Abstract
Many mutations of the housekeeping gene encoding glucose-6-phosphate dehydrogenase (G6PD) cause G6PD deficiency in humans. Some underlie severe forms of chronic nonspherocytic hemolytic anemia (CNSHA) for which there is no definitive treatment. By using retroviral vectors pseudotyped with the vesicular stomatitis virus G glycoprotein that harbor the human G6PD (hG6PD) complementary DNA, stable and lifelong expression of hG6PD was obtained in all the hematopoietic tissues of 16 primary bone marrow transplant (BMT) recipient mice and 14 secondary BMT recipients. These findings demonstrate the integration of a functional gene in totipotent stem cells. The average total G6PD in peripheral blood cells of these transplanted mice, measured as enzyme activity, was twice that of untransplanted control mice. This allowed the inference that the amount of G6PD produced by the transduced gene must be therapeutically effective. With the same vectors both the cloning efficiency and the ability to form embryoid bodies were restored in embryonic stem cells, in which the G6PD gene had been inactivated by targeted homologous recombination, thus effectively rescuing their defective phenotype. Finally, expression of normal human G6PD in hG6PD-deficient primary hematopoietic cells and in human hematopoietic cells engrafted in nonobese diabetic/severe combined immunodeficient mice was obtained. This approach could cure severe CNSHA caused by G6PD deficiency.
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) is a highly conserved cytosolic nictotinaminde adenine dinucleotide phosphate (NADP)–linked dehydrogenase essential in defense against oxidative stress.1,2 G6PD is present in almost all living organisms3 and it is expressed in all tissues and cell types from higher animals and plants4; it can be therefore regarded as a prototype example of a housekeeping gene. In humans and in mice, G6PD is a homodimeric molecule encoded by an X-linked gene. G6PD deficiency is an inherited condition highly prevalent in humans.5 More than 100 missense mutations in theG6PD gene are known to date.6 Most of these mutations cause little or no disease, except when patients are challenged by oxidative drugs or fava beans. However, some mutations cause severe instability of the dimeric molecule and, as a result, lifelong chronic nonspherocytic hemolytic anemia (CNSHA).7,8 Patients with CNSHA are, by definition, anemic and jaundiced, but often tolerate their condition well. However, in the more severe cases, patients have anemia that impairs their quality of life, requiring periodic blood transfusions; they are also at risk from life-threatening exacerbation of hemolysis concomitant with infections. In addition, the granulocytes are so severely deficient in G6PD in some of these patients that they are susceptible to infections,9,10 with a pattern resembling that characteristic of chronic granulomatous disease.11Currently there is no definitive treatment for severe G6PD deficiency. Because in this condition the clinical manifestations are confined to red blood cells (RBCs) and white blood cells (WBCs), all of which in turn derive from hematopoietic stem cells (HSCs), it is in principle an excellent candidate for gene therapy.
Vectors based on murine retroviruses are, in principle, suitable for this purpose because they are able to transfer genes permanently into mammalian cells.12,13 The efficiency of retroviral-mediated gene transfer depends crucially on 2 major processes: binding of the viruses to cellular receptors and cell division.14,15 Both of these processes are somewhat problematic when using retroviruses for transducing HSCs.16 Indeed, most HSCs express low levels of conventional retroviral receptors17,18 and they are relatively quiescent cells.19,20 The first problem could be overcome by pseudotyping a retrovirus with the vesicular stomatitis virus G glycoprotein (VSV-G),21 which, by binding to ubiquitous cell surface glycolipids,22 provides the virus with an alternative mode of entry into cells. The efficiency of gene transfer into quiescent HSCs can be increased by stimulating them from quiescence with different combinations of hematopoietic cytokines.23-25 However, a major concern is that these manipulations may reduce the repopulating ability and the totipotency of HSCs.26-29 It remains uncertain whether short-term ex vivo culture with a suitable combination of cytokines could provide enough HSC division to make gene transfer efficient, but not so much as to compromise their ability to reconstitute hematopoiesis and their totipotency.30 We have constructed a set of VSV-G pseudotyped retroviral vectors harboring human G6PD (hG6PD) driven by different long terminal repeats (LTR). After transducing murine HSCs with these vectors by the use of short-term ex vivo culture, we have obtained stable, lifelong expression of physiologic levels of hG6PD in peripheral blood cells after serial bone marrow transplantation (BMT). With human cells we have satisfied the criteria for transduction of bona fide HSCs in the most stringent surrogate assay; that is, we have obtained expression of functional hG6PD 2 months after engraftment in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. In addition, we have corrected the defective phenotype of G6PD null embryonic stem (ES) cells.
Materials and methods
Construction of retroviral vectors encoding hG6PD
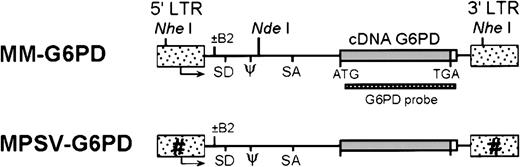
Vector construction (Figure 1) was based on the SFG-plasmid backbone,31 a splicing vector harboring the Moloney murine leukemia virus (MMLV) LTR. The hG6PD complementary DNA (cDNA; 1659-bp fragment containing the entire coding region plus 110 bp of the adjacent 3′UTR) was excised by partialNcoI-SmaI digestion from the pKK233-2G6PD we previously constructed32 and inserted by blunt-end ligation into SFG-vector digested with NcoI-HincII to generate the MM-G6PD vector. To construct MMb2-G6PD, anEagI-EcoRI 4176-bp fragment from MM-G6PD was cloned into the same restrictions sites of the SFGb2 vector harboring the b2 mutation (G→A at position +160 of the MMLV sequence31,33). MPSV-G6PD and MPSVb2-G6PD were constructed cloning the 4316-bp NheI fragment from MM-G6PD and from MMb2G6PD, respectively, in the NheI site of the MPSV-ADA vector,31 which contains the 3′LTR derived from the myeloproliferative sarcoma virus (MPSV). To construct MPSVb2-G6PDA anNcoI fragment of hG6PD A cDNA was released from the plasmid pRx-G6PD A and cloned in the same restriction sites of MPSVb2-G6PD. G6PD A differs from G6PD B by a single substitution (376A→G, cDNA numbering), causing an amino acid replacement (126Asn→Asp).34
Retroviral vector constructs for the transfer of hG6PD.
Different shadings indicate modifications of the LTR as indicated by the designation of the vectors. Arrows indicate origin and direction of transcription. NheI and NdeI are restriction sites used for Southern blot analysis of retroviral integration. LTR indicates long terminal repeat; SD, splice donor; SA, splice acceptor; ψ, packaging sequence.
Retroviral vector constructs for the transfer of hG6PD.
Different shadings indicate modifications of the LTR as indicated by the designation of the vectors. Arrows indicate origin and direction of transcription. NheI and NdeI are restriction sites used for Southern blot analysis of retroviral integration. LTR indicates long terminal repeat; SD, splice donor; SA, splice acceptor; ψ, packaging sequence.
Production of viral supernatant
Ψ-CRE is an NIH3T3-derived ecotropic packaging cell line35; 293GPG is a VSV-G pseudotyped packaging cell line.36 Both Ψ-CRE and 293GPG were transfected with the retroviral vectors by the calcium phosphate precipitation method. Stable transfectant was selected and resistant clones were pooled. Both packaging cell lines produced hG6PD and were tested by Southern blotting to rule out gross rearrangements. Viral production from the transfected 293GPG cells was induced by tetracycline withdrawal and high-titer viral stocks were prepared by ultracentrifugation.21 Titers of viral supernatants were estimated by Southern blot analysis after transduction of NIH3T3 cells.
Transduction of murine hematopoietic stem cells and bone marrow transplantation
Bone marrow (BM) was flushed from femurs and tibiae of male (donor) 10- to 12-week-old C57BL/6J (B6) mice (Jackson Laboratories, Bar Harbor, ME) 6 days after the injection through the tail vein with 5-fluorouracil (150 mg/kg). After RBC hypotonic lysis, BM cells were washed, pooled, and transduced in the presence of murine recombinant cytokines (R&D Systems, Minneapolis, MN) and 8 μg/mL polybrene: (1) 3.5 × 106 cells were cocultured with 30 Gy irradiated viral producer Ψ-CRE on a 10-cm dish in the presence of 200 U/mL of interleukin (IL)-3 and 10 ng/mL of IL-1α for 48 hours; (2) 3.5 × 106 cells were transduced in the presence of 200 U/mL of IL-3 and 10 ng/mL of stem cell factor (SCF) with 3 mL of VSV-G pseudotyped virus stock (5 × 106 viral particles/mL) for 2 cycles of 12 hours; between the 2 cycles the cells were maintained for 12 hours in virus-free medium. Transduced BM cells were transplanted via tail-vein injection into lethally irradiated (2 5-Gy fractions of 137Cs-γ-ray, 4 hours apart) female (recipients) 10- to 12-week-old B6 mice: 5 × 104 cells for spleen colonies, 106 cells for long-term studies. All experimental procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee of our institution.
Detection of hG6PD expression
The WBCs from peripheral blood, BM, and other hematopoietic tissues were purified from RBCs by hypotonic lysis and cell extracts were prepared by 4 cycles of flash freezing–thawing in lysis buffer (Tris HCl, pH 7.4, 10 mmol/L, EDTA 1 mmol/L, EACA 1 μmol/L, NaCl 10 mmol/L, MgCl2 3 mmol/L, NADP 20 μmol/L). RBC extracts were obtained by lysis in 5 volumes of 20 μmol/L NADP. Lysates were cleared by spinning and assayed for G6PD activity by a spectrophotometric assay as previously described at 30°C.37 Mouse and human G6PD were resolved by cellulose acetate gel electrophoresis (Helena Laboratories, Beaumont, TX), followed by specific G6PD activity staining.38 By this method the mouse G6PD is visualized as a purple band running faster than hG6PD; an intermediate band, consisting of a human-mouse heterodimer will be seen whenever both genes are coexpressed within the same cells. Relative activity of human and mouse G6PD was calculated by the intensity of each band quantified by densitometry.
Analysis of chimerism in mice
Southern blot of NheI digested genomic DNA was hybridized with a human cDNA probe. The same filter was rehybridized with a Y chromosome-specific fragment from the pY353/B plasmid39 to assess the extent of repopulation of hematopoiesis by donor-derived cells. To calculate the percentage of donor cells (D) we used the ratio (Rn) of the Y signal in each transplanted female mouse to the Y signal in a male control, normalized to the intensity of the signal from the endogenousG6PD gene. The Rn was further corrected to take into account the fact that in hematopoietic cells of transplanted animals the average number of X chromosomes (where the G6PDgene is located) varies in proportion to the contribution of donor cells between 1 (100% male cells) and 2 (100% female cells). Therefore we have used the following formula:
The average proviral copy number (PCN) per cell after BMT was calculated on the same autoradiograph normalizing the density of the proviral G6PD to the sum of the densities of the 2 endogenous G6PD bands (mG6PD). This number was corrected for the average number of the X chromosomes, thus:
The average copy number in donor-derived cells (ie, in the cells that had been exposed to transduction) is the ratio 100 × PCN/D between the proviral copy number and the percentage of donor cells.
Transduction of human hematopoietic stem cells
Bone marrow and peripheral blood stem cells from normal donors were obtained at the Memorial Sloan-Kettering Cancer Center and cord blood at the New York Blood Center in accordance with institutional procedures. Mononuclear cells were collected by density gradient centrifugation and enriched for progenitors by depletion of differentiated cells by using antibodies directed against lineage-specific antigens (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, glycophorin A) with a StemSep device (StemCell Technologies, Vancouver, BC, Canada). Lineage negative (Lin−) cells were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 20% BIT 9500 serum substitute (StemCell Technologies) and human recombinant cytokines (R&D Systems): IL-3 (20 ng/mL), IL-6 (20 ng/mL), and SCF (50 ng/mL) for 9 hours. Then 3 × 106Lin− cells were transduced on dishes coated with fibronectin (CH-296, TaKara Co, Shiga, Japan) for 2 cycles of 12 hours with 1.5 mL VSV-G pseudotyped MPSVb2-G6PD A retroviral supernatant (108 viral particles/mL) supplemented with the cytokine combination described above. The transduced cells were washed and used for in vitro assay and for injection in NOD/SCID mice. Long-term culture-initiating cell assays were performed as described40 plating 105 transduced Lin− cells in Human MyeloCult media (StemCell Technology) supplemented with 1 μmol/L hydrocortisone on MS-5 murine stromal cells.41 The cells were cultured at 37°C in 5% CO2 for 6 weeks with weekly half-medium change. For colony-forming cell assay 103 transduced Lin−cells, 103 cells from the long-term BM culture, or 106 BM cells from transplanted NOD/SCID mice were plated in 1 mL methylcellulose medium in 35-mm dishes containing the following recombinant human cytokines (R&D Systems): erythropoietin (EPO; 3 U/mL), granulocyte colony-stimulating factor (G-CSF; 50 ng/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL), IL-3 (20 ng/mL), and SCF (20 ng/mL). G-CSF was omitted for colonies from BM of engrafted NOD/SCID mice.42 After 14 days colonies were analyzed for the presence of provirus by polymerase chain reaction (PCR).
Transplantation in NOD/SCID mice
Transduced Lin− cells (2.5 × 105) were injected via tail vein into sublethally irradiated (3 Gy of 137Cs-γ-ray) 8- to 10-week-old NOD/LtSz-scid/scid (NOD/SCID) mice (Jackson Laboratory). Human cells in NOD/SCID mice were detected by flow cytometry using antibodies to human CD45 and CD59.
Detection of provirus and of viral RNA after transplantation in NOD/SCID mice
The presence of the hG6PD-harboring provirus was tested by PCR on individual colonies picked from methylcellulose cultures. Genomic DNA was extracted as previously described.43 Cycling conditions: 35 cycles of 94°C for 45 seconds, 61°C for 30 seconds, and 72°C for 45 seconds. Forward primer (FP), retroviral sequence 5′-CCCCACCGCCCTCAAAGTAG-3′; reverse primer (RP), hG6PD exon 5 sequence 5′-GGCAAGGCCAGGTAGAAGAGGCG-3′ producing a 523-bp fragment. RNA was isolated from BM cells of transplanted NOD/SCID mice using RNA Insolator Kit (Genosys, The Woodlands, TX). RNA was reverse transcribed using random hexamers. The cDNA was amplified by PCR as described for genomic DNA. When human cells were less than 10% an additional amplification cycle was performed: 35 cycles of 94°C for 45 seconds, 61°C for 30 seconds, and 72°C for 45 seconds. FP, retroviral sequence 5′-GAAGTCTGGAGACCTCTGGCGGC-3′; RP, hG6PD exon6 sequence 5′-TGTGGTTGGACAGCCGGTCA-3′). Control samples in which reverse transcriptase (RT) was omitted did not yield any visible band.
Transduction of embryonic stem cells
CJ7 is an ES cell line derived from 129Sv mice.44 The ES cell clone 302 is a G6PD null derivative previously generated.45 ES cells (4 × 105) were grown in 35-mm dishes on a monolayer of primary embryonic fibroblasts as previously described46; after 24 hours the ES cells were transduced for 3 cycles of 24 hours with 3 mL ES cell medium containing 8 μg/mL polybrene and VSV-G psudotyped virus (5 × 107 viral particles/mL). After 72 more hours the ES cells were trypsinized and analyzed for hG6PD expression, cloning efficiency, and embryoid body (EB) formation. ES cell cloning efficiency was measured by plating 100 ES cells and counting the clones after 7 days. EB formation was measured plating 6 × 103ES cells in methylcellulose as previously described43 and counting the EB after 7 to 10 days.
Results
Retroviral-mediated transfer and expression of hG6PD in murine hematopoietic cells
We have constructed retroviral vectors in which thehG6PD gene is driven by either of 2 strong constitutive retroviral promoters: the MMLV-LTR and the MPSV-LTR.31First, by using NIH3T3 cells as a test system, we established that all recombinant viruses are competent for transfer and expression of hG6PD and that they do not undergo genomic rearrangements (data not shown). Next, we proceeded to transduce murine BM cells by conventional coculture with irradiated ecotropic virus producer cells and by adding cell-free VSV-G pseudotyped virus stock. In 7 of 7 experiments hematopoietic colonies collected after 7 to 14 days of culture in methylcellulose expressed hG6PD. The transduced cells were injected into lethally irradiated syngeneic mice. We found intact provirus and hG6PD expression in 53% of 57 spleen colonies in the recipients at 12 days. Integrated provirus was not detected by Southern blot analysis in the 27 colonies that did not express hG6PD.
Long-term expression of hG6PD is achieved in primary and secondary transplanted mice
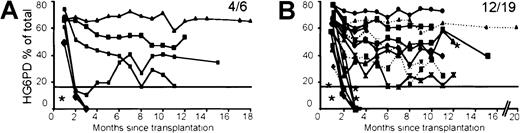
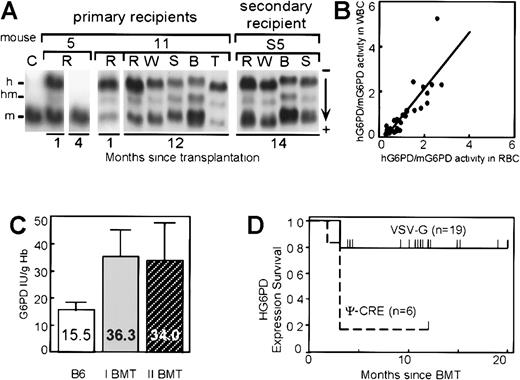
Long-term expression was studied by monthly analysis after BMT in 26 animals that showed donor hematopoiesis. One mouse never expressed hG6PD and no integrated provirus was detected by Southern blot analysis in the hematopoietic cells (Table 1). The remaining 25 mice (96%) had levels of hG6PD in circulating RBCs approximating that of the mouse endogenous enzyme (Figure2). Of these 25 mice, 9 lost expression within 4 months (Figure 2). At this time integrated provirus was not detected by Southern blot analysis in these mice either (Table 1), indicating that the transplant did not contain transduced long-lived stem cells. The remaining 16 transplanted mice showed stable and lifelong expression (8-20 months) of hG6PD (Figure 2), providing evidence that HSCs had been transduced. Moreover, hG6PD expression was also seen in WBCs (the majority of which in the mouse are lymphocytes), as well as in the BM, spleen, and thymus (Figure3A), further supporting the notion that stem cells were their source. To further corroborate this, 8 primary recipient mice were killed (3 mice after 4 months and 5 mice after 9-12 months since BMT) to carry out a second round of BMT. Of the 26 secondary recipients, 22 expressed hG6PD for at least 2 months, and 14 continued to express the hG6PD gene in RBCs and in all their hematopoietic tissues for their whole life (Figure 3A and Table 1). Integrated provirus was not detected by Southern blot analysis in the secondary recipients that either do not express or have lost hG6PD expression. In our transplant experiments, we observed life-long hG6PD expression in only 17% of mice transplanted with Ψ-CRE transduced cells, but in 79% of mice transplanted with VSV-G transduced cells (P = .012 by Fisher exact test) (Figure3D). The precise mechanism for the better result obtained with VSV-G pseudotyped viruses has not yet been investigated.
Analysis of long-term expression of human glucose-6-phosphate dehydrogenase after syngeneic bone marrow transplantation with transduced hematopoietic stem cells
| . | Primary BMT . | Secondary BMT . |
|---|---|---|
| Mice transplanted, n | 26* | 26† |
| Mice expressing hG6PD, n | 25 | 22 |
| Mice with long-term expression, n (follow-up, mo) | 16 (4-20) | 14 (6-16) |
| Mice with short-term‡ expression, n | 9 | 8 |
| Provirus1-153/nonexpressing mice1-155 | 0/10 | 0/12 |
| Chimerism1-155%, mean ± SD (range) | 40 ± 14 (22-66) | ND |
| Provirus copy number per donor cell,1-154 mean ± SD (range) | 2.8 ± 2.1 (0.9-7.6) | ND |
| . | Primary BMT . | Secondary BMT . |
|---|---|---|
| Mice transplanted, n | 26* | 26† |
| Mice expressing hG6PD, n | 25 | 22 |
| Mice with long-term expression, n (follow-up, mo) | 16 (4-20) | 14 (6-16) |
| Mice with short-term‡ expression, n | 9 | 8 |
| Provirus1-153/nonexpressing mice1-155 | 0/10 | 0/12 |
| Chimerism1-155%, mean ± SD (range) | 40 ± 14 (22-66) | ND |
| Provirus copy number per donor cell,1-154 mean ± SD (range) | 2.8 ± 2.1 (0.9-7.6) | ND |
BMT indicates bone marrow transplantation; hG6PD, human glucose-6-phosphate dehydrogenase; ND, not determined.
Mice with donor hematopoiesis.
From 8 primary recipients.
Mice that lost hG6PD expression within 3 to 4 months.
Presence of the provirus detected by Southern blotting.
Mice that failed or ceased to express hG6PD.
See “Materials and methods.”
Long-term follow-up of hG6PD expression in mouse peripheral blood RBCs.
Each line represents one mouse. (A) MMb2-G6PD mice. (B) MPSV-G6PD (…) and MPSVb2-G6PD (—) mice. In each panel the fractional number indicates the number of mice still expressing at 8 to 20 months/number of mice originally expressing at 1 month. Mice marked with asterisk received BM cells transduced by coculture; all others received BM cells transduced with VSV-G supernatants. In each panel a horizontal line is traced at 17% of the total G6PD activity, which is equivalent to 20% of the endogenous murine activity (see text). The relative quantitation of hG6PD expression was obtained by cellulose acetate gel electrophoresis.
Long-term follow-up of hG6PD expression in mouse peripheral blood RBCs.
Each line represents one mouse. (A) MMb2-G6PD mice. (B) MPSV-G6PD (…) and MPSVb2-G6PD (—) mice. In each panel the fractional number indicates the number of mice still expressing at 8 to 20 months/number of mice originally expressing at 1 month. Mice marked with asterisk received BM cells transduced by coculture; all others received BM cells transduced with VSV-G supernatants. In each panel a horizontal line is traced at 17% of the total G6PD activity, which is equivalent to 20% of the endogenous murine activity (see text). The relative quantitation of hG6PD expression was obtained by cellulose acetate gel electrophoresis.
Analysis of expression of hG6PD in hematopoietic cells of reconstituted mice.
(A) Analysis of hG6PD expression by cellulose acetate gel electrophoresis; h indicates human G6PD homodimer; m, mouse G6PD homodimer; hm, human-mouse heterodimer. C, lysate from an untransplanted control mouse. Mouse 5 illustrates loss of expression 4 months after BMT. Mouse 11 illustrates stable long-term expression in RBCs (R), WBCs (W), spleen (S), BM (B), and thymus (T). Mouse S5 was analyzed 14 months after receiving a transplant from a primary recipient that served as donor 11 months after the primary BMT. (B) Similar levels of hG6PD expression in RBCs and WBCs in individual transplanted mice. For both cell types hG6PD activity was normalized to that of the endogenous mouse G6PD. The slope of the regression line (y = 1.2 × −0.3) is very close to 1. (C) Expression of hG6PD doubles the overall G6PD level in peripheral blood RBCs. Average and SD values are shown for untransplanted control mice (B6) (n = 8) and for long-term expressing primary (I) BMT recipients (n = 16) and secondary (II) BMT recipients (n = 14). (D) Survival of hG6PD expression (Kaplan-Meyer method): VSV-G pseudotyped versus ecotropic virus. Each individual mouse still expressing hG6PD is shown by a short vertical line above each survival curve.
Analysis of expression of hG6PD in hematopoietic cells of reconstituted mice.
(A) Analysis of hG6PD expression by cellulose acetate gel electrophoresis; h indicates human G6PD homodimer; m, mouse G6PD homodimer; hm, human-mouse heterodimer. C, lysate from an untransplanted control mouse. Mouse 5 illustrates loss of expression 4 months after BMT. Mouse 11 illustrates stable long-term expression in RBCs (R), WBCs (W), spleen (S), BM (B), and thymus (T). Mouse S5 was analyzed 14 months after receiving a transplant from a primary recipient that served as donor 11 months after the primary BMT. (B) Similar levels of hG6PD expression in RBCs and WBCs in individual transplanted mice. For both cell types hG6PD activity was normalized to that of the endogenous mouse G6PD. The slope of the regression line (y = 1.2 × −0.3) is very close to 1. (C) Expression of hG6PD doubles the overall G6PD level in peripheral blood RBCs. Average and SD values are shown for untransplanted control mice (B6) (n = 8) and for long-term expressing primary (I) BMT recipients (n = 16) and secondary (II) BMT recipients (n = 14). (D) Survival of hG6PD expression (Kaplan-Meyer method): VSV-G pseudotyped versus ecotropic virus. Each individual mouse still expressing hG6PD is shown by a short vertical line above each survival curve.
Effective levels of hG6PD expression are achieved in recipients of primary and secondary transplants
In each one of the transplanted mice that showed life-long expression, the level of hG6PD remained relatively uniform over time (Figure 2), and it was remarkably similar in RBCs and in WBCs (Figure3B). The overall G6PD activity was, on average, twice that of untransplanted control mice (Figure 3C). In addition, in every case hG6PD expression was such as to provide on its own an enzyme activity between 20% and 300% of the endogenous murine G6PD activity. There was no significant difference among the vectors we have used in the levels of G6PD expression (data not shown).
Effective transfer and expression of hG6PDin human hematopoietic stem cells engrafted in NOD/SCID mice
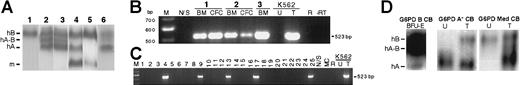
To test G6PD gene transfer into human HSCs, we replaced the wild-type G6PD cDNA in the MPSV-G6PD vector (Figure 1) with cDNA encoding the nondeficient G6PD A variant, which is electrophoretically fast. After transduction of human HSCs from a normal donor, we see clearly the expression of this G6PD variant in methylcellulose colonies harvested after 14 days, as well as after 6 weeks in culture, indicating successful transduction of human hematopoietic progenitor cells (Figure 4A). Next, samples of either peripheral blood stem cells or cord blood cells, depleted of cells expressing lineage differentiation markers, were transduced with the MPSV-G6PD A vector and injected into sublethally irradiated NOD/SCID mice. After 8 weeks, 9 mice had human cells in their BM (range, 1%-41%). In these mice the presence of G6PD messenger RNA (mRNA) originating from the integrated provirus was detected by RT-PCR in the BM and in the colonies obtained by plating BM cells (Figure 4B). At this time, 20.9% of individual hematopoietic colonies showed the presence of provirus at the genomic level (Figure 4C). Moreover, in these colonies enzymatically active G6PD A was also demonstrated (Figure 4D, left). Finally, we were able to use as recipients for our transfer experiments HSCs from 2 samples of G6PD-deficient cord blood: one G6PD A−47 and one G6PD Mediterranean (which has a B-like electrophoretic mobility).48 In both cases we achieved transfer and expression of the appropriate G6PD type (G6PD B and G6PD A, respectively; Figure 4D, right) after 6 weeks in long-term culture. Thus, we effectively corrected G6PD deficiency.
Transfer and expression of hG6PD in human hematopoietic cells.
(A) Expression in short-term and long-term cultures in vitro. Human BM cells were transduced with retrovirus harboring the cDNA of hG6PD A, and then processed for methylcellulose colony assays and long-term cultures. 1, Untransduced human BM cells. Transduced human BM cells: 2, cells after 7 days in liquid culture; 3, pooled white and erythroid colonies; 4, adherent cells obtained at week 6 of a long-term BM culture (the m band derives from mouse MS-5 stromal cells). Controls: 5, mixture of human RBC G6PD B and of murine MS-5 cells; 6, RBCs from a woman heterozygous for G6PD B and G6PD A. hB, human G6PD B. hA, human G6PD A; hA-B, heterodimer of human G6PD A and B; m, murine G6PD. (B) Transfer and expression of hG6PD in human HSCs in vivo. RT-PCR analysis for the presence of hG6PD mRNA transcribed from integrated provirus in BM and human BM colonies (CFC) from 3 NOD-SCID mice transplanted with human HSCs.1,2 3 Positive control: transduced (T) human K562 cells. Negative controls: untransduced (U) K562 cells; untreated NOD/SCID mouse BM cells (N/S); R: reagent control; −RT: omission of reverse transcriptase. M, molecular weight marker. (C) PCR analysis of individual hematopoietic colonies from mouse 1 (Figure 4B): integrated provirus was present in 5 of 25 colonies. MC indicates methylcellulose control. (D) Expression of functional G6PD in vivo and correction of human G6PD deficiency. (Left) Electrophoretic analysis of human erythroid colonies (BFU-E) obtained from NOD/SCID mice 8 weeks after engraftment of G6PD B human cord blood transduced with an hG6PD A vector. (Right) Electrophoretic analysis of human HSCs from G6PD-deficient cord blood samples (G6PD A−and G6PD Mediterranean, respectively) after transduction with an hG6PD vector (G6PD B and G6PD A, respectively). In the case of G6PD Mediterranean, which entails a more severe quantitative defect than G6PD A−, the activity from the transferred gene is clearly higher than from the endogenous gene.
Transfer and expression of hG6PD in human hematopoietic cells.
(A) Expression in short-term and long-term cultures in vitro. Human BM cells were transduced with retrovirus harboring the cDNA of hG6PD A, and then processed for methylcellulose colony assays and long-term cultures. 1, Untransduced human BM cells. Transduced human BM cells: 2, cells after 7 days in liquid culture; 3, pooled white and erythroid colonies; 4, adherent cells obtained at week 6 of a long-term BM culture (the m band derives from mouse MS-5 stromal cells). Controls: 5, mixture of human RBC G6PD B and of murine MS-5 cells; 6, RBCs from a woman heterozygous for G6PD B and G6PD A. hB, human G6PD B. hA, human G6PD A; hA-B, heterodimer of human G6PD A and B; m, murine G6PD. (B) Transfer and expression of hG6PD in human HSCs in vivo. RT-PCR analysis for the presence of hG6PD mRNA transcribed from integrated provirus in BM and human BM colonies (CFC) from 3 NOD-SCID mice transplanted with human HSCs.1,2 3 Positive control: transduced (T) human K562 cells. Negative controls: untransduced (U) K562 cells; untreated NOD/SCID mouse BM cells (N/S); R: reagent control; −RT: omission of reverse transcriptase. M, molecular weight marker. (C) PCR analysis of individual hematopoietic colonies from mouse 1 (Figure 4B): integrated provirus was present in 5 of 25 colonies. MC indicates methylcellulose control. (D) Expression of functional G6PD in vivo and correction of human G6PD deficiency. (Left) Electrophoretic analysis of human erythroid colonies (BFU-E) obtained from NOD/SCID mice 8 weeks after engraftment of G6PD B human cord blood transduced with an hG6PD A vector. (Right) Electrophoretic analysis of human HSCs from G6PD-deficient cord blood samples (G6PD A−and G6PD Mediterranean, respectively) after transduction with an hG6PD vector (G6PD B and G6PD A, respectively). In the case of G6PD Mediterranean, which entails a more severe quantitative defect than G6PD A−, the activity from the transferred gene is clearly higher than from the endogenous gene.
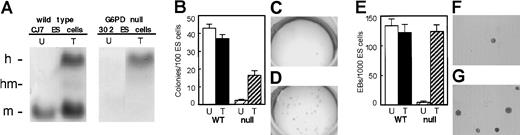
Retrovirally transferred hG6PD corrects the phenotype of G6PD null embryonic stem cells
We have previously produced G6PD null ES cells by targeted homologous recombination, and we have characterized their growth defects.45 46 After injecting these G6PD null ES cells into blastocysts, we found that G6PD-deficient mice die in utero (Longo and coworkers, manuscript in preparation). Therefore, a suitable mouse model of G6PD deficiency is not available. Nevertheless, we were able to use the G6PD null ES cells as a biologic assay of the ability of our vectors to correct severe G6PD deficiency. After retroviral transduction with both VSV-G pseudotyped viruses, we found restoration of hG6PD activity (Figure 5A). In addition, we carried out 2 functional assays. (1) When G6PD null ES cells are plated at low density, their cloning efficiency is about 5% of that of control ES cells. After retroviral transduction, the cloning efficiency is restored to about 40% of normal (Figure 5B-D). (2) When G6PD null ES cells are plated in methylcellulose in the presence of appropriate growth factors, their capacity to produce EB is reduced to less than 2% compared to control ES cells. However, when cells from any of the clones rescued by hG6PD transduction were used to produce EB, the yield is restored to normal (Figure 5E-G).
Transfer and expression of hG6PD in mouse G6PD null ES cells.
(A) Biochemical correction of the G6PD null phenotype. After retroviral transduction wild-type (WT) ES cells show an hG6PD band of comparable intensity to the endogenous murine band. In G6PD null ES cells no G6PD activity is visible before transduction; after transduction only the hG6PD band is seen. (B-D) Functional rescue of the G6PD null phenotype in undifferentiated ES cells. (B) For WT ES cells (n = 5) the cloning efficiency (%) was very similar in untransduced (43 ± 2.4) and in transduced cells (37.1 ± 3.1). By contrast, for G6PD null ES cells the cloning efficiency (n = 5) is only 2.4 ± 0.5 (C), but it is restored (n = 5) to 16.2 ± 2.2 after transduction (D). (E-G) Functional rescue of the G6PD null phenotype in differentiating EB. (E) WT ES cells (n = 2) formed high numbers of EB (133.3 ± 11.8 and 121.7 ± 14/1000 cells in untransduced and in transduced cells, respectively). By contrast, G6PD null ES cells formed only 3.9 ± 1.9 EB/1000 cells (F), but this was restored (n = 6) to 123 ± 12 after transduction (G). U indicates untransduced; T, transduced; WT, wild-type ES cells; null, G6PD null ES cells.
Transfer and expression of hG6PD in mouse G6PD null ES cells.
(A) Biochemical correction of the G6PD null phenotype. After retroviral transduction wild-type (WT) ES cells show an hG6PD band of comparable intensity to the endogenous murine band. In G6PD null ES cells no G6PD activity is visible before transduction; after transduction only the hG6PD band is seen. (B-D) Functional rescue of the G6PD null phenotype in undifferentiated ES cells. (B) For WT ES cells (n = 5) the cloning efficiency (%) was very similar in untransduced (43 ± 2.4) and in transduced cells (37.1 ± 3.1). By contrast, for G6PD null ES cells the cloning efficiency (n = 5) is only 2.4 ± 0.5 (C), but it is restored (n = 5) to 16.2 ± 2.2 after transduction (D). (E-G) Functional rescue of the G6PD null phenotype in differentiating EB. (E) WT ES cells (n = 2) formed high numbers of EB (133.3 ± 11.8 and 121.7 ± 14/1000 cells in untransduced and in transduced cells, respectively). By contrast, G6PD null ES cells formed only 3.9 ± 1.9 EB/1000 cells (F), but this was restored (n = 6) to 123 ± 12 after transduction (G). U indicates untransduced; T, transduced; WT, wild-type ES cells; null, G6PD null ES cells.
Discussion
The definitive correction of an inherited genetic disease that affects blood cells requires the stable transfer of a functional gene into the self-renewing HSCs and their mature progeny. In principle, oncoretrovirus-based vectors are well suited for this purpose. However, certain of their characteristics limit their efficacy. (1) Retroviral-mediated gene transfer depends on binding of viruses to cellular receptors, and HSCs express only low levels of amphotropic retroviral receptors.49 (2) Vector integration requires the active division of the target cell,14 and HSCs are mainly quiescent cells. (3) Retroviral LTRs are often susceptible to transcriptional silencing in mammalian cells, particularly on cell differentiation,50 51 and it is in the mature progeny of HSCs that gene expression is usually needed.
Transduction of bona fide murine hematopoietic stem cells
The long-term expression of hG6PD obtained in both primary and secondary BMT recipients (Figures 2 and 3A) is solid evidence that we have transduced bona fide HSCs, because the repopulation of a myeloablated host after serial transplantation is the paramount characteristic of these elusive cells. We have not yet investigated the mechanism for the higher proportion of life-long hG6PD expression achieved with the VSV-G pseudotyped virus. It is possible that, although Ψ-CRE was used in coculture, the lower success rate was due at least in part to the number of the infectious viral particles in the Ψ-CRE supernatant being approximately 5 times less than in the VSV-G supernatant. In practice VSV-G pseudotyped virus has been superior, probably for several reasons. First, the viral particles bind to ubiquitous cell surface glycolipids22rather than to one of the specific proteins that usually act as retroviral receptors15; second, by using ultracentrifugation we can increase the viral titer virtually at will.21 It is possible that high titer and perhaps rapid entry are features that have enabled us to reduce the ex vivo culture time, thus helping to prevent loss of totipotency and homing ability.30
Absence of transcriptional silencing
A major concern in the use of retroviral vectors has been the extinction of LTR promoter-driven gene transcription.51,52However, by Southern blot analysis we have never detected proviral sequences in any hematopoietic tissue of primary and secondary recipient mice that either failed to express or had ceased to express hG6PD (Table 1). Recently, long-term expression of transferred housekeeping genes in mouse HSCs has been reported for Jak-3,53 dihydrofolate reductase (DHFR),54and ferrochelatase.55 In all of these systems cell selection was operating—either naturally in vivo in the case of Jak-3 or artificially in vivo in the case of DHFR; or artificially in vitro (by sorting of GFP-expressing BM cells) in the case of ferrochetalase. In the present study long-term expression in mice was obtained without any kind of selection.
Successful gene transfer in ES cells has been previously reported56,57; however, ES cells have a marked tendency to silence retroviral promoters58 and, invariably, expression ceases with cell differentiation.50 In fact, our vectors have produced in ES cells hG6PD sufficient to correct the G6PD null ES cell phenotype. Moreover, hG6PD activity was retained in EB undergoing hematopoietic differentiation, as well as in heterozygous embryos obtained by blastocyst injection (L.L. et al, unpublished data, 2000). A possible explanation of this unexpected result may be that, because G6PD is required for the growth of ES clones and of EB, this very growth has selected for cells in which the viral integration site permits constitutive expression.
Adequate levels of transgene expression
The final goal of retroviral gene transfer for the purpose of gene therapy of an inherited disorder is the expression of adequate levels of the transferred gene in the appropriate target cells, in the case of hemolytic anemia that means RBCs. We have obtained lifelong and stable expression of hG6PD in RBCs of both primary and secondary BMT recipient mice. In these mice the overall G6PD activity was, on average, twice that of untransplanted control mice (Figure 3C) and it was proportional in RBCs and in WBCs (Figure 3B). The level of enzyme activity in RBCs was relatively uniform during the follow-up and it was always more than 20% of that of the endogenous murine G6PD (Figure 2). This level is significant for 2 reasons. First, it is twice the level of G6PD activity, 10% of normal, that, according to the World Health Organization classification, is the cutoff point between “mild to moderate” and “severe” G6PD deficiency.59 Second, there was no evidence of hemolysis in a G6PD-deficient mutant mouse with about 15% of the normal activity in the RBCs.60 In addition, the fact that the severely compromised G6PD null ES cells can be corrected by retroviral-transferred hG6PD proves that gene transfer is functionally efficacious. Based on the above findings, the level of retroviral-transferred hG6PD obtained in RBCs can be expected to be therapeutic because it will prevent chronic hemolysis, the main consequence of severe G6PD deficiency.
Long-term expression in both primary and secondary recipients has been reported for gp91phox.61 Although the expression level was low (5%-10% of normal), it was sufficient to correct the mouse deficient phenotype. By contrast, we obtained expression of integrated provirus at physiologic levels in every case. This must reflect (1) a high efficiency of gene transfer in our experiments (average copy number per donor derived cell was 2.8; Table1) and (2) expression resisting extinction regardless of integration sites. In the case of adenosine deaminase long-term expression was obtained in blood cells,31 but the level was lower with an MMLV vector than with an MPSV vector. In contrast, we found similar levels of expression with all of our vectors, which are slightly different from those used by Rivière and colleagues.31
Transduction of human hematopoietic stem cells
The final targets of gene therapy for hematologic disorders are the human HSCs. The closest surrogates of bona fide human HSCs are primitive HSCs able to engraft into NOD/SCID mice (NOD/SCID repopulating cells [SRC]).62 SRC are a difficult target for retroviral gene transfer63; until now, expression in SRC has been demonstrated only with reporter genes.64,65 Recently, transfer of the selectableMDR gene66,67 and expression of the selectableneo gene into SRC has been reported.64 Our data indicate that, using a high titer of VSV-G-pseudotyped virus and a short transduction protocol, a substantial proportion of very primitive human HSCs can be successfully transduced, and that after engraftment into recipient mice they are able to express the retroviral-transferred hG6PD, documented both in terms of mRNA and in terms of enzymatically active protein. These results are at variance with those recently reported by Miyoshi and colleagues,68 probably due to the fact that we have used a transduction protocol more suitable for oncoretroviruses.
Our preclinical work shows that hG6PD gene transfer into HSCs may be a viable strategy for treatment of severe CNSHA due to G6PD deficiency. In our preclinical experiments we did not have the benefit of selection in favor of corrected cells, because the mice had normal G6PD activity. On the other hand, self-selection of autologous stem cells, once they have been genetically corrected by retroviral-mediated integration of a normal hG6PD gene, may well take place in patients with CNSHA. We think this is more than wishful thinking for 2 reasons. (1) Because G6PD inheritance is X-linked, the heterozygous mothers of these (male) patients are genetic mosaics as a result of X-chromosome inactivation. It has been observed that often the G6PD level in the blood cells of these heterozygotes is normal, suggesting somatic cell selection in favor of the hematopoietic cells with the normal G6PD allele on the active X chromosome.69 (2) In our experiments with severely G6PD-deficient ES cells, we observed strong selection in favor of the phenotypically corrected cells. Therefore, it would seem reasonable to entertain a human protocol for the treatment of CNSHA that would avoid the potentially life-threatening infectious and toxic complications of a myeloablative treatment.
Acknowledgments
We are extremely grateful to K. Nafa, A. Karadimitris, L. Longo, D. Tabarini, and C. Tan for much support and advice; A. Wong, C. Murphy, and K. K. Kee for help in the laboratory; we thank C. Stevens and P. Rubinstein for providing cord blood samples, P. Mason for the pRx-G6PDA plasmid, M. J. Mitchell for the pY353/B plasmid, and M. Moore for the MS-5 cell line. A.R. present address: Instituto de Biologı́a Fundamental, Universidad Autónoma de Barcelona, Bellaterra, Spain. M.D.A. was on a leave of absence from the IIGB-CNR, Napoli, Italy. R.N. is on leave of absence from the Division of Hematoloy, Federico II University, Napoli, Italy.
Supported by grants HL59312 and HL57612 from the National Institutes of Health and by the Dewitt Wallace Foundation. M.D.A. was supported by grants from the Italian National Research Council (CNR) and Telethon Foundation.
A.R. and M.D.A. have contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lucio Luzzatto, Istituto Scientifico Tumori, Largo Rosanna Benzi, 16100 Genova, Italy; e-mail:luzzatto@hp380.ist.unige.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal