Abstract
Because human CD34+ and murine Sca-1+hematopoietic stem–progenitor cells (HSPCs) express platelet-binding sialomucin P-selectin (CD162) and integrin Mac-1 (CD11b–CD18) antigen, it was inferred that these cells might interact with platelets. As a result of this interaction, microparticles derived from platelets (PMPs) may transfer many platelet antigens (CD41, CD61, CD62, CXCR4, PAR-1) to the surfaces of HSPCs. To determine the biologic significance of the presence of PMPs on human CD34+ and murine Sca-1+ cells, their expressions on mobilized peripheral blood (mPB) and on nonmobilized PB- and bone marrow (BM)–derived CD34+ cells were compared. In addition, the effects of PMPs on the proliferation of CD34+ and Sca-1+ cells and on adhesion of HSPCs to endothelium and immobilized SDF-1 were studied. Finally, the hematopoietic reconstitution of lethally irradiated mice receiving transplanted BM mononuclear cells covered or not covered with PMPs was examined. It was found that PMPs are more numerous on mPB than on BM CD34+cells, do not affect the clonogenicity of human and murine HSPCs, and increase adhesion of these cells to endothelium and immobilized SDF-1. Moreover, murine BM cells covered with PMPs engrafted lethally irradiated mice significantly faster than those not covered, indicating that PMPs play an important role in the homing of HSPCs. This could explain why in a clinical setting human mPB HSPCs (densely covered with PMPs) engraft more rapidly than BM HSPCs (covered with fewer PMPs). These findings indicate a new role for PMPs in stem cell transplantation and may have clinical implications for the optimization of transplantations.
Introduction
Peripheral blood mobilized (mPB) by various means (cytokines, chemotherapy, or both) has become a source of hematopoietic stem–progenitor cells (HSPC) for autologous and allogeneic transplantation.1 Hematopoietic recovery after transplantation with mPB HSPCs is faster than with bone marrow (BM), but the mechanism(s) responsible for this are not completely understood.1-3 To explain this, we compared the surface expression of adhesion molecules in mPB CD34+ cells to that of BM-derived CD34+ cells. We found to our surprise that mPB CD34+ cells expressed a significantly higher level of αIIbβ3 glycoprotein (CD41 antigen) than CD34+ cells isolated from either nonmobilized PB or BM. Although the expression of the CD41 antigen on the surfaces of human CD34+ cells had been previously observed and attributed to the presence of platelets attached to their surfaces,4 the biologic importance of this phenomenon remains unknown. Because cytokine mobilization and leukapheresis are known to activate platelets, we predicted that CD34+ cells separated from the leukapheresis products of mPB would be covered by activated platelets. Thorough examination of these cells under an electron microscope, however, did not reveal intact platelets despite high surface expression of the CD41 antigen. Hence, we hypothesized that the presence of the CD41 antigen on mPB CD34+ cells results from the binding of platelet-derived microparticles (PMPs) to their surfaces.
PMPs are released on the activation of platelets and express functional adhesion receptors, including αIIbβ3 (CD41), P-selectin (CD62P), and other platelet membrane receptors such as CXCR4. and PAR-1.5 6 We speculated that PMPs released by activated platelets could subsequently bind to the membranes of human CD34+ or murine Sca-1+ cells. Based on these assumptions, we investigated mechanisms responsible for the presence of PMPs on HSPC and the biologic significance of this phenomenon.
We now report evidence suggesting that HSPCs interact with platelets and subsequently display on their surfaces PMP-derived antigens. PMPs covering the surfaces of human or murine HSPCs do not affect their proliferation but increase their adhesion to endothelium and, most important, significantly improve murine HSPC engraftment after transplantation into lethally irradiated mice. We suggest that PMPs displayed on HSPCs may regulate the trafficking and homing of these cells to hematopoietic organs.
Materials and methods
Human CD34+ cells and platelets
Light-density bone marrow mononuclear cells (BM MNCs) were obtained from consenting healthy donors, depleted of adherent cells and T lymphocytes (A−T− MNC) and enriched for CD34+ cells by immunoaffinity selection with MiniMACS paramagnetic beads (Miltenyi Biotec, Auburn, CA) as described previously.7-9 The purity of isolated BM CD34+cells was more than 95% as determined by fluorescence-activated cell sorter (FACS) analysis. Gel-filtered platelets were prepared from healthy donors as previously described.10,11 BM and PB cells were obtained from healthy volunteer donors with their informed consent and approval from the institutional review boards. PB was also obtained by leukapheresis using the Cobe Spectra from patients diagnosed with breast cancer (stage II-III) and mobilized with granulocyte–colony-stimulating factor (CSF) (Filgastrim; Amgen, Thousand Oaks, CA). Informed patient consent and approval from the institutional ethics committees were obtained for this procedure. Cells were enriched for CD34+ cells using immunoaffinity MidiMACS paramagnetic beads (Miltenyi Biotec) as described previously in detail2; the purity of the isolated mPB CD34+cells was greater than 97%.
Human umbilical vein endothelial cells
Human umbilical vein endothelial cells (HUVECs) were isolated as previously described in detail.8 Cells suspended in growth medium (90% M199, 10% fetal calf serum, 1 mg/mL BBE, 1 ng/mL human epithelial growth factor, 1 mg/mL hydrocortisone, 10 U/mL heparin) at a density of 2.5 × 103/mL were seeded in 96-well plates coated with fibronectin 24 hours before the experiment. Culture plates covered with these cells were subsequently used in adhesion assays as described below.
Murine BM MNCs, Sca-1+ cells, and platelets
Murine MNCs were isolated from BM flushed from the femurs of pathogen-free, 4- to 6-week-old female B.6SJL-Ptprca Pep3b/BoyJ–Ly5.1 mice (The Jackson Laboratory, Bar Harbor, ME), depleted of adherent cells (A−) and enriched for light-density MNC by Ficoll-Hypaque centrifugation as described.12Sca-1+ cells were isolated by using paramagnetic mini-beads (Miltenyi Biotec) according to the manufacturer's protocol. Murine platelets were prepared using differential centrifugation of whole anticoagulated blood. Briefly, blood samples obtained by venipuncture from the inferior vena cava were anticoagulated with 3.8% sodium citrate and adjusted to a final volume of 8 mL with Tyrode buffer (containing 1 mg/mL albumin; 5 U/mL apyrase; and 1 mM EGTA, pH 6.5). Platelet-rich plasma (PRP) was isolated from blood by centrifugation for 7 minutes at 150g at room temperature. Prostaglandin E1 was added to PRP to a final concentration of 1 μM. Platelets were pelleted by centrifugation of the PRP (at room temperature for 10 minutes at 800g) and resuspended (in HEPES buffer, pH 7.5, to a concentration of 8 × 108 platelets/mL.
Platelet microparticles
PMPs were prepared from platelets as described earlier.13 14 Before the experiments, platelet concentrates were activated by thrombin (2 U/mL) for 10 minutes and centrifuged at 800g for 20 minutes, and the supernatants enriched in PMPs were collected. Supernatants were examined by flow cytometry analysis using phycoerythrin (PE)-conjugated anti-human αIIbβ3 antibody (Coulter-Immunotech, Marseilles, France) and were PE-conjugated anti-human P-selectin (CD62) antibody (Becton Dickinson, Franklin Lakes, NJ) or PE- or fluorescein isothiocyanate (FITC)–conjugated anti-mouse αIIbβ3 antibody and anti-mouse CD62P (P-selectin) antibody (BD PharMingen, San Diego, CA).
Human myeloid, erythroid, and megakaryocytic precursor cells
CD34+ cells were cloned in serum-free methylcellulose cultures as previously described.7Briefly, CD34+ A−T− MNCs (104/mL) covered or not covered by PMPs were suspended in Iscoves MEM (Gibco BRL, Grand Island, NY) supplemented with 50% artificial serum containing 1% delipidated, deionized, and charcoal-treated bovine serum albumin, 270 μg/mL iron-saturated transferrin, 20 μg/mL insulin, and 2 mM L-glutamine (all from Sigma, St Louis, MO) and were cultured in 1% methylcellulose. Granulocyte macrophage (GM)–colony-forming unit (CFU) growth was stimulated with 10 ng/mL recombinant human (rh) interleukin (IL)-3 and 5 ng/mL rhGM-CSF. Erythroid burst-forming unit (BFU-E) growth was stimulated with 2 U/mL rh erythropoietin and 10 ng/mL rh kit ligand (KL). Megakaryocyte CFU (CFU-Meg) growth was stimulated with 50 ng/mL rh thrombopoietin and 10 ng/mL rh IL-3. Mixed CFU (CFU-Mix) growth was stimulated by 10 ng/mL KL, 10 ng/mL IL-3, 50 ng/mL thrombopoietin, 2 U/mL erythropoietin, and 5 ng/mL GM-CSF). All recombinant human cytokines and growth factors were obtained from R & D Systems (Minneapolis, MN). Cultures were incubated at 37°C in a fully humidified atmosphere supplemented with 5% CO2. Colonies were counted under an inverted microscope on day 11 for CFU-GM and CFU-Meg and on day 14 for CFU-Mix and BFU-E.
FACS analysis
Binding of platelets or PMPs to CD34+, Sca-1+, BFU-E–derived erythroblasts, or UT-7 and HL-60 cells was evaluated using flow cytometry. Cells were incubated with platelets or PMPs for 10 minutes to 2 hours at room temperature and centrifuged at 800 rpm to remove unbound platelets; the resultant cell pellets were resuspended in Tyrode buffer containing 2 mg/mL of PE–anti-αIIbβ3 antibody (anti-CD41 antibody; Coulter-Immunotech). Expression of αIIbβ3 was evaluated by FACS analysis (FACScan; Becton Dickinson) as described earlier.8 Fluorescence was measured in the gate characteristic for CD34+ cells or Sca-1+ cells. Data are presented as the percentage of cells labeled with anti-CD41 antibody. Similar experiments were performed for murine BM MNCs isolated from B.6SJL-Ptprca Pep3b/BoyJ–Ly5.1 mice.
Expression of PSGL-1 (CD162), Mac-1 (CD11b-CD18), CD62, CXCR4, and PAR-1 was evaluated on hematopoietic cells by FACS after staining with antigen-specific antibodies. We used the following monoclonal antibodies: PE–anti-PSGL-1 (Research Diagnostic, Flanders, NJ), PE–anti-CD62, and PE–anti-Mac-1 (Becton Dickinson, San Juan, CA), PE–anti-CXCR4 (BD PharMingen), and anti–PAR-1 (Coulter-Immunotech).
Adhesion to HUVECs and immobilized SDF-1
Adherence assays of human CD34+ cells that were metabolically labeled by S35-methionine and were incubated for 10 minutes with platelets or PMPs were performed as described.15 Briefly, 96-well microtiter plates (Becton Dickinson, Oak Park, MA) covered with HUVECs (as described above) or immobilized SDF-1 were incubated for 30 minutes at 37°C in culture media. Subsequently, cell suspensions (1 × 105 cells/100 μL) were applied to the wells and incubated for 3 hours at 37°C. The number of adherent cells was estimated in a scintillation counter as described.15
Marrow transplantation in lethally irradiated mice
Female mice (4-6 weeks old) congenic in murine CD45 at the Ly5 locus (C57BL/6J-Ly 5.2 and B.6SJL-Ptprca Pep3b/BoyJ Ly5.1) were obtained from Jackson Laboratory. For the transplantation experiments, C57BL/6J-Ly 5.2 mice were irradiated with a lethal dose of γ-irradiation (900 cGy [900 rads]). After 24 hours, the mice received transplanted cells by tail vein injection with 5 × 105 BM MNCs from B.6SJL-Ptprca Pep3b/BoyJ-Ly5.1 mice covered or not covered with PMPs. Mice receiving transplanted cells were bled at various intervals from the retro-orbital plexus to obtain samples for leukocyte, platelet, and hematocrit counts using Unopette Microcollection (Becton Dickinson, Rutherford, NJ) and heparinized microhematocrit capillary tubes (Oxford Labware, St Louis, MO) as described.12
Evaluation of chimerism after transplantation
Hematologic engraftment in mice that received transplanted cells was evaluated by staining bone marrow cells with Ly5.1 (clone A20) and Ly5.2 (clone 104) donor- and host-specific antibodies, respectively (PharMingen) as described.16
CFU-S assay
For the splenic colony-forming unit (CFU-S) assays, C57BL/6J-Ly 5.2 mice received transplanted 2 × 105 marrow cells from B.6SJL-Ptprca Pep3b/BoyJ-Ly5.1 mice covered or not covered with murine PMPs. At day 9 or 12, spleens were removed and fixed in Tellysyniczky fixative, and CFU-S were counted on the surface of the spleen using magnification glass as described.17
Statistical analysis
Arithmetic means and standard deviations of our FACS data were calculated on a Macintosh computer PowerBase 180 (Apple, Cupertino, CA), using Instat 1.14 (GraphPad, San Diego, CA) software. Data were analyzed using the Student t test for unpaired samples. Statistical significance was defined asP < .05.
Results
Human mPB CD34+ cells highly express platelet-characteristic antigens
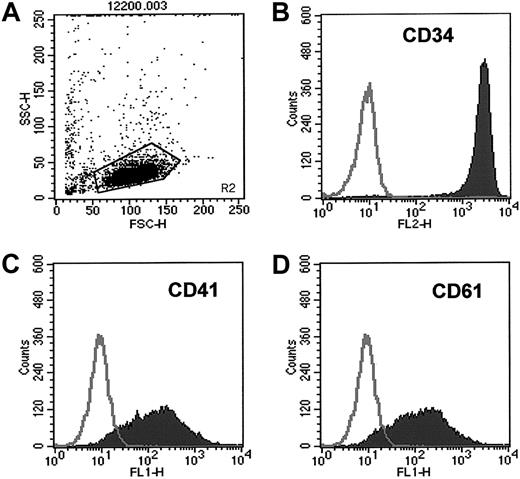
We found that human CD34+ cells isolated from mobilized PBs, in contrast to CD34+ cells obtained from nonmobilized PBs (collected from whole blood without leukapheresis), highly expressed antigens characteristic for platelets such as CD41 and CD61 integrins (Figure 1, Table1) and the thrombin receptor PAR-1 (data not shown). Only 23% ± 7% of nonmobilized PB CD34+ cells expressed the CD41 antigen compared with 83% ± 18% of the mPB CD34+ cells (P = .0017). Hence, we asked whether these mPB CD34+ cells were covered by platelets activated during leukapheresis. We performed electron-microscopic studies to address this issue and to assess the presence of platelets on the CD34+ cells. To our surprise, no intact platelets were found on the surfaces of either mobilized or nonmobilized PB CD34+ cells (data not shown).
CD34+ cells isolated from mPB highly express CD41 and CD61.
Expression of CD41 and CD61 antigens on CD34+ cells isolated from mPB was evaluated by FACS. (A) Cytogram of mPB CD34+ cells. (B) Purity of CD34+ cells. (C) Expression of CD41 on mPB CD34+ cells. (D) Expression of CD61 on mPB CD34+ cells. One study representative of 6 is shown.
CD34+ cells isolated from mPB highly express CD41 and CD61.
Expression of CD41 and CD61 antigens on CD34+ cells isolated from mPB was evaluated by FACS. (A) Cytogram of mPB CD34+ cells. (B) Purity of CD34+ cells. (C) Expression of CD41 on mPB CD34+ cells. (D) Expression of CD61 on mPB CD34+ cells. One study representative of 6 is shown.
Expression of platelet antigens on human CD34+cells derived from unmobilized whole PB, mPB, or BM
| Antigens . | PB CD34+ cells mean ± SD (%) (n = 3) . | mPB CD34+ cells mean ± SD (%) (n = 5) . | BM CD34+ cells mean ± SD (%) (n = 3) . |
|---|---|---|---|
| CD41 | 23 ± 7 | 83 ± 18* | 19 ± 4 |
| CD61 | 62 ± 9 | 81 ± 16 | 59 ± 6 |
| Antigens . | PB CD34+ cells mean ± SD (%) (n = 3) . | mPB CD34+ cells mean ± SD (%) (n = 5) . | BM CD34+ cells mean ± SD (%) (n = 3) . |
|---|---|---|---|
| CD41 | 23 ± 7 | 83 ± 18* | 19 ± 4 |
| CD61 | 62 ± 9 | 81 ± 16 | 59 ± 6 |
P = .0017 and P = .0011 compared to PB and BM, respectively.
Human BM-derived CD34+ cells display platelet-binding ligands on their surfaces, activate platelets, and subsequently incorporate PMPs
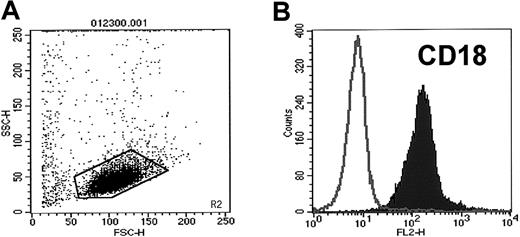
Because we could not demonstrate intact platelets on the surfaces of human CD34+ cells, we hypothesized that the high expression of platelet-characteristic antigens on these cells results from binding of PMPs to their membranes. It is known that PMPs are released from platelets activated by platelet agonists.6,8,18 19 We confirmed that human CD34+ cells express CD162 and Mac-1 (CD11b-CD18) (Figure2), both of which may potentially bind or activate platelets after binding to platelet selectin (CD62P) and platelet glycoprotein (Ibα), respectively.
CD34+ cells isolated from mPB highly express CD18.
Expression of CD18 on human mPB CD34+ cells was evaluated by FACS. (A) Cytogram of mPB CD34+ cells. (B) Expression of CD18 on mPB CD34+ cells. One study representative of 3 is shown.
CD34+ cells isolated from mPB highly express CD18.
Expression of CD18 on human mPB CD34+ cells was evaluated by FACS. (A) Cytogram of mPB CD34+ cells. (B) Expression of CD18 on mPB CD34+ cells. One study representative of 3 is shown.
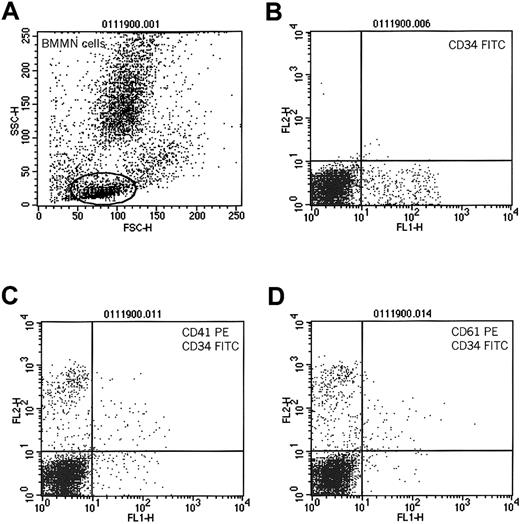
Consistent with previous findings, we observed that human BM CD34+ cells also expressed CD414; however, in contrast to mPB CD34+ cells, expression of CD41 on these cells was significantly lower (19% ± 4% versus 83% ± 18%;P = .0011) (Table 1). This could be explained by the fact that, in steady state hematopoiesis, BM CD34+ cells reside in bone marrow niches and probably interact with platelets and PMPs circulating in the peripheral blood to a lesser extent and thus have fewer PMPs on their surfaces. To test the hypothesis that CD34+ cells interact with platelets and that PMPs subsequently bind to their surfaces, we compared the expression of CD41 on the surfaces of BM-derived CD34+ cells before and after incubation with platelets. Accordingly, we incubated BM-derived MNCs with platelets freshly isolated from PB (5 × 109platelets/μL). We found that after incubation with platelets, BM CD34+ cells expressed significantly more CD41 (73% ± 21% for cells incubated vs 27% ± 8% for cells not incubated with platelets; P < .0001). We also found that CD41 expression could be partly inhibited after pre-incubating CD34+ cells with monoclonal antibodies blocking CD162 (73% ± 21% for cells not exposed and 42% ± 19% for cells exposed to anti-CD162 antibody; P < .002). Figure3 shows the expression of CD41 and CD61 antigens on BM CD34+ cells that have been pre-incubated with platelets.
Expression of CD41 and CD61 on human CD34+cells after incubation with platelets.
Human bone marrow-derived MNCs were incubated with platelets and subsequently evaluated for coexpression of CD34 and CD41 or CD61 antigens. (A) Cytogram of BM MNCs. R1 lymphocytic gate. (B) Cells in R1 stained for CD34 antigen. (C) Expression of CD41 on CD34+cells incubated with platelets. (D) Expression of CD61 on CD34+ cells incubated with platelets. One study representative of 3 is shown.
Expression of CD41 and CD61 on human CD34+cells after incubation with platelets.
Human bone marrow-derived MNCs were incubated with platelets and subsequently evaluated for coexpression of CD34 and CD41 or CD61 antigens. (A) Cytogram of BM MNCs. R1 lymphocytic gate. (B) Cells in R1 stained for CD34 antigen. (C) Expression of CD41 on CD34+cells incubated with platelets. (D) Expression of CD61 on CD34+ cells incubated with platelets. One study representative of 3 is shown.
Human erythroblasts, HL-60, and UT-7 cells may interact with platelets and bind PMPs
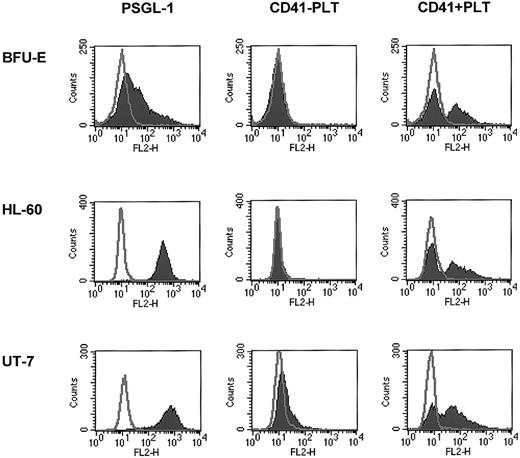
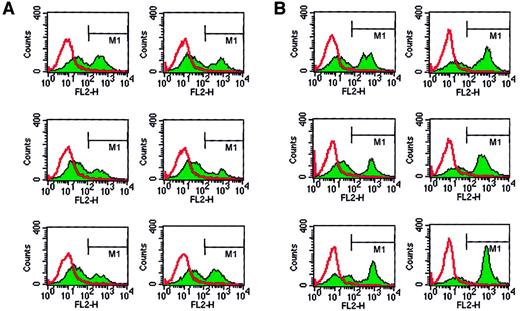
Next we tested whether, in addition to human CD34+cells, other hematopoietic cells may bind PMPs to their membranes. Because we found that human erythroblasts, HL-60 cells, and UT-7 cells expressed CD162 on their surfaces, we incubated these cells with platelets and found that after incubation they expressed CD41 on their surfaces (Figure 4). This interaction was again partly inhibited by anti-CD162 blocking monoclonal antibody (data not shown). Hence, platelet binding on hematopoietic cell surfaces, followed by the binding of PMPs to the cell membranes, seems to be a common mechanism for platelet interaction with hematopoietic cells of various lineages.
Human BFU-E–derived cells, HL-60 cells, and UT-7 cells express PSGL-1 (CD162) and become covered with PMPs after incubation with platelets.
Normal human BFU-E–derived cells (upper row), HL-60 cells (middle row), and UT-7 cells (lower row) were stained with PE–anti-PSGL-1 monoclonal antibody (left panels) and PE–anti-CD41 monoclonal antibody before (middle panels) or after (right panels) incubation with platelets. One study representative of 3 is shown.
Human BFU-E–derived cells, HL-60 cells, and UT-7 cells express PSGL-1 (CD162) and become covered with PMPs after incubation with platelets.
Normal human BFU-E–derived cells (upper row), HL-60 cells (middle row), and UT-7 cells (lower row) were stained with PE–anti-PSGL-1 monoclonal antibody (left panels) and PE–anti-CD41 monoclonal antibody before (middle panels) or after (right panels) incubation with platelets. One study representative of 3 is shown.
Murine Sca-1+ cells activate platelets and incorporate PMPs into cell membranes
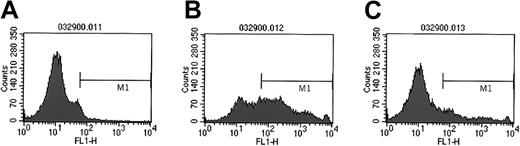
Next we investigated whether similar effects occur in murine hematopoietic cells, and we isolated HSPCs expressing the Sca-1 antigen from murine bone marrow. We found that these cells weakly express CD41 on their surfaces (10% ± 4%) (Figure5A). Subsequently, Sca-1+cells were incubated with the murine platelets. As in human CD34+ cells, we found that murine Sca-1+ cells expressed CD41 highly on the surface after incubation with platelets (62% ± 8%) (Figure 3B). This binding was inhibited after the pre-incubation of cells with monoclonal antibodies against platelet CD62P (after pre-incubation with anti-CD62 antibody, 47% ± 12% of cells expressed CD41; P < .0002), suggesting that in murine, as in human, HSPCs, there is at least some involvement of the CD162–CD62P axis in platelet binding. In addition, though murine Sca-1+ cells highly expressed CD41 antigen on their surfaces, electron microscopy revealed the absence of intact platelets (not shown). This supports our assumption that high expression of CD41 antigen on Sca-1+ cells, as for human mPB CD34+cells, is the result of PMP binding to the cell membranes.
Murine Sca-1+ cells activate platelets in a PSGL-1–dependent manner.
Expression of CD41 on the surfaces of murine Sca-1+ cells not incubated with platelets (A), incubated with platelets (B), and incubated with platelets in the presence of anti-CD162 blocking antibodies (C). Experiment was repeated twice with similar results.
Murine Sca-1+ cells activate platelets in a PSGL-1–dependent manner.
Expression of CD41 on the surfaces of murine Sca-1+ cells not incubated with platelets (A), incubated with platelets (B), and incubated with platelets in the presence of anti-CD162 blocking antibodies (C). Experiment was repeated twice with similar results.
PMPs do not affect proliferation of either human CD34+ or murine A− BM MNC cells
Next we examined whether PMPs may affect the proliferation of human CD34+ cells. The latter were or were not pre-incubated with PMPs and were assayed in in vitro clonogenic assays. We found that the presence of PMPs on the surfaces of CD34+cells did not affect their clonogenicity. CD34+ cells covered with PMPs formed 112 ± 27 of BFU-E–derived, 241 ± 53 of CFU-GM–derived, 98 ± 11 of CFU-Meg–derived, and 19 ± 7 of CFU-Mix–derived colonies. Cells not covered with PMPs formed 127 ± 20 of BFU-E–derived, 237 ± 67 of CFU-GM–derived , 102 ± 17 of CFU-Meg–derived, and 17 ± 6 of CFU-Mix–derived colonies, respectively (n = 6). Similarly, we did not observe any effects of PMPs on the cloning efficiency of murine A− BM MNCs (data not shown).
Human CD34+ cells covered with PMPs adhere better to endothelium and immobilized SDF-1
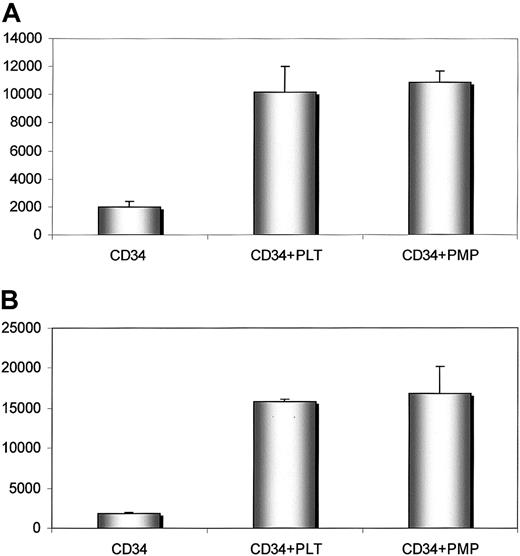
Our data suggest that PMPs transfer various platelet-endothelium attachment receptors (glycoprotein [GP] IIb/IIIa [CD41], GPIb, GPIaIIa, P-selectin, chemokine CXCR4) to the cell membranes of CD34+ cells.5,6 This has an important role in the adhesion of CD34+ cells to the endothelial cells in bone marrow.5 Because the SDF-1 chemokine is preferentially expressed by bone marrow endothelium,3,20 21 we investigated whether cells covered with PMPs adhere better to endothelial cells and immobilized SDF-1 cells and found that human BM CD34+ cells covered with PMPs adhered significantly better to HUVECs and immobilized SDF-1 cells than those that were not covered (Figure6).
Human CD34+ cells covered with PMPs adhere to HUVECs and immobilized SDF-1.
Adhesion of human CD34+ cells to HUVECs (upper panel) and SDF-1 (lower panel). Before adhesion, CD34+ cells were not incubated (left columns) or were incubated with fresh platelets (middle columns) or PMPs (right columns). Experiment was repeated twice with similar results.
Human CD34+ cells covered with PMPs adhere to HUVECs and immobilized SDF-1.
Adhesion of human CD34+ cells to HUVECs (upper panel) and SDF-1 (lower panel). Before adhesion, CD34+ cells were not incubated (left columns) or were incubated with fresh platelets (middle columns) or PMPs (right columns). Experiment was repeated twice with similar results.
Murine BM HSPCs covered with PMPs engraft faster
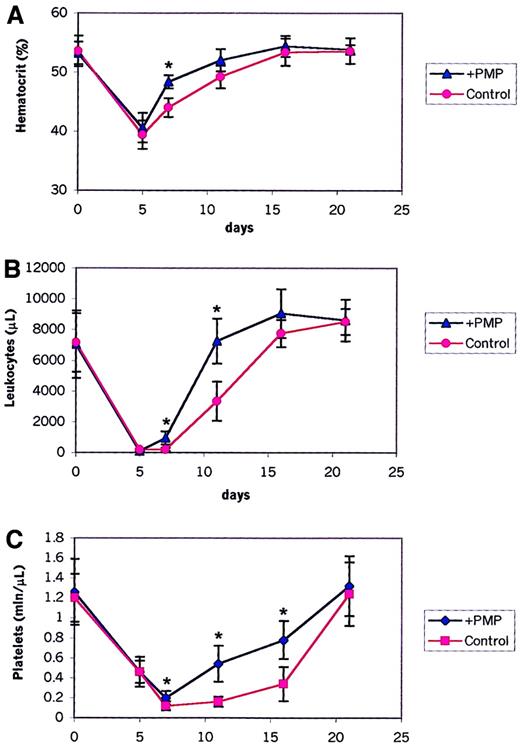
We found that HSPCs covered with PMPs expressed several new platelet-expressed adhesion molecules and, as shown above, adhered significantly better to endothelial cells and immobilized SDF-1. Thus, we asked whether the presence of PMPs on the HSPC surface has any effect on their engraftment and on the kinetics of hematopoietic reconstitution after transplantation. To address this, we performed transplantations between congenic C57BL mice that differed in the Ly5 locus, a murine homolog of the CD45 antigen. Ly5.2 mice received transplanted BM cells derived from congenic Ly5.1 animals covered or not covered with PMPs. After transplantation, the recovery of peripheral blood cells was observed in these mice (Figure7). Mice receiving transplanted cells covered with PMPs showed a statistically significant shorter recovery time for leukocytes and platelets by 3 to 4 days than mice receiving transplanted cells not covered. This difference was statistically significant (P < .0001 for days 7 and 11 for leukocytes; P < .0001 for days 7, 11, and 16 for platelets). We also observed faster hematocrit recovery (P < .0001 for day 7).
Hematopoietic recovery in lethally irradiated mice receiving transplanted bone marrow cells covered (+ PMPs) or not covered (control) with PMPs.
Shown are percentages of peripheral blood hematocrit (A), leukocyte counts (B), and platelet counts (C) in lethally irradiated mice receiving transplanted donor cells not covered (control) or covered with PMPs (+ PMP). Data are pooled from 2 independent experiments, each with 5 to 8 mice in the each of the 2 groups (control and experimental). *P < .0001.
Hematopoietic recovery in lethally irradiated mice receiving transplanted bone marrow cells covered (+ PMPs) or not covered (control) with PMPs.
Shown are percentages of peripheral blood hematocrit (A), leukocyte counts (B), and platelet counts (C) in lethally irradiated mice receiving transplanted donor cells not covered (control) or covered with PMPs (+ PMP). Data are pooled from 2 independent experiments, each with 5 to 8 mice in the each of the 2 groups (control and experimental). *P < .0001.
We also looked for the number of day 9 and day 12 CFU-S colonies after transplantation of murine A− BMNCs covered or not covered with PMPs. In 2 independent experiments, we found that mice receiving transplanted BM cells covered with PMPs had approximately 1.5 to 2 times more spleen colonies (CFU-S) at days 9 and 12 after transplantation than mice receiving transplanted cells not covered with PMPs (Table 2).
Number of day 9 and day 12 CFU-S in mice receiving transplanted marrow cells covered or not covered with PMPs
| Cells . | Experiment . | Day 9 CFU-S . | Day 12 CFU-S . |
|---|---|---|---|
| Not covered with PMPs | 1 | 5.4 ± 4.4* | 27.8 ± 4.3‡ |
| 2 | 15.2 ± 4† | 41.2 ± 62-153 | |
| Covered with PMPs | 1 | 10 ± 2.7 | 42.6 ± 8 |
| 2 | 23.6 ± 8 | 63.8 ± 17 |
| Cells . | Experiment . | Day 9 CFU-S . | Day 12 CFU-S . |
|---|---|---|---|
| Not covered with PMPs | 1 | 5.4 ± 4.4* | 27.8 ± 4.3‡ |
| 2 | 15.2 ± 4† | 41.2 ± 62-153 | |
| Covered with PMPs | 1 | 10 ± 2.7 | 42.6 ± 8 |
| 2 | 23.6 ± 8 | 63.8 ± 17 |
P < .002 compared to cells covered with PMPs;
P < .003 compared to cells covered with PMPs;
P < .003 compared to cells covered with PMPs;
P < .01 compared to cells covered with PMPs.
Moreover, the changes in the number of CFU-S colonies paralleled the changes in the degree of chimerism detected in the bone marrows of Ly 5.1+ animals receiving transplanted Ly5.2+ marrow cells. Accordingly, we found that Ly 5.1+ mice receiving transplanted cells covered with PMPs had significantly more Ly5.2+ cells in their bone marrow cavities at day 12 after transplantation (Figure8, Table3).
Detection of donor cells in the bone marrow of mice that received transplanted cells.
Bone marrow cells were not covered (A) or were covered (B) with PMPs, were isolated from animals receiving transplanted cells, and subsequently were stained with α-Ly5 monoclonal antibodies. Because donor and host mice differed in the Ly5 locus, donor and host cells were easily detectable by Ly5.1- or Ly5.2-specific monoclonal antibodies. A representative study of 2 is shown.
Detection of donor cells in the bone marrow of mice that received transplanted cells.
Bone marrow cells were not covered (A) or were covered (B) with PMPs, were isolated from animals receiving transplanted cells, and subsequently were stained with α-Ly5 monoclonal antibodies. Because donor and host mice differed in the Ly5 locus, donor and host cells were easily detectable by Ly5.1- or Ly5.2-specific monoclonal antibodies. A representative study of 2 is shown.
Chimerism of bone marrow cells examined in Ly5.1+ mice 12 days after transplantation
| Cells . | Donor-derived Ly5.2+ cells (%) . |
|---|---|
| Not covered with PMPs | Experiment 1: 32.5 ± 4.23-150 |
| Experiment 2: 21.2 ± 8.33-151 | |
| Covered with PMPs | Experiment 1: 57.2 ± 14.6 |
| Experiment 2: 49.7 ± 23.1 |
| Cells . | Donor-derived Ly5.2+ cells (%) . |
|---|---|
| Not covered with PMPs | Experiment 1: 32.5 ± 4.23-150 |
| Experiment 2: 21.2 ± 8.33-151 | |
| Covered with PMPs | Experiment 1: 57.2 ± 14.6 |
| Experiment 2: 49.7 ± 23.1 |
P < .001 compared to cells covered with PMPs;
P < .009 compared to cells covered with PMPs.
Finally, at day 30, we evaluated hematopoietic reconstitution after the transplantation of 5 × 105 BM MNCs per mouse. By this time, there were no differences in hematopoietic chimerism, suggesting that both types of cells (covered and not covered with PMPs) reconstituted mice in a similar way.
Discussion
PMPs generated from peripheral blood platelets activated by agonists are believed to play an important role in various physiological processes, such as facilitating the interaction of leukocytes or monocytes with endothelial cells5,6 and in the aggregation and accumulation of leukocytes on selectin-expressing substrates,14 or in many pathologic states including heparin-induced thrombocytopenia.18 19 In this study we report that both human and murine HSPCs are covered with PMPs after incubation with platelets, and we discuss mechanisms and biologic consequences of this phenomenon.
In view of the observations by others22-24 that PB CD34+ cells express PSGL-1 (CD162) and to explain the high expression of CD41/CD61 on these cells, we initially hypothesized that PB CD34+ cells bind platelets to their surfaces. In fact, we were able to confirm the presence of platelet-activating and -binding PSGL-1 on these cells; in addition, we demonstrated that they express another platelet-binding molecule, Mac-1 (CD11b-CD18). The fact that monoclonal antibodies against CD162 partly prevented the interaction of platelets with human CD34+ cells and that antibodies against murine CD62P selectin partly inhibited the interaction of murine Sca-1 cells with platelets suggests that the CD162–CD62P axis does indeed play an important role in platelet binding to human and murine HSPCs.25
Because we could not detect platelets on the surfaces of CD34+ cells by electron microscopy, we postulate that the expression of platelet-derived antigens such as CD41, CD62, CXCR4, and PAR-1 is related to the presence of PMPs on CD34+ cells rather than intact platelets. Given that platelets are exposed to many platelet agonists (eg, chemokines, cytokines) during mobilization and that platelets in mPB are additionally activated while they circulate in the plastic tubing used during leukapheresis, we set out to demonstrate that CD34+ cells isolated from the leukapheresis products of mPB are more highly covered with PMPs than their counterparts harvested from nonmobilized PB or BM. Moreover, it has been reported that during leukapheresis platelets not only become activated but also release PMPs,5 6 so it is likely that mobilized CD34+ cells bind circulating PMPs to their surfaces.
The other major observation emanating from our work is that hematopoietic cells covered with PMPs adhere more efficiently to endothelial cells and immobilized SDF-1, which suggests that PMP-derived molecules expressed on CD34+ cells increase these effects. Potential candidates for these interactions are PMP-derived molecules such as the platelet-endothelium attachment receptors GPIIb/IIIa, GPIb, and GPIa/IIa and the chemokine receptor CXCR4.27-29 Moreover, we postulate that because not all HSPCs express endogenous CXCR4 on the surfaces,30PMP-derived CXCR4 may regulate the homing of such CXCR4−HSPCs. The SDF-1 chemokine on the BM endothelial cell surface plays an important role in the tethering of circulating CD34+cells,20,21 and the interaction of CXCR4 from PMPs covering CD34+ cells with SDF-1 of the BM endothelium, in addition to CXCR4 constitutively expressed by CD34+ cells, facilitates homing of these cells to the bone marrow.20,21,27-29 Furthermore, it is likely that PMPs may also directly activate or up-regulate certain adhesion molecules on human CD34+ cells. In support of this, it has been reported that arachidonic acid released from PMPs directly activates Mac-1 and ICAM-1 on monocytes and P- and E-selectins on endothelial cells.5 6 Hence, the mechanism(s) whereby PMPs regulate the expression of genes encoding adhesion molecules on human CD34+ cells requires further study.
Most important, we demonstrated in our in vivo animal model that murine bone marrow-derived HSPCs covered with PMPs engraft significantly faster after transplantation into lethally irradiated mice than marrow cells not covered with PMPs. This observation may explain why in a clinical setting we observe faster early-hematopoietic recovery after transplantation of mPB stem–progenitor cells than of BM.1In this study, we found that human HSPCs obtained from mPB are more densely covered with PMPs than their nonmobilized BM counterparts. Because murine HSPCs covered with PMPs showed faster homing after transplantation, we suggest that “painting” of human HSPCs with PMPs could potentially accelerate early engraftment.
The activation/binding of platelets on the surfaces of human CD34+ cells we describe here is an interesting biologic phenomenon and represents an example of cross-talk between platelets and hematopoietic cells. We suggest that incorporation of PMPs into the membranes of mobilized CD34+ cells can be important in directing these cells from the peripheral blood back to the bone marrow and possibly in other physiological processes. For example, a similar mechanism may direct PMP-covered lymphocytes from areas of inflammation to the lymphopoietic organs in which they would proliferate and expand. This possibility is under investigation in our laboratory.
There are potential disadvantages associated with PMP-binding to human CD34+ cells. First, in patients with immunothrombocytopenia, it is possible that some platelet-associated antigens may be displayed by PMPs on the surfaces of HSPCs and thus may be recognized by the antiplatelet antibodies. Second, PMPs rich in phosphatidylserine may “mark” CD34+ cells as apoptotic cells that may then become targets for immune response.31Both these questions require further study. It has been reported recently that activation of PSGL-1 (CD162) expressed on human CD34+ cells by CD62P delivers negative signals in human hematopoietic progenitors26 and could result in inhibition of the growth of these cells. In our work, however, the addition of platelets or PMPs to cultured in vitro human or murine progenitors did not affect their proliferation. We believe that these discrepancies can be explained by the fact that CD162 expressed on CD34+cells interacts with platelet- or PMP-derived CD62P differently than with purified recombinant CD62P protein.26
In conclusion, our study demonstrated that CD34+cells interact with platelets and bind PMPs to their cell membranes, and we present evidence that such PMPs increase HSPC homing into bone marrow. We postulate that the so-called painting of CD34+cells with PMPs may prove to be a strategy for accelerating engraftment after transplantation. The potential role of PMPs in the homing of lymphohematopoietic cells to lymphohematopoietic organs may also be of great importance and requires further investigation.
Supported by National Institutes of Health grant R01 HL61796-01 (M.Z.R.) and by a Canadian Blood Services Research and Development grant (A.J.-W.).
A.J.-W. and M.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mariusz Z. Ratajczak, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 405A Stellar Chance Labs, 422 Curie Blvd, Philadelphia, PA 19104; e-mail: mariusz@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal