Abstract
To investigate whether the migratory ability of peripheral blood-derived CD34+ cells of patients undergoing autologous peripheral blood stem cell transplantation is related to the homing efficiency of these cells, the migration in vitro of these cells was determined and correlated with in vivo hematopoietic recovery. Large inter-individual differences of the in vitro migratory ability of the CD34+ cells were observed, ranging from 1.1% to 16.4% for spontaneous migration and 6.2% to 40.8% for SDF-1–induced (100 ng/mL) migration. Significantly faster hematologic recovery was observed in those patients who received transplanted CD34+cells that showed high spontaneous and SDF-1–induced migration in vitro (P < .05). Moreover, CD34+ cells from healthy G-CSF–mobilized donors exhibited significantly higher spontaneous and SDF-1–induced (P < .01) migration than CD34+ cells from patients mobilized with chemotherapy and G-CSF. The lower migratory capacity in vitro of patient-derived CD34+ cells was not due to lower expression of CXCR-4 but probably reflected decreased motogenic behavior of the cells. These results indicate that the migratory capacity of the cells is important for hematopoietic recovery. The data suggest that the engraftment potential of autologous stem cells is more or less impaired by treatment before or during the mobilization procedure and might possibly be restored by in vitro manipulation of the cells. In addition, an exponential relation between CXCR-4 expression and number of CD34+ cells that mobilized to the peripheral blood was found (P < .001), suggesting that CXCR-4 expression plays a role in the mobilization of CD34+ cells.
Introduction
Homing of hematopoietic progenitor cells (HPCs) can be considered a multistep process in which various adhesion molecules on HPCs and endothelial cells are involved. This is similar to what has been found for the transendothelial migration of leukocytes at places of inflammation.1 This multistep process, involving the different adhesion molecules that are activated by a range of modifiers, may explain selective migration at specific places. Recently, the first powerful chemoattractant for CD34+cells was described—stromal cell–derived factor-1 (SDF-1)—produced by stromal cells, including those from the bone marrow (BM).2,3 It has also been shown that SDF-1 activates integrins on HPCs and induces transendothelial migration of HPCs in vitro.2,4,5 SDF-1 is classified as a CXC-chemokine and is also a chemoattractant for monocytes and lymphocytes.6 The receptor for SDF-1 is a G-protein–coupled receptor, called Fusin, LESTR, or CXCR-4.7-10 In SDF-1 or CXCR-4 knockout mice, hematopoietic precursors do not shift to the bone marrow during fetal development, suggesting that SDF-1 plays an important role in the migration of HPC to the bone marrow.11-13 Recently, Peled et al found SDF-1 and CXCR-4 to be critical for murine bone marrow engraftment by SCID-repopulating stem cells.14 They also demonstrated that migration to SDF-1 of CD34+CD38−/low cells to SDF-1 in vitro correlated with in vivo engraftment and stem cell function in NOD/SCID mice.
To investigate which factors may be involved in the homing of CD34+ cells derived from the peripheral blood (PB) of patients undergoing autologous peripheral blood stem cell transplantation (PBSCT), we determined CXCR-4 expression and migration in vitro of these cells. We hypothesized that reinfused CD34+ cells that have a high migratory capacity may home more efficiently to the bone marrow compartment and that this may lead to a more rapid hematopoietic recovery after PBSCT.
Patients, materials, and methods
Patient characteristics
The CD34+ cells of 62 patients undergoing autologous PBSCT and of 6 healthy donors for allogeneic transplantation were used in this study. Patients were treated for the following diseases: metastatic breast cancer (BC1), breast cancer with 4 or more tumor-positive axillary lymph nodes (BC4+), multiple myeloma, ovarian cancer, non-Hodgkin lymphoma (NHL), Hodgkin disease (HD), acute lymphoid leukemia (ALL), germ-cell cancer, acute myeloid leukemia, paraganglioma, neuroblastoma, and Ewing sarcoma. The mean age of the cancer patients was 45 years (range, 3-66 years). Among 6 healthy donors for allogeneic transplantation, the mean age was 34 years (range, 17-43 years). Author protocols have been approved by the Institutional Review Board of the Academic Medical Center, University of Amsterdam, and Netherlands Cancer Institute. Informed consent was provided according to the Declaration of Helsinki.
Mobilization procedure, PBSC harvest, and reinfusion
Hematopoietic progenitor cells from patients were mobilized by chemotherapy followed by daily 5 μg/kg (leukemia patients, 10 μg/kg) subcutaneous granulocyte colony-stimulating factor (G-CSF) (Filgastrim; Amgen, Thousand Oaks, CA) until the completion of leukapheresis. Mobilization regimens, high-dose regimens, and reinfusion procedures are only described for the 28 patients analyzed for in vitro migration and in vivo recovery. For patients with breast cancer, the chemotherapy regimen consisted of 5-fluorouracil (500 mg/m2), epirubicin (120 mg/m2), and cyclophosphamide (500 mg/m2) on day 1; treatment with G-CSF started on day 2.15 For patients with ovarian cancer, PBSCs were mobilized by ifosfamide (4 g/m2 on day 1), followed by G-CSF treatment on day 2.16 Patients with multiple myeloma were pretreated with 0.4 mg vincristine, 9 mg/m2 doxorubicin, and 40 mg dexamethasone as initial therapy.17 Thereafter, 4 g/m2 cyclophosphamide was given and G-CSF was started on day 4. Patients with HD were mobilized by G-CSF after treatment with 40 mg/d dexamethasone on days 1 to 4, 4 g/m2 high-dose Ara-C on day 2, and 100 mg/m2 platinum on day 1 (DHAP). Patients with NHL were mobilized with different regimens: 750 mg/m2cyclophosphamide, 50 mg/m2 Adriamycin, VM26, and 50 mg/m2 prednisone (CHVmP) or DHAP followed by G-CSF. The patient with ALL was mobilized by 10 mg/m2 mitoxantrone and 2 × 3 g/m2 Ara-C.
Healthy donors were treated with G-CSF (2 × 5 μg/kg·d) for 4 to 5 days. When the white blood cell count exceeded 3.0 × 109/L after G-CSF administration and an unequivocal increase in CD34+ cell percentage was observed, leukapheresis procedures were started. Leukapheresis was performed as an outpatient procedure with a continuous-flow blood cell separator on 1 to 5 consecutive days, depending on the number of CD34+cells procured (determined at the end of each pheresis day). Cells were cryopreserved in physiological saline solution containing 0.1% glucose, 0.38% trisodium citrate, 10% (wt/vol) human serum albumin and 10% (vol/vol) dimethyl sulfoxide at a cell concentration of approximately 50 × 106/mL mononuclear cells. After the leukaphereses bags were emptied, residual cells in the bags were collected and used in our experiments. Cell suspensions were frozen at a controlled rate and were subsequently stored in the vapor phase of liquid nitrogen until reinfusion.
Patients with nonhematologic malignancies received high-dose chemotherapy consisting of 1600 mg/m2 carboplatin, 480 mg/m2 thiotepa, and 6000 mg/m2 cyclophosphamide (CTC) intravenously, divided over 4 days.18 Patients with malignant lymphoma received 300 mg/m2 carmustine, 800 mg/m2 etopside, 800 mg/m2 cytarabine, and 140 mg/m2 melphalan (BEAM).19 Patients with multiple myeloma were treated with 60 mg/kg cyclophosphamide on days −5 and −4, followed by 4.5 Gy total body irradiation (TBI) on days −2 and −1 (CYTBI) before PBSCT. The patient with ALL received 4 mg/kg busulfan on days −7 to −4 and cyclophosphamide on days −3 and −2 before PBSCT.
For reinfusion, the cryopreserved products were thawed rapidly by the bedside and were reinfused through an indwelling subclavian catheter. After transplantation, patients received G-CSF 300 μg/d, irrespective of body weight, which was started on the day of PBSC transplantation and was continued until the white blood cell count in the peripheral blood was higher than 5 × 109/L.
After high-dose chemotherapy, a mean number of 11.4 × 106 autologous CD34+cells/kg (range, 3.5 to 31.8 × 106 CD34+cells/kg) was reinfused in 25 patients. Eighteen of 25 patients had recovered to neutrophil counts of at least 0.1 × 109/L at day 11 after reinfusion; the day of reinfusion was considered day 0 (n = 25; mean, 11 days; range, 7-30 days). No significant differences in the rate of neutrophil recovery were observed between the CTC and BEAM chemotherapy regimens. Recovery in patients after CYTBI was significantly delayed compared to treatment with CTC and BEAM. No significant differences in the rate of neutrophil recovery were found between the various diagnoses, and the recovery in the group of patients with multiple myeloma (n = 3) was significantly slower than it was in other diagnoses. Consequently, we excluded the 3 patients with multiple myeloma from the engraftment study; the remaining group consisted of 22 patients. In this group, a mean of 10.9 × 106 CD34+ cells/kg (range, 3.5 to 31.8 × 106 CD34+ cells/kg) was reinfused. From all 22 patients, data on spontaneous migration were available. For 21 of 22 patients, data on SDF-1–induced migration were available. The mean of reinfused CD34+ cells in this group was 11.1 × 106 CD34+ cells/kg (range, 3.5 to 31.8 × 106 CD34+ cells/kg).
Neutrophil recovery was determined as the number of days needed for the neutrophil count in a patient to reach 0.1 × 109/L (gran 100); the day of reinfusion was considered day 0. Platelet recovery was determined as the number of days needed to reach platelet-transfusion independence, ie, when platelet counts reached 20 × 109/L (plat 20) without transfusion. We did not observe any correlation between in vitro migration and in vivo platelet recovery. Most likely, this was because we could not correct for the number of reinfused CD34+/CD41+ cells, which is known to vary strongly between patients and to be highly predictive for platelet recovery, as we previously showed.20
CD34+ cell purification
Freshly obtained PB mononuclear cells were enriched by density gradient centrifugation over Ficoll-Hypaque (1.077 g/mL; Pharmacia Biotech, Uppsala, Sweden). Mononuclear cells were resuspended in PBE buffer, containing phosphate-buffered saline, 0.5% (wt/vol) bovine serum albumin, and 5 mM EDTA. CD34+ cells were isolated with a hapten-labeled CD34 antibody (QBEND 10) with the VarioMacs System according to the manufacturer's instructions (Miltenyi Biotec GmbH, Gladbach, Germany). At least 95% of the cells isolated from PB expressed CD34 as determined by FACS analysis (Immunocytometry Systems; Becton-Dickinson, San Jose, CA).
Flowcytrometric analysis
CXCR-4 expression was determined as mean fluorescence intensity (MFI) of all CD34+ cells and is given after correction for the phycoerythrin (PE)-labeled IgG2a isotype control. CXCR-4 expression was determined by PE-labeled anti-human Fusin (12G5; Pharmingen, Hamburg, Germany). In all cases a similar homogeneous distribution of CXCR-4 was seen without an indication of subpopulations of extra bright or dull cells.21
Migration assay
Migration assays were performed in Transwell plates (Costar, Cambridge, MA) of 6.5-mm diameter, with 5 μm pore filters, as previously described.5 The upper and lower compartments of the Transwells were separated by filters coated overnight at 4°C with (bovine) fibronectin (obtained from Sigma, St Louis, MO) at a concentration of 20 μg/mL in phosphate-buffered saline. Before cells were added to the upper compartment, the coated Transwells were washed 3 times with assay medium (IMDM with 0.25% bovine serum albumin [fraction V; Sigma]). Then 20 000 to 100 000 freshly isolated CD34+ cells, in 0.1 mL assay medium, were added to the upper compartment, and 0.6 mL assay medium in the presence or absence of SDF-1 (in indicated concentrations) was added to the lower compartment. SDF-1α was purchased from Strathmann Biotech GmbH (Hannover, Germany). A 0.1-mL sample containing cells in assay medium was diluted with 0.5 mL assay medium and was kept as an input control for the quantitation of the number of migrated cells (see below). Transwell plates were incubated at 37°C, 5% CO2, for 4 hours. Preliminary experiments showed that after 4 hours a substantial fraction of the CD34+ cells had migrated. Cells that had migrated to the lower compartment were collected in a FACS tube to which a fixed number of control cell-line cells (HL-60) had been added and labeled with Calcine am, according to the manufacturer's instructions (Molecular Probes, Leiden, The Netherlands). The HL-60 cells were added to the FACS tubes just before FACS analysis. FACScan analysis was used to determine the ratio between labeled cells and unlabeled cells, with characteristic light scatter parameters, in the migrated fraction as previously described.5 By comparing this ratio to that in the input control, the number of migrated cells was quantitated. Using this method, we were able to determine reliably at least 200 migrated cells. All migration assays were performed using freshly obtained CD34+ cells. In a control study we did not observe any difference in migration between purified fresh or cryopreserved CD34+ cells (data not shown).
Statistical analysis
For normal distribution values the arithmetic mean and the standard deviation were used. Differences were tested with the Student t test. For multiple comparisons ordinary ANOVA and the Student-Newman-Keuls Multiple Comparisons post-test (only performed when P < .05) were used. Correlation was determined by Pearson test or linear regression analysis. P < .05 was considered significant.
Results
Migration of peripheral blood CD34+ cells from various patient groups
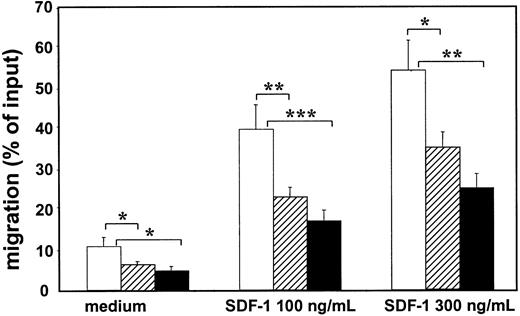
Migration assays were performed with mobilized PB CD34+ cells obtained from 6 healthy donors, from 18 patients with nonhematologic malignancies (BC1, n = 9; BC4+, n = 7; ovarian cancer, n = 2), and from 10 patients with hematologic malignancies (multiple myeloma, n = 6; HD, n = 1; NHL, n = 2; ALL, n = 1) (Figure 1). The patients were divided into these groups because the hematologic patients had received higher cumulative doses of chemotherapy and because in previous studies a correlation between stem cell function after PBSCT and the prior use of stem cell-toxic drugs was observed.22,23 Spontaneous migration and SDF-1–induced migration with 100 and 300 ng/mL SDF-1 over fibronectin-coated transwell filters were analyzed. The data represent the migration of the total CD34+ cell population. There are no clear indications that CD34+ subsets differ in their migratory behavior (data not shown).2,5 24 A broad range in percentages of migrated CD34+ cells was observed, varying not only within, but also between, the 3 groups. For all 3 groups together, a mean of 6.6% spontaneous migration (range, 1.1%-19.0%), 24.8% SDF-1–induced migration by 100 ng/mL SDF-1 (range, 6%-61%), and 36% by 300 ng/mL (range, 13%-72%) was observed. When tested in the Student-Newman-Keuls multiple comparisons test, it was found that in all experimental settings the PB CD34+ cells from mobilized healthy donors migrated significantly better than PB CD34+ cells harvested from patients with either nonhematologic or hematologic malignancies (P < .05). No significant differences were detected between the 2 patient groups in the 3 migration assays, though CD34+ cells from patients with hematologic malignancies seemed to migrate less than those from patients with nonhematologic malignancies (Figure 1).
PB CD34+ cells obtained from mobilized patients show reduced spontaneous and SDF-1–induced migration across fibronectin-coated filters compared to PB CD34+ cells of healthy mobilized donors.
Spontaneous and SDF-1–induced migration (100 ng/mL and 300 ng/mL) of PB-derived CD34+ cells after 4 hours of 6 healthy mobilized donors (■), 18 patients with nonhematologic malignancies (▨), and 10 patients with hematologic malignancies (▪). Spontaneous migration and SDF-1–induced migration (100 ng/mL and 300 ng/mL SDF-1) of PB CD34+ cells derived from healthy donors was significantly higher than from patients with nonhematologic or hematologic malignancies (* = P < .05; ** = P < .01; *** = P < .001). No significant differences in spontaneous and SDF-1–induced migration were observed between the 2 patient groups. Each bar represents the mean ± SEM.
PB CD34+ cells obtained from mobilized patients show reduced spontaneous and SDF-1–induced migration across fibronectin-coated filters compared to PB CD34+ cells of healthy mobilized donors.
Spontaneous and SDF-1–induced migration (100 ng/mL and 300 ng/mL) of PB-derived CD34+ cells after 4 hours of 6 healthy mobilized donors (■), 18 patients with nonhematologic malignancies (▨), and 10 patients with hematologic malignancies (▪). Spontaneous migration and SDF-1–induced migration (100 ng/mL and 300 ng/mL SDF-1) of PB CD34+ cells derived from healthy donors was significantly higher than from patients with nonhematologic or hematologic malignancies (* = P < .05; ** = P < .01; *** = P < .001). No significant differences in spontaneous and SDF-1–induced migration were observed between the 2 patient groups. Each bar represents the mean ± SEM.
Because the higher percentage of SDF-1–induced migrated cells in healthy donors might have been the result of a higher level of CXCR-4 expression on these cells, the CXCR-4 expression on CD34+cells obtained from the different groups was determined. CXCR-4 expression, defined by the MFI, on CD34+ cells did not significantly differ among the 3 groups (healthy donors, 33 ± 17; patients with nonhematologic malignancies, 28 ± 11; patients with hematologic malignancies, 28 ± 17). In addition, the percentage of CD34+ cells from each group that were positive for CXCR-4 did not significantly differ among the groups of mobilized healthy donors (85% ± 10%), patients with nonhematologic malignancies (76% ± 13%), and patients with hematologic malignancies (77% ± 19%). Freeze-thawing and dimethyl sulfoxide exposure did not influence CXCR-4 expression on CD34+ cells (mean MFI fresh, 24 ± 8; mean MFI of these cells after cryopreservation, 26 ± 7; n = 9).
Correlation between CXCR-4 expression and migration
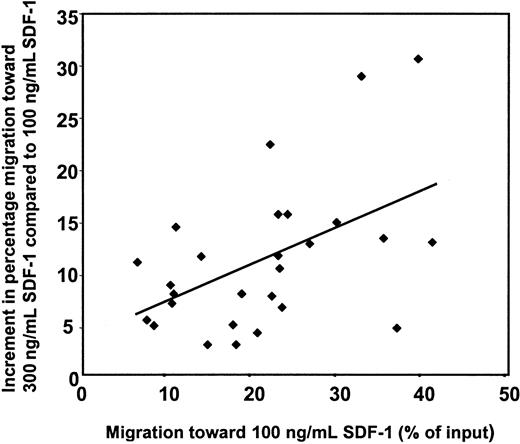
To determine whether the broad range in percentages of migrated CD34+ cells could be explained by a variable expression of the CXCR-4 receptor, we correlated data from the migration assay to the MFI of CXCR-4 receptor of the CD34+ cells. First, the group of healthy mobilized donors was investigated. As expected, no correlation between CXCR-4 expression and spontaneous migration was observed (data not shown). A strong correlation between CXCR-4 expression and migration toward a concentration of SDF-1 of 100 ng/mL or 300 ng/mL was found (r = 0.82 andr = 0.92, respectively; P < .05 andP < .01; data not shown). In contrast, no correlation was observed between the CXCR-4 expression and the SDF-1–induced migration when the CD34+ cells of a group (n = 27) of mobilized cancer patients was analyzed. The lack of correlation between migration in vitro and CXCR-4 expression on CD34+ cells from mobilized patients suggests that the differences in sensitivity for SDF-1 form just one of the factors responsible for variance in SDF-1–induced migration between patients. In a previous study it was found that maximal migration of PB CD34+ cells is induced by 600 to 1000 ng/mL SDF-1 without much difference between patients.5 The concentrations of 100 and 300 ng/mL are both in the linear part of the dose-response curve and were, therefore, used during this study to obtain insight into possible differences in dose-response curves between patients. The migratory capacity of patient-derived PB CD34+ cells was determined by subtracting the percentage of migration induced by 100 ng/mL SDF-1 from the percentage of migration induced by 300 ng/mL SDF-1. Had a decreased SDF-1 response been only due to a shift to the right of the dose-response curve, the increment in migration would have been the same for all patients. However, as shown in Figure2 for a group of 27 patients, greater increment was observed for CD34+ cells that showed a relatively higher migration toward 100 ng/mL SDF-1 (r = 0.5; P < .01). These results indicate that the migratory ability of the CD34+ cells is increased for cells with a relatively high migration to 100 ng/mL SDF-1 rather than that the sensitivity of the CD34+ cells for SDF-1 is increased in these patients.
Migratory capacity of patient-derived PB CD34+ cells.
The migratory capacity of patient-derived PB CD34+ cells was determined by subtracting the percentage of migration induced by 100 ng/mL SDF-1 from the percentage of migration induced by 300 ng/mL SDF-1. A positive correlation (r = 0.5;P < .01) was observed between the increment in migration and the percentage migration induced by 100 ng/mL SDF-1 for a group of 27 patients.
Migratory capacity of patient-derived PB CD34+ cells.
The migratory capacity of patient-derived PB CD34+ cells was determined by subtracting the percentage of migration induced by 100 ng/mL SDF-1 from the percentage of migration induced by 300 ng/mL SDF-1. A positive correlation (r = 0.5;P < .01) was observed between the increment in migration and the percentage migration induced by 100 ng/mL SDF-1 for a group of 27 patients.
Correlation between in vivo recovery and in vitro migration
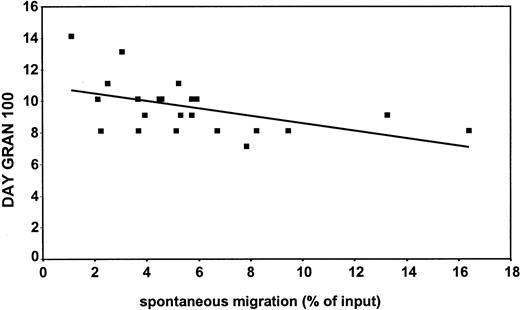
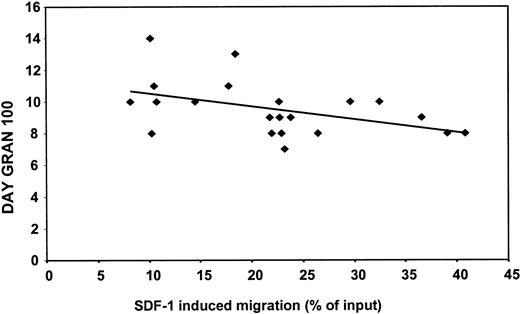
Recently, Peled et al14 demonstrated that human stem cell engraftment and repopulation of NOD/SCID mice is dependent on the ability of CD34+ cells to migrate to SDF-1. Therefore, the in vitro migratory capacity of CD34+ cells of mobilized patients was investigated and correlated to neutrophil recovery in these patients after stem cell transplantation. Figure3 shows the negative correlation (r = −0.5; P < .05) between spontaneous migration in vitro and neutrophil recovery (gran 100) in vivo for a group of 22 patients (ie, the faster the cells migrate, the sooner neutrophil recovery is reached). This was also observed when we analyzed the same parameters obtained from 21 patients and compared these to SDF-1–induced (100 ng/mL) migration in vitro (r = −0.46; P < .05) (Figure4). Similarly, the correlation coefficient was −0.4 when the recovery was related to the migration to 300 ng/mL SDF-1 (P = .07). The observed correlation between spontaneous and SDF-1–induced migration in vitro and neutrophil recovery in vivo was independent of the number of reinfused CD34+ cells (data not shown).
Negative correlation between spontaneous migration in vitro and neutrophil recovery in vivo in patients undergoing autologous PBSCT.
On the y-axis is depicted the day on which the neutrophil count in the patients in circulation reached 0.1 × 109 cells/L (day gran 100). The x-axis represents the spontaneous migration (percentage input). A negative correlation (r = −0.5; P < .05) was found between spontaneous migration in vitro and neutrophil recovery in vivo for a group of 22 patients.
Negative correlation between spontaneous migration in vitro and neutrophil recovery in vivo in patients undergoing autologous PBSCT.
On the y-axis is depicted the day on which the neutrophil count in the patients in circulation reached 0.1 × 109 cells/L (day gran 100). The x-axis represents the spontaneous migration (percentage input). A negative correlation (r = −0.5; P < .05) was found between spontaneous migration in vitro and neutrophil recovery in vivo for a group of 22 patients.
Negative correlation between SDF-1–induced migration in vitro and neutrophil recovery in vivo in patients undergoing autologous PBSCT.
On the y-axis is depicted the day on which the neutrophil count in the patients in circulation reached 0.1 × 109 cells/L (day gran 100). The x-axis represents the SDF-1–induced migration (percentage input). A negative correlation (r = −0.5; P < .05) was found between SDF-1–induced (100 ng/mL) migration in vitro and neutrophil recovery in vivo for a group of 21 patients.
Negative correlation between SDF-1–induced migration in vitro and neutrophil recovery in vivo in patients undergoing autologous PBSCT.
On the y-axis is depicted the day on which the neutrophil count in the patients in circulation reached 0.1 × 109 cells/L (day gran 100). The x-axis represents the SDF-1–induced migration (percentage input). A negative correlation (r = −0.5; P < .05) was found between SDF-1–induced (100 ng/mL) migration in vitro and neutrophil recovery in vivo for a group of 21 patients.
CXCR-4 expression during mobilization
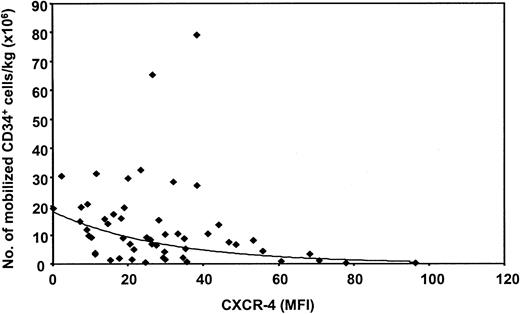
It has been suggested that CXCR-4 expression is down-regulated during mobilization because the expression of CXCR-4 is significantly higher on bone marrow CD34+ cells than on PB CD34+ cells.5,25 26 Therefore, whether a correlation existed between the number of mobilized CD34+cells and their CXCR-4 expression during the first leukapheresis was investigated. For this purpose, CXCR-4 expression was determined on the CD34+ cells from 54 patients undergoing autologous PBSCT. As shown in Figure 5, an exponential relation between CXCR-4 expression and the number of CD34+cells that mobilized to the peripheral blood was found by regression analysis (P < .001). The higher the level of CXCR-4 expression on mobilized CD34+ cells, the lower the number of mobilized cells. However, only 26% of the variation in the number of mobilized CD34+ cells can be explained by this model, indicating that other factors are involved.
Correlation between CXCR-4 expression and mobilization of CD34+ cells per kilogram body weight during the first course of leukapheresis.
An exponential decrease of mobilized CD34+ cells is observed when the MFI of CXCR-4 on the CD34+ cells is higher. Linear regression analysis was performed with the natural logarithm (Ln) of the number of CD34+ cells as a dependent variable and MFI of CXCR-4 as an independent variable. The following function describes this model: CD34 = 18.062xe(−0.033 × (CXCR-4)). The coefficient of CXCR-4 is significantly different from 0 (P < .0001). n = 54.
Correlation between CXCR-4 expression and mobilization of CD34+ cells per kilogram body weight during the first course of leukapheresis.
An exponential decrease of mobilized CD34+ cells is observed when the MFI of CXCR-4 on the CD34+ cells is higher. Linear regression analysis was performed with the natural logarithm (Ln) of the number of CD34+ cells as a dependent variable and MFI of CXCR-4 as an independent variable. The following function describes this model: CD34 = 18.062xe(−0.033 × (CXCR-4)). The coefficient of CXCR-4 is significantly different from 0 (P < .0001). n = 54.
To evaluate whether CXCR-4 expression changes under the influence of continuous G-CSF stimulation, PB CD34+ cells were obtained on subsequent days of leukapheresis procedures from 8 patients receiving treatment for NHL,2 HD,2BC1,2 multiple myeloma, or paraganglioma. CXCR-4 expression on the PB CD34+ cells was determined starting on the first day of leukapheresis. In only 1 of 8 patients (patient 1) was a clear increase in CXCR-4 expression observed on the second day of leukapheresis (Figure 6). In 2 patients (patients 4 and 6), a decrease in CXCR-4 expression on day 2, followed by an increase on day 3, was measured. However, no overall clear or consistent differences in CXCR-4 expression were found during the several days of leukapheresis. These results indicate that G-CSF–induced mobilization did not further down-regulate or up-regulate the CXCR-4 receptor on the PB CD34+ cells.
CXCR-4 expression on PB CD34+ cells during mobilization in 8 patients undergoing autologous PBSCT.
On the y-axis the CXCR-4 expression in MFI of all CD34+cells is given after correction for the PE-labeled IgG2a isotype control. On the x-axis, the day of leukapheresis is depicted.
CXCR-4 expression on PB CD34+ cells during mobilization in 8 patients undergoing autologous PBSCT.
On the y-axis the CXCR-4 expression in MFI of all CD34+cells is given after correction for the PE-labeled IgG2a isotype control. On the x-axis, the day of leukapheresis is depicted.
Discussion
During the process of homing of HPCs to bone marrow, the HPCs must cross the bone marrow endothelium to engraft. This migration process is a complex interplay of transient interactions, directed by chemoattractants and mediated by various adhesion molecules, and it requires motogenic behavior of the HPCs.
In the current study we observed large differences between the in vitro migratory ability of the CD34+ cells obtained from patients undergoing autologous PBSCT, ranging from 1.1% to 16.4% for spontaneous migration and 6.2% to 40.8% for SDF-1–induced migration (100 ng/mL). Peled et al14 have shown in the NOD/SCID model that only CD34+ cells able to migrate to SDF-1 have SCID-repopulating activity. Consequently, it was found relevant to investigate whether the observed differences in migratory ability of CD34+ cells of patients were correlated with their hematopoietic recovery after stem cell transplantation. Indeed, a significantly faster neutrophil recovery was observed in those patients who underwent transplantation with CD34+ cells that showed a high SDF-1–induced migration percentage in vitro (P < .05). This correlation was found despite the fact that, because of the high number of transplanted CD34+cells (3.5-31.8 × 106 cells /kg), the hematologic recovery within the analyzed patient group was relatively fast and barely variable. Previously it was shown that because of the flat end of the dose-effect curve, it is hard to enhance neutrophil recovery by increasing cell dose.27 28
CD34+ cells from healthy G-CSF–mobilized donors exhibited a significantly higher SDF-1–induced (P < .01) migration than CD34+ cells from patients mobilized with chemotherapy and G-CSF. This may indicate that for the group of patients with malignancies, the treatment before or during the mobilization procedure is responsible for the lower migratory capacity of these cells. It has been shown in other studies22 23 that the exposure of stem cells to stem cell toxic drugs before mobilization affects the engraftment potential of PBSC grafts.
What causes the difference in migration between mobilized CD34+ cells obtained from healthy donors and cancer patients and the differences between the patients? The activity of chemokines can be regulated by chemokine receptor expression, modification of signaling pathways, or both.29 The lower migratory in vitro capacity of patient-derived CD34+ cells seemed not to result from down-regulation of CXCR-4 because the mean CXCR-4 expression on the CD34+ cells of the various patient groups was not significantly different from the expression on CD34+ cells of healthy donors. Moreover, no correlation between CXCR-4 expression and SDF-1–induced migration was observed for PB CD34+ cells from mobilized patients. In contrast, a strong correlation between CXCR-4 expression and SDF-1–induced migration was observed for CD34+ cells derived from healthy G-CSF–mobilized donors. Presumably, for CD34+ cells obtained from mobilized patients, SDF-1–induced migration is not dependent on CXCR-4 expression level alone. We have previously described that cord blood (CB)–derived CD34+ cells show enhanced migration in comparison to bone marrow-derived CD34+ cells, though their CXCR-4 expression is similar.5 In addition, for other cell types, it is known that receptor expression is often not the sole determinant of responses to chemokines. For example, few CXCR-4–expressing T cells respond to SDF-1 with a rise in calcium influx.29 In developing human bone marrow B cells, SDF-1 responsiveness does not correlate with CXCR-4 expression levels.30 This lack of correlation between expression and function may result from differences in CXCR-4 function among the different cells.
An alternative explanation might be that CXCR-4 signaling is identical between the different cells but that not all cells are equally capable of translating the CXCR-4 signal into a migratory response caused by intrinsic differences in motility of the cells. Recently, Pilarski et al31 studied RHAMM-mediated motility of HPCs and observed marked differences in motogenic behavior between mobilized CD34+ cells from different patients. Our results also support this latter explanation. Similar to the SDF-1–induced migration, the spontaneous migration of patient-derived CD34+ cells was significantly lower (P < .05) than that of CD34+ cells derived from healthy mobilized donors. Comparable with the SDF-1–induced migration, a correlation between spontaneous migration in vitro and hematopoietic recovery in vivo was observed (P < .05). Furthermore, CD34+ cells that showed a relatively high percentage of migration to 100 ng/mL SDF-1 also had the capacity to show augmented migration to 300 ng/mL SDF-1. Thus, for CD34+ cells from cancer patients, the intrinsic migratory capacity of the cells might be of larger importance for the migratory response than CXCR-4–induced signaling. Moreover, the control of actin assembly and disassembly has been described to be important for cell migration.32 Our previous results indicated that SDF-1–induced actin polymerization is higher in the better-migrating CB CD34+ cells than in bone marrow CD34+ cells.5 It is possible that the ability of the CD34+ cells obtained from mobilized patients to reorganize the cytoskeleton is diminished because of pretreatment with various drugs.
Mobilization and homing can be seen as “mirror images” of each other—they differentially use similar classes of molecules and receptors.33 Indeed, Lapidot et al34 suggested a role for SDF-1 in the mobilization of hematopoietic stem cells. Our data support this presumed involvement of SDF-1 because a significant correlation was observed between CXCR-4 expression and the number of mobilized CD34+ cells. An exponential decrease in the amount of mobilized CD34+ cells per kilogram body weight was observed when the cells expressed higher amounts of CXCR-4.
In conclusion, the precise analysis of patient data, in combination with functional in vitro assays, can give insight into the mechanisms of stem cell homing and mobilization.35 Our results indicate that the intrinsic migratory capacity of the cells is important for hematopoietic recovery. This intrinsic migratory capacity seems to be influenced by pretreatment of the patients with cytotoxic drugs. These results suggest that the engraftment potential of stem cells obtained from pretreated cancer patients is more or less impaired. An improvement in migratory capacity of CD34+cells in autologous stem cell grafts, such as by in vitro manipulation, may lead to enhanced recovery or to the use of smaller grafts.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. E. van der Schoot, Department of Immunohematology, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Plesmanlaan 125, 1066 CX Amsterdam, Netherlands; e-mail: Schoot@clb.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal