Abstract
The regulatory roles of the common cytokine receptor γ chain (γc)– and Jak3-dependent signaling in the proliferation and survival of mast cells were determined using γc-deficient (γc−) and Jak3-deficient (Jak3−) mice. Although the mast cells in γc− and Jak3− mice were morphologically indistinguishable from those in wild-type mice, the number of peritoneal mast cells was decreased in γc− and Jak3− mice as compared with that in wild-type mice. Among γc-related cytokines, interleukin (IL)-4 and IL-9, but not IL-2, IL-7, or IL-15, enhanced the proliferation and survival of bone marrow–derived mast cells (BMMCs) from wild-type mice. However, the effects of IL-4 and IL-9 were absent in BMMCs from γc− and Jak3−mice. In addition, IL-4Rα, γc, and Jak3, but not IL-2Rβ or IL-7Rα, were expressed in BMMCs. In contrast, IL-13 did not significantly induce the proliferation and survival of BMMCs even from wild-type mice, and IL-13Rα1 was not expressed in BMMCs. Furthermore, IL-4 phosphorylated the 65-kd isoform of Stat6 in BMMCs from wild-type mice but not from γc− and Jak3− mice. These results indicate that γc- and Jak3-dependent signaling is essential for IL-4– and IL-9–induced proliferation and survival of murine mast cells, that the effects of IL-4 are mediated by type I IL-4R and that type II IL-4R is absent on mast cells, and that IL-4 phosphorylates the 65-kd isoform of Stat6 in mast cells in a γc- and Jak3-dependent manner.
Introduction
Mast cells are recognized as the major effector cells of the type I hypersensitivity reactions by virtue of their possessing high-affinity receptors for immunoglobulin (Ig) E and are known to play a pivotal role in allergic diseases, such as atopic rhinitis, asthma, and atopic dermatitis.1,2 Mature mast cells are distributed throughout all vascularized tissues, and the development and proliferation of mast cells require proper signaling from several cytokines, among which the c-kit/stem cell factor (SCF) system and interleukin (IL)-3 are the best studied.1-4
The common cytokine receptor γ chain (γc) is a shared component of the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15.5,6 Mutations in γc result in X-linked severe combined immunodeficiency (XSCID) in humans.7 Like humans with XSCID, mice in which the γc gene has been disrupted by homologous recombination exhibit a hypoplastic thymus and are compromised in their ability to respond to antigenic stimuli.8-10 Janus kinase 3 (Jak3) is a tyrosine kinase that is known to transduce γc-dependent signals.11 Mutation of the Jak3 gene results in a form of severe combined immunodeficiency (SCID) that is clinically and immunologically indistinguishable from XSCID, except for the autosomal recessive inheritance pattern in Jak3-deficient SCID,12,13which suggests the essential role of Jak3 in γc-dependent signaling for lymphocyte development. IL-7 appears to be the most important cytokine for T-cell and B-cell development,14-16whereas IL-15 seems to be important for natural killer (NK) cell and NK T-cell development.17-19 Among γc-related cytokines, IL-4,20-23IL-9,24,25 and IL-1526 have been reported to support the proliferation and survival of mast cells.
IL-4 exerts a number of biologic activities in the hematopoietic and immune system.27 IL-4 transduces the signals through 2 types of IL-4 receptors (IL-4Rs): Type I IL-4R is a heterodimer of IL-4Rα and γc, and type II IL-4R is a heterodimer of IL-4Rα and IL-13Rα1.28,29 Type I IL-4R is preferentially expressed in hematopoietic cells, whereas type II IL-4R is preferentially expressed in nonhematopoietic cells.28,29 However, in some hematopoietic cells, including human B cells30,31 and murine macrophages,32 both types of IL-4R are expressed, and IL-4 can transduce the signals in the absence of γc through type II IL-4R. Recently, it has been shown that IL-13, a cytokine that shares many biologic activities with IL-4,33 also uses type II IL-4R for signal transduction.29 Although previous studies have shown that IL-4 is a potent stimulator of mast cell proliferation and survival,20-23 it remains unknown which type of IL-4R is functional on mast cells. Furthermore, the role of IL-13 in mast cell growth also remains unclear.
IL-15 is a cytokine that shares many functional properties with IL-2.34 Analysis of IL-15Rα-deficient mice revealed that IL-15 played important roles in NK cell development and generation of memory CD8+ T cells.19 Recently, it was demonstrated that IL-15 induced mast cell proliferation through an undefined receptor, IL-15R X.26 Interestingly, Tagaya et al found that IL-15 activated Jak2 in mast cells through IL-15R X,26 whereas IL-15 activated Jak1 and Jak3 in T cells and NK cells through a heterodimer of IL-2Rβ and γc.34 However, the nature of IL-15R X and IL-15 signaling in mast cell proliferation is still largely unknown.
Although analyses of mice lacking γc and Jak3 have revealed the importance of γc and Jak3 in T-cell and NK cell development in vivo,8-11,35 36 the roles of γc and Jak3 in mast cell development remain unclear. We present data that demonstrate an important regulatory role of γc- and Jak3-dependent signals in the proliferation and survival of murine mast cells.
Materials and methods
Mice and genetic analysis
γc-Deficient (γc−) mice9 and Jak3-deficient (Jak3−) mice35 were back-crossed to BALB/c mice (Jackson Laboratory, Bar Harbor, ME) for at least 6 generations. The mice were genotyped by polymerase chain reaction (PCR) as described previously,36 and littermate wild-type (WT) mice were used as controls. Mice were housed in microisolator cages under pathogen-free conditions. All experiments followed the guidelines of Chiba University.
Culture of bone marrow–derived mast cells (BMMCs)
Primary culture of IL-3–dependent BMMCs was prepared from 8- to 12-week-old WT, γc−, or Jak3−mice and maintained as described previously.37 Briefly, the mice were killed and bone marrow was flushed aseptically from femurs and tibias into RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS), 50 μmol/L β-mercaptoethanol, 2 mmol/L l-glutamine, 1 mmol/L sodium pyruvate, 0.1 mmol/L nonessential amino acids, antibiotics, and 10% (vol/vol) of murine IL-3 transfectant X63 cell conditioned medium38 (X63–IL-3; kindly provided by Dr H. Karasuyama, Tokyo Metropolitan Institute of Medical Science) as a source of IL-3. The nonadherent bone marrow cells were then maintained at 37°C at a density of 2 to 5 × 105 cells/mL in the same medium, with biweekly replacement of old media with fresh ones. BMMCs obtained after 4 weeks of culture were more than 98% mast cells, as demonstrated by morphologic analysis, and BMMCs were used for the following experiment at 4 to 6 weeks of culture. In preliminary experiments, the maximum proliferation of BMMCs was observed at 2% to 20% of X63–IL-3 conditioned medium.
Peritoneal lavage cells
Peritoneal lavage was performed by injecting 10 mL of ice-cold phosphate-buffered saline (PBS) into the peritoneal cavity of the mouse. After the cells were centrifuged (400g), resuspended in 1 mL of PBS, and counted using a hemocytometer, differential cell counts were performed on cytospin cell preparations stained with Wright-Giemsa solution. Peritoneal mast cells were identified morphologically according to the criteria described previously.2 39 A fraction of the cells was subjected to flow cytometric analysis as follows.
Flow cytometric analysis
Cells from the peritoneal cavity and BMMCs were stained and analyzed on a FACScaliber (Becton Dickinson, San Jose, CA) with CELLQuest software. The following antibodies were purchased: anti-CD117 (c-kit) fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC) (2B8; PharMingen, San Diego, CA), anti-CD122 (IL-2Rβ) PE (TM-β1; PharMingen), anti-γc PE (4G2; PharMingen), anti-CDw124 (IL-4Rα) (1688-01; Genzyme Corp, Cambridge, MA), anti-CD127 (IL-7Rα) biotin (B12-1; PharMingen), anti-CD16/32 (FcγII/III) PE (2.4G2; PharMingen), anti–Gr-1 APC (RB6-8C5; PharMingen), anti-CD4 APC (RM4-5; PharMingen), anti-CD8 APC (53-6.7; PharMingen), anti-CD45R/B220 APC (RA3-6B2; PharMingen), and anti-rat IgG2a FITC (RG7/1.30; PharMingen). Before staining, Fc receptors were blocked with anti-CD16/32 antibody (2.4G2; PharMingen) excepting the detection of anti-CD16/32 PE staining. Negative controls consisted of isotype-matched, directly conjugated, nonspecific antibodies (PharMingen).
IgE receptors on mast cells
To quantify the levels of IgE receptors expressed on the cell surface, we first incubated the cells with mouse antitrinitrophenol IgE (IgE3; PharMingen) at 4°C for 60 minutes to saturate the IgE receptors, and then labeled them with anti-IgE FITC (R35-72; PharMingen). In some experiments, IgE receptors on BMMCs were visualized directly by FITC-conjugated mouse IgE (IgE3; PharMingen).
Cell survival assay
BMMCs were washed 3 times with PBS and cultured at 1 × 106 cells/mL in triplicate at 37°C for 1 to 5 days in RPMI 1640 medium without IL-3 in the presence of the indicated cytokines: murine IL-4 (20 ng/mL; Genzyme Corp), murine IL-9 (20 ng/mL; PeproTech Inc, Rocky Hill, NJ), murine IL-13 (20 ng/mL; R&D Systems, Minneapolis, MN), or human IL-15 (20 ng/mL and 129 ng/mL; DIACLONE Research, Besançon Cedex, France). Cells were harvested and viability was determined by fluorescence-activated cell sorting (FACS) with 5 μg/mL of propidium iodide (PI) (Boehringer Mannheim, Indianapolis, IN).40 To examine the survival of peritoneal mast cells, we cultured freshly isolated cells from the peritoneal cavity for 24 hours in RPMI 1640 medium without IL-3 in the presence of murine IL-4 (20 ng/mL) or murine IL-9 (20 ng/mL). The viability of c-kit+ cells was determined by FACS with the use of anti–c-kit FITC and 5 μg/mL of PI.
Proliferation assay
BMMCs (2 × 105/well) were cultured in triplicate at 37°C for 36 hours in 96-well plates in RPMI 1640 medium containing 10% (vol/vol) of X63–IL-3 conditioned medium with the indicated cytokines: human IL-2 (20 ng/mL; R&D Systems), murine IL-4 (20 ng/mL), murine IL-7 (20 ng/mL; R&D Systems), murine IL-9 (20 ng/mL), murine IL-13 (20 ng/mL), or human IL-15 (20 ng/mL and 129 ng/mL), with 0.5 μCi of [3H] thymidine added for the final 12 hours.
Degranulation assay of mast cells
BMMCs were cultured at 37°C for 4 days in the presence or absence of murine IL-4 (20 ng/mL) in RPMI 1640 medium containing 10% (vol/vol) of X63–IL-3 conditioned medium. BMMCs were then stimulated with A23187 (200 ng/mL; Sigma, St Louis, MO) at 37°C for 30 minutes. Enzyme activity of β-hexosaminidase was evaluated for both the supernatant and the cell lysate using p-nitrophenyl-N-acetyl β-d-glucosamine (Sigma) as a substrate.41The percentage of specific β-hexosaminidase release was expressed as: 100 × supernatant activity/(supernatant activity + cell lysate activity).
Western blotting
After BMMCs were starved for 2 hours from IL-3, the cells were stimulated with IL-4 (20 ng/mL) or IL-13 (20 ng/mL) at 37°C for 15 minutes. As a control, freshly isolated murine splenocytes or human peripheral blood lymphocytes were stimulated with IL-4 or IL-13. The cells were washed with PBS and lysed in lysis buffer (1% Nonidet P-40, 20 mmol/L Tris-HCl [pH 8.0], 50 mmol/L NaCl, 2 mmol/L dithiothreitol, 4 mmol/L EGTA, 10 mmol/L NaF, 1 mmol/L Na3VO4, 5 μg/mL aprotinin, 5 μg/mL leupeptin, 2 μg/mL pepstatin, 0.5 mmol/L phenylmethylsulfonyl fluoride, and 10% glycerol) on ice for 30 minutes, and cell lysates were prepared by centrifugation. A total of 15 μg of cell lysate was separated on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels and transferred to Immobilon-P membranes (Millipore Corp, Bedford, MA). After blocking with PBS containing 0.15% Tween 20 and 3% bovine serum albumin (BSA) for 1 hour at room temperature, the membranes were incubated with antisera to mouse Stat6 (M-20) (Santa Cruz Biotechnology Inc, Santa Cruz, CA), mouse Stat6 (M-200) (Santa Cruz Biotechnology), phospho-Stat6 (New England Biolabs Inc, Beverly, MA), mouse Stat5 (Transduction Laboratories, Lexington, KY), phospho-Stat5 (New England Biolabs), and mouse Jak3 (Upstate Biotechnology, Lake Placid, NY) for 1 hour at room temperature. After washing 3 times with PBS containing 0.15% Tween 20, the membranes were incubated with anti-mouse IgG or anti-rabbit IgG antibodies conjugated with horseradish peroxidase (Amersham Pharmacia Biotech, Little Chalfont, UK) in PBS containing 0.15% Tween 20 and 3% BSA for 1 hour at room temperature. The membranes were then washed with PBS containing 0.15% Tween 20 and developed with an enhanced chemiluminescent substrate (Roche Diagnostics GmbH, Mannheim, Germany).
Reverse transcriptase–PCR assay
BMMCs and splenocytes were washed twice with PBS, and total RNA was extracted using Isogen reagent (Nippon Gene Co, Tokyo, Japan). The first-strand complementary DNA (cDNA) was then synthesized from total RNA using moloney murine leukemia virus reverse transcriptase (RT) and oligo(dT) primers (Pharmacia Biotech, Buckinghamshire, UK). cDNAs encoding IL-13Rα142 and β-actin (as a control) were amplified by PCR.
Data analysis
Data are summarized as mean ± SD. Statistical analysis of the results was performed by the unpaired t test.P < .05 was considered significant.
Results
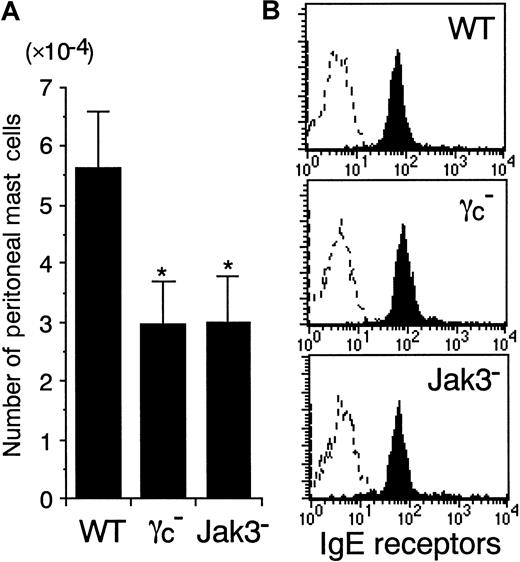
The number of peritoneal mast cells is decreased in γc− and Jak3− mice
Whereas the importance of SCF and IL-3 in mast cell development is well documented,1-4 the role of γc-dependent cytokines in mast cell development is less understood. To determine whether γc and Jak3 are required for mast cell development in vivo, we analyzed the number of peritoneal mast cells in γc-deficient (γc−) and Jak3-deficient (Jak3−) mice. The total cell numbers recovered from the peritoneal cavity were decreased in γc− and Jak3− mice, resulting mainly from the diminished number of peritoneal CD5+ B cells (B-1 cells) (Suzuki et al, article in preparation). In contrast, the number of peritoneal macrophages was normal in γc− and Jak3− mice (data not shown), consistent with a previous report on γc− mice.32 Although the peritoneal mast cells in γc− and Jak3− mice were morphologically indistinguishable from those in WT mice, the number of mast cells recovered from the peritoneal cavity was significantly decreased in γc− and Jak3− mice (WT mice, 5.62 ± 0.98 × 104; γc−mice, 2.92 ± 0.71 × 104; and Jak3− mice, 2.96 ± 0.79 × 104; mean ± SD, n = 6-8 mice each; P < .01) (Figure 1A). Consistent with the diminished number of peritoneal mast cells in γc− and Jak3− mice, FACS analysis revealed that c-kit+ cells in the peritoneal cavity were decreased in γc− and Jak3− mice (data not shown). However, when electrically gated on c-kit+ peritoneal cells, the intensity of IgE receptors was indistinguishable among WT, γc−, and Jak3− mice (Figure1B). The expression levels of Bcl-2 as well as the number of apoptotic cells (Annexin V binding–positive cells) were also indistinguishable among c-kit+ peritoneal cells from WT, γc−, and Jak3− mice (data not shown). In addition, the number of mast cells (c-kit+IgER+Gr-1−B220−CD4−CD8−population) was also decreased in the spleen in γc− and Jak3− mice (WT mice, 1.36 ± 0.15 × 104; γc−mice, 0.88 ± 0.09 × 104; and Jak3− mice, 0.81 ± 0.04 × 104; 3-week-old mice, n = 5 each;P < .01). These results suggest that γc- and Jak3-dependent signals play an important role in the proliferation of mast cells, but not in the maturation of mast cells in vivo. However, because the numbers of T cells, NK cells, and NK T cells are severely diminished in γc− and Jak3− mice,8-11,35 36 the decreased cytokine production from these cells may also contribute to the impairment in mast cell development. Therefore, we then studied the role of γc-dependent signals in the proliferation and survival of cultured mast cells from γc− and Jak3− mice.
The number of peritoneal mast cells is decreased in γc− and Jak3− mice.
(A) Peritoneal mast cells were recovered by lavage and counted in 8- to 12-week-old wild-type (WT), γc-deficient (γc−), and Jak3-deficient (Jak3−) mice. Peritoneal mast cells were identified morphologically on cytospin cell preparations stained with Wright-Giemsa solution. Data are means ± SD for 6 to 8 mice in each group. The mean values for γc− mice and Jak3− mice are significantly different from the mean value for WT mice, *P < .01. (B) Expression of IgE receptors on c-kit+ peritoneal cells. IgE receptors on peritoneal mast cells were quantified by first incubating the cells recovered by peritoneal lavage with mouse IgE, then labeling with anti-IgE FITC, and analyzing by FACS gating on c-kit+ cells. Shown are representative FACS profiles of IgE receptor staining and control staining (dashed lines) on c-kit+ peritoneal cells from WT, γc−, and Jak3− mice (n = 6-8 mice in each group).
The number of peritoneal mast cells is decreased in γc− and Jak3− mice.
(A) Peritoneal mast cells were recovered by lavage and counted in 8- to 12-week-old wild-type (WT), γc-deficient (γc−), and Jak3-deficient (Jak3−) mice. Peritoneal mast cells were identified morphologically on cytospin cell preparations stained with Wright-Giemsa solution. Data are means ± SD for 6 to 8 mice in each group. The mean values for γc− mice and Jak3− mice are significantly different from the mean value for WT mice, *P < .01. (B) Expression of IgE receptors on c-kit+ peritoneal cells. IgE receptors on peritoneal mast cells were quantified by first incubating the cells recovered by peritoneal lavage with mouse IgE, then labeling with anti-IgE FITC, and analyzing by FACS gating on c-kit+ cells. Shown are representative FACS profiles of IgE receptor staining and control staining (dashed lines) on c-kit+ peritoneal cells from WT, γc−, and Jak3− mice (n = 6-8 mice in each group).
Development of IL-3–dependent BMMCs is normal in γc− and Jak3− mice
Murine mast cell lines can be established from bone marrow hematopoietic progenitors in the presence of IL-3.37 To determine the role of γc-dependent signals in mast cell development, we prepared primary cultures of IL-3–dependent BMMCs from WT, γc−, and Jak3− mice. BMMCs obtained after 4 weeks of culture were more than 98% mast cells and were morphologically indistinguishable among these mice (data not shown). The number of BMMCs recovered per mouse was also indistinguishable among these mice (data not shown). Moreover, IL-3–induced proliferation of BMMCs was normal in γc− and Jak3− mice (Figure2A). The expression levels of c-kit, FcγII/III, and IgE receptor were also indistinguishable among BMMCs from WT, γc−, and Jak3− mice (Figure 2B). These results indicate that γc- and Jak3-dependent signals are not essential for IL-3–induced mast cell development in vitro.
Development of IL-3–dependent bone marrow–derived mast cells (BMMCs) is normal in γc− and Jak3− mice.
(A) BMMCs (2 × 105/well) were cultured in the presence of IL-3 at 37°C for 36 hours, and the proliferative responses were evaluated by the addition of [3H] thymidine for the final 12 hours. Data are means ± SD for 5 mice in each group. (B) BMMCs from WT, γc−, and Jak3− mice were stained with anti–c-kit APC, anti-FcγII/III PE, and IgE FITC, as described in “Materials and methods.” Shown are representative FACS profiles for c-kit, FcγII/III, and IgE receptor staining from 5 independent experiments. Dashed lines are FACS profiles of negative controls.
Development of IL-3–dependent bone marrow–derived mast cells (BMMCs) is normal in γc− and Jak3− mice.
(A) BMMCs (2 × 105/well) were cultured in the presence of IL-3 at 37°C for 36 hours, and the proliferative responses were evaluated by the addition of [3H] thymidine for the final 12 hours. Data are means ± SD for 5 mice in each group. (B) BMMCs from WT, γc−, and Jak3− mice were stained with anti–c-kit APC, anti-FcγII/III PE, and IgE FITC, as described in “Materials and methods.” Shown are representative FACS profiles for c-kit, FcγII/III, and IgE receptor staining from 5 independent experiments. Dashed lines are FACS profiles of negative controls.
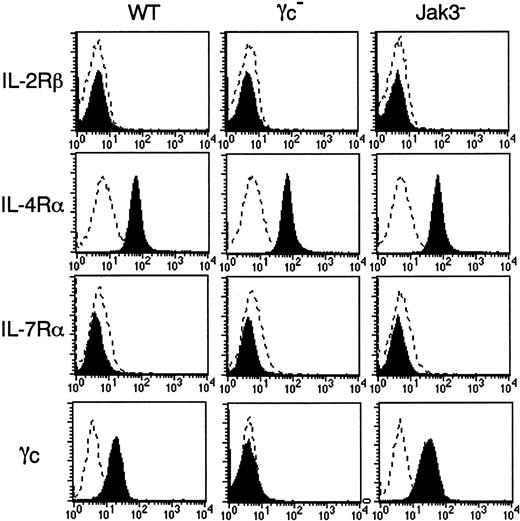
Expression of IL-4Rα, γc, and Jak3 in BMMCs
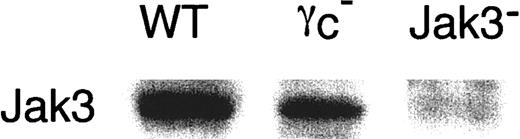
We next examined the expression of γc, γc-related cytokine receptors, and Jak3 in BMMCs. As shown in Figure 3, the expression of IL-2Rβ and IL-7Rα was absent in BMMCs from WT, γc−, and Jak3− mice. On the other hand, IL-4Rα was equally expressed in BMMCs from WT, γc−, and Jak3− mice (Figure 3). As expected, BMMCs from γc− mice lacked γc expression, confirming the correct recognition by anti-γc monoclonal antibody (Figure 3). Interestingly, expression levels of γc were significantly increased in BMMCs from Jak3− mice as compared with those from WT mice (WT mice, 20.5 ± 2.6; Jak3− mice, 36.2 ± 3.0; mean fluorescent intensities for γc staining, n = 4 each; P < .01) (Figure3). Increased γc expression was also observed in c-kit+ peritoneal cells from Jak3− mice (data not shown). In contrast, the expression of Jak3 in BMMCs was significantly lower in γc− mice than that in WT mice (Figure 4). As expected, Jak3 was undetectable in Jak3− mice (Figure 4).
Expression of IL-4Rα and γc in BMMCs.
BMMCs from WT, γc−, and Jak3−mice were analyzed for the expression of IL-2Rβ, IL-4Rα, IL-7Rα, and γc on c-kit+ cells. Shown are representative FACS profiles from 4 independent experiments. Dashed lines are FACS profiles for the isotype-matched controls.
Expression of IL-4Rα and γc in BMMCs.
BMMCs from WT, γc−, and Jak3−mice were analyzed for the expression of IL-2Rβ, IL-4Rα, IL-7Rα, and γc on c-kit+ cells. Shown are representative FACS profiles from 4 independent experiments. Dashed lines are FACS profiles for the isotype-matched controls.
Expression of Jak3 in BMMCs.
Jak3 expression was analyzed by Western blotting for BMMCs from WT, γc−, and Jak3− mice as described in “Materials and methods.” Shown is a representative anti-Jak3 Western blot from 4 independent experiments.
Expression of Jak3 in BMMCs.
Jak3 expression was analyzed by Western blotting for BMMCs from WT, γc−, and Jak3− mice as described in “Materials and methods.” Shown is a representative anti-Jak3 Western blot from 4 independent experiments.
IL-4 enhances the proliferation, survival, and degranulation of BMMCs through γc- and Jak3-dependent signaling
IL-4 has been shown to function as a mast cell growth factor.20-23 Although it has been shown that IL-4 can transduce the signals from 2 types of receptors and that the types of IL-4Rs expressed on cells differ depending on cell lineage,28,29 it is still unclear which type of IL-4R is functional on mast cells. To address this question, we examined the effect of IL-4 on the proliferation of BMMCs from γc− and Jak3− mice. Because IL-4 alone did not induce the proliferation of BMMCs from WT mice (data not shown), we examined the synergistic effect of IL-4 on IL-3–induced proliferation of BMMCs. IL-4 significantly enhanced IL-3–induced proliferation of BMMCs from WT mice (IL-3, 14.6 ± 0.8 × 103 cpm; IL-3 + IL-4, 44.8 ± 0.9 × 103 cpm; n = 5 each;P < .005) (Figure 5). IL-4 exhibited no effect on IL-3–induced proliferation of BMMCs from γc− and Jak3− mice (Figure 5). These results indicate that IL-4 induces the proliferation of BMMCs through γc- and Jak3-dependent signals and that the functional IL-4R on BMMCs is type I IL-4R. In contrast to IL-4, IL-13, a cytokine that exhibits IL-4–like functions on B cells43and endothelial cells44 through type II IL-4R, did not significantly enhance the IL-3–induced proliferation of BMMCs even in WT mice (Figure 5).
IL-4 induces the proliferation of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured in the presence of IL-3 for 36 hours, with [3H] thymidine added for the final 12 hours. Where indicated, recombinant murine IL-4 or recombinant murine IL-13 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. The mean value of IL-4–stimulated BMMCs is significantly different from the mean value of control BMMCs (IL-3 alone) in WT mice. *P < .005.
IL-4 induces the proliferation of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured in the presence of IL-3 for 36 hours, with [3H] thymidine added for the final 12 hours. Where indicated, recombinant murine IL-4 or recombinant murine IL-13 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. The mean value of IL-4–stimulated BMMCs is significantly different from the mean value of control BMMCs (IL-3 alone) in WT mice. *P < .005.
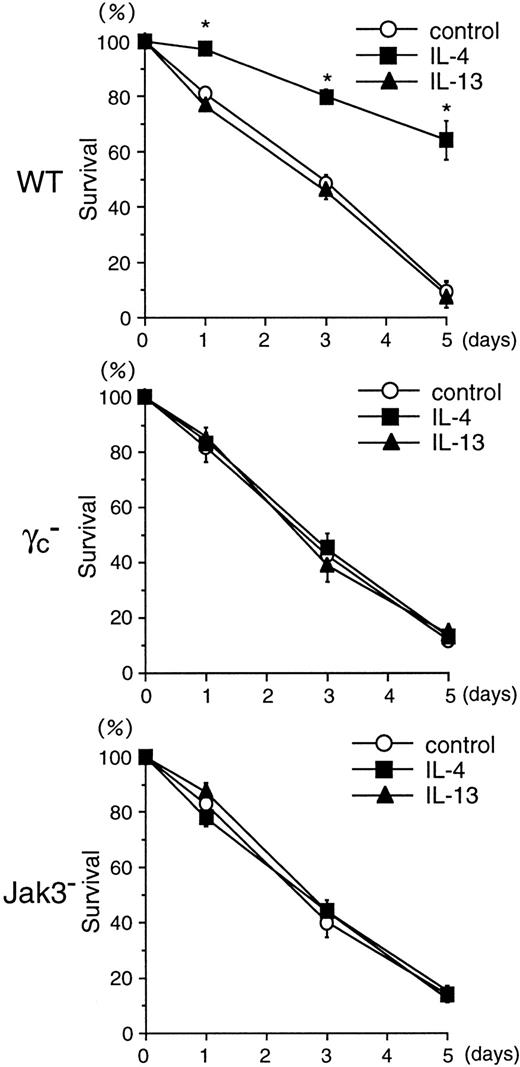
We next examined the antiapoptotic effect of IL-4 and IL-13 on BMMCs. IL-3–deprived BMMCs from WT, γc−, and Jak3− mice were cultured with IL-4 (20 ng/mL) or IL-13 (20 ng/mL) for 1 to 5 days, and the cell viability was then determined by FACS. As shown in Figure 6, the rate of survival after IL-3 withdrawal was similar among BMMCs from WT, γc−, and Jak3− mice. IL-4 significantly enhanced the survival of BMMCs from WT mice (control, 9.5% ± 0.5%; IL-4, 64.2% ± 7.0% at day 5; n = 5 each;P < .001) (Figure 6). However, IL-4 had no effect on the survival of BMMCs from γc− and Jak3− mice (Figure 6). IL-13 did not significantly affect the survival of BMMCs from WT, γc−, or Jak3− mice (Figure 6). These results indicate that IL-4, but not IL-13, enhances the proliferation and survival of BMMCs through γc- and Jak3-dependent signaling of type I IL-4R.
IL-4 induces the survival of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured in the absence of IL-3 for 5 days, and cell viability was determined by FACS using PI (5 μg/mL) at days 0, 1, 3, and 5. Where indicated, recombinant murine IL-4 or recombinant murine IL-13 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. The mean value of IL-4–stimulated BMMCs is significantly different from the mean value of control BMMCs in WT mice. *P < .001.
IL-4 induces the survival of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured in the absence of IL-3 for 5 days, and cell viability was determined by FACS using PI (5 μg/mL) at days 0, 1, 3, and 5. Where indicated, recombinant murine IL-4 or recombinant murine IL-13 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. The mean value of IL-4–stimulated BMMCs is significantly different from the mean value of control BMMCs in WT mice. *P < .001.
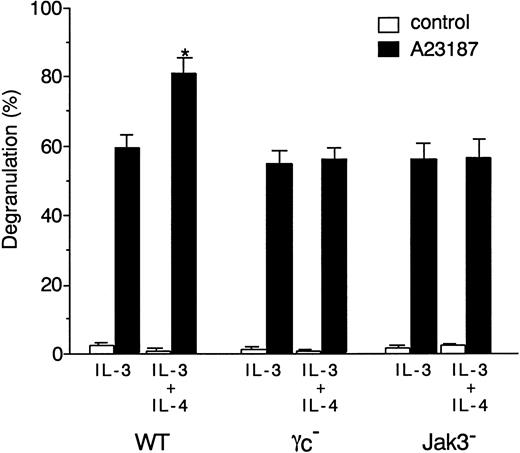
To determine whether γc and Jak3 signaling is involved in IL-4–induced enhancement of mast cell degranulation, we examined the effect of IL-4 on A23187-induced degranulation of BMMCs in WT, γc−, and Jak3− mice. Consistent with a previous finding on the role of IL-4 in human mast cell degranulation,45 IL-4 significantly enhanced A23187-induced degranulation of BMMCs from WT mice (P < .01) (Figure 7). In contrast, however, IL-4 did not increase the degranulation of BMMCs from γc− and Jak3− mice (Figure7). These results indicate that, in addition to its effect on proliferation and survival, γc and Jak3 signaling is essential for IL-4–induced enhancement of mast cell degranulation.
IL-4 induces the degranulation of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured for 4 days in the presence of IL-3 alone or IL-3 plus murine IL-4 (20 ng/mL). BMMCs were then stimulated with A23187 (200 ng/mL) for 30 minutes, and the percentage of specific β-hexosaminidase release was determined as described in “Materials and methods.” Data are means ± SD for 4 mice in each group. The mean value of IL-4–stimulated BMMCs is significantly different from the mean value of the corresponding BMMCs without IL-4 stimulation in WT mice. *P < .01.
IL-4 induces the degranulation of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured for 4 days in the presence of IL-3 alone or IL-3 plus murine IL-4 (20 ng/mL). BMMCs were then stimulated with A23187 (200 ng/mL) for 30 minutes, and the percentage of specific β-hexosaminidase release was determined as described in “Materials and methods.” Data are means ± SD for 4 mice in each group. The mean value of IL-4–stimulated BMMCs is significantly different from the mean value of the corresponding BMMCs without IL-4 stimulation in WT mice. *P < .01.
IL-4, but not IL-13, phosphorylates the 65-kd Stat6 isoform in BMMCs
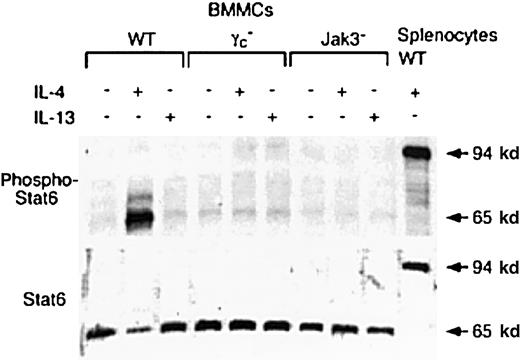
In the IL-4 signaling pathway, the best-studied signaling molecule is signal transducers and activators of transcription 6 (Stat6).11 Stat6 is also rapidly activated after cellular exposure to IL-13.11 Therefore, we analyzed IL-4– and IL-13–induced Stat6 phosphorylation in BMMCs from WT, γc−, and Jak3− mice. After BMMCs were deprived of IL-3 for 2 hours, BMMCs were stimulated with either IL-4 or IL-13 for 15 minutes, and tyrosine phosphorylation of Stat6 at tyrosine 641 was detected by anti-phospho Stat6 antibody. Surprisingly, IL-4 phosphorylated the 65-kd isoform of Stat6 in BMMCs from WT mice, whereas IL-4 phosphorylated the 94-kd isoform of Stat6 in WT splenocytes (Figure 8). Reblotting with anti-Stat6 antibody M200, which recognizes the middle part of murine Stat6 (AA280-AA480), revealed that BMMCs expressed the 65-kd isoform of Stat6 but not the 94-kd Stat6 (Figure 8). Interestingly, the 65-kd isoform of Stat6 was not detected by anti-Stat6 antibody M20, which recognizes the c-terminus of murine Stat6, whereas the 94-kd Stat6 in splenocytes was readily detected by M20 (data not shown). These results suggest that the 65-kd isoform of Stat6 lacks the c-terminus. Although the 65-kd isoform of Stat6 was equally expressed in BMMCs among WT, γc−, and Jak3− mice (Figure 8), IL-4 did not induce the phosphorylation of the 65-kd Stat6 in BMMCs from γc− or Jak3− mice (Figure 8). In addition, IL-13 did not induce the phosphorylation of 65-kd Stat6 in BMMCs even from WT mice (Figure 8), whereas murine IL-13 did phosphorylate the 94-kd Stat6 in human peripheral blood lymphocytes (data not shown).
IL-4–induced phosphorylation of 65-kd Stat6 isoform in BMMCs is γc- and Jak3-dependent.
BMMCs from WT, γc−, and Jak3−mice were washed with PBS, cultured for 2 hours in the absence of IL-3, and stimulated with IL-4 or IL-13 (20 ng/mL) for 15 minutes. After washing with PBS, cell lysates were prepared, separated on a 10% SDS gel, and blotted with antisera to either phospho-Stat6 or Stat6 (M200). As a control, cell lysates from IL-4–stimulated WT splenocytes were used. Shown is a representative blot from 4 independent experiments.
IL-4–induced phosphorylation of 65-kd Stat6 isoform in BMMCs is γc- and Jak3-dependent.
BMMCs from WT, γc−, and Jak3−mice were washed with PBS, cultured for 2 hours in the absence of IL-3, and stimulated with IL-4 or IL-13 (20 ng/mL) for 15 minutes. After washing with PBS, cell lysates were prepared, separated on a 10% SDS gel, and blotted with antisera to either phospho-Stat6 or Stat6 (M200). As a control, cell lysates from IL-4–stimulated WT splenocytes were used. Shown is a representative blot from 4 independent experiments.
IL-13Rα1 is not expressed in BMMCs
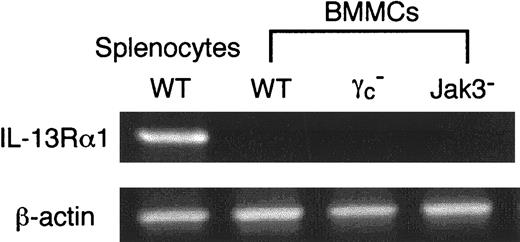
To determine whether IL-13Rα1 is expressed on BMMCs, we performed RT-PCR analysis for IL-13Rα1 mRNA expression in BMMCs from WT, γc−, and Jak3− mice. As shown in Figure 9, IL-13Rα1 was not detected in BMMCs from WT, γc−, or Jak3− mice. In contrast, IL-13Rα1 was expressed in splenocytes from WT mice (Figure 9). The absence of IL-13Rα1 expression on BMMCs is consistent with our results presented earlier: that IL-4, but not IL-13, functioned on BMMCs and that IL-4–induced effects on BMMCs depended on γc and Jak3.
IL-13Rα1 is not expressed on murine BMMCs.
Total RNA was prepared from BMMCs, and RT-PCR analysis for IL-13Rα1 and β-actin (as a control) was performed as described in “Materials and methods.” As a control, splenocytes from WT mice were used. Shown are representative data from 5 independent experiments.
IL-13Rα1 is not expressed on murine BMMCs.
Total RNA was prepared from BMMCs, and RT-PCR analysis for IL-13Rα1 and β-actin (as a control) was performed as described in “Materials and methods.” As a control, splenocytes from WT mice were used. Shown are representative data from 5 independent experiments.
IL-9, but not IL-15, enhances the proliferation and survival of BMMCs through γc- and Jak3-dependent signaling
As described earlier, IL-4 enhanced the proliferation (Figure 5) and survival (Figure 6) of BMMCs through γc- and Jak3-dependent signaling. However, because the number of peritoneal mast cells was reported to be normal in IL-4–deficient mice,46 defects of IL-4 signals in γc− and Jak3− mice may not be responsible for the diminished mast cell numbers in γc− and Jak3− mice. Therefore, we determined the role of other γc-related cytokines—IL-2, IL-7, IL-9, and IL-15—in the proliferation of BMMCs. Among these cytokines, only IL-9 significantly enhanced the IL-3–induced proliferation of BMMCs from WT mice (IL-3, 14.6 ± 1.0 × 103 cpm; IL-3 + IL-9, 24.3 ± 1.8 × 103 cpm; n = 5 each;P < .005) (Figure 10). IL-9 had no effect on the proliferation of BMMCs from γc− and Jak3− mice (Figure 10). Interestingly, and inconsistent with a previous report,26IL-15 did not significantly enhance the proliferation of BMMCs from WT mice (Figure 10), even when a high concentration of IL-15 (129 ng/mL) was added to the BMMC culture (data not shown). The biologic activity of IL-15 was confirmed by IL-2–dependent CTLL-2 proliferation assay, and the addition of IL-15 achieved a half-maximal proliferation of CTLL-2 cells at approximately 0.01 ng/mL (data not shown).
IL-9 induces the proliferation of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured in the presence of IL-3 for 36 hours, with [3H] thymidine added for the final 12 hours. Where indicated, recombinant human IL-2, recombinant murine IL-7, recombinant murine IL-9, or recombinant human IL-15 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. *P < .005.
IL-9 induces the proliferation of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured in the presence of IL-3 for 36 hours, with [3H] thymidine added for the final 12 hours. Where indicated, recombinant human IL-2, recombinant murine IL-7, recombinant murine IL-9, or recombinant human IL-15 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. *P < .005.
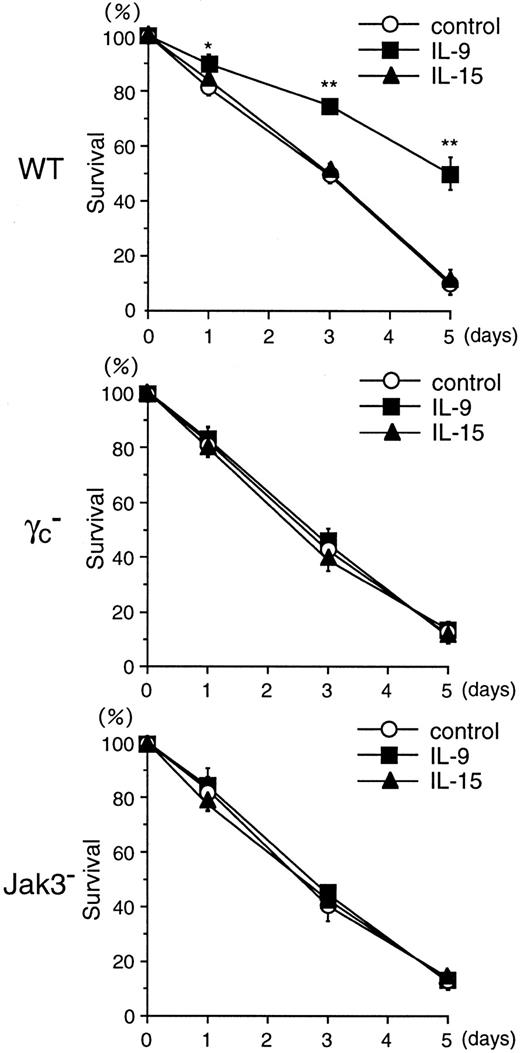
We then determined the effects of IL-9 and IL-15 on the survival of IL-3–deprived BMMCs. Whereas IL-9 enhanced the survival of BMMCs from WT mice (control, 9.7% ± 0.6%; IL-9, 50.1% ± 6.5% at day 5; n = 5; P < .005), IL-9 did not significantly affect the survival of BMMCs from γc− and Jak3− mice (Figure 11). These results indicate that the antiapoptotic effect of IL-9 on BMMCs depends on γc and Jak3. Again, IL-15 did not significantly enhance the survival of BMMCs even in WT mice (Figure11).
IL-9 induces the survival of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured in the absence of IL-3 for 5 days, and cell viability was determined by FACS using PI (5 μg/mL) at days 0, 1, 3, and 5. Where indicated, recombinant murine IL-9 or recombinant human IL-15 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. *P < .05, **P < .005.
IL-9 induces the survival of BMMCs through γc- and Jak3-dependent signaling.
BMMCs from WT, γc−, and Jak3−mice were cultured in the absence of IL-3 for 5 days, and cell viability was determined by FACS using PI (5 μg/mL) at days 0, 1, 3, and 5. Where indicated, recombinant murine IL-9 or recombinant human IL-15 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. *P < .05, **P < .005.
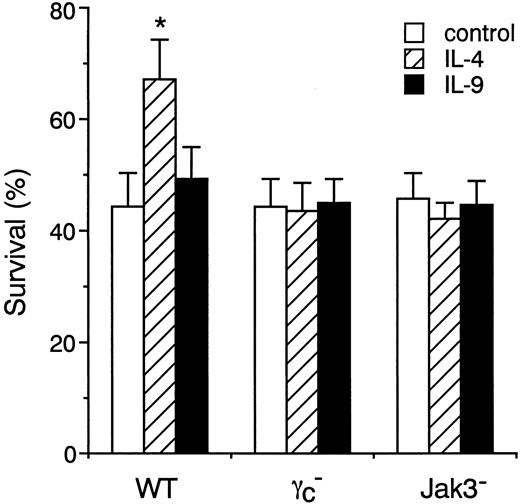
IL-4 enhances the survival of peritoneal mast cells through γc- and Jak3-dependent signaling
Finally, we studied the role of γc and Jak3 signaling in IL-4– and IL-9–induced survival of freshly isolated peritoneal mast cells. As shown in Figure12, IL-4 significantly increased the survival of peritoneal mast cells from WT mice (control, 44.4% ± 6.1%; IL-4, 67.3% ± 7.0% at 24 hours; n = 4 each;P < .01). In contrast, IL-9 exhibited only a marginal effect on the survival of peritoneal mast cells from WT mice. Consistent with the in vitro experiments on BMMCs (Figures 6 and 11), IL-4 as well as IL-9 exhibited no effect on the survival of peritoneal mast cells from γc− and Jak3−mice (Figure 12). These results indicate that IL-4 enhances the survival of peritoneal mast cells through γc- and Jak3-dependent signaling.
IL-4 enhances the survival of peritoneal mast cells through γc- and Jak3-dependent signaling.
Peritoneal cells from WT, γc−, and Jak3− mice were cultured in the presence of IL-4 or IL-9 for 24 hours, and cell viability of c-kit+ cells was determined by FACS as described in “Materials and methods.” Data are means ± SD for 4 mice in each group. The mean value of IL-4–stimulated peritoneal mast cells is significantly different from the mean value of control peritoneal mast cells in WT mice. *P < .01.
IL-4 enhances the survival of peritoneal mast cells through γc- and Jak3-dependent signaling.
Peritoneal cells from WT, γc−, and Jak3− mice were cultured in the presence of IL-4 or IL-9 for 24 hours, and cell viability of c-kit+ cells was determined by FACS as described in “Materials and methods.” Data are means ± SD for 4 mice in each group. The mean value of IL-4–stimulated peritoneal mast cells is significantly different from the mean value of control peritoneal mast cells in WT mice. *P < .01.
Discussion
Previous studies have shown that γc- and Jak3-dependent signals play the essential regulatory roles in T-cell and NK cell development.8-11,35 36 In this study, we show that γc- and Jak3-dependent signaling induces the proliferation and survival of murine mast cells. We found that, among cytokines that use γc as a shared receptor component, IL-4 and IL-9, but not IL-2, IL-7, or IL-15, enhanced the proliferation and survival of BMMCs from WT mice and that the effects of IL-4 and IL-9 were absent in BMMCs from γc− and Jak3− mice (Figures 5, 6, 10, and 11). We also found that IL-4Rα, γc, and Jak3, but not IL-2Rβ, IL-7Rα, or IL-13Rα1, were expressed in BMMCs (Figures 3, 4, and 9). Our findings of the decreased number of peritoneal mast cells in γc− and Jak3− mice also suggest an important role of γc- and Jak3-dependent signals in the proliferation and survival of mast cells in vivo (Figure1).
Our results indicate that IL-4–induced proliferation and survival of murine mast cells are mediated through type I IL-4R. It has been shown recently that type I IL-4R is a heterodimer of IL-4Rα and γc, that type II IL-4R is a heterodimer of IL-4Rα and IL-13Rα1, and that the usage of IL-4R differs depending on cell lineage.28,29 We found that IL-4 enhanced the proliferation and survival of BMMCs from WT mice but not from γc− or Jak3− mice (Figures 5and 6) and that IL-4Rα and γc were expressed in BMMCs (Figure 3). In contrast, although a previous study reported that IL-13 induced the proliferation of a murine mast cell line,47 we found that IL-13 did not significantly enhance the proliferation and survival of BMMCs even from WT mice (Figures 5 and 6) and that IL-13Rα1 was not expressed in BMMCs (Figure 9). Therefore, although increasing evidence suggests that IL-13 may play an important role in allergic inflammation,48,49 mast cells may not be a target of IL-13. In addition to type I and type II IL-4Rs, the experiments using chimeric proteins that were constructed with the cytoplasmic domain of IL-4Rα and an extracellular domain of erythropoietin,50,51 CD8,51 or c-kit52 suggested that the homodimerization of IL-4Rα could transduce IL-4 signals. However, our finding that γc-deficient BMMCs, which express IL-4Rα, did not respond to IL-4 stimulation also indicates that the expression of IL-4Rα alone is not sufficient to transduce IL-4 signals in mast cells.
The best-characterized molecule downstream of γc/Jak3 in IL-4 signals is Stat6.11 Interestingly, whereas Stat6 is essential for IL-4–induced proliferation of T cells,53-55IL-4–induced proliferation of BMMCs is slightly increased in the absence of Stat6.56 In addition, mastocytosis induced by in vivo administration of IL-4 was rather enhanced in Stat6-deficient mice.57 In the present study, we showed that the 65-kd isoform of Stat6, which lacked the c-terminus, was predominantly expressed in mast cells (Figure 8). Moreover, we found that the 65-kd isoform of Stat6 was phosphorylated by IL-4 in a γc- and Jak3-dependent manner (Figure 8). Our findings are in agreement with the recent observation by Sherman et al58 that murine mast cells preferentially express 65-kd Stat6. The 65-kd isoform of Stat6 is apparently different from the previously reported Stat6 isoforms in human fibroblast, Stat6b and Stat6c, which encode an NH2-terminal truncation and an SH2-domain deletion, respectively.59 Because deletion mutants of Stat6 that lack the c-terminus still bind to the Stat6-recognition site of DNA but lose transcriptional activity,60 it is possible that the 65-kd isoform of Stat6 may function as a negative regulator of transcription. At present, it is unknown how the 65-kd isoform of Stat6 is generated. In preliminary experiments, we found that when the extract from splenocytes was mixed with the extract from BMMCs and incubated at 37°C, the 94-kd Stat6 in splenocytes was cleaved to 65 kd (our unpublished data), suggesting that the 65-kd Stat6 in mast cells is generated by cleavage of mature protein rather than by alternative splicing of mRNA. In addition to Stat6, it has been reported recently that IL-4 induces the proliferation of murine pro–B-cell line Ba/F3 through activation of Stat5.61However, we observed no Stat5 phosphorylation in IL-4–stimulated BMMCs (our unpublished data). Thus, it is unlikely that Stat5 activation is responsible for IL-4–induced proliferation and survival of BMMCs. Taken together, our results indicate that IL-4 enhances the proliferation and survival of mast cells in a γc- and Jak3-dependent manner, but not in a Stat6- or Stat5-dependent manner.
We also show that IL-9 enhances the proliferation and survival of BMMCs through γc- and Jak3-dependent signaling (Figures 10 and11). IL-9 is a Th2-cell–derived cytokine with pleiotropic effects on various cell types, including mast cell growth.24,25,62,63Because the number of peritoneal mast cells was reported to be normal in IL-4–deficient mice,46 it is conceivable that the diminished peritoneal mast cell numbers in γc− or Jak3− mice (Figure 1A) may result from the absence of signaling via IL-9 or an undefined γc/Jak3-dependent cytokine. IL-9 or IL-9R knockout mice could be valuable in further investigating this issue.
IL-15 was also previously shown to function as a mast cell growth factor through an undefined IL-15R X, but not through an IL-2Rβ/γc heterodimer.26 However, we found that IL-15 had no significant effect on the proliferation and survival of BMMCs even in WT mice (Figures 10 and 11). In addition, in parallel experiments, we found that IL-15 did induce the proliferation of CTLL-2 cells at 0.01 ng/mL, but IL-15 did not enhance the proliferation of BMMCs even at 129 ng/mL. Because Tagaya et al26 reported that the activity of IL-15 on mast cell proliferation differed between batches of human IL-15, unknown factor(s) possibly contaminated in some batches of IL-15 might induce the expression of IL-15R X, and thereby IL-15 might enhance the proliferation of BMMCs in a γc-independent manner.
In summary, we have shown that γc- and Jak3-dependent signaling is essential for IL-4– and IL-9–induced proliferation and survival of murine mast cells. We have also shown that the effects of IL-4 on mast cells are mediated by type I IL-4R and that IL-13 exhibits no IL-4–like effects on mast cells because of the absence of IL-13Rα1. In addition, IL-4 phosphorylates the 65-kd isoform of Stat6 in mast cells through γc- and Jak3-dependent signaling. These results suggest that γc- and Jak3-dependent signaling has an important role in the mast cell expansion of Th2-cell–mediated inflammatory responses.
Acknowledgments
We thank Warren J. Leonard for γc-deficient mice, Hajime Karasuyama for X63–IL-3 cells, Miki Nishimura and Maki Watanabe for technical help, and Kenji Izuhara and Kazuhiro Kurasawa for valuable discussion.
Supported in part by grants from the Ministry of Education, Science and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroshi Nakajima, Department of Internal Medicine II, Chiba University School of Medicine, 1-8-1 Inohana, Chiba City, Chiba 260-8670, Japan; e-mail: nakajimh@intmed02.m.chiba-u.ac.jp.

![Fig. 2. Development of IL-3–dependent bone marrow–derived mast cells (BMMCs) is normal in γc− and Jak3− mice. / (A) BMMCs (2 × 105/well) were cultured in the presence of IL-3 at 37°C for 36 hours, and the proliferative responses were evaluated by the addition of [3H] thymidine for the final 12 hours. Data are means ± SD for 5 mice in each group. (B) BMMCs from WT, γc−, and Jak3− mice were stained with anti–c-kit APC, anti-FcγII/III PE, and IgE FITC, as described in “Materials and methods.” Shown are representative FACS profiles for c-kit, FcγII/III, and IgE receptor staining from 5 independent experiments. Dashed lines are FACS profiles of negative controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2172/5/m_h81800149002.jpeg?Expires=1769100346&Signature=T4cv0MnyZjrAn-kFQkzfMidFRfYzEls32IOzW1yF4kdSfRKcOMobiW52sFW6bfg4Y2PauHRTVS2bkFEHG1u4WTMTrnXPWZnBnG1lLHdxpRoZ-ubnw35Nk0jFGH3mIjV~adCccdeQ9Jx6ViWZT9SU8LLoRywWrLFb7-eLvFzzdWydgFsYelIAa9cyDbdpI9IgGFlHzykY0kS7svdGfqYLLMyUPKNPpcqwtMqPVQATFz-sbXQtmGF0b6RsWs8nMAS15D4INlh54CrXPoG-aC3q4-3wNe-lGYMKIs5roRkDqbWdpa8ppXjlsxg3lw2nVzMwEoV9FH5pVmQ--HqHwjZZ9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. IL-4 induces the proliferation of BMMCs through γc- and Jak3-dependent signaling. / BMMCs from WT, γc−, and Jak3−mice were cultured in the presence of IL-3 for 36 hours, with [3H] thymidine added for the final 12 hours. Where indicated, recombinant murine IL-4 or recombinant murine IL-13 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. The mean value of IL-4–stimulated BMMCs is significantly different from the mean value of control BMMCs (IL-3 alone) in WT mice. *P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2172/5/m_h81800149005.jpeg?Expires=1769100346&Signature=ah58cJUrFrA3eMr4CQmD3-F5OEigow3FmvW9hywRg~7CpzoHXh8VzHzKgbSBrmstqMyBFTj4QqZ3ENn6RSD42HLeIJEC3-cav8iFecfdMIvDq6PfXLkh2Iysa7LbMlueg6SD9H7lGGAGzOxgJKCNeg0NPjqghAOpIo40NqoyVKwLSp~7TuDZoFRIEvR0N3PJVOBM5Y9bmT0GI6PJXIFLtdtMBNkWWeuLiEVRT-8PRthCzJBf9wcX-DPBH9QToODzScZBc6SaRxXyG2~LM2OuXJY1RKk6b~IKOLP79aBvtfoQPlgcp1zjQu89Q-LE7-ojrc1vmoYdECIyMb1Rm~63aA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 10. IL-9 induces the proliferation of BMMCs through γc- and Jak3-dependent signaling. / BMMCs from WT, γc−, and Jak3−mice were cultured in the presence of IL-3 for 36 hours, with [3H] thymidine added for the final 12 hours. Where indicated, recombinant human IL-2, recombinant murine IL-7, recombinant murine IL-9, or recombinant human IL-15 was added at 20 ng/mL. Data are means ± SD for 5 mice in each group. *P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2172/5/m_h81800149010.jpeg?Expires=1769100346&Signature=K86G~R7~6CJsErmxSnZK93f~GbFIuihfr--QoHGafrIhrtqzbsqn9qboUz6PAsBDmIyP0wyBJKvfJwrlMDuGH3XYZg1FiSVJ8lL4jVZj4BokJGWo-bUk7sJGvxczg-7GQgiAR-rllfN9aw9YtxCd3FS50mMgzRfq5FjLadtH~zk-R6hNdLNm7yzHE1HeyqnwSVdRLKQiJ9TTza5rIss8KyV8qx9jtaLsRJkZFWRXTJIsK6QcEsdTLa14Sn4tySHmPEB~VBr86wrFuHJcLKBU4Va-v8VCbIJ6erNo3snRPY4gWApH1AapUNWEFNvJwjbu5oDOP-W6TEyza8Hv5Tf2Ow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal