Abstract
We have previously shown that pretreatment of mice with keratinocyte growth factor (KGF), an epithelial tissue repair factor, can ameliorate graft-versus-host disease (GVHD) after intensive chemoradiotherapeutic conditioning and allogeneic bone marrow transplantation (BMT). To determine whether this effect was dependent on a KGF-mediated mechanism affecting repair of conditioning-induced epithelial cell injury, we studied GVHD in the absence of conditioning using BALB/c severe combined immune-deficient (SCID) recipients given C57BL/6 T cells. KGF (5 mg/kg per day, subcutaneously) given either before or after T-cell transfer enhanced body weights and extended survival. KGF-treated recipients had elevated serum levels of the Th2 cytokine interleukin 13 (IL-13) on day 6 after T-cell transfer concomitant with reduced levels of the inflammatory cytokines tumor necrosis factor-α (TNF-α) and interferon gamma (IFN-γ). A 3-day KGF pretreatment also depressed the secondary in vitro mixed lymphocyte response (MLR) of C57BL/6 splenocytes taken 7 days after in vivo alloimmunization with irradiated BALB/c spleen cells. To determine whether KGF would inhibit host-antidonor–mediated BM rejection, pan-T-cell–depleted BALB/c BM cells were infused into sublethally irradiated C57BL/6 mice and administered KGF either before or before and after BMT. Surprisingly, all KGF schedules tested actually resulted in enhanced alloengraftment. The presence of KGF receptor on donor antihost alloreactive T cells could not be detected by binding studies with radiolabeled KGF, reverse transcriptase–polymerase chain reaction, and Western blotting. Therefore, the mechanism of action of KGF on inhibiting T-cell–mediated immune effects may not be due to a direct effect of KGF on T cells. These studies demonstrate that KGF, by mechanisms independent of repair of conditioning-induced injury, has great potential as an anti-GVHD therapeutic agent with the added benefit of inhibiting the rejection of pan-T-cell–depleted donor BM allografts.
Introduction
Since its discovery in 1989,1keratinocyte growth factor (KGF), a mediator of epithelial cell growth,2 has been the subject of intense investigation because of its potential as a cytoprotective agent against chemoradiotherapeutic toxicities.3-6 We recently demonstrated in a murine model that KGF administration, completed before conditioning, could ameliorate manifestations of graft-versus-host disease (GVHD) and idiopathic pneumonia syndrome (IPS) after allogeneic bone marrow transplantation (BMT).7-9 These BMT complications are significant causes of morbidity and mortality in human BMT and are usually related to the intensity of the conditioning regimen used and the degree of alloreactivity of the donor graft.10 11
Our laboratory has shown that in vivo administration of exogenous KGF, completed before conditioning, ameliorates GVHD-induced weight loss and mortality after allogeneic BMT in mice.7 In addition, KGF diminished GVHD-induced lesions in the target organs, especially the skin and the lungs, of long-term allogeneic BMT survivors. Furthermore, we demonstrated that the lungs of allogeneic BMT recipient mice may benefit from KGF pretreatment by preservation of alveolar epithelialization and attenuation of immune-mediated injury due to a failure to up-regulate costimulatory B7 ligands and cytolytic molecules, and the induction of anti-inflammatory Th2 cytokines in situ.9 Using a different allogeneic murine BMT model, Krijanovsky and coworkers12 have recently demonstrated that KGF given both before and after BMT ameliorates GVHD while preserving the graft-versus-leukemia (GVL) properties of the donor graft.
The purpose of this study was to determine whether the benefits of KGF on the inhibition of GVHD manifestations were dependent on a KGF-mediated mechanism affecting repair of conditioning-induced epithelial cell injury. Because of the potential clinical use of KGF in BMT, we also wished to determine whether KGF adversely affected engraftment. Our data show that KGF is an effective anti-GVHD therapeutic agent in a nonconditioning murine GVHD model and can facilitate engraftment of lymphoid and myeloid cells.
Materials and methods
Mice
BALB/c (H2d), BALB/c severe combined immune deficient (SCID) and C57BL/6 (H2b) mice were purchased from the National Institutes of Health (Bethesda, MD). B10.BR (H2k) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in microisolator cages in the SPF facility of the University of Minnesota and cared for according to the Research Animal Resources guidelines of our institution. For BMT, donors were 8 to 12 weeks of age, and recipients were used at 8 to 10 weeks of age.
Keratinocyte growth factor production
Recombinant human KGF (rhKGF) produced in Escherichia coli was prepared as previously described13 at Amgen (Thousand Oaks, CA) with an ED50 of 40.02 ng/mL, as assessed by BALB/MK cell proliferation. Endotoxin level of the working solution was less than 0.77 EU/mL.
Graft-versus-host disease studies
To determine whether KGF could increase survival in allogeneic recipients not receiving heavy doses of conditioning therapy, we used a model in which nonconditioned BALB/c SCID mice were given highly purified lymph node (LN) T cells. Inguinal, mesenteric, and axillary LN T cells from C57BL/6 donors were purified using columns containing goat-antirat and goat-antimouse Ig-coated beads (Biotex, Edmonton, AB, Canada) and infused (0.5, 1, or 2 × 106 cells) via tail vein on day 0 into BALB/c SCID mice. The composition of the donor T-cell suspension infused was 54% CD4+ T cells, 42% CD8+ T cells, 2% Mac-1+ cells, and 0% CD19+ cells as determined by flow cytometry. Before T-cell infusion, recipient mice were treated with anti-asialoGM1 antibody (Wako Pharmaceuticals, Richmond, VA) on days −3 and −1 to remove natural killer (NK) cells. BALB/c SCID mice received phosphate-buffered saline (PBS) or KGF (5 mg/kg per day subcutaneously [sc]) at either of 4 different dose schedules (for comparison): (1) days −6, −5, and −4 pretransfer; (2) days −3, −2, and −1 pretransfer; (3) days 0 to 3 postdonor T-cell transfer every day; and (4) days 0 to 6 posttransfer, every day. Recipient mice were monitored for survival and total body weights over a 100-day observation period. In some experiments, a separate cohort of mice (n = 6 per group) was killed on day 0 or day 6 after transfer for serum cytokine analysis.
Serum cytokine analysis
At the time of death, blood was collected by cardiac puncture, placed immediately at 4°C, and the serum separated at 4°C and stored at −80°C. Serum levels of interleukin 13 (IL-13) (assay sensitivity 1.5 pg/mL), tumor necrosis factor alpha (TNF-α) (sensitivity 5.1 pg/mL), and interferon gamma (IFN-γ) (sensitivity 2.0 pg/mL) were determined by enzyme-linked immunosorbent assay (ELISA) using commercial kits (R&D Systems, Minneapolis, MN).
Allogeneic mixed lymphocyte response
For in vivo mixed lymphocyte response (MLR), C57BL/6 mice were injected sc (hind flank) with either PBS or KGF (5 mg/kg per day) on days −3, −2, and −1, followed by 5 × 107 irradiated (2500 cGy) BALB/c splenocytes injected intraperitoneally on day 0. Spleens were harvested from the recipients on day 7, and secondary MLR was set up in vitro, in triplicate, at stimulator:responder ratios of 1:1, 0.5:1, 0.25:1, and 0.125:1. Cultures were pulsed with3H-thymidine on day 3, harvested on day 4, and analyzed by scintillation counting. The specific response was calculated as the mean counts per minute (cpm) of the syngeneic response subtracted from the mean cpm of the allogeneic responses.
Engraftment studies
C57BL/6 mice were sublethally irradiated (600 cGy TBI by x-ray at a dose rate of 0.41 Gy/min) on the day before BMT as described.14 Donor BALB/c BM was T-cell depleted (TCD) with anti-Thy 1.2 monoclonal antibody (mAb) (clone 30-H-12, rat IgG2b, kindly provided by Dr David Sachs, Charlestown, MA) plus complement (Nieffenegger Co, Woodland, CA). Recipient mice were transplanted via caudal vein with 10 × 106 TCD BALB/c BM. C57BL/6 mice received PBS or KGF (5 mg/kg per day, sc) at either of 3 different dose schedules (for comparison): (1) days −4, −3, and −2 before BMT; (2) days −4, −3, −2, and 0 to 14 every day; (3) days −4, −3, −2, and then 3 times per week from days 0 to 14.
Peripheral blood leukocyte and bone marrow typing by flow cytometry
Initial chimerism of peripheral blood mononuclear cells was evaluated on day 57 by quantitation of donor cells using FITC-labeled anti-H2d mAb (clone 34-5-8S, mouse IgG2b) and host cells using PE-labeled anti-H2b (clone EH144, mouse IgG). For determination of stability of donor cell engraftment and to examine the chimerism levels in various hematopoietic lineages, chimerism also was evaluated for peripheral blood leukocyte (PBL) and BM of some mice on day 105. The T-cell, B-cell, and monocyte composition of peripheral blood and BM on day 105 after BMT was determined using fluorochrome-labeled mAb (FITC or PE) directed to CD4 (clone GK1.5, rat IgG2b provided by Dr Frank Fitch, Chicago, IL), CD8 (clone 53-6.72, rat IgG2a provided by Dr Jeffrey Ledbetter, Seattle, WA), CD19 (clone 1D3, rat IgG2a, Pharmingen, San Diego, CA), and CD11b (Mac-1, clone M1/70, rat IgG2b, Pharmingen), respectively. Flow cytometry was performed on a FACScalibur (Becton Dickinson, Mountain View, CA) with 10 000 events analyzed (determined by forward and side scatter).
KGF receptor studies
To determine whether KGF could directly bind to T cells, KGFR expression was analyzed on alloreactive donor T cells obtained after in vivo priming. B10.BR mice were lethally irradiated (8 Gy) on day −1 and infused the following day with 8 × 106 C57BL/6 BM and 25 × 106 C57BL/6 spleen cells to induce GVHD. Donor T cells were harvested by thoracic duct cannulation15 on day 6 after BMT and analyzed for expression of KGFR (FGFR2 splice variant) by radiolabeled binding studies, reverse transcriptase–polymerase chain reaction (RT-PCR) and Western blot. KGF receptor binding assays were performed essentially as previously described.16 Briefly, T cells harvested from the murine thoracic duct lymphatics were maintained on ice in complete DMEM overnight before assay. They were subsequently washed twice with HEPES binding buffer (100 mmol/L HEPES, 150 mmol/L NaCl, 5 mmol/L KCl, 1.2 mmol/L MgSO4, 8.8 mmol/L Dextrose, 0.1% BSA, pH 7.4) and resuspended in this buffer at a concentration of 2.9 million per milliliter. BALB/MK mouse keratinocytes were trypsinized, and then washed and resuspended in the same manner. Triplicate aliquots of cells (250 μL) were incubated for 3 hours at 16°C in binding buffer containing 0.3 μg/mL of heparin (12 kd from porcine intestine; Fisher, Pittsburgh, PA), 0.0037 MBq (0.1 μCi)125I-KGF, and varying concentrations of unlabeled KGF as indicated in the figure (Figure 6). Cells were washed 3 times with cold phosphate-buffered saline (PBS) and radioactivity of the cell pellets was measured in a gamma counter. KGFR transcript was detected by RT-PCR as previously described.17 RNA was prepared from T cells harvested from the murine thoracic duct and bone marrow and from BALB/MK cells, which served as a positive control. After generation of cDNA, PCR was performed with a sense primer corresponding to the KGFR-specific IIIb exon and an antisense primer corresponding to a sequence common to both KGFR and other FGFR isoforms. Amplification products were resolved by electrophoresis in an agarose gel and visualized by ethidium bromide staining.
Statistical analysis
Survival data were analyzed by life table methods using the Mantel-Peto-Cox summary of χ2. Other data were analyzed by analysis of variance (ANOVA) or Student t test. Probability (P) values less than or equal to 0.05 were considered statistically significant.
Results
KGF ameliorates GVHD in the absence of conditioning
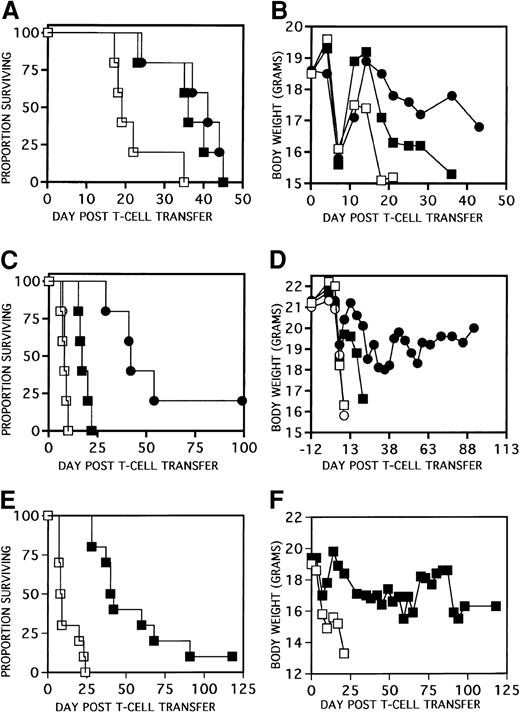
To determine whether our previous findings of GVHD inhibition7 were solely due to a KGF-mediated mechanism affecting repair of conditioning-induced epithelial cell injury, GVHD studies were performed in the absence of chemoradiotherapy conditioning regimens. NK-depleted (αAsGM1-treated) BALB/c SCID (H2d) recipients were administered PBS or KGF (5 mg/kg per day sc) on days −3, −2, and −1 before infusion of 0.5 or 2 × 106C57BL/6 T cells. In other injury models, the optimal effect of KGF is obtained when administered in a 3-day schedule before injury3 6 and, in mice, rhKGF is cleared to nondetectable levels within 24 hours after infusion (Catherine Ferrell and Anne Heatherington, Amgen, unpublished data, 1998). Figure1A,B show that KGF-pretreatment on days −3, −2, and −1 pretransfer of 2 × 106 T cells significantly delayed mortality (P < .007 vs PBS) and enhanced body weights after T-cell transfer. Similar results were obtained when KGF was administered every day from either days 0 to 3 (Figure 1C,D) or days 0 to 6 (Figure 1E,F) in recipients of 0.5 to 2 × 106 T cells (P < .001 for survival with either cell dose) with weights especially improved in recipients of the lower T-cell doses. Side-by-side comparison of KGF administered on days −3, −2, and −1 versus days 0 to 6 to recipients of 2 × 106 T cells revealed no statistical differences between schedules (Figure 1A,B). In contrast, administration of KGF on days −6, −5, and −4 before T-cell transfer did not affect actuarial survival or body weights in recipients of 2 × 106allogeneic T cells and only marginally prolonged survival in allogeneic recipients of the lower dose of 0.5 × 106 T cells (not shown). Therefore, the amelioration of GVHD by KGF administration can be achieved in the absence of conditioning, indicating that the mechanism involved need not be exclusively due to the KGF-mediated repair of conditioning-induced epithelial tissue injury resulting in a less potent immune response “accelerant.” The data confirm that a 3-day course of KGF is sufficient to achieve the effect of KGF provided it is not given too far in advance of the T-cell transfer.
KGF ameliorates GVHD in a nonconditioned SCID model.
Different KGF schedules and T-cell transfer doses are shown. In panels A (survival) and B (weights), BALB/c SCID mice received PBS (■) or KGF on days −3, −2, and −1 pretransfer (▪) of 2 × 106 C57BL/6 LN T cells or days 0 to 6 after transfer (●) as shown (P < .007 for survival of KGF mice vs PBS, n = 5 per group). In panels C (survival) and D (weights), BALB/c SCID mice received PBS or KGF on days 0, 1, 2, and 3 after transfer of either 0.5 (○, PBS; ●, KGF) or 2 × 106 (■, PBS; ▪, KGF) C57BL/6 LN T cells as shown (P < .001 for survival of KGF vs non-KGF counterparts, n = 5 per group). In panels E (survival) and F (weights), BALB/c SCID mice received PBS (■) or KGF (▪) on days 0 to 6 after transfer of 106 C57BL/6 LN T cells (P < .001 for survival of KGF vs PBS, n = 10 per group).
KGF ameliorates GVHD in a nonconditioned SCID model.
Different KGF schedules and T-cell transfer doses are shown. In panels A (survival) and B (weights), BALB/c SCID mice received PBS (■) or KGF on days −3, −2, and −1 pretransfer (▪) of 2 × 106 C57BL/6 LN T cells or days 0 to 6 after transfer (●) as shown (P < .007 for survival of KGF mice vs PBS, n = 5 per group). In panels C (survival) and D (weights), BALB/c SCID mice received PBS or KGF on days 0, 1, 2, and 3 after transfer of either 0.5 (○, PBS; ●, KGF) or 2 × 106 (■, PBS; ▪, KGF) C57BL/6 LN T cells as shown (P < .001 for survival of KGF vs non-KGF counterparts, n = 5 per group). In panels E (survival) and F (weights), BALB/c SCID mice received PBS (■) or KGF (▪) on days 0 to 6 after transfer of 106 C57BL/6 LN T cells (P < .001 for survival of KGF vs PBS, n = 10 per group).
KGF increases the expression of an anti-inflammatory Th2 cytokine and decreases Th1 proinflammatory cytokine production after donor T-cell transfer
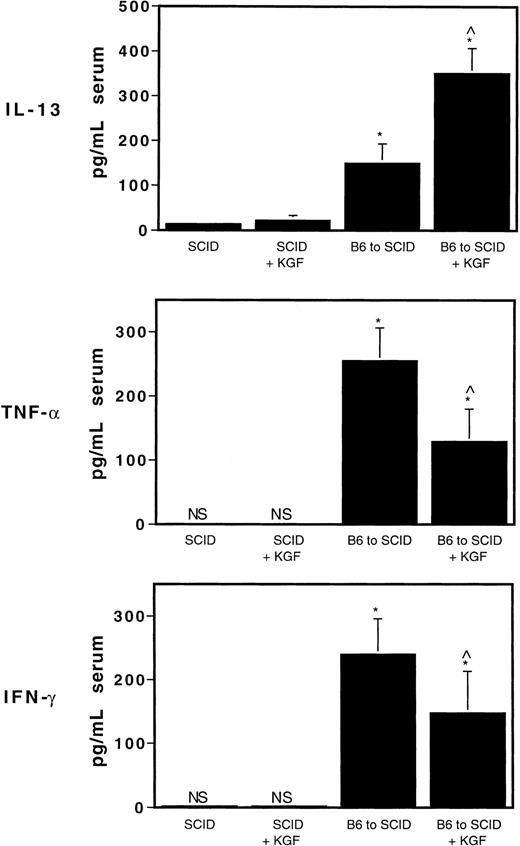
The production of proinflammatory Th1 cytokines such as TNF-α is normally associated with increased severity of GVHD, whereas the production of anti-inflammatory Th2-type cytokines such as IL-4 and IL-10 often is associated with reduced GVHD-induced lethality.18 We have previously shown that increased serum levels of the anti-inflammatory Th2 cytokine IL-13 are induced by KGF in our allogeneic BMT model using lethally irradiated recipients, implicating a role for IL-13, or the induction of Th2 cells, in the mechanism responsible for the effects of KGF on ameliorating GVHD.9 To determine whether Th2 cytokines were induced by KGF in our nonconditioned GVHD model, serum levels of IL-13 were measured by ELISA on day 6 in BALB/c SCID recipients of B6 T cells given KGF on days 0 through 6, every day (mice were bled 2 hours after last injection of KGF on day 6). Figure 2shows that the circulating levels of IL-13 on day 6 after T-cell transfer were significantly elevated in allogeneic T-cell recipients treated with KGF (P < .0006 compared with non-KGF counterparts and control SCID mice). The increased IL-13 was particularly noted with donor T-cell infusion because KGF-treated (day 0-6) SCID mice that were not infused with allogeneic T cells did not exhibit increased circulating IL-13 levels on day 6. We did, however, see a moderate, yet statistically significant increase in serum IL-13 levels after a 3-day course of KGF in SCID mice, ie, KGF given on days −3, −2, and −1 and serum taken on day 0 (44.6 ± 3.9 vs 14.8 ± 3.6 pg/mL, KGF vs non-KGF,P = 1.0−9). Furthermore, Figure 2 shows that recipients of KGF day 0 to 6 had decreased levels of the inflammatory mediators TNF-α and IFN-γ on day 6 after T-cell transfer (P < .02 vs non-KGF). These data indicate that KGF, either directly or indirectly, induced the production of the Th2-like anti-inflammatory cytokine IL-13, a potent down-regulator of macrophage differentiation and function, and decreased the production of the Th1 cytokines TNF-α and IFN-γ, consistent with recent findings in our lethally irradiated BMT model.8 9
KGF increases IL-13 and decreases TNF-α and IFN-γ after allogeneic T-cell transfer in SCID mice.
Serum levels of IL-13, TNF-α and IFN-γ were measured by ELISA on day 6 in BALB/c SCID recipients of 2 × 106 C57BL/6 T cells given KGF on days 0 through 6, every day (mice were bled 2 hours after the last injection of KGF on day 6). For IL-13, *P < .00000007 vs SCID controls; ^P < .0006 compared with non-KGF counterparts and control SCID mice. For TNF-α and IFN-γ, ^P < .02 versus non-KGF counterpart. Mean values of 6 to 9 mice per group ± SD are shown. NS indicates not significant, levels below assay sensitivity.
KGF increases IL-13 and decreases TNF-α and IFN-γ after allogeneic T-cell transfer in SCID mice.
Serum levels of IL-13, TNF-α and IFN-γ were measured by ELISA on day 6 in BALB/c SCID recipients of 2 × 106 C57BL/6 T cells given KGF on days 0 through 6, every day (mice were bled 2 hours after the last injection of KGF on day 6). For IL-13, *P < .00000007 vs SCID controls; ^P < .0006 compared with non-KGF counterparts and control SCID mice. For TNF-α and IFN-γ, ^P < .02 versus non-KGF counterpart. Mean values of 6 to 9 mice per group ± SD are shown. NS indicates not significant, levels below assay sensitivity.
KGF administration depresses the in vivo allogeneic mixed lymphocyte response
To determine whether KGF may be inhibiting the in vivo T-cell alloresponse, C57BL/6 mice were given KGF sc on days −3, −2, and −1, followed by injection of irradiated BALB/c spleen cells intraperitoneally on day 0. This schedule was chosen because we had determined (above) that the effect of KGF on inhibiting GVHD was temporally related to T-cell infusion. Figure3, depicting one of 3 experiments, shows that the secondary in vitro MLR response of splenocytes taken 7 days after in vivo alloimmunization is depressed in KGF-treated mice at all stimulator:responder ratios used (P < .001 by ANOVA). The same result was obtained when data were calculated as either stimulation index (allogeneic/syngeneic) or difference in response (allogeneic-syngeneic). Furthermore, KGF did not affect the response to syngeneic stimulators (not shown) demonstrating that the calculated decreased allogeneic response was not due to an increase in syngeneic response. These data implicate KGF as playing a direct or indirect role in the dampening of the alloresponse during GVHD in vivo.
KGF suppresses the in vivo allogeneic MLR.
C57BL/6 mice were given KGF (5 mg/kg per day) sc on days −3, −2, and −1, followed by injection of 5 × 107 irradiated BALB/c spleen cells intraperitoneally on day 0. Splenocytes were taken 7 days after in vivo alloimmunization, and secondary in vitro MLR was set up at the indicated responder:stimulator ratios. Data are presented as mean ± SE for 3 mice per group. P < .001 for KGF (●) versus PBS (○) by ANOVA. One of 3 reproducible experiments is shown.
KGF suppresses the in vivo allogeneic MLR.
C57BL/6 mice were given KGF (5 mg/kg per day) sc on days −3, −2, and −1, followed by injection of 5 × 107 irradiated BALB/c spleen cells intraperitoneally on day 0. Splenocytes were taken 7 days after in vivo alloimmunization, and secondary in vitro MLR was set up at the indicated responder:stimulator ratios. Data are presented as mean ± SE for 3 mice per group. P < .001 for KGF (●) versus PBS (○) by ANOVA. One of 3 reproducible experiments is shown.
KGF facilitates engraftment after allogeneic BMT
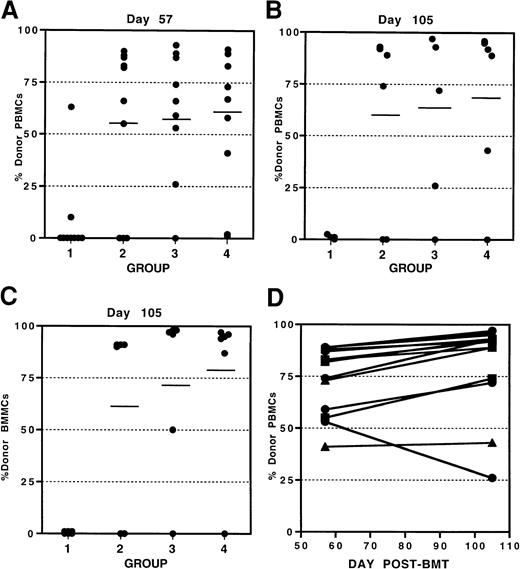
Because KGF inhibited alloresponses in vivo, we proceeded to determine whether T-cell–mediated host rejection of donor grafts would be affected by KGF pretreatment. We used our established sublethal alloengraftment model in which 10 × 106pan-T-cell–depleted BALB/c (H2d) BM cells are infused into sublethally irradiated (6 Gy) C57BL/6 (H2b) mice. Under these conditions, host T cells resist the T-cell–depleted donor BM grafts. KGF (5 mg/kg per day, sc) was administered on days −4, −3, and −2, ie, ending 1 day before conditioning. To determine whether rejection would be affected in the presence of KGF, and because KGF is cleared within 24 hours in the mouse, 2 additional schedules were compared that used both pre- and post-BMT KGF administration: days −4, −3, −2, and 0 to 14 (every day) and days −4, −3, −2, and 3 times per week from day 0 to 14. Engraftment of donor cells (H2d) in peripheral blood and BM was evaluated on day 57 after BMT by FACS. Figure 4A shows that all 3 KGF schedules resulted in enhanced alloengraftment (mean ± SD 58% ± 33% donor engraftment vs 8% ± 19% for controls,P < .001) and did not differ from each other. This comparison of KGF dose schedules further extends our previous findings that a 3-day dosing before conditioning and BMT is sufficient for the effects of KGF to be manifested in vivo. The administration of KGF both before and after BMT does not significantly further enhance engraftment beyond that obtained with the 3-day pre-BMT dosing.
KGF facilitates engraftment postallogeneic BMT.
C57BL/6 recipient mice were sublethally irradiated (6 Gy) (day −1) and given T-cell–depleted BALB/c BM (day 0). Three KGF treatment schedules were compared (5 mg/kg per day, sc). Group 1: control BMT group; group 2: KGF given on days −4, −3, and −2 before BMT; group 3: KGF given on days −4, −3, −2, and 0 to 14 (every day); group 4: KGF given on days −4, −3, −2, and 3 times per week from day 0 through 14. Engraftment of donor cells was evaluated by FACS as described in “Materials and methods.” Results for individual mice are shown for donor cell engraftment in peripheral blood on day 57 (A) and day 105 (B) as well as in bone marrow on day 105 (C). Maintenance of engraftment in peripheral blood for individual KGF-treated mice is shown in panel D.
KGF facilitates engraftment postallogeneic BMT.
C57BL/6 recipient mice were sublethally irradiated (6 Gy) (day −1) and given T-cell–depleted BALB/c BM (day 0). Three KGF treatment schedules were compared (5 mg/kg per day, sc). Group 1: control BMT group; group 2: KGF given on days −4, −3, and −2 before BMT; group 3: KGF given on days −4, −3, −2, and 0 to 14 (every day); group 4: KGF given on days −4, −3, −2, and 3 times per week from day 0 through 14. Engraftment of donor cells was evaluated by FACS as described in “Materials and methods.” Results for individual mice are shown for donor cell engraftment in peripheral blood on day 57 (A) and day 105 (B) as well as in bone marrow on day 105 (C). Maintenance of engraftment in peripheral blood for individual KGF-treated mice is shown in panel D.
To determine whether the KGF-facilitated engraftment was maintained, chimerism was evaluated on day 105 after BMT in these same recipient mice (n = 6 sentinel mice per group). KGF-treated mice had significantly high levels of engraftment in both the peripheral blood (Figure 4B) and BM (Figure 4C). Again, the 3 KGF dose schedules compared did not differ from each other. The relationship between the level of donor cell engraftment achieved in the periphery and the BM exhibited a high degree of correlation (r2 = 0.938) indicating the ability of KGF to enhance engraftment in both primary and peripheral lymphohematopoietic compartments. In addition, Figure 4D shows that 13 of 14 (93%) of KGF-treated mice that had engrafted to varying degrees (41% to 89%) on day 57, exhibited either maintained or increased levels of donor cell engraftment on day 105 after BMT. This demonstrates that the effects of KGF on facilitating engraftment are long-term.
Although KGF facilitated overall engraftment of donor cells, we wanted to determine whether KGF preferentially affected engraftment of cells involved in cellular (monocytes, CD4+, and CD8+T cells) or humoral (B cells, CD4+ T cells) immune responses that would have implications for reconstitution of immune competence after BMT. Figure 5A shows that, on day 105 after BMT, in the peripheral blood, KGF facilitated engraftment of CD4+ and CD8+ T cells, B cells (as determined by CD19 expression) and cells of the monocyte lineage (as determined by expression of Mac-1). In the bone marrow, KGF facilitated engraftment of B cells and monocytes as would be expected for this compartment (Figure 5B).
KGF facilitates engraftment of T cells, B cells and monocytes.
Recipient mice from Figure 4 are shown. KGF schedules are as follows: KGF-1, days −4, −3, and −2 before BMT; KGF-2, days −4, −3, −2, and 0 to 14 (every day); KGF-3, days −4, −3, −2, and 3 times a week from day 0 through 14. Engraftment of donor T cells (CD4, CD8), B cells (CD19), and monocytes (Mac-1) was evaluated on day 105 after BMT by FACS as described in “Materials and methods.” Mean values are indicated for 6 mice per group. PBS indicates non-KGF–treated C57BL/6 recipients of BALB/c BM, most of which reject the BM grafts.
KGF facilitates engraftment of T cells, B cells and monocytes.
Recipient mice from Figure 4 are shown. KGF schedules are as follows: KGF-1, days −4, −3, and −2 before BMT; KGF-2, days −4, −3, −2, and 0 to 14 (every day); KGF-3, days −4, −3, −2, and 3 times a week from day 0 through 14. Engraftment of donor T cells (CD4, CD8), B cells (CD19), and monocytes (Mac-1) was evaluated on day 105 after BMT by FACS as described in “Materials and methods.” Mean values are indicated for 6 mice per group. PBS indicates non-KGF–treated C57BL/6 recipients of BALB/c BM, most of which reject the BM grafts.
Alloactivated T cells do not express KGF receptor (FGFR2 splice variant)
KGFR is normally found on epithelial cells and KGF has also been shown to have direct effects on endothelium.19 The expression of KGFR on T cells has not been addressed formally. Because of the effects of KGF on ameliorating T-cell–mediated GVHD and graft rejection, we wanted to know whether KGF could be directly binding to and affecting T cells. Otherwise, KGF would most likely be affecting T cells via an indirect mechanism. To answer this question, B10.BR mice were lethally irradiated (8 Gy) and infused with 8 × 106C57BL/6 BM and 25 × 106 spleen cells to induce GVHD. Donor T cells were harvested by thoracic duct cannulation on day 6 after BMT, the time of maximal expansion of these cells in the lymphatics containing more than 98% donor T cells (23% CD4+, 72% CD8+). Figure6 shows that KGF specific binding could not be detected on these T cells compared with KGFR+control BALB/MK epithelial cells. Similar results were obtained with in vitro alloactivated spleen cells from day 4 through 5 MLR cultures (data not shown). The presence of KGFR (FGFR splice variant) on these cells also could not be detected by either RT-PCR or Western blot (4 reproducible experiments, data not shown). Therefore, the mechanism of action of KGF on inhibiting in vivo T-cell–mediated immune effects appears not to be due to a direct effect of KGF on T cells, insofar as to the level of detection with the methods we used. Also, we saw no direct effects on in vitro MLR (data not shown). However, we do not exclude the possibility that KGF can bind to T cells via another surface ligand (not FGFR2) present at low density.
KGF receptor is not detected on alloactivated T cells.
B10.BR recipient mice were lethally irradiated with TBI (8 Gy, day −1) and given C57BL/6 BM with spleen cells the following day. Donor T cells were harvested by thoracic duct cannulation on day 6 after BMT and incubated with radiolabeled 125I-KGF in the presence of increasing amounts of unlabeled (cold) KGF. The BALB-MK KGFR+ control epithelial cell line was used as a control. Mean values ± standard error of triplicate aliquots of cells pooled from 6 recipient mice are indicated.
KGF receptor is not detected on alloactivated T cells.
B10.BR recipient mice were lethally irradiated with TBI (8 Gy, day −1) and given C57BL/6 BM with spleen cells the following day. Donor T cells were harvested by thoracic duct cannulation on day 6 after BMT and incubated with radiolabeled 125I-KGF in the presence of increasing amounts of unlabeled (cold) KGF. The BALB-MK KGFR+ control epithelial cell line was used as a control. Mean values ± standard error of triplicate aliquots of cells pooled from 6 recipient mice are indicated.
Discussion
These studies demonstrate that murine GVHD can be ameliorated in the absence of conditioning in SCID recipients of allogeneic T cells when KGF is administered either before or after T-cell transfer. We also found that KGF can depress the in vivo alloresponse and shift the Th1/Th2 cytokine balance away from the inflammatory Th1 type perhaps through induction of IL-13. Furthermore, KGF has the added benefit of inhibiting the rejection of pan-T-cell–depleted donor BM allografts. These effects on T-cell–mediated responses are likely indirect, as we could not demonstrate specific KGF receptor on alloreactive T cells. Our data also show that KGF given before BMT only was sufficient to facilitate engraftment of lymphoid and myeloid cells.
KGF has been studied mainly as a cytoprotective agent in several chemotherapy- and irradiation-induced injury models. Investigations on its mechanisms of action have described effects on enhancement of DNA repair, inhibition of apoptosis, and induction of antioxidants (reviewed in Werner20). We recently described that KGF suppressed the up-regulation of inflammatory immune mediators in the post-BMT lung,9 and our current findings of GVHD inhibition in the absence of conditioning lend further evidence that KGF also has effects on the immune system. However, we do not rule out the possibility that, in this SCID mouse model of GVHD, KGF is still acting as a cytoprotective agent against inflammatory mediators and allospecific cytolytic cells in their effects on epithelial cell injury. Furthermore, the SCID mice we used did not appear histologically to have pre-existing epithelial cell damage in the gut (eg, enterocolitis) because of their immune deficiency. Nonetheless, KGF may still be acting in a cytoprotective manner by its previously described actions of enhancing intestinal stem cell survival and DNA repair in the face of subclinical infection or predisposition to damage.
The data confirm our observations, in an allogeneic lethally irradiated mouse BMT model,7 that a 3-day pretreatment course of KGF is sufficient to achieve the anti-GVHD effect provided that it is not given too far in advance of the allogeneic transfer. To determine whether KGF may be effective during the injury induction phase as has been suggested, KGF was administered after allogeneic T-cell transfer. Side-by-side comparison of a 3-day pretreatment course of KGF with a 7-day posttreatment course revealed no difference in the prolongation of survival, but body weights were higher in the mice given KGF after transfer. These findings are consistent with the recent study by Ferrara and coworkers showing additional benefit on recovery of murine intestinal mucosa when KGF is administered both before and after BMT.12
The KGF-mediated induction of the Th2 cytokine IL-13 in this SCID model is consistent with our previous findings of increased IL-13 levels at the time of BM infusion in our allo-BMT model in recipients that were pretreated with KGF.9 IL-13 is a potent down-regulator of many monocyte functions in vitro and in vivo.21-23 We have proposed that the induction of Th2-type cytokines in KGF-treated mice may be contributing to the decreased frequency of cells expressing the T-cell costimulatory molecules B7.1 and B7.2 and the decreased inflammatory (TNF-α, IFN-γ), and cytotoxic (granzyme B) T-cell mediators we found in the postallogeneic BMT lung. Consistent with this hypothesis is the current observation of increased serum levels of IL-13 concomitant with decreased TNF-α and IFN-γ levels. Although the cytokine profile appears to be shifted to a Th2 phenotype in KGF-treated mice and is T-cell dependent, the source of the increased IL-13 has not been identified. The major source of IL-13 is normally Th2 cells. Previously, we found increased serum IL-13 levels in KGF-pretreated T-cell–replete mice at the time of BMT. In the current study, we observed increased IL-13 in SCID mice on day 6 after transfer of allogeneic T cells and KGF (days 0 to 6). The source of IL-13 is presumably the donor T cells, because SCID mice given KGF (days 0 to 6) but no T cells did not exhibit increased IL-13 postcessation of KGF administration. However, we did find a modest increase in serum IL-13 in nontransferred SCID mice after a short 3-day course of KGF, suggesting that IL-13 is also being produced by other cell types.24 25
We show that the in vivo alloresponse is depressed in KGF-pretreated mice given irradiated allogeneic spleen cells. This is in contrast to a recent observation showing no effect on the MLR of splenic T cells taken 14 days postallogeneic murine BMT.12 However, the potential reasons for this discrepancy are many, including different time point (we analyzed splenocytes from day 7 after immunization), different immunization environment (lethally irradiated BMT vs nonconditioned, non-BMT), and different responder:stimulator ratios (statistically significant differences were observed at ratios lower than 1:1 in our study). We do not know whether KGF is directly or indirectly dampening the alloresponse in vivo. We have begun to investigate the hypothesis that, in response to KGF, epithelial cells produce a factor(s) that can dampen the MLR. Consistent with this hypothesis is a recent study26 by our group demonstrating that the post-BMT lungs in mice pretreated with KGF have increased surfactant protein A that has been shown to suppress T-cell responses (reviewed in Wright27). There may be parallel inductions of other epithelial-derived factors that are capable of suppressing T-cell responses in other GVHD target organs.
We observed that KGF facilitated engraftment by inhibiting the T-cell–mediated rejection of donor BM. Engraftment of lymphoid and myeloid cells in the peripheral blood and BM was maintained long-term in KGF-treated mice. In comparisons of KGF administration schedules, we confirmed our previous findings that a 3-day dosing before conditioning is sufficient for the effects of KGF to be manifested in vivo because there was no difference in engraftment when KGF was given both before and after BMT versus before BMT only. These findings support the conclusion that the inhibitory effects of KGF on GVHD are not due to suppressed donor-cell engraftment. This effect of pretreatment with cytokine or growth factor is quite unique in the bone marrow transplant setting because factors such as G-CSF are routinely used after transplant to facilitate neutrophil recovery. To our knowledge, only one other cytokine, IL-1, has been documented as a pretreatment in a murine allogeneic BMT model28 that enhanced alloengraftment. In addition to its hematopoietic activities, IL-1 has been identified as one of the radioprotective cytokines.29Its effect in facilitating engraftment has been described as being mediated by transient enhancement of endogenous hematopoiesis and not due to a direct effect on allogeneic stem cells.30Although we have not fully explored this mechanism in our model, we have not been able to detect any change in IL-1 protein levels in the sera or messenger RNA (mRNA) levels in the spleens of KGF-pretreated mice (unpublished data). Furthermore, it is possible that a mechanism of the effect of IL-1 on engraftment may involve the up-regulation of endogenous KGF because IL-1 is the strongest known inducer of KGF expression.31 32
KGFR (FGFR II splice variant) is found primarily on epithelial cells.33 KGF can also stimulate proliferation of microvascular endothelial cells, although KGFR mRNA could not be detected in these cells.19 This implies that KGF may bind to an, as yet unidentified, alternate receptor. Because of the in vivo effects of KGF on T-cell–mediated functions, we attempted to determine whether KGFR could be detected in alloreactive T cells, a question not formally addressed previously. The presence of KGFR (FGFR splice variant) could not be detected by either RT-PCR or Western blot. In addition, binding of radiolabeled KGF to these cells also was not seen. Therefore, the mechanism of action of KGF on inhibiting in vivo T-cell–mediated immune effects appears not to be direct, but we do not exclude the possibility that KGF can bind to T cells via another surface ligand (not FGFR2) present at low density.
In summary, these studies demonstrate that KGF is an effective anti-GVHD therapeutic agent by a mechanism independent of repair of conditioning-induced epithelial injury with the added benefit of inhibiting the rejection of pan-T-cell–depleted donor BM allografts.
Acknowledgments
The expert technical assistance of Chris Lees, John Hermanson, Naomi Fujioka, Stacey Hermanson, Melinda Berthold, and Kelly Coffee is greatly appreciated. We thank Dr Vladimir Wolf for performing the RT-PCR analysis of KGFR expression. We are grateful to Dr Paul Martin and Dr Dimitry Danilenko for helpful discussions.
Supported by the Children's Cancer Research Fund, the Viking Children's Fund, and National Institutes of Health grants RO1 AI 34495, RO1 HL 63452, R37 HL 56067, and N01-CO-56000.
The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Declaration of commercial interest: J.S.R. has declared a financial interest in the company whose potential product was studied in the present work. C.L.F. and D.L.L. are employed by Amgen, Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Angela Panoskaltsis-Mortari, University of Minnesota, Department of Pediatrics, Division of Hematology-Oncology, Blood and Marrow Transplant Program, Box 366 Mayo, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: panos001@tc.umn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal