results are somewhat surprising given the cell cycle–dependent expression of several integrins that direct the homing of stem and progenitor cells to the bone marrow.33-35 Efficient homing of intravenously transplanted cells presumably represents the first step in their timely engraftment. We have recently shown that CFCs generated in vitro under the conditions reported here exhibit a 10-fold reduction in their ability to home to the BM and spleen of irradiated mice compared with their freshly isolated counterparts.15This defect correlates with a 10-fold decrease in β1 integrin expression on ex vivo expanded cells that retain the Sca-1+c-kit+ phenotype (S.J.S, unpublished data, February 1999). Presumably, reduced levels of β1 would result in a decreased expression of all 6 adhesion molecules that use this common chain. One of these molecules, α4β1 (or very late activation antigen–4; VLA-4), is known to play a critical role in stem cell homing.36-38 VLA-4 expression has been observed to decrease during S phase.35 As a result, one might expect that cycling expanded cells should home less efficiently and engraft more slowly than quiescent cells expressing higher levels of this integrin. This was not observed, which suggests that down-regulated VLA-4 is partly compensated for by other integrins that may also be down-regulated during expansion, but whose expression is not cell cycle dependent. Potential candidates include CD43, CD44, and L-selectin, for which a correlation between rapid hematopoietic reconstitution potential and long-term marrow repopulating ability has previously been noted.39 40 Our current studies are directed toward addressing this issue in more detail.
Clearly the most interesting finding of this study was the ability of a single dose of AcSDKP to enhance early reconstitution when administered in vivo simultaneously with hematopoietic cells that had been expanded without the inhibitor. The mechanism of this action is not currently known but appears from our present data to be unrelated to AcSDKP's activity as a stem cell cycle inhibitor.20 More likely, AcSDKP improves the efficiency with which expanded cells home to bone marrow. AcSDKP has been reported to increase (1) the adherence of CFU-S to a cloned stromal cell line41 and (2) the number of CFU-GM and day 12 CFU-S that can be recovered from the BM of mice transplanted only 1 day earlier with whole normal marrow cells.22 To our knowledge, the potential effects of AcSDKP on the expression by mesenchymal cells of integrin receptors which may mediate these cellular associations have not been investigated. AcSDKP does not induce the secretion of cytokines, such as tumor necrosis factor–α (TNF-α), IL-1, IL-3, IL-6, or G-CSF, when added daily for several weeks to human long-term BM cultures (LTBMCs).42Nevertheless, prevailing evidence suggests that AcSDKP exerts its effects on hematopoietic progenitors indirectly through its action on stromal cells. Cashman et al24 reported that adherent cells were required for the inhibitory effects of AcSDKP on colony formation by normal marrow progenitors. AcSDKP-mediated inhibition of CFC cycling in LTBMCs was also blocked by the addition of macrophage inflammatory protein–1β (MIP-1β), an antagonist of stroma-derived MIP-1α which inhibits cycling of CFCs in the adherent layers of such cultures.24 43 Our present finding that AcSDKP in vivo does not hasten engraftment by highly enriched Sca-1+c-kit+Lin− stem cells also suggests that mature cells, which are depleted by stem cell sorting but then regenerated during ex vivo culture, may play an indirect role in the AcSDKP-mediated engraftment of expanded cells. Of course, the extended time required for small numbers of purified stem cells to generate detectable numbers of differentiated progeny in vivo may also be an intrinsic characteristic of their peak position in the hematopoietic cell hierarchy. Engraftment of these very primitive cells may thus not be amenable to further acceleration by AcSDKP, irrespective of the presence of accessory cells.
In summary, our studies indicate that cell cycle activation of hematopoietic cells during ex vivo expansion does not deleteriously affect their ability to mediate rapid engraftment in vivo, as demonstrated here and elsewhere for more primitive long-term repopulating stem cells. Engraftment kinetics are nevertheless amenable to manipulation by relatively simple means, exemplified here using the tetrapeptide AcSDKP. It is hoped that continued systematic analysis of the parameters influencing early engraftment will culminate in the successful clinical application of expanded human hematopoietic cells.
Acknowledgments
The authors thank Mr Michael Bass for technical assistance; Dr Pieter Wierenga, University of Groningen, Groningen, The Netherlands, for advice on in vitro studies of AcSDKP; Drs Edward Srour and Christie Traycoff, Indiana University, IN, for advice on Hoechst staining; Dr Sylviane Moreau, Biomeasure, Milford, MA, for generously providing AcSDKP in the later stages of this study; and Drs Gary Van Zant and Craig Jordan, the Blood and Marrow Transplant Program, University of Kentucky, Lexington, KY, for many stimulating discussions and for critically reviewing the manuscript.
Supported by the Department of Internal Medicine and the University of Kentucky Hospital, University of Kentucky Medical Center, Lexington, KY, and by grant R01-HL61392 to S.J.S. from the National Institutes of Health, Bethesda, MD. S.J.S. is supported by a Junior Faculty Scholar Award from The American Society of Hematology, Washington, D.C.
Reprints:Stephen J. Szilvassy, Hematology/Oncology-BMT, Lucille P. Markey Cancer Center, Room CC414, 800 Rose Street, Lexington, KY 40536-0093; e-mail: szilvas@pop.uky.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal