Abstract
The effects of activation of adult murine stem cells on their expression of CD38 were studied using a murine transplantation model. First, the published finding that the majority of long-term engrafting cells from normal adult steady-state marrow are CD38+ was confirmed. Next, it was determined that the majority of stem cells activated in vivo by injection of 5-fluorouracil (5-FU) or mobilized by granulocyte colony-stimulating factor are CD38−. Stem cells that were activated in culture with interleukin-11 and steel factor were also CD38−. Previous studies have shown that expression of CD34 by adult stem cells is also modulated by in vivo or in vitro activation. To determine whether there is reciprocal expression of CD38 and CD34, 4 populations of post–5-FU marrow cells were analyzed. The majority of the stem cells were in the CD38−CD34+ fraction. However, secondary transplantation experiments indicated that when the bone marrow reaches steady state, the majority of the stem cells become CD38+CD34−. In addition, the minority populations of CD34+ stem cells that occur in steady-state bone marrow are CD38−. This reversible and reciprocal expression of CD38 and CD34 by murine stem cells may have implications for the phenotypes of human stem cells.
Introduction
Identification of the surface phenotypes of hematopoietic stem cells and development of strategies for their purification have significant basic and clinical implications in hematology. Transplantation of purified human hematopoietic stem cells that are free of T lymphocytes could minimize graft-versus-host disease in allogeneic transplantation. Transplantation of cells that are free of tumor cells may reduce the risk of recurrence in autologous transplantation. For many years, it was generally accepted that human hematopoietic stem cells and primitive progenitors are CD34+CD38−/low.1 However, recent studies in 2 laboratories clearly demonstrated that the stem cells of fetal and adult mice are CD38+.2,3 There also has been controversy about CD34 expression by murine and human stem cells. Despite the long-held belief that human stem cells are CD34+,1 recent studies of a murine model clearly demonstrated that the majority of stem cells from normal adult mice are CD34−.4,5 In our laboratory, we observed that the majority of murine stem cells that are activated in vivo by 5-fluorouracil (5-FU) injection or in culture by a combination of early-acting cytokines are CD34+, whereas the stem cells in the steady-state marrow of normal mice are CD34−.6 We therefore entertained the hypothesis that stem cell expression of CD38 is also influenced by the activation state of the stem cells and tested 2 monoclonal anti-CD38 antibodies in the standard murine transplantation model. The results clearly demonstrated reversible changes in stem cell phenotype from CD38+ to CD38− induced by activation of the stem cells.
Materials and methods
Monoclonal antibodies and hybridomas
We used 2 monoclonal anti-CD38 antibodies. Fluorescein isothiocyanate (FITC)–conjugated NIM-R5 (anti-CD38; rat IgG2a) was purchased from Southern Biotechnology Associates (Birmingham, AL). FITC- or biotin-conjugated 90 (anti-CD38; rat IgG2a) was purchased from Pharmingen (San Diego, CA). The 2 antibodies revealed very similar staining patterns. The choice of anti-CD38 antibody is described in the individual figure legends. FITC-conjugated RAM34 (anti-CD34; rat IgG2a), phycoerythrin (PE)–conjugated D7 (anti–Ly-6A/E [anti–Sca-1]; rat IgG2a), allophycocyanin (APC)–conjugated 2B8 (anti–c-kit; rat IgG2b), biotin-conjugated RA3-6B2 (anti-CD45R/B220; rat IgG2a), biotin-conjugated RB6-8C5 (anti–Gr-1; rat IgG2b), biotin-conjugated M1/70 (anti–Mac-1; rat IgG2b), and biotin-conjugated 30-H12 (anti–Thy-1.2; rat IgG2b) were purchased from Pharmingen. Some antibodies were also either received as nonlabeled antibodies or prepared from hybridomas. Purified antibodies were conjugated to FITC or biotin in our laboratory. Hybridoma RB6-8C5 (anti–Gr-1; rat IgG2b) was provided by R. L. Coffman (DNAX, Palo Alto, CA). Monoclonal antibody TER-119 (antierythrocytes; rat IgG2b) was a gift from T. Kina (Kyoto University, Kyoto, Japan). Hybridomas 14.8 (anti-B220; rat IgG2b), M1/70.15.11.5 (anti–Mac-1; rat IgG2b), GK1.5 (anti-CD4; rat IgG2b), and 53-6.72 (anti-CD8; rat IgG2a) were purchased from American Type Culture Collection (Rockville, MD). Hybridoma A20 (anti–Ly-5.1; IgG1) was provided by H. Fleming (Emory University, Atlanta, GA).
Cell preparation
Ten- to 12-week-old male or female C57BL/6-Ly-5.1 mice (Jackson Laboratories, Bar Harbor, ME) were used as bone marrow and peripheral blood (PB) donors. Ten- to 14-week-old female C57BL/6-Ly-5.2 mice (Charles River Laboratories, Raleigh, NC) were used as irradiated recipients and as the source for radioprotective cells. In some experiments, the donor mice were treated with an intravenous injection of 5-FU at 150 mg/kg body weight 48 hours before sacrifice. Bone marrow cells were flushed from femurs and tibiae, pooled, and washed twice with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA). A single-cell suspension was prepared by repeated pipetting and filtering through 40-μm nylon mesh. In transplantation experiments of PB cells, recombinant human granulocyte colony-stimulating factor (G-CSF) (Filgrastim; Amgen, Thousand Oaks, CA) was used to mobilize stem cells. Mice were injected at 12-hour intervals subcutaneously with 125 μg/kg G-CSF in 0.1 mL PBS for 5 consecutive days. Three hours after the last injection, PB was collected by cardiac puncture under methoxyflurane anesthesia and pooled. Both bone marrow and PB cells with densities ranging from 1.063 to 1.077 g/mL were collected by gradient separation using Nycodenz (Accurate Chemical and Scientific, Westbury, NY). Lineage (Lin)− marrow cells of normal mice were prepared by incubating cells with anti–Mac-1, anti–Gr-1, anti-B220, anti-CD4, anti-CD8, and anti–TER-119 antibodies and immunomagnetic beads (Dynabeads M-450 coupled to sheep antirat IgG; Dynal, Great Neck, NY). For preparation of Lin− cells of 5-FU–treated mice and PB of G-CSF–treated mice, anti–Mac-1 was omitted from the cocktail because of possible Mac-1 expression by a population of stem cells.7 The resulting Lin− cells were stained with FITC-conjugated anti-CD38 (NIM-R5), PE-conjugated anti–Sca-1, and APC-conjugated anti–c-kit. In some experiments, we separated the light-density mononuclear bone marrow cells of normal mice based on CD38 and CD34 expression without prior removal of Lin+cells. The mononuclear cells were stained with FITC-conjugated anti-CD38 (NIM-R5) and biotin-conjugated anti-CD34 (RAM34), followed by streptavidin-PE. In secondary transplantation experiments, bone marrow cells harvested from the primary recipients were stained with FITC-conjugated anti-CD38 (NIM-R5), PE-conjugated anti–Sca-1, APC-conjugated anti–c-kit, and biotin-conjugated anti–Ly-5.1, followed by staining with streptavidin-conjugated PharRed (Pharmingen). After addition of propidium iodide (PI) at a concentration of 1 μg/mL, the cells were washed twice, resuspended in PBS containing 0.1% BSA, and kept on ice until cell sorting. In the experiments testing simultaneous expression of CD38 and CD34, Lin−cells were stained with PE-conjugated anti–Sca-1, APC-conjugated anti–c-kit, biotin-conjugated anti-CD38, and FITC-conjugated anti-CD34. The cells were then washed twice and stained with streptavidin-Red 613 (Life Technologies, Gaithersburg, MD). PI staining was omitted because its emission wavelength is similar to that of Red 613. Cell sorting was performed on a FACS Vantage (Becton Dickinson, San Jose, CA) with appropriate isotype-matched controls. The Lin− bone marrow or PB cells were first enriched for the c-kit+Sca-1+ population and then sorted again based on expression of CD38 or a combination of CD38 and CD34. Purity of the sorted cells, determined by reanalysis of the sorted cells, was higher than 96% throughout the experiments.
Suspension culture
Five thousand Lin−c-kit+Sca-1+CD38+cells were incubated in a 25-cm2 culture flask (Corning, Corning, NY). The culture medium contained α-modification of Eagle's Minimal Essential Medium (ICN Biomedicals, Aurora, OH), 20% fetal calf serum, 1% deionized fraction-V BSA, 1 × 10−4 M 2-mercaptoethanol, recombinant rat steel factor (SF; c-kit ligand), and interleukin (IL)-11. Recombinant SF (Amgen) was used at a concentration of 100 ng/mL. Human IL-11 was a gift from P. Schendel (Genetics Institute, Cambridge, MA) and was used at a concentration of 100 ng/mL. After a week of incubation, the cells were washed twice, stained with FITC-conjugated anti-CD38, and sorted for CD38− and CD38+ fractions.
Hematopoietic reconstitution
Ly-5.2 mice were administered a single 850-cGy dose of total-body irradiation using a 4 × 106 V linear accelerator. The test Ly-5.1 cells were injected into the tail vein of irradiated Ly-5.2 mice along with 2 × 105 autologous bone marrow cells. For analysis of engraftment, PB was obtained from the retro-orbital plexus using heparin-coated micropipettes (Drummond Scientific, Broomall, PA) 6 months after transplantation. Red cells were lysed by 0.15 M NH4Cl. The samples were stained with FITC-conjugated anti–Ly-5.1 and analyzed for donor-derived cells on a FACS Calibur (Becton Dickinson). Donor cells in T-cell, B-cell, granulocyte, and monocyte/macrophage lineages at 6 months after transplantation were analyzed by staining with biotin-conjugated anti–Thy-1.2, biotin-conjugated anti-B220, biotin-conjugated anti–Gr-1, and biotin-conjugated anti–Mac-1, followed by staining with streptavidin-conjugated PE.
Secondary transplantation
Mice that had transplantation with Lin−c-kit+Sca-1+CD38+cells of 5-FU–treated mice were killed 6 months after transplantation. Ly-5.1 Lin−c-kit+Sca-1+CD38−cells and Ly-5.1 Lin−c-kit+Sca-1+CD38+cells were prepared from the bone marrows of these mice and injected into secondary Ly-5.2 recipients along with 2 × 105Ly-5.2 bone marrow cells. PB was analyzed for Ly-5.1 cells 6 months after the secondary transplantation.
Data presentation and statistical analyses
In all experiments, the number of cells transplanted reflects the ratios of cells in the original population determined by flow cytometry. Levels of significance were determined using the Studentt test.
Results
Transplantation with Lin−c-kit+Sca-1+CD38−cells or Lin−c-kit+Sca-1+CD38+cells of normal mice
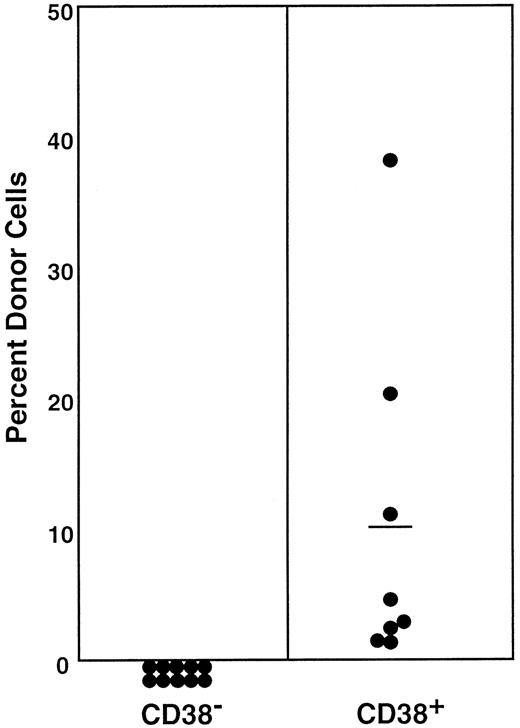
Lin−c-kit+Sca-1+ cells were separated into CD38− and CD38+ cell populations using the fluorescence-activated cell sorting (FACS) regions shown in Figure 1. Because the ratio of the CD38− (R2) cells to CD38+ (R3) cells was 2:1, we transplanted 200 CD38− cells or 100 CD38+ cells per mouse. The levels of engraftment were determined by measuring the percentage of donor (Ly-5.1) PB nucleated cells 6 months after transplantation. The results are presented in Figure2. Only the mice having transplantation with CD38+ cells revealed engraftment at 6 months after transplantation. The evidence for multilineage engraftment in individual mice is presented in Table 1. These results are in agreement with the published observation2 that stem cells in normal adult mice are CD38+.
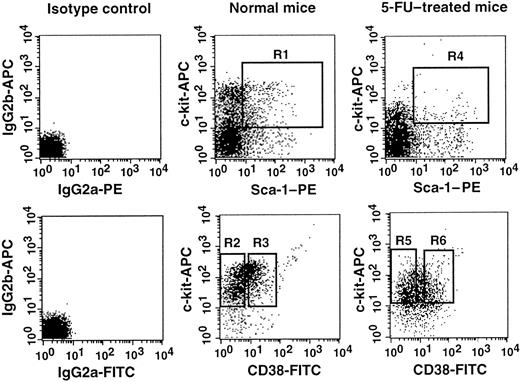
Sorting regions used for preparation of Lin−c-kit+Sca-1+CD38−and Lin−c-kit+Sca-1+CD38+cell populations.
The Lin− cells from normal mice were enriched for c-kit+Sca-1+ cells using the R1 window and sorted on the basis of CD38 expression using R2 and R3 sorting gates. The cells from 5-FU–treated mice were prepared using R4, R5, and R6 gates. The experiments were performed with NIM-R5 anti-CD38 antibody (Southern Biotechnology Associates).
Sorting regions used for preparation of Lin−c-kit+Sca-1+CD38−and Lin−c-kit+Sca-1+CD38+cell populations.
The Lin− cells from normal mice were enriched for c-kit+Sca-1+ cells using the R1 window and sorted on the basis of CD38 expression using R2 and R3 sorting gates. The cells from 5-FU–treated mice were prepared using R4, R5, and R6 gates. The experiments were performed with NIM-R5 anti-CD38 antibody (Southern Biotechnology Associates).
Percentage of donor nucleated cells in the blood of individual mice having transplantation with 200 Lin−c-kit+Sca-1+CD38−or 100 Lin−c-kit+Sca-1+CD38+marrow cells of normal mice 6 months before.
The numbers of transplanted CD38− and CD38+cells reflect the 2:1 ratio of the cells in R2 and R3 windows of Figure1. Results shown are representative of 3 experiments.
Percentage of donor nucleated cells in the blood of individual mice having transplantation with 200 Lin−c-kit+Sca-1+CD38−or 100 Lin−c-kit+Sca-1+CD38+marrow cells of normal mice 6 months before.
The numbers of transplanted CD38− and CD38+cells reflect the 2:1 ratio of the cells in R2 and R3 windows of Figure1. Results shown are representative of 3 experiments.
Multilineage reconstitution analyzed at 6 months after transplantation
| CD38+ cells from normal mice . | CD38− cells from 5-FU–treated mice . | ||||
|---|---|---|---|---|---|
| G+M . | T . | B . | G+M . | T . | B . |
| 1 | 2 | 3 | 4 | 3 | 2 |
| 5 | 4 | 4 | 55 | 61 | 55 |
| 6 | 16 | 27 | 76 | 82 | 88 |
| 0 | 0 | 1 | 38 | 30 | 22 |
| 11 | 0 | 3 | 10 | 9 | 95 |
| 78 | 32 | 32 | 6 | 10 | 5 |
| 42 | 3 | 9 | 17 | 20 | 89 |
| 1 | 1 | 2 | 21 | 35 | 39 |
| 19 | 42 | 39 | |||
| CD38+ cells from normal mice . | CD38− cells from 5-FU–treated mice . | ||||
|---|---|---|---|---|---|
| G+M . | T . | B . | G+M . | T . | B . |
| 1 | 2 | 3 | 4 | 3 | 2 |
| 5 | 4 | 4 | 55 | 61 | 55 |
| 6 | 16 | 27 | 76 | 82 | 88 |
| 0 | 0 | 1 | 38 | 30 | 22 |
| 11 | 0 | 3 | 10 | 9 | 95 |
| 78 | 32 | 32 | 6 | 10 | 5 |
| 42 | 3 | 9 | 17 | 20 | 89 |
| 1 | 1 | 2 | 21 | 35 | 39 |
| 19 | 42 | 39 | |||
Peripheral blood nucleated cells were stained with anti–Ly-5.1 and either anti–Gr-1 and anti–Mac-1, anti–Thy-1.2, or anti-B. Numbers indicate the percentage of cells within each lineage that expressed Ly-5.1.
G+M indicates granulocytes and/or macrophages; T, T lymphocytes; B, B lymphocytes.
Transplantation with Lin−c-kit+Sca-1+CD38−cells or Lin−c-kit+Sca-1+CD38+cells of 5-FU–treated mice
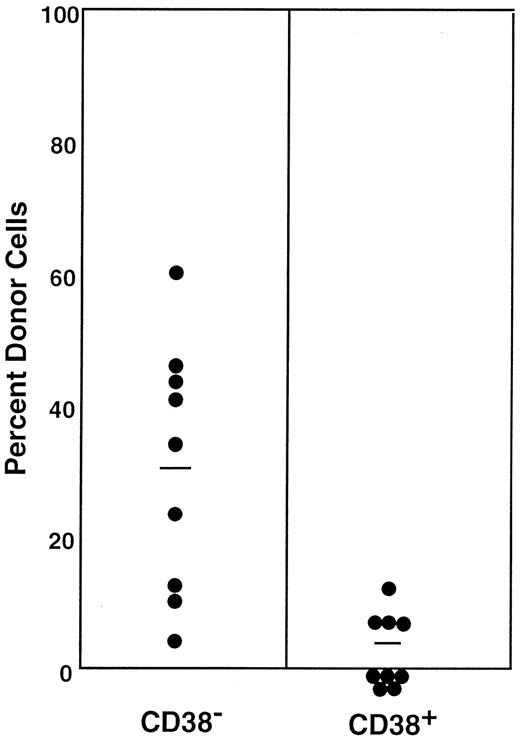
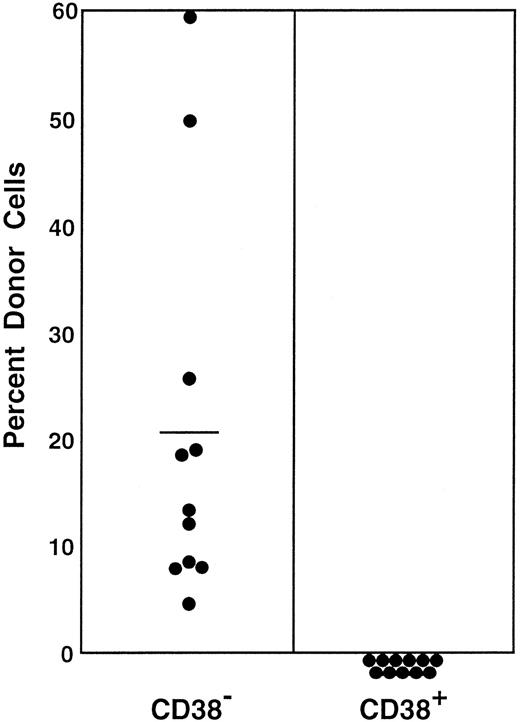
A number of stem cell surface markers, such as CD34, c-kit, and Mac-1,6 7 are modulated by activation. We tested the effects of stem cell activation on the expression of CD38. We used the bone marrow cells of 5-FU–treated mice as the source of activated stem cells. The sorting regions are presented in Figure 1. Because the ratio of the CD38− (R5) to CD38+ (R6) cell populations in the bone marrow cells of 5-FU–treated mice was approximately 2:1, we transplanted 400 CD38− or 200 CD38+ cells per mouse. In contrast to the cells from normal mice, all mice having transplantation with CD38− cells showed multilineage engraftment at 6 months after transplantation (Figure 3). Only 4 of the 9 mice having transplantation with CD38+ cells revealed low-level engraftment. The evidence for multilineage engraftment by the CD38− post–5-FU cell population is presented in Table 1.
Percentage of donor nucleated cells in the blood of individual mice having transplantation with 400 Lin−c-kit+Sca-1+CD38−or 200 Lin−c-kit+Sca-1+CD38+marrow cells of 5-FU–treated mice 6 months before.
The numbers of transplanted CD38− and CD38+cells reflect the 2:1 ratio of the cells in R5 and R6 windows of Figure1. The difference between the groups is significant atP < .001. Results shown are representative of 2 experiments.
Percentage of donor nucleated cells in the blood of individual mice having transplantation with 400 Lin−c-kit+Sca-1+CD38−or 200 Lin−c-kit+Sca-1+CD38+marrow cells of 5-FU–treated mice 6 months before.
The numbers of transplanted CD38− and CD38+cells reflect the 2:1 ratio of the cells in R5 and R6 windows of Figure1. The difference between the groups is significant atP < .001. Results shown are representative of 2 experiments.
Conversion of CD38+ stem cells to CD38−stem cells in culture
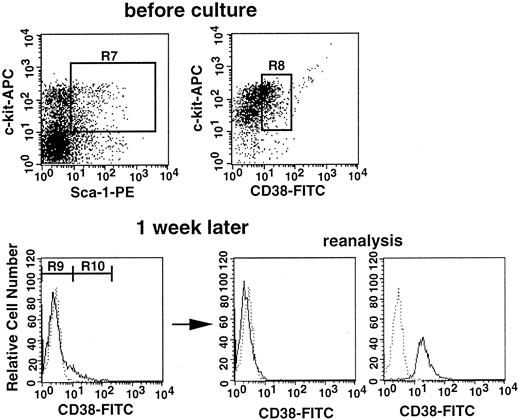
The results presented in Figures 2 and 3 suggest that stem cell CD38 expression is influenced by the activation state of the stem cells. We used cell culture to further test this premise. In the next experiment, Lin−c-kit+Sca-1+CD38+cells of normal mice were incubated in suspension culture for 1 week in the presence of SF and IL-11. The cells expanded from 5000 to 1.5 × 106 cells, and 95% of the cultured cells became CD38−, as shown in Figure4. The cultured cells were then separated on the basis of CD38 expression. Analysis of the sorted CD38− and CD38+ cells revealed greater than 99% purity (Figure 4). Because the ratio of CD38− (R9) to CD38+ (R10) cells was approximately 20:1 after incubation, we transplanted in each recipient either 60 000 CD38−cells or 3000 CD38+ cells. The levels of engraftment at 6 months after transplantation are shown in Figure5. None of the 11 mice having transplantation with CD38+ cultured cells revealed engraftment. In contrast, all mice having transplantation with CD38− cultured cells revealed long-term engraftment. These results clearly demonstrate that CD38+ stem cells that are stimulated in culture undergo phenotypic changes and become CD38−. All mice showed evidence of multilineage engraftment at 6 months after transplantation (data not shown).
Sorting regions and flow cytometric analyses of CD38 expression before and after culture.
Top: Sorting regions used for preparing the Lin−c-kit+Sca-1+CD38+population from bone marrow cells of normal mice. The Lin−cells were enriched for c-kit+Sca-1+ cells using R7 windows and again sorted on the basis of CD38 expression using the R8 window. The cells were incubated with SF and IL-11 for 1 week. Bottom: Sorting regions used for the cultured cells and analyses of purity of the sorted cells. Dotted and solid lines represent cells stained with IgG2a-FITC and CD38-FITC, respectively. The anti-CD38 antibody used in this experiment was purchased from Southern Biotechnology Associates.
Sorting regions and flow cytometric analyses of CD38 expression before and after culture.
Top: Sorting regions used for preparing the Lin−c-kit+Sca-1+CD38+population from bone marrow cells of normal mice. The Lin−cells were enriched for c-kit+Sca-1+ cells using R7 windows and again sorted on the basis of CD38 expression using the R8 window. The cells were incubated with SF and IL-11 for 1 week. Bottom: Sorting regions used for the cultured cells and analyses of purity of the sorted cells. Dotted and solid lines represent cells stained with IgG2a-FITC and CD38-FITC, respectively. The anti-CD38 antibody used in this experiment was purchased from Southern Biotechnology Associates.
Percentage of donor nucleated cells in the blood of individual mice having transplantation with cultured cells 6 months before.
Because the ratio of CD38− (R9) to CD38+ (R10) cells in Figure 4 was approximately 20:1, 60 000 CD38− or 3000 CD38+ cultured cells were injected into individual recipient mice. Results shown are representative of 3 experiments.
Percentage of donor nucleated cells in the blood of individual mice having transplantation with cultured cells 6 months before.
Because the ratio of CD38− (R9) to CD38+ (R10) cells in Figure 4 was approximately 20:1, 60 000 CD38− or 3000 CD38+ cultured cells were injected into individual recipient mice. Results shown are representative of 3 experiments.
Transplantation with Lin−c-kit+Sca-1+ mobilized PB cells that were further separated on the basis of CD38 expression
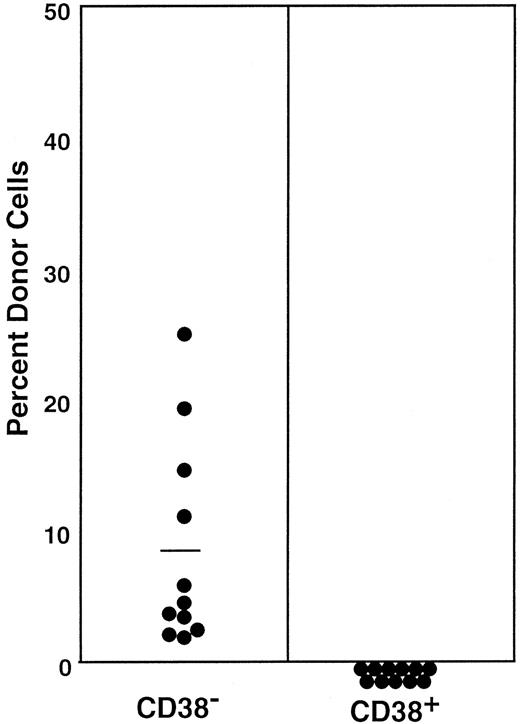
In a separate report,8 we documented that the majority of stem cells that are mobilized by G-CSF administration are activated and express CD34. In the next experiment, we tested the state of CD38 expression by G-CSF–mobilized stem cells. We separated Lin− c-kit+Sca-1+ cells into CD38− and CD38+ cell populations using the electronic sorting regions (R12 and R13) shown in Figure6. Because the ratio of CD38− (R12) cells to CD38+ (R13) cells was 9:5, we transplanted 3600 CD38− cells or 2000 CD38+ cells per mouse. The engraftment levels of individual mice at 6 months after transplantation are presented in Figure7. Only the mice having transplantation with CD38− cells revealed engraftment.
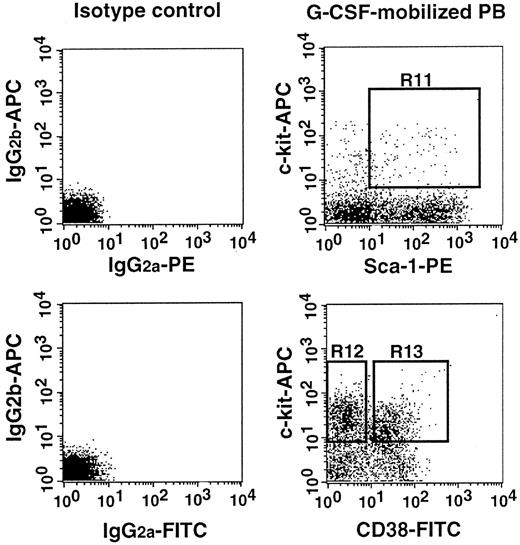
Sorting regions used for preparing Lin−c-kit+Sca-1+ PB cells of G-CSF–treated mice that were further separated on the basis of CD38.
First, Lin+ cells were removed from mononuclear PB cells with immunomagnetic beads. The Lin− cells were enriched for c-kit+Sca-1+ cells using R11 window and again sorted on the basis of CD38− (R12) and CD38+ (R13) windows. The experiments were performed with NIM-R5 anti-CD38 antibody.
Sorting regions used for preparing Lin−c-kit+Sca-1+ PB cells of G-CSF–treated mice that were further separated on the basis of CD38.
First, Lin+ cells were removed from mononuclear PB cells with immunomagnetic beads. The Lin− cells were enriched for c-kit+Sca-1+ cells using R11 window and again sorted on the basis of CD38− (R12) and CD38+ (R13) windows. The experiments were performed with NIM-R5 anti-CD38 antibody.
Percentage of donor nucleated cells in the blood of individual mice having transplantation with G-CSF–mobilized PB stem cells 6 months before.
Mice had transplantation with 3600 Lin−c-kit+Sca-1+CD38−or 2000 Lin−c-kit+Sca-1+CD38+G-CSF–mobilized PB cells, reflecting the 9:5 ratio of the cells in CD38− (R12) and CD38+ (R13) windows of Figure6. Results shown are representative of 2 experiments.
Percentage of donor nucleated cells in the blood of individual mice having transplantation with G-CSF–mobilized PB stem cells 6 months before.
Mice had transplantation with 3600 Lin−c-kit+Sca-1+CD38−or 2000 Lin−c-kit+Sca-1+CD38+G-CSF–mobilized PB cells, reflecting the 9:5 ratio of the cells in CD38− (R12) and CD38+ (R13) windows of Figure6. Results shown are representative of 2 experiments.
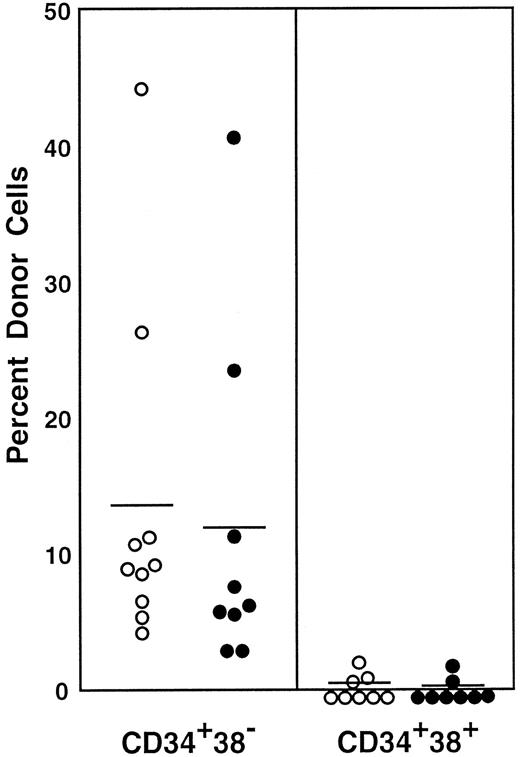
Transplantation with Lin−c-kit+Sca-1+ post–5-FU cells that were further separated on the basis of CD38 and CD34 expression
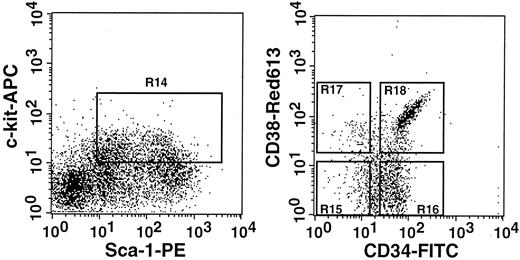
Earlier, we showed that CD34− stem cells become CD34+ upon activation.6 In the current study, we have shown conversion of CD38+ stem cells to CD38− stem cells by activation. To document the apparent reciprocal changes in CD38 and CD34 expression, we prepared 4 populations of post–5-FU marrow cells that differed in CD38 and CD34 expression and tested their long-term engrafting capabilities. The sorting regions are presented in Figure8. All cells in the 4 regions (R15-R18) were injected into 7 mice per group. The estimated cell numbers transplanted per mouse were 60, 200, 30, and 180 cells for the CD38−CD34−, CD38−CD34+, CD38+CD34−, and CD38+CD34+ cell populations. The results are presented in Figure 9. In complete agreement with our previous studies of CD34 expression6 and the results in the preceding sections of this paper, the majority of stem cells in the 5-FU–treated mice were CD38−CD34+. A minor population of stem cells was present in the CD38+CD34− fraction. There were no stem cells of CD38+CD34+ or CD38−CD34− phenotype.
Sorting regions used for preparing Lin−c-kit+Sca-1+ cells of 5-FU–treated mice that were further separated on the basis of CD38 and CD34.
The Lin− cells were enriched for c-kit+Sca-1+ cells using R14 window and again sorted on the basis of CD38 and CD34 expression using R15 to R18 windows. We used anti-CD38 (90) antibody from Pharmingen in this experiment.
Sorting regions used for preparing Lin−c-kit+Sca-1+ cells of 5-FU–treated mice that were further separated on the basis of CD38 and CD34.
The Lin− cells were enriched for c-kit+Sca-1+ cells using R14 window and again sorted on the basis of CD38 and CD34 expression using R15 to R18 windows. We used anti-CD38 (90) antibody from Pharmingen in this experiment.
Engraftment levels of mice having transplantation with cells positive or negative for CD34 and CD38.
The mice had transplantation with Lin−c-kit+Sca-1+ marrow cells of 5-FU–treated mice that were separated on the basis of CD38 and CD34 expression. All cells in the 4 sorting windows (R15-R18; Figure 8) were injected into 7 mice per group. It is estimated that 60 CD38−CD34−, 200 CD38−CD34+, 30 CD38+CD34−, or 180 CD38+CD34+ cells were injected per mouse. Results shown are representative of 2 experiments.
Engraftment levels of mice having transplantation with cells positive or negative for CD34 and CD38.
The mice had transplantation with Lin−c-kit+Sca-1+ marrow cells of 5-FU–treated mice that were separated on the basis of CD38 and CD34 expression. All cells in the 4 sorting windows (R15-R18; Figure 8) were injected into 7 mice per group. It is estimated that 60 CD38−CD34−, 200 CD38−CD34+, 30 CD38+CD34−, or 180 CD38+CD34+ cells were injected per mouse. Results shown are representative of 2 experiments.
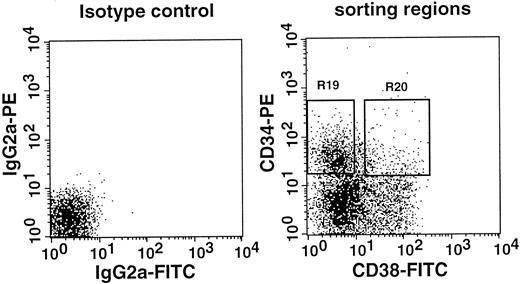
Examination of CD38 expression by CD34+ stem cells of normal mice
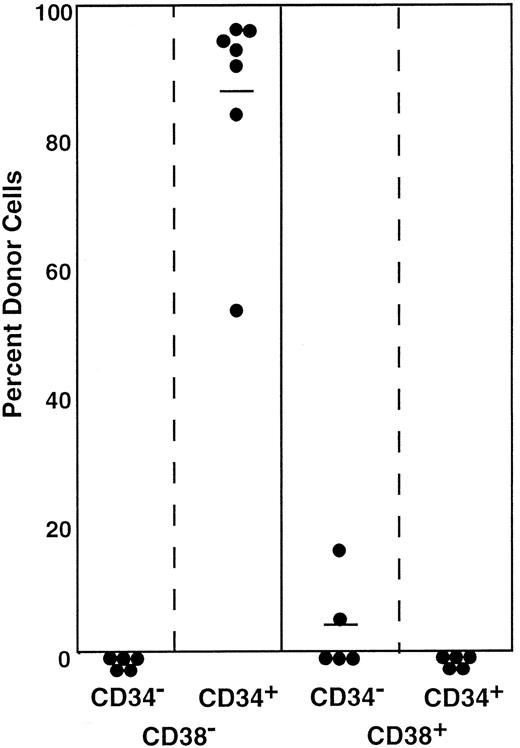
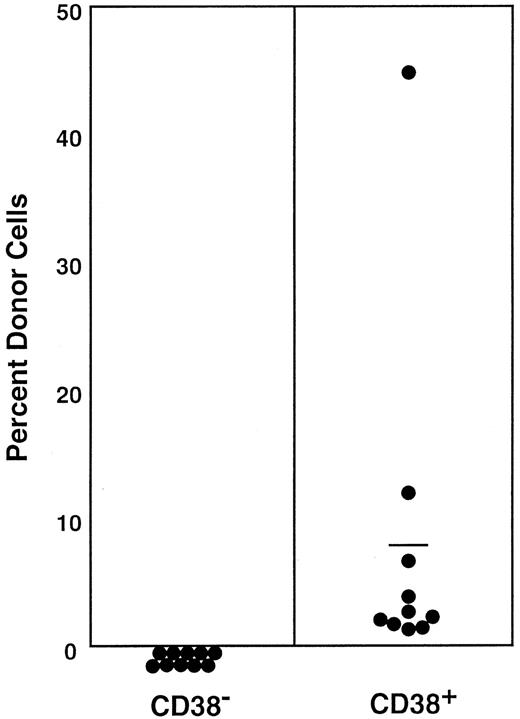
All stem cells in the Lin−Sca-1+c-kit+ marrow cells of normal adult mouse are CD34−4,6 and CD38+, as shown in this paper. In a separate report,9 we documented that approximately 15% to 20% of the stem cells in the bone marrow mononuclear cells of normal adult mice are CD34+. In the next experiment, we tested CD38 expression by the CD34+stem cells of normal adult mice. We sorted the marrow mononuclear cells into CD34+CD38− and CD34+CD38+ populations and tested their long-term engrafting capabilities. The sorting gates are shown in Figure 10. Because the ratio of CD34+CD38− cells (R19) to CD34+CD38+ cells (R20) was 27:5, we transplanted in each mouse 1.0 × 105CD34+CD38− cells or 1.8 × 104CD34+CD38+ cells. The results presented in Figure 11 and Table 2 clearly demonstrate that the minority population of CD34+ stem cells in the normal mice are CD38−. These findings suggest reciprocal expression of CD38 and CD34 by stem cells in the steady-state marrow.
Sorting regions used for preparation of CD34+CD38− and CD34+CD38+ mononuclear marrow cells of normal adult mice.
Mononuclear cells were sorted, without other enrichment methods, for CD34+CD38− and CD34+CD38+ populations using R19 and R20 electronic windows, respectively. The anti-CD38 antibody was purchased from Pharmingen.
Sorting regions used for preparation of CD34+CD38− and CD34+CD38+ mononuclear marrow cells of normal adult mice.
Mononuclear cells were sorted, without other enrichment methods, for CD34+CD38− and CD34+CD38+ populations using R19 and R20 electronic windows, respectively. The anti-CD38 antibody was purchased from Pharmingen.
Percentage of donor nucleated cells in the blood of individual recipient mice 4 and 6 months after transplantation.
Mice had transplantation with CD34+ mononuclear marrow cells of normal adult mice that were further separated on the basis of CD38 expression. Because the ratio of CD34+CD38− (R19) cells to CD34+CD38+ (R20) cells was 27:5 according to Figure 10, 1.0 × 105CD34+CD38− cells or 1.8× 104CD34+CD38+ cells were injected into individual mice. Results are shown at 4 months (○) and 6 months (●). The difference between the groups (6-month engraftment) is significant at P < .05. Results shown are representative of 2 experiments.
Percentage of donor nucleated cells in the blood of individual recipient mice 4 and 6 months after transplantation.
Mice had transplantation with CD34+ mononuclear marrow cells of normal adult mice that were further separated on the basis of CD38 expression. Because the ratio of CD34+CD38− (R19) cells to CD34+CD38+ (R20) cells was 27:5 according to Figure 10, 1.0 × 105CD34+CD38− cells or 1.8× 104CD34+CD38+ cells were injected into individual mice. Results are shown at 4 months (○) and 6 months (●). The difference between the groups (6-month engraftment) is significant at P < .05. Results shown are representative of 2 experiments.
Multilineage reconstitution by CD34+CD38− cells of normal adult mice analyzed at 6 months after transplantation
| Lineage . | Individual mice . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | |
| G+M | 1 | 63 | 4 | 3 | 3 | 5 | 1 | 5 | 2 |
| T | 4 | 41 | 7 | 11 | 14 | 32 | 4 | 35 | 17 |
| B | 3 | 26 | 10 | 7 | 5 | 8 | 4 | 28 | 4 |
| Lineage . | Individual mice . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | |
| G+M | 1 | 63 | 4 | 3 | 3 | 5 | 1 | 5 | 2 |
| T | 4 | 41 | 7 | 11 | 14 | 32 | 4 | 35 | 17 |
| B | 3 | 26 | 10 | 7 | 5 | 8 | 4 | 28 | 4 |
Nucleated blood cells were stained with anti–Ly-5.1 and with anti–Gr-1 and anti–Mac-1, anti–Thy-1.2, or anti-B220. Numbers indicate the percentage of cells within each lineage that expressed Ly-5.1.
G+M indicates granulocytes and/or macrophages; T, T lymphocytes; B, B lymphocytes.
Reversion of CD38+ stem cells to CD38− stem cells
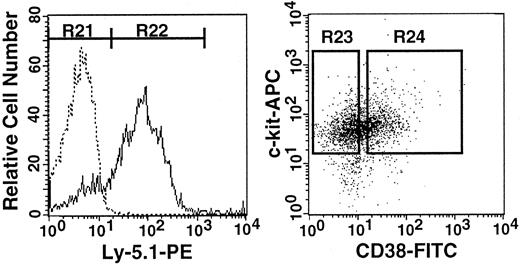
Previously, we have shown that stem cell CD34 expression is reversible.6 In the next experiment, we tested the reversibility of CD38 expression. We isolated the donor cells from the 7 Ly-5.2 primary recipients presented in Figure 9 that had had transplantation with Lin−c-kit+Sca-1+CD38−CD34+cells from Ly-5.1 5-FU–treated mice. Similar to the high levels of engraftment seen in the blood, 83.1% of the Lin− marrow cells of the primary recipients were of donor origin (Ly-5.1) (Figure12). The Ly-5.1 marrow cells were then separated on the basis of CD38 expression (Figure 12) and transplanted into secondary Ly-5.2 hosts for analysis of engraftment capabilities. Because the ratio of CD38− (R23) to CD38+(R24) cells was 1:1, individual mice received either 1000 Ly-5.1 CD38− cells or 1000 Ly-5.1 CD38+ cells. The levels of engraftment at 6 months after transplantation are presented in Figure 13. Only CD38+cells engrafted. Reflecting the nature of serial transplantation,10 the levels of engraftment were relatively low. However, this result clearly demonstrates that when the bone marrow of the primary recipients recovered from the radiation-induced hypoplasia and reached steady state, the stem cells again expressed CD38.
Flow cytometry and sorting regions used in serial transplantation experiment.
Left: Analysis of Ly-5.1 expression by Lin− marrow cells of Ly-5.2 primary recipients that had transplantation with Lin−c-kit+Sca-1+CD38−CD34+cells of Ly-5.1 5-FU–treated mice. Dotted and solid lines represent cells stained with isotype-matched Ig and Ly-5.1–specific antibody, respectively. The levels of engraftment measured with PB nucleated cells of the recipients are presented in Figure 9. Right: Sorting windows of Ly-5.1 Lin−c-kit+Sca-1+cells that were separated on the basis of CD38 expression. The ratio of the cells in R23 to R24 was 1:1. The anti-CD38 antibody used in this experiment was purchased from Southern Biotechnology Associates.
Flow cytometry and sorting regions used in serial transplantation experiment.
Left: Analysis of Ly-5.1 expression by Lin− marrow cells of Ly-5.2 primary recipients that had transplantation with Lin−c-kit+Sca-1+CD38−CD34+cells of Ly-5.1 5-FU–treated mice. Dotted and solid lines represent cells stained with isotype-matched Ig and Ly-5.1–specific antibody, respectively. The levels of engraftment measured with PB nucleated cells of the recipients are presented in Figure 9. Right: Sorting windows of Ly-5.1 Lin−c-kit+Sca-1+cells that were separated on the basis of CD38 expression. The ratio of the cells in R23 to R24 was 1:1. The anti-CD38 antibody used in this experiment was purchased from Southern Biotechnology Associates.
Percentage of donor nucleated cells in the blood of individual secondary recipients at 6 months after transplantation.
Reflecting the 1:1 ratio of CD38− and CD38+cells (Figure 12), 1000 Ly-5.1 CD38− or CD38+bone marrow cells of the primary recipients were transplanted into individual secondary Ly-5.2 recipients. Results shown are representative of 2 experiments.
Percentage of donor nucleated cells in the blood of individual secondary recipients at 6 months after transplantation.
Reflecting the 1:1 ratio of CD38− and CD38+cells (Figure 12), 1000 Ly-5.1 CD38− or CD38+bone marrow cells of the primary recipients were transplanted into individual secondary Ly-5.2 recipients. Results shown are representative of 2 experiments.
Discussion
CD38 is a transmembrane glycoprotein with a molecular weight of 42 to 46 kd. Initially, it was proposed as a T-lymphocyte differentiation marker11; however, subsequent studies revealed that it is expressed also by B lymphocytes, natural killer cells, and myeloid cells at various stages of development.12,13 The physiologic role of CD38 in hematopoiesis is unclear because CD38-null mice did not show defective engraftment capability.14However, Terstappen et al15 proposed that the most primitive human hematopoietic progenitors are CD34+CD38− based on sequential culture of colonies from single cells. Subsequently, investigators in several laboratories documented that the primitive human progenitors assayable in culture, such as long-term culture-initiating cells16and high proliferative potential–colony-forming cells,17are in the CD34+CD38− cell population. In agreement with these studies in culture, Civin et al18performed xenogeneic transplantation into fetal sheep and demonstrated that the CD34+CD38− populations of adult human marrow cells contain long-term engrafting cells, whereas CD34+CD38+ cells are limited in engraftment capabilities. These observations, however, were in apparent contradiction to the studies of murine stem cells by Randall et al,2 who reported that stem cells of adult mice are CD38+.
Studies presented in this paper may provide a solution to the current controversies about stem cell CD38 and CD34 expression. Here, we confirmed the earlier report2 that the stem cells in the Lin−c-kit+Sca-1+ fraction of normal adult murine marrow cells are CD38+. There is agreement in the literature that stem cells in the Lin−c-kit+Sca-1+ marrow cells of normal mice are CD34−.4,6 We then documented that activated stem cells, including G-CSF–mobilized stem cells, are CD38−. Earlier, we showed that activated stem cells in 5-FU–treated mice and G-CSF–mobilized PB are CD34+.6,8 This apparent reciprocity in stem cell CD38 and CD34 expression was confirmed by 4-color sorting of post–5-FU marrow cells. In a separate report, we described that a minor population (approximately 20%) of CD34+ stem cells exist in the mononuclear cell fraction but not in the selected Lin−c-kit+Sca-1+ marrow cells of normal adult mice.9 In the current study, we documented that the stem cells in the CD34+ marrow populations of normal mice are CD38−. This demonstration that the CD34+ stem cells of normal adult mice are CD38− is in agreement with the CD34+CD38− phenotype of primitive human progenitors and stem cells.15-18 However, the data also reiterate the fact that a majority of adult murine stem cells are CD34− and CD38+. The possibility that this reciprocity may apply to human stem cells must be considered in future strategies for stem cell purification.
We thank Dr Pamela N. Pharr and Anne G. Livingston for assistance in preparation of this manuscript, and the staff of the Radiation Oncology Department of the Medical University of South Carolina for assistance in irradiation of mice.
Supported by National Institutes of Health grants RO1-DK54197 and PO1-CA78582 and by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Makio Ogawa, Ralph H. Johnson VA Medical Center, 109 Bee St, Charleston, SC 29401-5799; e-mail: ogawam@musc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal