Abstract
Core-binding factor β (CBFβ) is the non–DNA-binding subunit of the heterodimeric CBFs. Genes encoding CBFβ (CBFB),and one of the DNA-binding CBFα subunits, Runx1 (also known as CBFα2, AML1, and PEBP2αB), are required for normal hematopoiesis and are also frequent targets of chromosomal translocations in acute leukemias in humans. Homozygous disruption of either the Runx1or Cbfb gene in mice results in embryonic lethality at midgestation due to hemorrhaging in the central nervous system, and severely impairs fetal liver hematopoiesis. Results of this study show that Cbfb-deficient mouse embryonic stem (ES) cells can differentiate into primitive erythroid colonies in vitro, but are impaired in their ability to produce definitive erythroid and myeloid colonies, mimicking the in vivo defect. Definitive hematopoiesis is restored by ectopic expression of full-length Cbfbtransgenes, as well as by a transgene encoding only the heterodimerization domain of CBFβ. In contrast, the CBFβ–smooth muscle myosin heavy chain (SMMHC) fusion protein generated by the inv(16) associated with acute myeloid leukemias (M4Eo) cannot rescue definitive hematopoiesis by Cbfb-deficient ES cells. Sequences responsible for the inability of CBFβ-SMMHC to rescue definitive hematopoiesis reside in the SMMHC portion of the fusion protein. Results also show that the CBFβ-SMMHC fusion protein transdominantly inhibits definitive hematopoiesis, but not to the same extent as homozygous loss of Runx1 orCbfb. CBFβ-SMMHC preferentially inhibits the differentiation of myeloid lineage cells, while increasing the number of blastlike cells in culture.
Introduction
Core-binding factors (CBFs) are heterodimeric transcription factors consisting of a DNA-binding subunit (CBFα) and a non–DNA-binding CBFβ subunit.1-3 Three genes in mammals encode CBFα subunits (Runx1, Runx2, Runx3, formerly known as Cbfa2, Cbfa1, and Cbfa3,respectively), whereas the CBFβ subunit is encoded by one gene,Cbfb.1-8 CBFβ heterodimerizes with all 3 CBFα subunits in vitro, but the functional significance of this interaction has been shown only for Runx1. Homozygous disruption of either the gene encoding Runx1 or CBFβ caused identical developmental defects, indicating that both subunits are essential for the Runx1-CBFβ heterodimer to function in vivo.9-13
The CBFs function in multiple developmental pathways in mammals and invertebrates. The Drosophila runt gene, which encodes a CBFα subunit, is involved in sex determination, segmentation, and neurogenesis.14-17 The related gene lozengeplays a role in developmental pathways involving the eye, antenna, and tarsal claws, and in the formation of crystal cells, a lineage of blood cells in the fly.18-20 The mammalian Runx2 gene is required for bone formation,21,22 and bothRunx1 and Cbfb are required for definitive hematopoiesis.9-13
Definitive hematopoiesis is the second wave of hematopoiesis in the developing vertebrate embryo and produces hematopoietic cells of the lymphoid, myeloid, and enucleated (definitive) erythroid lineages. Definitive hematopoiesis is preceded by primitive erythropoiesis, which occurs in the yolk sac and produces a transient population of nucleated primitive erythrocytes and macrophages. Progenitors and stem cells that give rise to the definitive hematopoietic lineages emerge in the yolk sac, the para-aortic splanchnopleure, the vitelline and umbilical arteries, and in the aorta/genital ridge/mesonephros (AGM) region in mammalian embryos.23-35 Homozygous disruption ofRunx1 or Cbfb does not appear to significantly impair the first stage of primitive erythropoiesis in the yolk sac, but blocks the second wave of definitive hematopoiesis.9-13
RUNX1 (AML1) and CBFB are frequent targets of chromosomal translocations in human leukemias.36RUNX1 is disrupted by the t(8;21)(q22;q22) and t(12;21)(p13;q22) in acute myeloid and lymphocytic leukemias,4,37,38 and by the t(3;21)(q26;q22) and t(16;21)(q24;q22) in therapy-related leukemias and myelodysplasias.39,40CBFB is disrupted in acute myeloid leukemias by inv(16)(p13;q22), t(16;16), and del(16)(q22).6 The translocations generate chimeric proteins that contain all or part of Runx1 or CBFβ fused to sequences encoded in the other chromosomal segment involved in the translocation. For example, the inv16 that disrupts CBFB results in a chimeric protein that contains most of the CBFβ protein fused to the C-terminal α-helical rod domain from a smooth muscle myosin heavy chain (SMMHC) encoded by theMYH11 gene.6 Genetic experiments in mice demonstrated that the CBFβ-SMMHC protein transdominantly inhibits wild-type Runx1:CBFβ function in vivo, in that mice heterozygous for a “knocked-in” CBFB-MYH11 allele die at midgestation from essentially the same defect exhibited by mice deficient forRunx1 or Cbfb.41 Expression of aCBFB-MYH11 transgene in myeloid lineage cells impaired neutrophilic differentiation and this effect was enhanced by an activated N-ras oncogene.42 The CBFβ-SMMHC protein is thought to transdominantly inhibit Runx1:CBFβ function by heterodimerizing with RUNX1 and sequestering it on actin filaments in the cytoplasm,43,44 or by functioning as a dedicated transcriptional repressor in the nucleus.45
A number of studies have demonstrated the utility of differentiating mouse embryonic stem (ES) cells in vitro to study hematopoiesis,46 and showed that ES cells deficient in proteins critical for hematopoiesis in the embryo often show similar hematopoietic impairments in vitro. For example, GATA-1–deficient ES cells fail to develop primitive erythroid precursors in vitro, and definitive erythropoiesis is blocked at the proerythroblast stage of development.47 Ectopic expression of GATA-1transgenes in an immature erythroid cell line derived from GATA-1–deficient ES cells restored terminal erythroid differentiation in vitro.48 SCL-deficient ES cells fail to generate either primitive or definitive hematopoietic cells of all lineages, and this defect could be rescued by ectopic expression of theScl transgene.49,50 ES cells homozygous for deletion of PU.1 produce no detectable myeloid cells in vitro, but expression of a PU.1 transgene restored myelopoiesis.51,52 Runx1-deficient ES cells cannot produce definitive hematopoietic progenitors, but a Runx1 transgene knocked into the Runx1 locus rescued definitive hematopoiesis.53 Here we demonstrate thatCbfb-deficient ES cells undergo primitive erythropoiesis in vitro, but are impaired in their ability to undergo definitive hematopoiesis. Ectopic expression of Cbfb transgenes from the murine stem cell virus (MSCV) restores definitive hematopoiesis of CBFβ-deficient ES cells. We demonstrate that the heterodimerization domain in CBFβ is sufficient for its function in this assay. In contrast, the CBFβ-SMMHC fusion protein is unable to rescue definitive hematopoiesis by CBFβ-deficient ES cells, and thus cannot provide CBFβ function in vivo. We also show that the CBFβ-SMMHC protein does not completely impair definitive hematopoiesis in cells containing one copy of the Cbfb-MYH11 allele and one wild-type Cbfb allele, indicating that Runx1-CBFβ function is not completely inhibited by the CBFβ-SMMHC protein.
Materials and methods
Retroviral transfer of Cbfb complementary DNAs toCbfb−/−ES cells
Runx1−/−,Cbfb+/−, Cbfb−/−, and CbfbCbfb-MYH11/+ ES cells were derived previously.10,11,41 Murine Cbfb complementary DNAs (cDNAs) were subcloned into a murine stem cell viral vector harboring a puromycin-N-acetyltransferase gene (MSCVpac)54 (subcloning details will be provided on request). Transfection of the packaging cell line and transduction of+/+, Cbfb+/−, andCbfb−/− ES cells were performed as described by Pear and coworkers.55 Briefly, BOSC23 cells were transiently transfected by the CaCl2 precipitate method in the presence of 25 mM chloroquine. Retroviral supernatants (3 mL) were harvested 48 hours later, passed through 0.2 μm filters, and added directly to subconfluent ES cell cultures. Polybrene (4 μg/mL; hexadimethrine bromide; Sigma, St Louis, MO) was added and cultures were incubated at 37°C for 3 hours. ES cell culture medium (described below) was added (7 mL) and the cultures were continued with daily changes of medium. After 48 hours, transduced ES cells were selected with 2 mg/mL puromycin and maintained in the presence of puromycin until cells in control cultures were no longer alive. Resistant cells were expanded and tested for transgene expression by Western blotting. Thereafter, cells were grown either in the absence or presence of puromycin, but no significant difference was observed in either protein expression or hematopoietic differentiation under the 2 conditions.
Western blot analyses
The ES cell lysates were collected in IP buffer (150 mM NaCl, 50 mM Tris [pH 8.0], 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.2 mM EDTA, 2.0 mM EGTA) plus protease inhibitors (1 mg/mL pepstatin A, 1 mM Pefablock, 2 mg/mL leupeptin, 2 mg/mL aprotinin). Lysates were boiled in SDS loading buffer, resolved on 13% SDS-polyacrylamide gels, and proteins were transferred to nitrocellulose. CBFβ proteins were detected with mouse monoclonal antibody β141.2 and enhanced chemiluminescence (ECL) reagents (Amersham, Piscataway, NJ).
Cell culture and in vitro differentiation
The ES cells were maintained on gelatinized flasks in Dulbecco modified Eagle medium (DMEM) with 15% fetal calf serum (FCS; Hyclone, Logan, UT), 1.5 × 10−4 M monothioglycerol (MTG), 50 U/mL penicillin G, 50 μg/mL streptomycin, and 1000 U/mL leukemia inhibitory factor (LIF) (Gibco/BRL, Rockville, MD). In vitro hematopoietic differentiation was performed as described. Two days before the initiation of differentiation, cells were transferred to Iscoves modified Dulbecco medium (IMDM) containing the above components. ES cells were trypsinized into a single-cell suspension and plated into primary differentiation media containing 1% methylcellulose (Fluka, Ronkonkoma, NY) in IMDM supplemented with 15% FCS, 2 mM l-glutamine, 1.5 × 10−4 M MTG, 50 μg/mL ascorbic acid, 200 μg/mL iron-saturated transferrin, interleukin 3 (IL-3) (20 ng/mL), IL-11 (10 ng/mL), and 5 ng/mL vascular endothelial growth factor (VEGF). After 6 or 10 days in culture, embryoid bodies (EBs) were disaggregated and replated into secondary methylcellulose cultures containing 10% plasma-derived serum (Antech, Tyler, TX), 5% protein-free hybridoma medium (PFHM2, Gibco/BRL) and cytokines. Primitive erythroid colony (EryP) precursors were enumerated by replating cells into medium containing erythropoietin (Epo; 2 U/mL). Epo (2 U/mL), granulocyte colony-stimulating factor (G-CSF; 2 ng/mL), granulocyte-macrophage CSF (GM-CSF; 5 ng/mL), macrophage CSF (M-CSF; 5 ng/mL), stem cell factor (SCF; 50 ng/mL), IL-1 (5 ng/mL), IL-3 (20 ng/mL), IL-6 (5 ng/mL), IL-11 (10 ng/mL), VEGF (5 ng/mL), and LIF (1 ng/mL) were added to secondary cultures to support differentiation of EryP, definitive erythroid (EryD), myeloid, and mixed lineage colonies. All cytokines and growth factors were purchased from R & D Systems (Minneapolis, MN). After 7 days of secondary culture, colonies were counted on an inverted light microscope. Cells were harvested, pelleted, washed once in serum-free IMDM, and counted before the preparation of cytospins. Slides were stained with Wright-Giemsa. Differential counts were performed by 2 individuals without knowledge of specimen identifications.
Two hematopoietic cell lines derived from ES cells expressing the CBFβ-SMMHC protein were generated by harvesting all colonies from a secondary differentiation plate, washing once with serum-free IMDM, then transferring cells to liquid cultures of RPMI 1640 media supplemented with 10% FCS, 2 mM l-glutamine, 50 U/mL penicillin G, 50 μg/mL streptomycin, 10 ng/mL IL-6, 10 ng/mL SCF, and 2 ng/mL IL-3.
Globin analysis
Globin gene expression patterns of colonies from secondary differentiation cultures were analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR). RNA was isolated using GlassMAX RNA Microisolation Spin Cartridges (Gibco/BRL) and following manufacturer's instructions. The Qiagen OneStep RT-PCR kit (Qiagen, Valencia, CA) was used for both reverse transcription and PCR amplification using previously published primers.56
FACS analysis
Cell surface antigens were detected by standard immunofluorescence assays using phycoerythrin (PE)-conjugated monoclonal antibodies to CD116 (Mac-1), CD117 (c-kit), CD44, CD45R (B220), and CD31 (Pecam-1). PE-conjugated streptavidin was used to detect biotinylated antibody to CD34. Appropriate isotype controls were included in all experiments. Reagents for flow cytometry were obtained from Pharmingen (San Diego, CA). Fluorescence was analyzed on a FACScan (Becton Dickinson, San Jose, CA).
Results
Definitive hematopoiesis is impaired in Runx1 and CBFβ-deficient ES cells
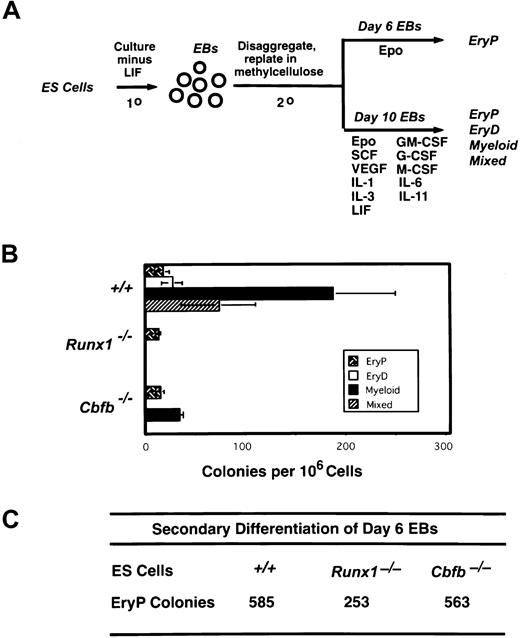
Keller and coworkers demonstrated that ES cells cultured under specific conditions generate differentiated hematopoietic cells in vitro.56 Both the kinetics of precursor development and the responsiveness to growth factors are similar to those found in yolk sac and fetal liver, paralleling hematopoietic development that occurs in the embryo. Large numbers of primitive erythroid precursors emerge first, peaking at day 7 of primary culture, followed by precursors for definitive erythroid, myeloid, and mixed erythroid/myeloid lineage colonies (peaking at days 8-10), and finally mast cell precursors (day 10). We cultured wild-type, Runx1−/−, andCbfb−/− ES cells in vitro to generate EBs, as schematically illustrated in Figure 1A, disaggregated the EBs 6 and 10 days after the establishment of the primary culture, and replated the cells in the presence of hematopoietic growth factors. Cells from day 6 EBs were cultured with Epo to enumerate primitive erythroid precursors, and cells from day 10 EBs were cultured in a combination of hematopoietic growth factors that support the differentiation of both primitive and definitive hematopoietic lineages. Day 6 EBs from wild-type,Runx1−/−, or Cbfb−/−ES cells contained large numbers of progenitors for primitive erythroid colonies (Figure 1C). Primitive erythroid colonies are smaller and brighter red and composed of larger cells than definitive erythroid colonies. Primitive erythroid progenitors were still present in secondary cultures of day 10 EBs from all 3 ES cell lines; however, as expected, their numbers dropped markedly.56 Only day 10 EBs from wild-type ES cells contained progenitors for all lineages of definitive colonies. Day 10 EBs from Runx1−/−ES cells contained only primitive erythroid progenitors, whereas day 10 EBs from Cbfb−/− ES cells contained primitive erythroid progenitors and reduced numbers of progenitors for myeloid colonies (Figure 1B). Thus, the hematopoietic defect in ES cell cultures mimics that seen in Runx1- and CBFβ-deficient mice, in that definitive hematopoiesis is blocked while primitive erythropoiesis is spared. The small number of myeloid progenitors inCbfb−/− ES cells also reflects data obtained in mice, where we observed the number of definitive hematopoietic progenitors was severely, but not completely, depressed in the fetal liver and yolk sac.11
Definitive hematopoiesis is impaired in Runx1- and CBFβ-deficient ES cells.
(A) Schematic diagram of the ES cell differentiation protocol. (B) Hematopoietic progenitor development in EBs from wild-type, Runx1−/−, andCbfb−/−ES cells. Colony assays were performed on EBs generated following 10 days of primary culture. The mean values (± SD) of 3 independent plates are shown from one experiment, representative of 3 independent experiments. EryP, primitive erythroid colonies; EryD, definitive erythroid colonies; mixed, erythroid/myeloid colonies. (C) Control cultures demonstrating that day 6 EBs from the experiment depicted in panel B contained primitive erythroid progenitors. Shown are total colony numbers per 106cells plated.
Definitive hematopoiesis is impaired in Runx1- and CBFβ-deficient ES cells.
(A) Schematic diagram of the ES cell differentiation protocol. (B) Hematopoietic progenitor development in EBs from wild-type, Runx1−/−, andCbfb−/−ES cells. Colony assays were performed on EBs generated following 10 days of primary culture. The mean values (± SD) of 3 independent plates are shown from one experiment, representative of 3 independent experiments. EryP, primitive erythroid colonies; EryD, definitive erythroid colonies; mixed, erythroid/myeloid colonies. (C) Control cultures demonstrating that day 6 EBs from the experiment depicted in panel B contained primitive erythroid progenitors. Shown are total colony numbers per 106cells plated.
Ectopic expression of Cbfb transgenes rescues definitive hematopoiesis by CBFβ-deficient ES cells
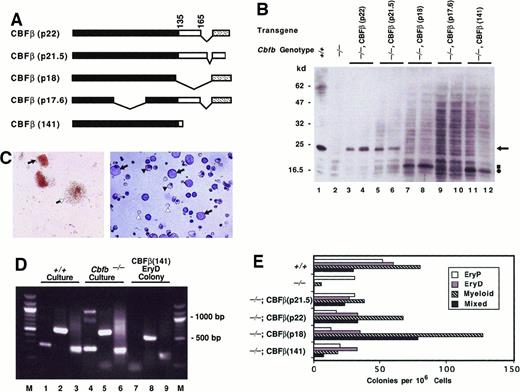
Four distinct cDNAs representing alternatively spliced isoforms of CBFβ have been isolated2,3 (Figure2A). Two isoforms, CBFβ(p22) and CBFβ(p21.5), encode CBFβ proteins containing 187 and 182 amino acids, respectively, that heterodimerize efficiently with CBFα subunits in vitro.2,3 The CBFβ(p17.6) isoform lacks amino acids encoded in exon 3 and fails to heterodimerize with CBFα in vitro.3 CBFβ(p18) lacks amino acids encoded in exon 5, including the last 2 amino acids (aa 134-135) in the domain that mediates heterodimerization with CBFα.57 TheCbfb−/− ES cells used in the in vitro differentiation assay contain a deletion in exon 5 of theCbfb gene.11 RT-PCR products encoding the CBFβ(p18) isoform were amplified from embryos produced from the sameCbfb−/− ES cells; however, a noticeable decrease in Cbfb messenger RNA level was observed compared to wild-type embryos.11 Western blotting with both embryos and ES cells showed that levels of the truncated CBFβ(p18) protein were greatly reduced compared with those of the endogenous CBFβ(p22) and CBFβ(p21.5) proteins.11Heterodimerization of CBFβ(p18) with CBFα on DNA in vitro was shown to be relatively weak.11 57 Specifically, CBFβ(p18) dissociates more readily from the CBFα–DNA complex than does CBFβ(p22) or CBFβ(p21.5) during the process of electrophoresis through polyacrylamide gels in electrophoretic mobility shift assays.
Ectopic expression of
Cbfb transgenes rescues definitive hematopoiesis by CBFβ-deficient ES cells. (A) Schematic diagram showing CBFβ isoforms generated by alternative splicing. Also shown is the experimentally truncated CBFβ(141) protein containing the heterodimerization domain for the CBFα proteins. Black rectangles indicate sequences within the heterodimerization domain. Sequences C-terminal to amino acid 165 in CBFβ(p22), CBFβ(p18), and CBFβ(p17.6) (stippled region) differ from those in CBFβ(p21.5). (B) Western blot analysis of CBFβ proteins produced by ectopic expression in Cbfb−/−ES cells. The genotype of the ES cell line and the protein produced from the ectopically expressedCbfb transgene is indicated above each lane. Protein lysates from 2 independently derived cell populations are shown. Arrow points to the endogenous CBFβ protein. Square and circle indicate positions of CBFβ(p18) and the truncated CBFβ(141) proteins, respectively. Molecular weight markers in kilodaltons are listed on the left. (C) Left panel: Definitive erythroid and mixed-lineage colonies generated in secondary cultures of EBs derived fromCbfb−/−ES cells ectopically expressing the CBFβ(p22) protein. Closed arrow indicates definitive erythroid colony; open arrow shows mixed-lineage colony. Right panel: Cytocentrifuge preparation of definitive mixed lineage colonies fromCbfb−/−ES cells ectopically expressing CBFβ(p22). Five mixed-lineage colonies were picked from secondary methylcellulose cultures, cytocentrifuged, and stained with Wright-Giemsa. Closed arrows identify myeloid cells, closed arrowheads show nucleated erythroid cells, and open arrowheads indicate enucleated red blood cells. (D) Globin expression analysis of secondary hematopoietic colonies. All colonies from one plate of a secondary culture of wild-type or Cbfb−/−EBs are compared to an individual definitive erythroid colony picked from a secondary culture of Cbfb−/−ES cells ectopically expressing CBFβ(141). Lanes 1, 4, and 7 show expression of βH1 (265 bp); lanes 2, 5, and 8 show βmajor (578 bp). HPRT expression (249 bp) is shown in lanes 3, 6, and 9 as a positive control. A 100-bp molecular weight ladder (M) flanks lanes 1 to 9. (E) Hematopoietic progenitor development in EBs from wild-type,Cbfb−/−, and Cbfb−/−ES cells expressing Cbfb transgenes. Colony assays were performed on EBs generated from 10 days of primary culture. The mean values of 2 or 3 independent plates are shown from one experiment. Similar results were obtained in 4 independent experiments. Differences in numbers of various colony types with expression of different transgenes were not statistically significant.
Ectopic expression of
Cbfb transgenes rescues definitive hematopoiesis by CBFβ-deficient ES cells. (A) Schematic diagram showing CBFβ isoforms generated by alternative splicing. Also shown is the experimentally truncated CBFβ(141) protein containing the heterodimerization domain for the CBFα proteins. Black rectangles indicate sequences within the heterodimerization domain. Sequences C-terminal to amino acid 165 in CBFβ(p22), CBFβ(p18), and CBFβ(p17.6) (stippled region) differ from those in CBFβ(p21.5). (B) Western blot analysis of CBFβ proteins produced by ectopic expression in Cbfb−/−ES cells. The genotype of the ES cell line and the protein produced from the ectopically expressedCbfb transgene is indicated above each lane. Protein lysates from 2 independently derived cell populations are shown. Arrow points to the endogenous CBFβ protein. Square and circle indicate positions of CBFβ(p18) and the truncated CBFβ(141) proteins, respectively. Molecular weight markers in kilodaltons are listed on the left. (C) Left panel: Definitive erythroid and mixed-lineage colonies generated in secondary cultures of EBs derived fromCbfb−/−ES cells ectopically expressing the CBFβ(p22) protein. Closed arrow indicates definitive erythroid colony; open arrow shows mixed-lineage colony. Right panel: Cytocentrifuge preparation of definitive mixed lineage colonies fromCbfb−/−ES cells ectopically expressing CBFβ(p22). Five mixed-lineage colonies were picked from secondary methylcellulose cultures, cytocentrifuged, and stained with Wright-Giemsa. Closed arrows identify myeloid cells, closed arrowheads show nucleated erythroid cells, and open arrowheads indicate enucleated red blood cells. (D) Globin expression analysis of secondary hematopoietic colonies. All colonies from one plate of a secondary culture of wild-type or Cbfb−/−EBs are compared to an individual definitive erythroid colony picked from a secondary culture of Cbfb−/−ES cells ectopically expressing CBFβ(141). Lanes 1, 4, and 7 show expression of βH1 (265 bp); lanes 2, 5, and 8 show βmajor (578 bp). HPRT expression (249 bp) is shown in lanes 3, 6, and 9 as a positive control. A 100-bp molecular weight ladder (M) flanks lanes 1 to 9. (E) Hematopoietic progenitor development in EBs from wild-type,Cbfb−/−, and Cbfb−/−ES cells expressing Cbfb transgenes. Colony assays were performed on EBs generated from 10 days of primary culture. The mean values of 2 or 3 independent plates are shown from one experiment. Similar results were obtained in 4 independent experiments. Differences in numbers of various colony types with expression of different transgenes were not statistically significant.
We introduced transgenes encoding all 4 CBFβ isoforms intoCbfb−/− ES cells using the MSCV retroviral vector.54 Pools of drug-resistant ES cells were isolated and screened for CBFβ expression by Western blot (Figure 2B). Ectopically expressed CBFβ(p22) and CBFβ(p21.5) comigrate with endogenous CBFβ and accumulate at levels similar to that of the endogenous protein(s) in cells. Ectopically expressed CBFβ(p18) migrates faster than endogenous CBFβ through SDS-polyacrylamide gels. The steady-state level of ectopically expressed CBFβ(p18) (Figure 2B, lanes 7-8) was similar to that of the endogenous CBFβ(p22) and/or CBFβ(p21.5) protein in wild-type ES cells (Figure 2B, lane 1), but much greater than that of endogenous CBFβ(p18) in eitherCbfb+/+ or Cbfb−/− ES cells (Figure 2B, lanes 1-2). Ectopically expressed CBFβ(p17.6), as well as endogenous CBFβ(p17.6), were not detectable by Western blot.
Ectopic expression of CBFβ(p22) and CBFβ(p21.5) inCbfb−/− ES cells rescued definitive hematopoiesis, indicating that both of these CBFβ isoforms are functional in vivo (Figure 2C-E). Ectopic expression of CBFβ(p18) also restored definitive hematopoiesis (Figure 2E). Although theCbfb−/− ES cells may produce the CBFβ(p18) isoform from the mutated Cbfb locus,11 its concentration in Cbfb−/− cells and embryos was apparently insufficient for in vivo function. However, the residual CBFβ(p18) activity in Cbfb−/− cells may account for the small numbers of definitive hematopoietic progenitors found in both ES cell cultures and mouse embryos.11
The CBFβ(p17.6) failed to rescue definitive hematopoiesis (not shown). This result cannot be interpreted as an intrinsic failure of CBFβ(p17.6) to rescue because the protein was not detectable.
The heterodimerization domain for the CBFα proteins is contained within the N-terminal 135 amino acids of CBFβ(p22) and CBFβ(p21.5).57 We previously showed that an amino acid 1 to 141 fragment of CBFβ, referred to as CBFβ(141), binds to a Runx1-DNA complex with a Kd equivalent to that of full-length CBFβ(p22).58 CBFβ(141) also assumes a folded structure indistinguishable from that which it assumes in the context of the full-length protein.58Ectopic expression of CBFβ(141) restores definitive hematopoiesis by Cbfb−/− ES cells (Figure 2E). The presence of definitive erythroid colonies was confirmed by RT-PCR analysis of β-globin expression (Figure 2D). Primitive erythroid cells express both embryonic (βH1) and adult type (β major) β-globin, whereas definitive erythroid cells express only theβ major gene. Individual definitive erythroid colonies isolated from secondary cultures of Cbfb−/− ES cells expressing CBFβ(141) transcribed the β major, but not the βH1 gene (Figure 2D, lanes 7-8). Therefore, the heterodimerization domain of CBFβ, which is common to both the CBFβ(p21.5) and CBFβ(p22) isoforms, is sufficient for CBFβ function in hematopoiesis in this assay system.
One limitation of the rescue assay is that numbers of definitive colonies produced from both Cbfb+/+ ES cells andCbfb−/− ES cells expressing various transgenes varied from 5- to 20-fold between experiments, making quantitative comparisons of the ability to rescue difficult. Although the rescue assay is not quantitative, under no circumstances did we detect progenitors for definitive erythroid or mixed erythroid/myeloid lineage colonies in EBs derived from Cbfb−/− ES cells. Therefore, we scored the appearance of definitive erythroid and mixed erythroid/myeloid lineage progenitors as rescue, and the absence of such progenitors as failure to rescue.
CBFβ-SMMHC fails to rescue hematopoiesis byCbfb−/−ES cells
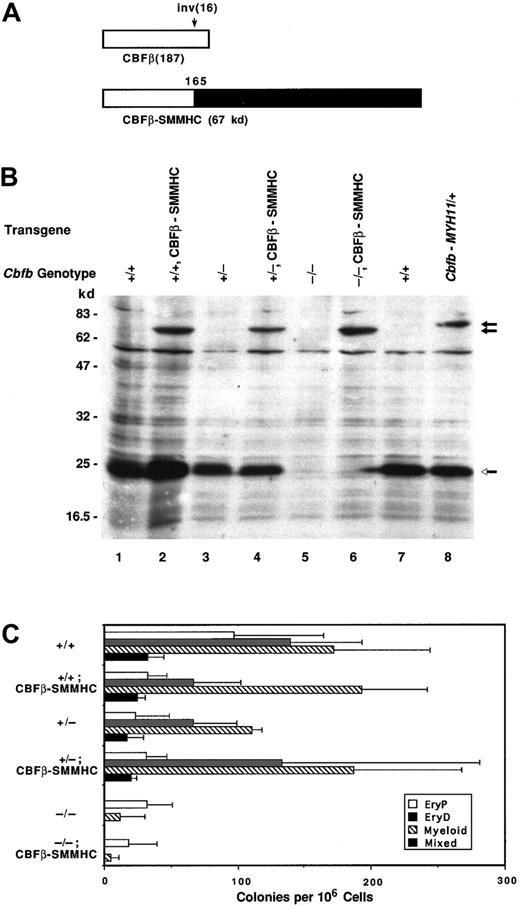
The CBFβ-SMMHC fusion protein generated as a result of the inv(16) was shown in mouse “knock-in” studies to transdominantly inhibit Runx1-CBFβ function in vivo.41CBFβ-SMMHC contains the N-terminal 165 amino acids of CBFβ, including the intact Runx1 heterodimerization domain, fused to the SMMHC tail.6 We ectopically expressed a 67-kd isoform of CBFβ-SMMHC in Cbfb+/+,Cbfb+/−, and Cbfb−/−ES cells (Figure 3). Western blot analysis confirms expression of CBFβ-SMMHC in all 3 cell lines (Figure 3B). The CBFβ-SMMHC protein concentration appears to be lower than that of endogenous CBFβ (Figure 3B, lanes 2 and 4), but the transfer efficiency of the 2 proteins may differ, making direct comparisons difficult. Progenitors for definitive erythroid and mixed lineage colonies were present in EBs derived fromCbfb+/+ and Cbfb+/− ES cells expressing the CBFβ-SMMHC transgene (Figure 3C), indicating that ectopic expression of the CBFβ-SMMHC protein from the MSCV enhancer cannot significantly impair definitive hematopoiesis in this assay system.
Ectopically expressed CBFβ-SMMHC does not inhibit definitive hematopoiesis.
(A) Schematic diagram of the wild-type CBFβ(p22) protein and one of the CBFβ-SMMHC proteins generated by the inv(16). (B) Western blot documenting ectopic expression of the 67-kd CBFβ-SMMHC protein in Cbfb+/+, Cbfb+/−, andCbfb−/−ES cells, and expression of the 71-kd CBFβ-SMMHC protein in cells heterozygous for a “knocked-in”CbfbCbfb-MYH11 allele.41 Open arrow indicates the endogenous CBFβ proteins; closed arrows indicate the ectopically expressed CBFβ-SMMHC proteins. (C) Hematopoietic progenitor development in EBs derived from ES cells expressing the CBFβ-SMMHC protein. The Cbfb genotype of the ES cell is indicated, followed by the protein expressed from the transgene. Colony assays were performed on EBs generated from 10 days of primary culture. The mean values (± SD) of total colony numbers in 3 independent experiments are shown. The differences in colony numbers in cells with or without the transgene were not statistically significant.
Ectopically expressed CBFβ-SMMHC does not inhibit definitive hematopoiesis.
(A) Schematic diagram of the wild-type CBFβ(p22) protein and one of the CBFβ-SMMHC proteins generated by the inv(16). (B) Western blot documenting ectopic expression of the 67-kd CBFβ-SMMHC protein in Cbfb+/+, Cbfb+/−, andCbfb−/−ES cells, and expression of the 71-kd CBFβ-SMMHC protein in cells heterozygous for a “knocked-in”CbfbCbfb-MYH11 allele.41 Open arrow indicates the endogenous CBFβ proteins; closed arrows indicate the ectopically expressed CBFβ-SMMHC proteins. (C) Hematopoietic progenitor development in EBs derived from ES cells expressing the CBFβ-SMMHC protein. The Cbfb genotype of the ES cell is indicated, followed by the protein expressed from the transgene. Colony assays were performed on EBs generated from 10 days of primary culture. The mean values (± SD) of total colony numbers in 3 independent experiments are shown. The differences in colony numbers in cells with or without the transgene were not statistically significant.
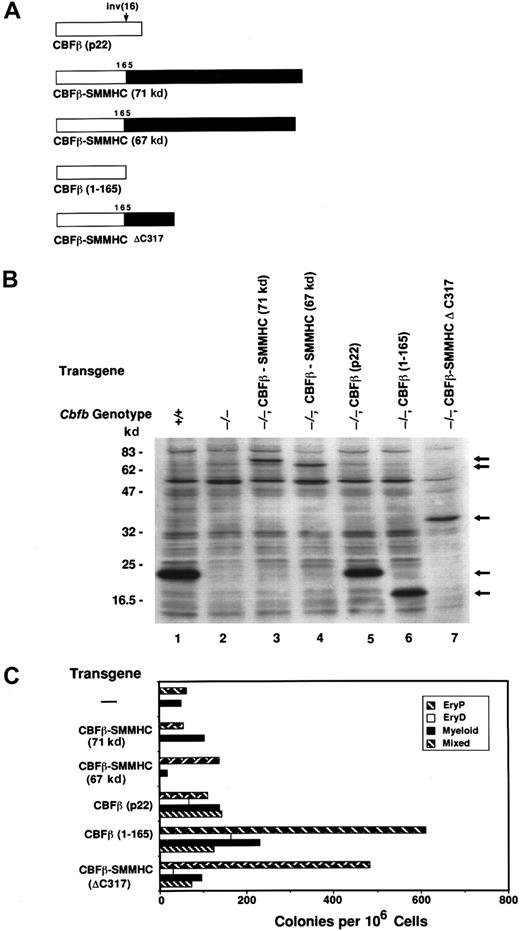
We also determined whether ectopic expression of CBFβ-SMMHC fusion proteins could restore definitive hematopoiesis byCbfb−/− ES cells. Expression of either the 67-kd (Figures 3C and 4C) or the 71-kd (Figure4C) isoforms of CBFβ-SMMHC failed to rescue definitive hematopoiesis. This indicates either that expression levels of these proteins were insufficient to restore CBFβ function, or that the CBFβ-SMMHC proteins are intrinsically defective in providing CBFβ function in vivo. However, a truncated form of the CBFβ-SMMHC protein lacking the C-terminal 317 amino acids of the SMMHC tail region, CBFβ-SMMHCΔC317 (Figure 4A), when ectopically expressed at approximately equivalent levels as the full-length CBFβ-SMMHC proteins (Figure 4B, lanes 3, 4, and 7), rescued definitive hematopoiesis of Cbfb−/− ES cells (Figure 4C). CBFβ-SMMHCΔC317 lacks SMMHC sequences that enable it to associate with and sequester CBFα subunits on cytoplasmic actin filaments.44 We therefore conclude that the full-length CBFβ-SMMHC protein is intrinsically deficient in its ability to restore CBFβ function inCbfb−/− ES cells, due to the presence of SMMHC sequences.
Sequences in the SMMHC portion of CBFβ-SMMHC inhibit CBFβ function.
(A) Schematic diagram of human CBFβ(p22), the CBFβ-SMMHC proteins generated by the inv(16), and experimentally truncated derivatives of human CBFβ and CBFβ-SMMHC. (B) Western blot documenting expression of the proteins illustrated in panel A inCbfb−/−ES cells. (C) Colony formation byCbfb−/−ES cells expressing the transgenes shown in panels A and B. Assays were performed with EBs following 10 days of primary differentiation. The mean values from 2 or 3 independent plates are shown from one experiment, representative of 3 independent experiments.
Sequences in the SMMHC portion of CBFβ-SMMHC inhibit CBFβ function.
(A) Schematic diagram of human CBFβ(p22), the CBFβ-SMMHC proteins generated by the inv(16), and experimentally truncated derivatives of human CBFβ and CBFβ-SMMHC. (B) Western blot documenting expression of the proteins illustrated in panel A inCbfb−/−ES cells. (C) Colony formation byCbfb−/−ES cells expressing the transgenes shown in panels A and B. Assays were performed with EBs following 10 days of primary differentiation. The mean values from 2 or 3 independent plates are shown from one experiment, representative of 3 independent experiments.
CBFβ-SMMHC impairs myeloid cell differentiation
To address the possibility that the concentration of ectopically expressed CBFβ-SMMHC was not adequate to overcome endogenous wild-type CBFβ function in this assay, we analyzed the hematopoietic potential of ES cells heterozygous for a Cbfb-MYH11“knock-in” allele. This allele was generated by replacing a portion of exon 5 from the mouse Cbfb gene with exon 5 sequences from the human CBFB gene, fused to MYH11sequences.41 Both the normal CBFβ proteins and the 71-kd CBFβ-SMMHC fusion protein are expressed from the endogenousCbfb promoters in the “knock-in” (CbfbCbfb-MYH11/+) ES cells (Figure 3B, lane 8). It was previously demonstrated that embryos heterozygous for the CbfbCbfb-MYH11allele die at midgestation and display impaired definitive hematopoiesis, with severely depressed numbers of definitive hematopoietic progenitors in fetal livers and yolk sacs.41
The ES cells heterozygous for the CbfbCbfb-MYH11allele produced significantly more definitive hematopoietic progenitors than either Cbfb−/−orRunx1−/− ES cells in vitro (Figures 5A and 1B). The number of definitive progenitors generated byCbfbCbfb-MYH11/+ ES cells was in fact only modestly reduced, ranging from 0- to 10-fold in independent experiments, relative to progenitor numbers from wild-type ES cells. Thus, the same ratio of CBFβ-SMMHC to CBFβ sufficient to cause embryonic lethality in mice is insufficient to completely block definitive hematopoiesis by CbfbCbfb-MYH11/+ ES cells cultured in vitro.
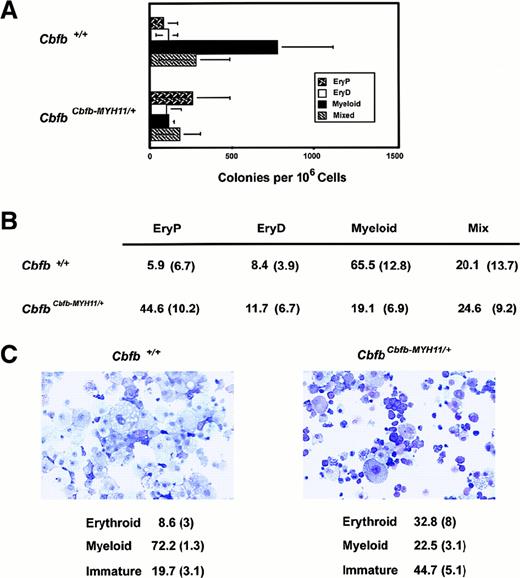
Impaired myelopoiesis by
CbfbCbfb-MYH11/+ ES cells. (A) Hematopoietic colony formation by ES cells heterozygous for a “knocked-in” Cbfb-MYH11 allele, from which the 71-kd CBFβ-SMMHC fusion protein is expressed.41 The average values (± SD) from 5 independent experiments are presented. (B) Colony types formed from Cbfb+/+ andCbfbCbfb-MYH11/+ ES cells. Data represent the mean percentage of colonies of specific lineages per total colonies, followed by SD in parentheses, from 5 independent experiments. The differences between the number of myeloid and EryP colonies formed by the Cbfb+/+ andCbfbCbfb-MYH11/+ ES cells are significant atP < .005 by Student t test. There is no significant difference between the number of EryD or mixed lineage colonies. (C) Cytocentrifuge preparations of hematopoietic cells derived from secondary cultures of Cbfb+/+ andCbfbCbfb-MYH11/+ ES cells. All cells from one plate were collected, washed, counted, and used for cytocentrifugation. The percentage of each cell type (average of 4 experiments ± SD) is indicated below. The differences in erythroid and immature cells between Cbfb+/+ andCbfbCbfb-MYH11/+ ES cells is significant atP < .01 by Student t test. The difference in myeloid cells in the 2 populations is significant atP < .0001.
Impaired myelopoiesis by
CbfbCbfb-MYH11/+ ES cells. (A) Hematopoietic colony formation by ES cells heterozygous for a “knocked-in” Cbfb-MYH11 allele, from which the 71-kd CBFβ-SMMHC fusion protein is expressed.41 The average values (± SD) from 5 independent experiments are presented. (B) Colony types formed from Cbfb+/+ andCbfbCbfb-MYH11/+ ES cells. Data represent the mean percentage of colonies of specific lineages per total colonies, followed by SD in parentheses, from 5 independent experiments. The differences between the number of myeloid and EryP colonies formed by the Cbfb+/+ andCbfbCbfb-MYH11/+ ES cells are significant atP < .005 by Student t test. There is no significant difference between the number of EryD or mixed lineage colonies. (C) Cytocentrifuge preparations of hematopoietic cells derived from secondary cultures of Cbfb+/+ andCbfbCbfb-MYH11/+ ES cells. All cells from one plate were collected, washed, counted, and used for cytocentrifugation. The percentage of each cell type (average of 4 experiments ± SD) is indicated below. The differences in erythroid and immature cells between Cbfb+/+ andCbfbCbfb-MYH11/+ ES cells is significant atP < .01 by Student t test. The difference in myeloid cells in the 2 populations is significant atP < .0001.
Although the absolute numbers of definitive colonies produced by theCbfbCbfb-MYH11/+ ES cells were only modestly reduced compared to wild-type ES cells, expression of the CBFβ-SMMHC fusion protein preferentially inhibited the formation of myeloid colonies, relative to primitive and/or definitive erythroid colonies (Figure 5B). Cytospins of secondary cultures from wild-type and CbfbCbfb-MYH11/+ ES cells revealed significantly different ratios of individual cell types (Figure 5C). Most of the cells derived from wild-type ES cells were macrophage-like with prominent foamy cytoplasm. Hematopoietic cells from colonies derived from CbfbCbfb-MYH11/+ ES cells were more heterogeneous. Many more cells showed evidence of erythroid differentiation, including multinucleate forms. There was some maturation to macrophage-like cells. Occasional cells were granulated, suggesting mast cell differentiation. Many precursor cells with higher nuclear cytoplasmic ratios and nucleoli were also present. Enumeration of these various cell types indicated a decrease in the percentage of mature myeloid cells and an increase in the percentage of erythroid and blast-like cells in cultures derived fromCbfbCbfb-MYH11/+ ES cells (Figure 5C). Because we observed an increase in the number of primitive erythroid colonies, we believe that the overall increase in erythroid cells is due to an increase in primitive erythroid cells.
Establishment and characterization of aCbfbCbfb-MYH11/+cell line
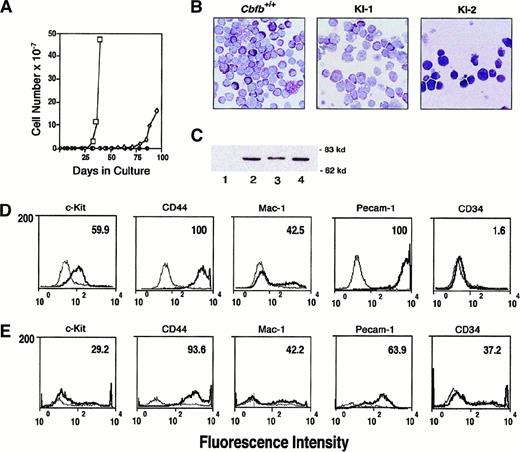
Hematopoietic colonies from secondary cultures ofCbfb+/+, Cbfb−/−, andCbfbCbfb-MYH11/+ ES cells were recovered from methylcellulose cultures, disaggregated, and grown in liquid cultures in the presence of IL-3, IL-6, and SCF, following the procedure described by Okuda and coworkers.59 Two cell lines were independently derived from the CbfbCbfb-MYH11/+ES cells. After approximately 25 days in culture, one cell line (KI-1) began to expand, reaching logarithmic growth after 35 days (Figure6A). The second line (KI-2) began to actively proliferate after approximately 75 days in culture. Viable cells persisted in liquid cultures derived fromCbfb+/+ and Cbfb−/− ES cells for up to 70 days but active proliferation was never observed and the cell numbers declined steadily over the course of the experiment. Growth and survival of all cells was dependent on the presence of hematopoietic growth factors, and rapid cell death was observed in their absence. Morphologically, cells derived from wild-type ES cells displayed mast cell differentiation with prominent purple granules (Figure 6B). Precursor cells with sparse granules, larger nuclei, and prominent nucleoli as well as a few nongranulated precursors were observed. The KI-1 cell line derived fromCbfbCbfb-MYH11/+ ES cells showed similar morphology with most cells displaying some maturation toward mast cells, but occasional large foamy macrophages were also present. The morphology of the KI-2 cells was very distinct from that of either the KI-1 cell line or Cbfb+/+ cells. Most KI-2 cells were fairly undifferentiated with prominent nucleoli and large nuclear cytoplasmic ratios. Very few cells possessed granules and several had vacuoles (Figure 6B). Both KI-1 and KI-2 cells continued to express the CBFβ-SMMHC protein (Figure 6C).
Morphology and cell surface phenotype of cell lines derived from secondary cultures of CbfbCbfb-MYH11/+ES cells.
(A) Growth curve showing expansion of cells derived from secondary cultures of wild-type (WT, ●) andCbfbCbfb-MYH11/+ ES cells (KI-1, ■, and KI-2, ⋄). (B) Giemsa stains of cells derived from secondary cultures ofCbfb+/+ andCbfbCbfb-MYH11/+ ES cells (KI-1, KI-2). Cytocentrifuge preparations of the Cbfb+/+ and KI-1 cells were made after 42 days in liquid culture. KI-2 preparations were made after 85 days in liquid culture. (C) Western blot analysis documenting continued expression of the CBFβ-SMMHC protein in the KI-1 and KI-2 cell lines. Lanes: 1, +/+ ES cells; 2,CbfbCbfb-MYH11/+ ES cells; 3, KI-1 cells; 4, KI-2 cells. Molecular weight markers are indicated on the right. (D) Flow cytometric analysis of surface antigen expression on KI-1 cells expanded in liquid culture. Heavy black line represents staining obtained with antibodies specific for the indicated hematopoietic antigen. Lighter line corresponds to the signal obtained with a control antibody. Number in upper right hand corner indicates percentage of cells positive for surface expression of each marker. (E) Flow cytometric analysis of surface antigen expression on KI-2 cells.
Morphology and cell surface phenotype of cell lines derived from secondary cultures of CbfbCbfb-MYH11/+ES cells.
(A) Growth curve showing expansion of cells derived from secondary cultures of wild-type (WT, ●) andCbfbCbfb-MYH11/+ ES cells (KI-1, ■, and KI-2, ⋄). (B) Giemsa stains of cells derived from secondary cultures ofCbfb+/+ andCbfbCbfb-MYH11/+ ES cells (KI-1, KI-2). Cytocentrifuge preparations of the Cbfb+/+ and KI-1 cells were made after 42 days in liquid culture. KI-2 preparations were made after 85 days in liquid culture. (C) Western blot analysis documenting continued expression of the CBFβ-SMMHC protein in the KI-1 and KI-2 cell lines. Lanes: 1, +/+ ES cells; 2,CbfbCbfb-MYH11/+ ES cells; 3, KI-1 cells; 4, KI-2 cells. Molecular weight markers are indicated on the right. (D) Flow cytometric analysis of surface antigen expression on KI-1 cells expanded in liquid culture. Heavy black line represents staining obtained with antibodies specific for the indicated hematopoietic antigen. Lighter line corresponds to the signal obtained with a control antibody. Number in upper right hand corner indicates percentage of cells positive for surface expression of each marker. (E) Flow cytometric analysis of surface antigen expression on KI-2 cells.
Flow cytometric analysis using a panel of monoclonal antibodies directed against hematopoietic cell surface antigens was used to further characterize the CbfbCbfb-MYH11/+ KI-1 and KI-2 cell lines (Figures 6D and E, respectively). Both cell lines were uniformly positive for high levels of CD44. CD44 is the receptor for hyaluronate and is normally expressed at low levels on B cells, monocytes, macrophages, and subsets of T cells. Subpopulations of both cell lines expressed similar levels of CD11b (Mac-1), a marker of myeloid lineage commitment. Approximately 60% of the KI-1 cells and 30% of the KI-2 cells expressed CD117 (c-kit), the transmembrane tyrosine kinase receptor for SCF, and a common marker for hematopoietic progenitor cells. Expression of CD34, another common marker of progenitor cells, was not detected on the KI-1 cells but 37% of the KI-2 cells were positive for this antigen. CD31 (Pecam-1 or platelet endothelial cell adhesion molecule) is normally expressed constitutively on endothelial cells, at high levels on definitive hematopoietic progenitor cells, and only weakly expressed on peripheral lymphoid cells and platelets. CD31 was expressed at very high levels in 100% of the KI-1 cells and on 64% of the KI-2 cells. Approximately 5% of both lines expressed CD45R (B220). The cells did not express CD90 (Thy-1), CD16/CD32 (FcγRII/FcγRIII receptor), or CD3 (data not shown).
Discussion
CBFβ is the non–DNA-binding subunit of the CBF complexes. CBFβ heterodimerizes with the DNA-binding Runt domain of the CBFα proteins, and increases their affinity for DNA. No other biochemical functions of the CBFβ protein have thus far been demonstrated. Here we describe an assay for CBFβ function based on the ability of ectopically expressed Cbfb transgenes to rescue definitive hematopoiesis by CBFβ-deficient ES cells in culture. Using this assay, we show that 2 CBFβ isoforms previously shown to form stable heterodimers with CBFα in vitro, CBFβ(p22) and CBFβ(p21.5),2,3 are both capable of providing CBFβ function in vivo. A third CBFβ isoform, CBFβ(p18), which forms an unstable complex with CBFα and DNA in vitro,11,57 also restores definitive hematopoiesis by Cbfb−/−ES cells. In addition, we demonstrate that the heterodimerization domain of CBFβ, which mediates its interaction with the CBFα Runt domain, is sufficient for its in vivo function with Runx1 in this assay. Kanno and colleagues also showed that the CBFβ heterodimerization domain can support transactivation by Runx1, using a transient expression and reporter gene assay in Jurkat T cells.43 We both confirm and extend their analyses by demonstrating the heterodimerization domain of CBFβ is sufficient to carry out a developmental program that requires CBFβ.
The ability of CBFβ(p18) to rescue hematopoiesis was somewhat unexpected, given that CBFβ(p18) is synthesized from the endogenous mutated Cbfb allele in Cbfb−/− ES cells.11 CBFβ(p18) lacks exon 5–encoded sequences, including 2 amino acids (aa 134-135) at the C-terminus of the heterodimerization domain defined by Kagoshima and coworkers.57 Amino acids 134 to 135 reside in the middle of an α helix spanning amino acids 129 to 138.60,61Truncation of this α helix in the CBFβ(p18) isoform appears to destabilize the heterodimerization domain to some extent, as evidenced by the instability of ternary Runx1-CBFβ(p18)-DNA complexes in electrophoretic mobility shift assays.11,57 However, CBFβ(p18) contains all the amino acids that comprise the interaction surface for the CBFα Runt domain,60 and when present at sufficiently high concentrations can provide CBFβ function in vivo. Thus, it appears that the hematopoietic block resulting from the mutation we and others introduced into the Cbfbgene11 12 was primarily due to insufficient levels of the CBFβ(p18) protein in Cbfb−/− ES cells and embryos.
We show that the emergence or differentiation of definitive hematopoietic progenitors from ES cells heterozygous for a “knocked-in” CbfbCbfb-MYH11 allele is only modestly impaired in vitro. This was somewhat unexpected given the dramatic phenotype observed in mice heterozygous for theCbfbCbfb-MYH11 allele.CbfbCbfb-MYH11/+ mice die at midgestation with central nervous system (CNS) hemorrhaging and a severe block in fetal liver hematopoiesis.41 However, more recent results indicate that hematopoietic stem cells (HSCs) hemizygous for theCbfbCbfb-MYH11 allele do persist in adult chimeric mice generated withCbfbCbfb-MYH11/+ ES cells.62CbfbCbfb-MYH11/+ HSCs may have emerged in chimeric mice because of the incomplete repression of normal Runx1-CBFβ function by the CbfbCbfb-MYH11allele. The CbfbCbfb-MYH11/+ HSCs were found to give rise to mature erythrocytes efficiently but were unable to differentiate along myeloid and lymphoid lineages.62Transgenic mice in which expression of CBFβ-SMMHC was specifically targeted to myeloid cells showed impairment but not a complete block of neutrophil differentiation.42 The in vivo findings are consistent with ES cell differentiation results reported here and suggest that CBFβ-SMMHC preferentially impairs the differentiation of myeloid lineage cells.
An incomplete block in definitive hematopoiesis was also seen in the t(8;21) knock-in mouse models, in which cDNAs encoding the AML1-ETO fusion protein were introduced into the Runx1gene.59,63 For example, Yergeau and associates demonstrated that although no definitive hematopoietic colonies of any kind could be cultured from yolk sacs derived from 10.5 days post coitus (dpc) Runx1-deficient embryos, macrophage-like colonies differentiated from the yolk sacs of embryos heterozygous for the t(8;21) knock-in allele.63 More recently and dramatically, Okuda and coworkers reported that in their independently derived t(8;21) knock-in mouse strain, dysplastic progenitors for mixed lineage colonies could be found in the fetal livers of day 11.5 to 13.5 embryos, and that these progenitors had an abnormally high self-renewal capacity.59 We recently found that the hematopoietic block in Runx1−/− mice occurs during the emergence of definitive hematopoietic progenitors in the embryo64(North and colleagues, unpublished results, 2000). The in vivo data from the t(8;21) knock-in mice generated by Okuda and coworkers suggest that some definitive hematopoietic progenitors escape the early block associated with loss of Runx1 function and emerge, but are unable to complete differentiation into mature myeloid and erythroid cells.59 The impaired differentiation of myeloid lineage cells may be an important step in leukemogenesis associated with the t(8;21) and inv(16). Incomplete repression of wild-type Runx1-CBFβ function by the transdominant negative AML1-ETOand CBFB-MYH11 alleles may allow for the expansion and persistence of a population of myeloid progenitors that then become targets for the secondary mutations required for leukemic transformation.
Continued culture of the colonies derived fromCbfbCbfb-MYH11/+ ES cells resulted in the outgrowth of 2 cell lines, with different morphologic features and cell surface phenotypes. We do not understand the significance of the differences between the 2 cell lines. It is unclear whether the outgrowth from secondary ES cell cultures is directly caused by the presence of the CBFβ-SMMHC protein, and additional experiments are needed to more completely assess the effects of CBFβ-SMMHC on proliferative potential. However, the kinetics of cell outgrowth suggest that the CBFβ-SMMHC protein by itself was insufficient to immortalize primary hematopoietic cells. This is consistent with the demonstration that CbfbCbfb-MYH11/+ chimeric mice developed leukemias only at a low frequency and after a long latent period.62
It is interesting to note that although biallelic loss of function mutations in the RUNX1 gene have been found in leukemias,65 the frequency of chromosomal rearrangements of RUNX1 and CBFB in leukemias is greater. Furthermore, biallelic mutations were observed in M0 leukemias, which are minimally differentiated acute myeloid leukemias, whereas the (8;21) and inv(16) are found in the somewhat more differentiated M2 and M4 acute myeloid leukemia subtypes. The difference in frequency and diagnostic subtypes suggests that the translocations and loss of function mutations involvingRUNX1 and CBFB are not equivalent in leukemogenesis. The inability of CBFβ-SMMHC and AML1-ETO to completely inhibit Runx1:CBFβ activity may be an essential feature of their distinct leukemogenic properties.
We were also able to document an intrinsic inability of CBFβ-SMMHC to provide CBFβ function in vivo, based on its failure to restore definitive hematopoiesis by Cbfb−/− ES cells. This observation could form the basis of an assay for evaluating the biochemical properties of the SMMHC sequences that inhibit its function in vivo, and for testing candidate drugs targeted against the CBFβ-SMMHC protein.
We gratefully acknowledge Stuart Orkin and members of his laboratory, especially Catherine Porcher, for their technical advice. We thank Norman Levy and Letha Mills for histopathology consultations, Robert Hawley for providing the MSCV vector, Neeraj Adja for the CBFβ-SMMHC constructs, and Ken Orndorff for help with taking pictures. Flow cytometry was done at Dartmouth Medical School in The Herbert C. Englert Cell Analysis Laboratory, established by a grant from the Fannie E. Rippel Foundation and supported in part by the Core Grant of the Norris Cotton Cancer Center (CA 23108).
N.A.S. is supported by Public Health Service grants R01 CA58343 and CA75611. J.D.M. was supported by T32 AI 07363 from the National Institutes of Health/Agency for International Development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nancy A. Speck, Department of Biochemistry, Dartmouth Medical School, Hanover, NH 03755; e-mail:nancy.speck@dartmouth.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal