Abstract
The marrow repopulating potential (MRP) of different sources of human hematopoietic stem cells (HSCs) was directly compared using an in vivo assay in which severe combined immunodeficient disease (SCID) mice were implanted with human fetal bones. HSCs from 2 human lymphocyte antigen (HLA)-mismatched donors were injected individually or simultaneously into the fetal bones of a 3rd distinct HLA type and donor and recipient myeloid and lymphoid cells were identified after 8 to 10 weeks. The study compared the MRP of umbilical cord blood (CB) and adult bone marrow (ABM) CD34+ cells as well as grafts of each type expanded ex vivo. Equal numbers of CB and ABM CD34+ cells injected individually demonstrated similar abilities to establish multilineage hematopoiesis. However, when CB and ABM cells were transplanted simultaneously, the engraftment of CB cells was markedly superior to ABM. CB and ABM CD34+ cells were expanded ex vivo using either a porcine microvascular endothelial cell (PMVEC)-based coculture system or a stroma-free expansion system. Primary CB CD34+ cells or CD34+ cells expanded in either culture system demonstrated a similar ability to engraft. However, the MRP of expanded grafts simultaneously injected with primary CB cells was uniformly inferior to primary CB cells. CD34+ cell grafts expanded in the stroma-free system, furthermore, outcompeted CD34+ cells expanded using the PMVEC coculture system. The triple HLA-mismatched SCID-hu model represents a novel in vivo stem cell assay system that permits the direct demonstration of the functional consequences of ex vivo HSC expansion and ontogeny-related differences in HSCs.
Introduction
Human hematopoietic stem cells (HSCs) represent rare cell populations that are characterized by their unique ability to self-renew, differentiate into multiple lineages, and rescue myeloablated hosts.1-3 Important differences in the functional properties of different tissue sources of HSCs have been well documented.2,3 Some of these functional differences appear to be based on intrinsic, ontogeny-related properties.4-7 In addition, stem cells that have been exposed ex vivo to cytokines possess an impaired ability to repopulate myeloablated hosts.8-12 These acquired defects have been attributed to a cytokine-induced reduction in stem cell self-renewal (replicative senescence) or an acquired, cell cycle-specific defect in homing to the marrow.8 13-15
Many of the functional properties characteristic of the various tissue sources of human HSCs are readily apparent with their use as grafts during clinical transplantation.16-23 Cord blood (CB) grafts are associated with delayed times to hematologic reconstitution when compared to marrow grafts, whereas mobilized stem cell grafts are associated with shortened periods required for hematologic reconstitution.16,17,20-23 These functional properties have been further characterized using a variety of in vitro and in vivo stem cell assays.24-33 These assays include in vitro long-term stem cell cultures and a variety of in vivo assays in which human stem cells engraft and differentiate in severe combined immunodeficient disease (SCID) mice or early gestation sheep fetuses, producing xenogenic chimerism that persists for months to years.24-33 A direct comparison of the functional properties of these various sources of human stem cell populations has not, however, previously been possible.
Studies of the long-term functional capacity of various sources of murine stem cells, however, have been achieved with the use of competitive repopulation assays.34-40 Stem cell function of a donor with a particular genotype has been assayed by mixing its marrow with a constant number of marrow cells from a second donor with a distinguishable phenotypic marker and measuring the relative ability of each donor cell population to reconstitute stem cell-depleted recipients.34 Such competitive repopulation assays have been useful for the study of the behavior of various sources of murine HSCs and their behavior after ex vivo expansion.34-40 In this report, we describe a new in vivo assay system that now permits the direct assessment of the relative marrow repopulating capacity and differentiative capacity of various sources of human HSCs.
Materials and methods
Cell collection and separation
Samples of CB were obtained from normal full-term deliveries after gaining informed parental consent according to guidelines established by the University of Illinois at Chicago Institutional Review Board. CB samples were collected by drainage of blood into sterile polypropylene tubes containing preservative-free sodium heparin (ICN, Aurora, OH) at a final concentration of 20 U/mL. CB samples were diluted in Hank balanced salt solution (HBSS) (BioWhittaker, Walkersville, MD) supplemented with 2% heat-inactivated fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT), 1 mmol/L HEPES, and 10 U/mL preservative-free sodium heparin. CB mononuclear cells (MNC) were isolated using Isoprep (1.077 g/mL) (Robbins Scientific, Sunnyvale, CA) density centrifugation. Human adult bone marrow (ABM) aspirates were obtained from the iliac crests of healthy normal adult volunteers after informed consent was provided, according to guidelines established by the University of Illinois at Chicago Institutional Review Board. Low-density MNC were separated from ABM by density centrifugation (Ficoll-Paque; Pharmacia LKB, Uppsala, Sweden). CB or ABM low-density MNC were washed twice in HBSS plus 2% heat-inactivated FBS and cryopreserved in Iscove modified Dulbecco medium (IMDM) (BioWhittaker) with 40% FBS and 10% dimethyl sulfoxide (DMSO) (Sigma, St Louis, MO). Cryopreserved samples were stored in liquid nitrogen. A sample of each tissue was reserved from human lymphocyte antigen (HLA)–type determination.
Isolation of cord blood and adult bone marrow CD34+ cells
Cryopreserved CB and ABM CD34+ cells were rapidly thawed at 37°C and slowly diluted in IMDM containing 10% FBS and 0.1 mg/mL Dnase I (Boehringer Mannheim, Indianapolis, IN) before further purification. CD34+ cells were immunomagnetically enriched from CB or ABM samples using the MACS CD34 Progenitor Cell Isolation Kit (Miltenyi Biotech, Inc., Auburn, CA) according to the manufacturer's instructions. Briefly, the MNC were washed and resuspended in Ca++-free and Mg++-free Dulbecco modified phosphate-buffered saline (dPBS) (BioWhittaker) supplemented with 0.5% bovine serum albumin (BSA) (Fraction V; Sigma) and 2 mmol/L EDTA. Cells were incubated with hapten-labeled anti-CD34 antibody (QBEND-10) in the presence of a blocking reagent and then with antihapten coupled to MACS microbeads. Labeled cells were filtered through a 30-μm nylon mesh and separated using a high-gradient magnetic separation column placed in a strong magnetic field. Magnetically retained cells were eluted and their purity was determined by flow cytometry to be more than 85%.
Ex vivo expansion cultures of cord blood and adult bone marrow CD34+ cells
The porcine microvascular endothelial cell line (PMVEC) is a primary cell line derived from 4- to 6-month-old Yucatan minipig brains (Sus scrofa).41 The ability of PMVECs to support the proliferation of early human marrow cells has been previously reported by our group.11,41-43 PMVEC (passages 22 through 29) were maintained and used for hematopoietic cell expansions as previously described.11 41-43 Triplicate cultures of CB or ABM CD34+ cells were seeded into 6-well tissue culture plates (Corning-Costar, Cambridge, MA) at 3 to 15 × 104cells/well with pre-established PMVEC monolayers (PMVEC cocultures) or into plates without PMVEC monolayers (stroma cell-free cultures). All cultures were maintained in IMDM with 10% FBS, 2 mmol/Ll-glutamine, 100 U/mL penicillin, 1 mg/mL streptomycin (all from BioWhittaker) in a humidified incubator maintained at 37°C with 5% CO2. Cultures were supplemented with a combination of recombinant human cytokines including interleukin (IL)-3 at 10 ng/mL (R&D Systems, Minneapolis, MN), IL-6 at 10 ng/mL (R&D Systems), granulocyte-macrophage colony-stimulating factor (GM-CSF) at 10 ng/mL (R&D Systems), stem cell factor (SCF) at 100 ng/mL (R&D Systems), and FLT-3 ligand (FLT-3L) at 100 ng/mL (R&D Systems). Cultures were replenished twice weekly by replacing half of the medium and cytokines and expanded into additional culture wells with or without pre-established PMVEC monolayers as required. At days 7, 14, and 21, aliquots of cells were harvested for the performance of cell counts, phenotypic analysis, and in vivo assays. The trypan blue exclusion method was used to determine the total viable cell content of expansion cultures. The large size and distinct appearance of PMVECs permitted their exclusion during cell counting and flow cytometric analysis.
Phenotypic characterization and human lymphocyte antigen–typing of expanded and nonexpanded cells
Primary CB and ABM cells and cells expanded ex vivo were phenotypically analyzed. Cells were preincubated with 1 mg/mL of human gamma globulin in staining buffer to block nonspecific binding. To determine the CD34+ and CD34+CD38−cell content of each sample, cells were then incubated with phycoerythrin (PE)-conjugated monoclonal antibody against CD38 (Becton Dickinson, San Jose, CA) and fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against CD34 (Becton Dickinson). A portion of each sample was incubated with the appropriate PE- and FITC-conjugated isotype control antibodies to establish the background level of nonspecific staining. To determine HLA allele expression, samples were incubated with BB7.2, GAP A3, MA2.1, BB7.1, and MB40.2 monoclonal antibodies derived from ATCC hybridomas (ATCC, Rockville, MD) or appropriate isotype control antibodies followed by the incubation with PE-conjugated rabbit antimouse (H+L) (Zymed, South San Francisco, CA). All staining was performed in dPBS staining buffer supplemented with 2% FBS, 10 U/mL preservative-free heparin, and 1 mg/mL human gamma globulin. Propidium iodide (PI; 1 μg/mL) (Sigma) was used during analysis to identify and exclude dead cells. Flow cytometric analysis was performed using a FACSCalibur cytometer (Becton Dickinson). At least 10 000 PI-negative events were collected. Acquired data were analyzed using CELLQuest 3.1 software (Becton Dickinson).
Triple mismatched SCID-human bone model
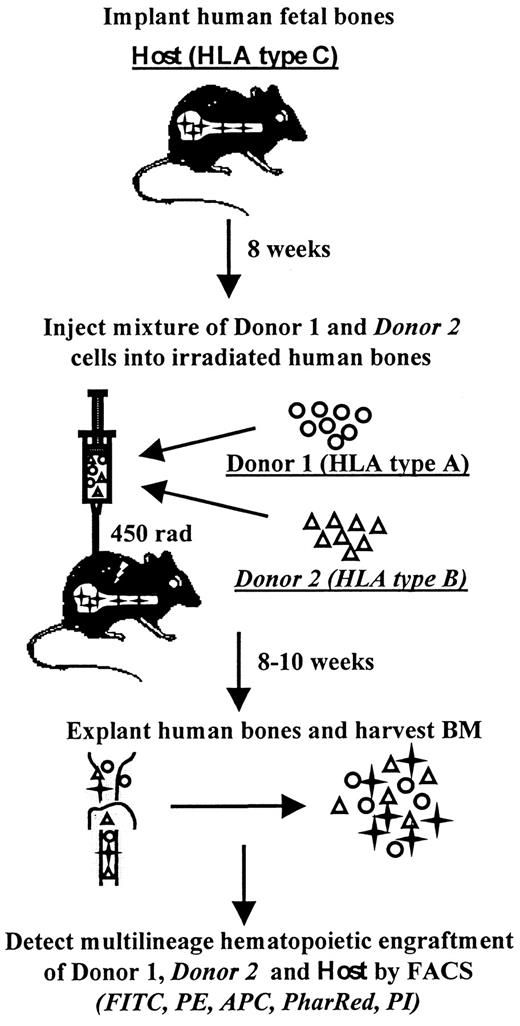
The marrow repopulating potential of primary CB and ABM as well as corresponding ex vivo expanded cells was assessed using an in vivo triple HLA-mismatched SCID-human (SCID-hu) bone assay system44,45 (Figure 1). SCID-hu bone mice were constructed as previously described.42 43 Briefly, human fetal bone fragments obtained from elective abortions (Advanced Bioscience Resources, Alameda, CA) were subcutaneously implanted in 6- to 8-week old C.B-17/Icr-scid mice (Charles River Laboratories, Wilmington, MA) and allowed to vascularize for 8 weeks (SCID-hu bone mice). A small portion of human fetal bone marrow was reserved for HLA typing. Primary ABM and CB cells, expanded ABM, or expanded CB were individually or in various combinations injected into fetal bone grafts after the mice received 450 cGy of total body irradiation. Tissues injected into the same bone simultaneously differed in HLA allele expression from each other and the host bone. For all in vivo assays, different tissues were normalized for CD34+ cell content: equivalent numbers of CD34+ from each donor source were injected into each bone. To minimize the impact of tissue variability on the experimental data, various CB populations were tested as follows: half of the bone grafts, for example, were injected with the mixture of primary CB#1 and expanded CB#2 and the other half of bone grafts were injected with the mixture of primary CB#2 and similarly expanded CB#1. Each of the sources of HSCs was assayed in SCID-hu assays individually as well. Eight to 10 weeks after injection of the grafts, ABM cells were harvested from the fetal bone grafts and analyzed by flow cytometry for the hematopoietic contribution of the host and each injected donor. The presence of myeloid cells, lymphoid cells, and hematopoietic progenitor cells was detected with allophycocyanin (APC)-conjugated anti-CD33, CD19, and CD34 antibodies (Becton Dickinson), respectively. Donor and host cells were detected by a 5-color flow-cytometric assay using FITC, PE, and biotin-conjugated W6/32 (Leinco Technologies, St Louis, MO) and appropriate HLA allele monoclonal antibodies with FITC, PE, and biotin-conjugated goat antimouse IgG1, IgG2a, IgG2b (Southern Biotechnology Associates, Birmingham, AL) and streptavidin-conjugate PharRed (Pharmingen, San Diego, CA). Grafts were analyzed using FACSVantage flow cytometer. Immediately prior to analysis, 1 μg/mL PI was added for the identification and exclusion of dead cells. Uninjected grafts were used as controls for nonspecific staining. Grafts were considered positive if they contained more than 1% of cells expressing a particular donor HLA antigen.
Schematic representation of triple HLA-mismatched SCID-hu bone model.
In the SCID-hu bone assay, a candidate stem cell population is microinjected into an HLA-mismatched fetal bone fragment implanted in an immunodeficient mouse. The bone fragment is retrieved and flow cytometrically analyzed 8 to 10 weeks later for the presence of donor-derived multilineage hematopoiesis. In the triple HLA-mismatched SCID-hu bone model, 2 different donors bearing distinct HLA alleles are injected into the same fetal bone graft with a third distinct HLA type. At the termination of the assay, the multilineage hematopoietic contribution of each of the 2 donors as well as the contribution of the recipient is determined in a 5-color flow-cytometric assay using monoclonal antibodies specific for human HLA allotypes and for various hematopoietic markers.
Schematic representation of triple HLA-mismatched SCID-hu bone model.
In the SCID-hu bone assay, a candidate stem cell population is microinjected into an HLA-mismatched fetal bone fragment implanted in an immunodeficient mouse. The bone fragment is retrieved and flow cytometrically analyzed 8 to 10 weeks later for the presence of donor-derived multilineage hematopoiesis. In the triple HLA-mismatched SCID-hu bone model, 2 different donors bearing distinct HLA alleles are injected into the same fetal bone graft with a third distinct HLA type. At the termination of the assay, the multilineage hematopoietic contribution of each of the 2 donors as well as the contribution of the recipient is determined in a 5-color flow-cytometric assay using monoclonal antibodies specific for human HLA allotypes and for various hematopoietic markers.
Results
Competitive repopulation of cord blood and marrow CD34+ cells
Initially, we directly assessed the ability of 2 different sources of HSCs obtained at different times of ontogeny to competitively engraft and differentiate into multiple hematopoietic lineages within the same SCID-hu bone. Equal numbers (9 × 104) of ABM and CB CD34+ cells containing similar numbers of CD34+ CD38− cells bearing distinct HLA alleles were directly injected individually and simultaneously into SCID-hu bones. After 10 weeks the grafts were harvested and flow cytometrically analyzed for the presence of ABM and CB progeny. As can be seen in Table 1, when injected individually, both ABM and CB CD34+ cells were each capable of producing significant and comparable levels of multilineage engraftment in virtually all fetal bones injected. However, when equal numbers of CB and ABM CD34+ cells containing equal numbers of CD34+CD38− cells were injected simultaneously into the same fetal bone grafts, the CB and ABM behaved quite differently. CB cells of multiple hematopoietic lineages were found in every fetal bone injected; however, evidence of ABM engraftment was present in only 50% of injected fetal bones. The percentage of ABM-derived cells in those bones where engraftment was confirmed was far less than the percentage of CB-derived cells in the same bones. In addition, the degree of engraftment when only ABM CD34+cells served as the graft was far greater than when the same dose of ABM cells were cotransplanted with CB cells. CB CD34+ cells were able to outcompete ABM CD34+ cells in this competitive repopulation assay. These data provide a direct demonstration of the ontogeny-related changes in human HSCs that had been previously inferred from the behavior of purified stem cell population in a variety of in vitro and in vivo assay systems.4-7,32 33
Competitive engraftment of primary cord blood and adult bone marrow CD34+ cells in triple HLA-mismatched SCID-hu bone model
| Population* . | No. of cells injected per bone† . | ABM positive grafts‡ . | Primary ABM % positive cells1-153 . | CB positive grafts‡ . | Primary CB % positive cells1-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| ABM | 9 × 104 | 1.14 × 103 | 5/6 | 29 ± 13 | 49 ± 19 | 47 ± 18 | ||||
| CB | 9 × 104 | 1.13 × 103 | 6/6 | 31 ± 9 | 30 ± 13 | 36 ± 10 | ||||
| ABM + CB | See above | 5/10 | 3.6 ± 1.6 | 5.8 ± 1.7 | 4.1 ± 1.3 | 10/10 | 36 ± 5 | 30 ± 5 | 27 ± 7 | |
| Population* . | No. of cells injected per bone† . | ABM positive grafts‡ . | Primary ABM % positive cells1-153 . | CB positive grafts‡ . | Primary CB % positive cells1-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| ABM | 9 × 104 | 1.14 × 103 | 5/6 | 29 ± 13 | 49 ± 19 | 47 ± 18 | ||||
| CB | 9 × 104 | 1.13 × 103 | 6/6 | 31 ± 9 | 30 ± 13 | 36 ± 10 | ||||
| ABM + CB | See above | 5/10 | 3.6 ± 1.6 | 5.8 ± 1.7 | 4.1 ± 1.3 | 10/10 | 36 ± 5 | 30 ± 5 | 27 ± 7 | |
ABM indicates adult bone marrow; CB, cord blood.
HLA-mismatched immunomagnetically selected CD34+ ABM and CB cells were injected individually or simultaneously into human fetal bone fragment (bearing a third distinct HLA allele) implanted in the SCID mouse (SCID-hu bone assay) to determine their competitive marrow repopulating ability.
Ten weeks after injection, fetal bone implants were removed and analyzed for the presence of ABM and CB HLA-bearing cells and CD19, CD33, and CD34 cells in a 5-color flow-cytometric assay. Uninjected grafts were used to determine the level of nonspecific binding of monoclonal antibodies.
Positive grafts indicate number of grafts containing donor-derived multilineage hematopoiesis per total number of injected grafts.
Percent positive cells represent donor-derived to total cells of the particular cell lineage present in the bone 10 weeks after injection. Numbers are expressed as mean % ± SEM.
Competitive repopulation of primary and expanded cord blood grafts
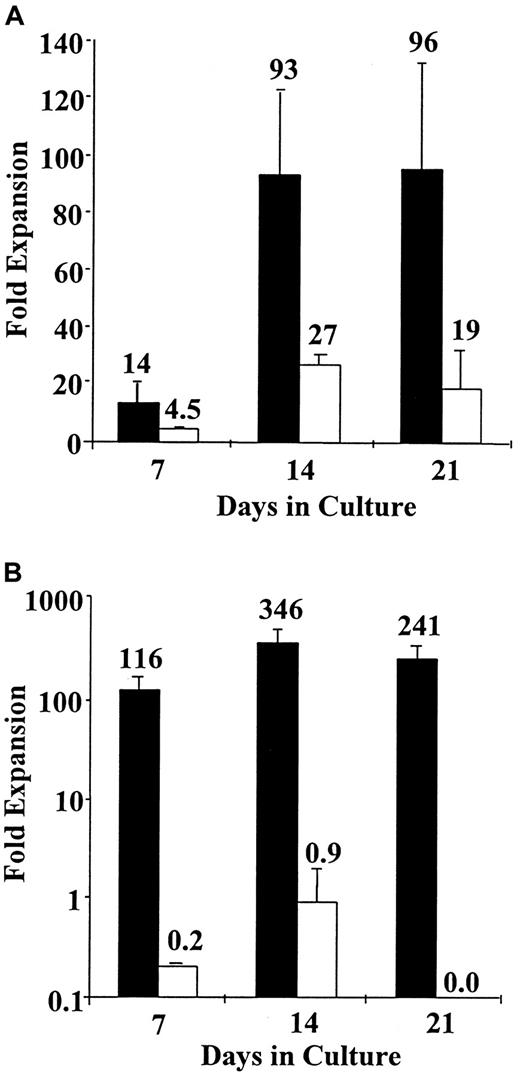
Recently, a number of laboratories have demonstrated that ex vivo expanded HCS possess inferior engraftment capabilities as compared to primary stem cells.8-12 This defect has been attributed to either replicative senescence or acquisition of a cell cycle-related homing defect.8-12,14 15 We next attempted to directly demonstrate the functional properties of expanded and primary HSCs using the triple mismatched SCID-hu model. Cord blood CD34+cells were expanded in a stroma-free system or a PMVEC-based system to which SCF, IL-3, GM-CSF, IL-6, and FLT-3L were added. The degree of expansion of CD34+ cells and the CD34+CD38− cells in each of these culture systems after 7 to 21 days of incubation is shown in Figure2. After 21 days of expansion in a PMVEC expansion system, a 5-fold greater expansion of CD34+ cells and over a 241-fold greater expansion of CD34+CD38− cells was observed as compared to the stroma-free cultures supplemented with a similar cytokine combination.
Ex vivo CD34+ and CD34− cell expansion in porcine microvascular endothelial cell-based coculture system and stroma-free culture system.
CB CD34+ cells were isolated using Miltenyi CD34+ selection kits and expanded in PMVEC cocultures (▪) or stroma-free cultures (■) supplemented with recombinant human IL3, IL6, GM-CSF, SCF, and FLT-3L. At days 7, 14, and 21, CD34+ (A) and CD34+CD38− (B) cell content of resulting cultures was assessed flow cytometrically with the aid of appropriate monoclonal antibodies. Very similar total cellular expansion was obtained in both culture systems. (A) A significantly greater expansion of CD34+ cells was achieved in PMVEC cocultures—at day 21 PMVEC cocultures contain 5 times more CD34+ cells than stroma-free cultures. (B) No expansion of CD34+CD38− cells was observed in stroma-free expansion cultures, whereas PMVEC cocultures at day 14 contained 346 times the input number of this primitive phenotype.
Ex vivo CD34+ and CD34− cell expansion in porcine microvascular endothelial cell-based coculture system and stroma-free culture system.
CB CD34+ cells were isolated using Miltenyi CD34+ selection kits and expanded in PMVEC cocultures (▪) or stroma-free cultures (■) supplemented with recombinant human IL3, IL6, GM-CSF, SCF, and FLT-3L. At days 7, 14, and 21, CD34+ (A) and CD34+CD38− (B) cell content of resulting cultures was assessed flow cytometrically with the aid of appropriate monoclonal antibodies. Very similar total cellular expansion was obtained in both culture systems. (A) A significantly greater expansion of CD34+ cells was achieved in PMVEC cocultures—at day 21 PMVEC cocultures contain 5 times more CD34+ cells than stroma-free cultures. (B) No expansion of CD34+CD38− cells was observed in stroma-free expansion cultures, whereas PMVEC cocultures at day 14 contained 346 times the input number of this primitive phenotype.
We then compared the engraftment capacity of primary CB cells and CB CD34+ cells expanded in a PMVEC coculture system or in a stroma-free culture system. The grafts were normalized prior to injection into the triple HLA mismatched SCID-hu bone assay so as to contain equal numbers of CD34+ cells (1.5 × 104 cells/tissue), and donor contribution was determined by flow cytomety 10 weeks later. Both primary CB cells and CB cells expanded in either culture system engrafted in a similar fashion; when injected individually each was able to establish a significant degree of donor-derived multilineage hematopoiesis in virtually all injected fetal bones (Tables2 and 3). However, when the primary CB cells and the PMVEC expanded CB cells were injected into the same fetal bone grafts, the primary CB cells clearly outcompeted the PMVEC expanded CB cells (Table 2). It is important to note that the primary CB grafts contained 50% of the number of the CD34+CD38− cells present in the PVVEC expanded graft. Only 2 of 20 simultaneously injected bones contained cells derived from PMVEC coculture expanded CB, whereas significant levels of multilineage primary CB-derived cells were present in every bone. Similar data were obtained when the engraftment capability of primary CB cells and CB CD34+ cells expanded in a stroma-free expansion system (Table 3) was compared in the same SCID-hu bone assay. When competed with primary CB cells, engraftment capacity of stroma-free expanded CB was greatly reduced, resulting in only a few grafts demonstrating the presence of expanded CB cells.
Competitive engraftment of primary and PMVEC coculture expanded cord blood CD34+ cells in triple HLA-mismatched SCID-hu bone model
| Population* . | No. of cells injected per bone† . | Primary positive grafts‡ . | Primary CB % positive cells2-153 . | PMVEC positive grafts‡ . | PMVEC expanded CB % positive cells2-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| Primary CB | 1.5 × 104 | 8.8 × 102 | 20/20 | 53 ± 7 | 59 ± 9 | 62 ± 8 | ||||
| PMVEC expanded CB | 1.5 × 104 | 1.8 × 103 | 20/21 | 49 ± 7 | 72 ± 7 | 59 ± 7 | ||||
| Primary CB + PMVEC | ||||||||||
| expanded CB | See above | 20/20 | 40 ± 7 | 47 ± 8 | 47 ± 7 | 2/20 | 1.4, 42 | 2, 1 | 5, 4 | |
| Population* . | No. of cells injected per bone† . | Primary positive grafts‡ . | Primary CB % positive cells2-153 . | PMVEC positive grafts‡ . | PMVEC expanded CB % positive cells2-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| Primary CB | 1.5 × 104 | 8.8 × 102 | 20/20 | 53 ± 7 | 59 ± 9 | 62 ± 8 | ||||
| PMVEC expanded CB | 1.5 × 104 | 1.8 × 103 | 20/21 | 49 ± 7 | 72 ± 7 | 59 ± 7 | ||||
| Primary CB + PMVEC | ||||||||||
| expanded CB | See above | 20/20 | 40 ± 7 | 47 ± 8 | 47 ± 7 | 2/20 | 1.4, 42 | 2, 1 | 5, 4 | |
CB indicates cord blood; primary, primary CB; PMVEC and PMVEC expanded, PMVEC coculture expanded CB.
CB CD34+ cells were immunomagnetically isolated and cultured for 17 days on pre-established monolayers of porcine microvascular endothelial cells (PMVEC) with IL3, IL6, GM-CSF, SCF, and FLT-3L. PMVEC coculture-expanded CB cells were injected individually or simultaneously with HLA-mismatched primary CB cells into human fetal bone fragment (bearing a third distinct HLA allele) implanted in the SCID mouse (SCID-hu bone assay) to determine their competitive marrow repopulating ability. Grafts were normalized for CD34+ cell content prior to injection.
Ten weeks after injection, fetal bone implants were removed and analyzed for the presence of primary or PMVEC coculture-expanded CB HLA-bearing cells and CD19, CD33, and CD34 cells in a 5-color flow-cytometric assay. Uninjected grafts were used to determine the level of nonspecific binding of monoclonal antibodies.
Positive grafts indicate number of grafts containing donor-derived multilineage hematopoiesis per total number of injected grafts.
Percent positive cells represent donor-derived to total cells of the particular cell lineage present in the bone 10 weeks after injection. Numbers are expressed as mean % ± SEM.
Competitive engraftment of primary and stroma-free expanded cord blood CD34+ cells in triple HLA-mismatched SCID-hu bone model
| Population3-150 . | No. of cells injected per bone3-151 . | Primary positive grafts3-152 . | Primary CB % positive cells3-153 . | Expanded positive grafts3-152 . | S-F expanded CB % positive cells3-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| Primary CB | 1.5 × 104 | 8.8 × 102 | 20/20 | 53 ± 8 | 59 ± 9 | 62 ± 8 | ||||
| S-F expanded CB | 1.5 × 104 | Not detected | 22/23 | 59 ± 7 | 62 ± 8 | 63 ± 7 | ||||
| Primary CB + S-F | ||||||||||
| expanded CB | See above | 20/22 | 41 ± 7 | 48 ± 9 | 50 ± 9 | 5/22 | 18 ± 16 | 16 ± 14 | 20 ± 17 | |
| Population3-150 . | No. of cells injected per bone3-151 . | Primary positive grafts3-152 . | Primary CB % positive cells3-153 . | Expanded positive grafts3-152 . | S-F expanded CB % positive cells3-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| Primary CB | 1.5 × 104 | 8.8 × 102 | 20/20 | 53 ± 8 | 59 ± 9 | 62 ± 8 | ||||
| S-F expanded CB | 1.5 × 104 | Not detected | 22/23 | 59 ± 7 | 62 ± 8 | 63 ± 7 | ||||
| Primary CB + S-F | ||||||||||
| expanded CB | See above | 20/22 | 41 ± 7 | 48 ± 9 | 50 ± 9 | 5/22 | 18 ± 16 | 16 ± 14 | 20 ± 17 | |
CB indicates cord blood; primary, primary CB; S-F expanded and expanded, stroma-free expanded.
CB CD34+ cells were immunomagnetically isolated and expanded for 17 days in S-F cultures supplemented with IL3, IL6, GM-CSF, SCF, and FLT-3L. S-F expanded CB cells were injected individually or simultaneously with HLA-mismatched primary CB cells into human fetal bone fragment (bearing a third distinct HLA allele) implanted in the SCID mouse (SCID-hu bone assay) to determine their competitive marrow repopulating ability. Grafts were normalized for CD34+ cell content prior to injection.
Ten weeks after injection, fetal bone implants were removed and analyzed for the presence of primary or S-F expanded CB HLA-bearing cells and CD19, CD33, and CD34 cells in a 5-color flow-cytometric assay. Uninjected grafts were used to determine the level of nonspecific binding of monoclonal antibodies.
Positive grafts indicate number of grafts containing donor-derived multilineage hematopoiesis per total number of injected grafts.
Percent positive cells represent donor-derived to total cells of the particular cell lineage present in the bone 10 weeks after injection. Numbers are expressed as mean % ± SEM.
We next tested the competitive repopulating potential of CB CD34+ cells that had been expanded in either the PMVEC-based or stroma-free expansion systems (Table4). Both expansion products, when transplanted alone, successfully engrafted all injected human fetal bones and were capable of generating significant levels of donor-derived CD34+, CD19+, and CD33+ cells. However, when these 2 expansion products containing equal numbers of CD34+ cells (1.5 × 104 cells) were cotransplanted into the same fetal bones, to our surprise, the cells produced in the stroma-free system were present in 40% more fetal bones than the cells expanded in the PMVEC cocultures (Table 4). Both expanded products, however, clearly, retained the ability to differentiate into multiple hematopoietic lineages (Figure 3). These findings are somewhat surprising because the PMVEC expanded grafts contained significant numbers of CD34+CD38− cells in contrast to the graft expanded in stroma-free cultures, which did not contain any CD34+CD38− cells.
Competitive engraftment of stroma-free and PMVEC coculture expanded cord blood CD34+ cells in triple HLA-mismatched SCID-hu bone model
| Population4-150 . | No. of cells injected per bone4-151 . | S-F positive grafts‡ . | S-F expanded CB % positive cells4-153 . | PMVEC positive grafts‡ . | PMVEC expanded CB % positive cells4-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| S-F expanded CB | 1.5 × 104 | not detected | 22/23 | 59 ± 7 | 62 ± 7 | 63 ± 7 | ||||
| PMVEC expanded CB | 1.5 × 104 | 1.8 × 103 | 20/21 | 49 ± 7 | 72 ± 7 | 59 ± 7 | ||||
| S-F expanded CB + PMVEC | ||||||||||
| expanded CB | See above | 18/18 | 33 ± 4 | 46 ± 6 | 42 ± 5 | 11/18 | 29 ± 6 | 24 ± 6 | 26 ± 6 | |
| Population4-150 . | No. of cells injected per bone4-151 . | S-F positive grafts‡ . | S-F expanded CB % positive cells4-153 . | PMVEC positive grafts‡ . | PMVEC expanded CB % positive cells4-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| S-F expanded CB | 1.5 × 104 | not detected | 22/23 | 59 ± 7 | 62 ± 7 | 63 ± 7 | ||||
| PMVEC expanded CB | 1.5 × 104 | 1.8 × 103 | 20/21 | 49 ± 7 | 72 ± 7 | 59 ± 7 | ||||
| S-F expanded CB + PMVEC | ||||||||||
| expanded CB | See above | 18/18 | 33 ± 4 | 46 ± 6 | 42 ± 5 | 11/18 | 29 ± 6 | 24 ± 6 | 26 ± 6 | |
CB indicates cord blood; S-F and S-F expanded, stroma-free expanded, PMVEC and PMVEC expanded, PMVEC coculture expanded CB.
CB CD34+ cells were immunomagnetically isolated and expanded for 17 days in cultures with or without pre-established PMVEC monolayers. Cultures were supplemented with IL3, IL6, GM-CSF, SCF, and FLT-3L. HLA-mismatched PMVEC coculture and S-F expanded CB cells were injected individually or simultaneously into human fetal bone fragment (bearing a third distinct HLA allele) implanted in the SCID mouse (SCID-hu bone assay) to determine their competitive marrow repopulating ability. Grafts were normalized for CD34+ cell content before injection.
Ten weeks after injection, fetal bone implants were removed and analyzed for the presence of PMVEC coculture or S-F expanded CB HLA-bearing cells and CD19, CD33, and CD34 cells in a 5-color flow-cytometric assay. Uninjected grafts were used to determine the level of nonspecific binding of monoclonal antibodies.
Positive grafts indicate number of grafts containing donor-derived multilineage hematopoiesis per total number of injected grafts.
Percent positive cells represent donor-derived to total cells of the particular cell lineage present in the bone 10 weeks after injection. Numbers are expressed as mean % ± SEM.
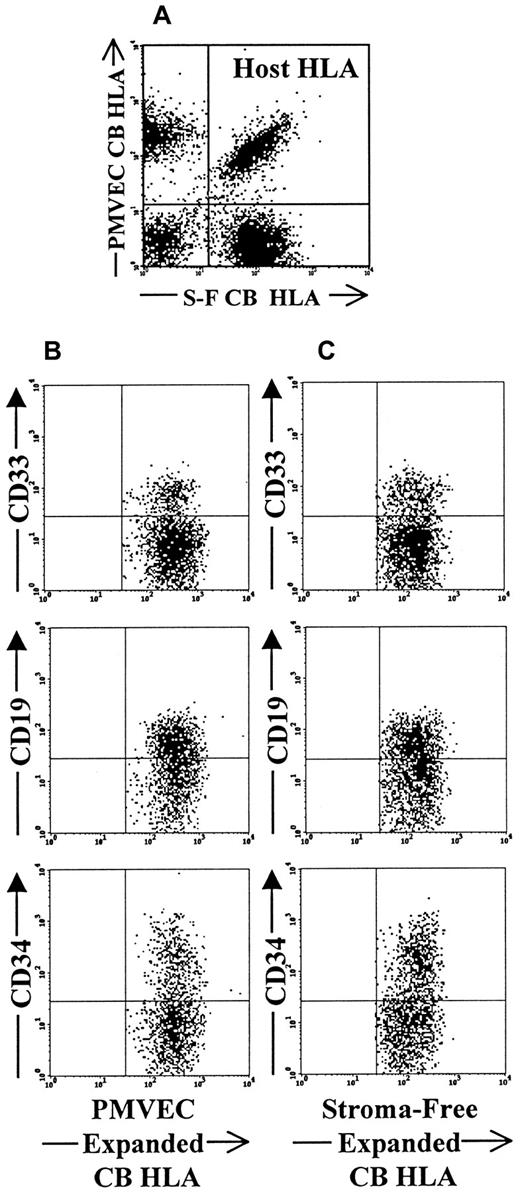
Flow cytometric analysis of competitive engraftment of PMVEC coculture and stoma-free culture-expanded CB CD34+cells in triple HLA-mismatched SCID-hu bone model.
(A) Dot-plot demonstrates the presence of 3 distinct HLA types within the same bone. Recipient-host cells in this case are double positive for each of the HLA markers (upper right quadrant of the plot). PMVEC coculture-derived cells lack the expression of stroma-free culture-derived HLA marker and are shown in the upper left quadrant of the plot. Stroma-free culture-derived cells do not express PMVEC coculture-derived cell HLA marker and are shown in the lower right quadrant of the plot. Panel B demonstrates the presence of CD33+ myeloid, CD19+ lymphoid, and CD34+ progenitor cells among PMVEC coculture-derived HLA marker-positive gated cells. Panel C documents presence of the same multiple hematopoietic lineages within the stroma-free culture derived population gate.
Flow cytometric analysis of competitive engraftment of PMVEC coculture and stoma-free culture-expanded CB CD34+cells in triple HLA-mismatched SCID-hu bone model.
(A) Dot-plot demonstrates the presence of 3 distinct HLA types within the same bone. Recipient-host cells in this case are double positive for each of the HLA markers (upper right quadrant of the plot). PMVEC coculture-derived cells lack the expression of stroma-free culture-derived HLA marker and are shown in the upper left quadrant of the plot. Stroma-free culture-derived cells do not express PMVEC coculture-derived cell HLA marker and are shown in the lower right quadrant of the plot. Panel B demonstrates the presence of CD33+ myeloid, CD19+ lymphoid, and CD34+ progenitor cells among PMVEC coculture-derived HLA marker-positive gated cells. Panel C documents presence of the same multiple hematopoietic lineages within the stroma-free culture derived population gate.
Competitive repopulation of expanded cord blood and adult bone marrow grafts
The competitive repopulating ability of CB cells expanded for 17 days in PMVEC cocultures supplemented with IL3, IL6, GM-CSF, SCF, and FLT-3L was compared to that of ABM expanded under the identical conditions. Immunomagnetically reisolated CD34+ cells (1 × 104) from HLA-mismatched CB and ABM were simultaneously injected into the SCID-hu bone assay. The CB expanded grafts contained 5-fold greater numbers of CD34+CD38− cells than the ABM expanded grafts (Table 5). When injected individually, expanded ABM and CB cells each demonstrated a significant level of multilineage donor engraftment in all fetal bone grafts (Table 5). When identical numbers of ABM and CB PMVEC coculture expanded cells were assayed within the same SCID-hu bone graft, expanded CB cells outcompeted expanded ABM cells; none of the injected grafts contained ABM-derived cells, whereas each graft contained CB-derived multilineage cells, albeit at a somewhat lower level than observed when primary CB cells were assayed.
Competitive engraftment of PMVEC coculture expanded cord blood and adult bone marrow CD34+ cells in triple HLA-mismatched SCID-hu bone model
| Population5-150 . | No. of cells injected per bone5-151 . | ABM positive grafts5-152 . | ABM-PMVEC % positive cells5-153 . | CB positive grafts5-152 . | CB-PMVEC % positive cells5-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| ABM-PMVEC | 1 × 105 | 3.2 × 103 | 10/10 | 26 ± 9 | 39 ± 12 | 29 ± 11 | ||||
| CB-PMVEC | 1 × 105 | 1.7 × 104 | 9/9 | 42 ± 10 | 56 ± 12 | 40 ± 9 | ||||
| ABM-PMVEC + CB-PMVEC | See above | 0/6 | 0 | 0 | 0 | 6/6 | 0.3 ± 0.1 | 17 ± 7 | 5 ± 2 | |
| Population5-150 . | No. of cells injected per bone5-151 . | ABM positive grafts5-152 . | ABM-PMVEC % positive cells5-153 . | CB positive grafts5-152 . | CB-PMVEC % positive cells5-153 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38− . | CD33 . | CD19 . | CD34 . | CD33 . | CD19 . | CD34 . | |||
| ABM-PMVEC | 1 × 105 | 3.2 × 103 | 10/10 | 26 ± 9 | 39 ± 12 | 29 ± 11 | ||||
| CB-PMVEC | 1 × 105 | 1.7 × 104 | 9/9 | 42 ± 10 | 56 ± 12 | 40 ± 9 | ||||
| ABM-PMVEC + CB-PMVEC | See above | 0/6 | 0 | 0 | 0 | 6/6 | 0.3 ± 0.1 | 17 ± 7 | 5 ± 2 | |
CB indicates cord blood; ABM, adult bone marrow; ABM-PMVEC, PMVEC coculture expanded ABM, CB-PMVEC, PMVEC coculture expanded CB.
CB and ABM CD34+ cells were immunomagnetically isolated and expanded for 17 days in cultures with or without pre-established PMVEC monolayers. Cultures were supplemented with IL3, IL6, GM-CSF, SCF, and FLT-3L. HLA-mismatched PMVEC coculture-expanded CB and ABM cells were injected individually or simultaneously into human fetal bone fragment (bearing a third distinct HLA allele) implanted in the SCID mouse (SCID-hu bone assay) to determine their competitive marrow repopulating ability. CD34+ cells were reisolated prior to injection.
Ten weeks after injection, fetal bone implants were removed and analyzed for the presence of CB and ABM HLA-bearing cells and CD19, CD33, and CD34 cells in a 5-color flow cytometric assay. Uninjected grafts were used to determine the level of nonspecific binding of monoclonal antibodies.
Positive grafts indicate number of grafts containing donor-derived multilineage hematopoiesis per total number of injected grafts.
Percent positive cells represent donor-derived to total cells of the particular cell lineage present in the bone 10 weeks after injection. Numbers are expressed as mean % ± SEM.
Discussion
The direct evaluation of the repopulating potential of different ontogeny-related sources of murine HSCs has been facilitated by the use of competitive repopulation assays, which measure stem cell activity based on the demonstrated ability of cell populations to reconstitute hematopoiesis when transplanted into lethally irradiated recipients in competition with a defined population of cotransplanted cells that allow for the survival of the recipients receiving myeloablative radiation.34-39 The HSCs detected are termed competitive repopulating units (CRU) and have been quantitated in unseparated or purified donor cell populations by limiting dilution analysis techniques.34 The stem cell populations of interest can be tracked either by the expression of phenotypic markers or by retroviral gene marking. Because the genetic modification of HSCs by itself might potentially affect stem cell function, the use of phenotypic markers such as cell surface antigens, expression of intracellular isoenzymes, or hemoglobulin subtypes to track HSC progeny has permitted the identification of the progeny of different stem cell sources with this assay system.34-40 Such studies have demonstrated important ontogenic differences in HSC behavior.36-38Murine fetal liver HSCs possess a greater proliferative activity in vivo than ABM stem cells.36,38 Furthermore, the functional activity of HSCs from late fetal and newborn mice, which is similar to CB in humans, has also been compared to murine peripheral blood and marrow stem cells using competitive repopulation assays.37The repopulating potential of late fetal or newborn blood has been reported to be several times less than that found in a similar number of ABM cells, but far more than in normal adult blood.37
Direct assessment of the competitive repopulating potential of various sources of human HSCs has not been previously possible. A variety of in vitro and in vivo assays have, however, been used to assess the fundamental properties of human adult ABM and CB HSCs.29,31-33 CB progenitor cells have a higher in vitro plating and replating efficacy, which has been suggested to be a consequence of the greater self-renewal capacity of CB HSCs as compared to ABM HSCs.7,26,27,32 In addition, several xenogeneic animal systems using various murine models such as SCID, nonobese diabetic (NOD)/SCID, bg/nu/xid (BND) mice as well as an in utero sheep transplant model have been used for these same purposes.24,28,29,32,46-49 Transplanted human HSCs engraft in each of these models within a xenogeneic microenvironment. Use of these in vivo models has provided important information about the biologic properties of CB- and ABM-derived HSCs. CB, unlike adult HSCs, for instance, are capable of engrafting NOD/SCID mice without the administration of exogenous cytokines.28 To establish a competitive repopulation assay for human HSCs, we used a SCID-hu bone model in which human fetal bone fragments are implanted in the SCID mouse.44,45 The SCID-hu bone model permits assessment of the engraftment potential of a putative stem cell population within an appropriate human ABM microenvironment without the need for exogenous cytokines or carrier cells. The triple HLA-mismatched SCID-hu bone model is largely patterned after the competitive repopulation assay in mice.34-37 HSCs from 2 HLA-mismatched donors are injected into the same recipient and myeloid (CD33+), lymphoid (CD19+), and progenitor (CD34+) cells derived from each donor are identified after 8 to 10 weeks using antibodies specific for human major histocompatibility complex (MHC) class I alleles. The hematopoietic cells produced by each of the 2 grafts are not only competing with each other but also with cells produced from the endogenous fetal marrow that had previously been suppressed with sublethal irradiation. Equal numbers of CD34+ cells from the various ontogenic sources of HSCs served as grafts in cells to evaluate their relative proliferative capacity. The relative efficiency of engraftment and multilineage differentiation was judged by the number of grafts containing cells from each individual graft, and their ability to differentiate into multiple hematopoietic lineages. The triple HLA-mismatched SCID-hu model as presently described represents a qualitative assay of HSC function rather than a quantitative assay of HSC CRU. This assay, however, if performed in limiting dilution assay would be capable of generating a quantitative assessment of CRU as has been previously reported with the NOD/SCID stem cell assay.30
Engraftment is believed to involve the directed movement of HSCs to specific microenvironmental “niches” that favor HSC self-renewal and differentiation.50 At least in the marrow microenvironment of a fetal bone, CB stem cells appear clearly to have an advantage over ABM HSCs in occupying such theoretical “niches” and sustaining hematopoiesis for over 10 weeks. Because CB HSCs appear to have superior engraftment capabilities in comparison to ABM HSCs, the delayed engraftment patterns that are observed during clinical CB allogeneic transplantation appear not to be due to a qualitative defect of individual CB stem cells, but rather due to the infusion of a graft containing inadequate numbers of HSCs that are actually functionally more effective than ABM HSCs. This hypothesis is supported by Harrison and Astle who reported that the concentration of murine marrow repopulating cells were 2 to 4 times higher in ABM than in late fetal or newborn blood.37 Our studies imply that an effective strategy for overcoming the engraftment problems encountered with clinical CB transplantation is the infusion of increased numbers of these functionally superior CB HSCs. Strategies involving infusion of multiple CB grafts into a single recipient or the ex vivo expansion of CB stem cells are currently being explored to overcome these difficulties.51 52
Although the expansion of adult marrow repopulating stem cells has been reported to be clearly favored by cocultivation with endothelial cells, the optimal culture system with which to expand CB CD34+has not been yet defined. To our surprise, CB CD34+ cells expanded in the stroma-free system outcompeted equivalent numbers of CB CD34+ cells with an identical cytokine combination expanded in the PMVEC coculture system when coinfused in the triple HLA-mismatched SCID-hu assay system. These findings emphasize the unique properties of the different ontogenic sources of HSCs that apparently have an impact on their potential for ex vivo expansion. The observation that expanded CB had a competitive repopulating advantage as compared to expanded ABM does, however, indicate that CB represents a more viable target for ex vivo stem cell expansion than ABM.
In this report we describe an in vivo assay system that will hopefully be useful not only in examining the behavior of different sources of stem cells but also in defining improved strategies for genetic modification or expansion of HSCs with retention of their marrow repopulating potential. Correlation between the behavior of HSC sources in the triple HLA-mismatched SCID-hu bone assay and the behavior of comparable cell populations following transplantation in myeloablated large animal models will be required to evaluate this assay system as a surrogate human HSC assay.
Acknowledgments
The authors would like to thank the staff of Advanced Bioscience Resources Inc for their assistance in obtaining tissues for the construction of the model, and Judy Schnell and Priscilla Fitting for their assistance with flow cytometry.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ronald Hoffman, Eileen Heidrick Professor of Oncology, Chief, Section of Hematology-Oncology, University of Illinois at Chicago, 900 South Ashland Avenue, M/C 734, Chicago, IL 60607; e-mail: ronhoff@uic.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal