The promotion of alloengraftment in the absence of global immune suppression and multiorgan toxicity is a major goal of transplantation. It is demonstrated that the infusion of a single modest bone marrow dosage in 200 cGy-irradiated recipients treated with anti-CD154 (anti-CD40L) monoclonal antibody (mAb) resulted in chimerism levels of 48%. Reducing irradiation to 100 or 50 cGy permitted 24% and 10% chimerism, respectively. In contrast, pan–T-cell depletion resulted in only transient engraftment in 200 cGy-irradiated recipients. Host CD4+ cells were essential for alloengraftment as depletion of CD4+ cells abrogated engraftment in anti-CD154–treated recipients. Strikingly, the depletion of CD8+ cells did not further enhance engraftment in anti-CD154 mAb–treated recipients in a model in which rejection is mediated by both CD4+ and CD8+ T cells. However, anti-CD154 mAb did facilitate engraftment in a model in which only CD8+ T cells mediate rejection. Furthermore, CD154 deletional mice irradiated with 200 cGy irradiation were not tolerant of grafts, suggesting that engraftment promotion by anti-CD154 mAb may not simply be the result of CD154:CD40 blockade. Together, these data suggest that a CD4+regulatory T cell may be induced by anti-CD154 mAb. In contrast to anti-CD154 mAb, anti-B7 mAb did not promote donor engraftment. Additionally, the administration of either anti-CD28 mAb or anti-CD152 (anti–CTLA-4) mAb or the use of CD28 deletional recipients abrogated engraftment in anti-CD154 mAb–treated mice, suggesting that balanced CD28/CD152:B7 interactions are required for the engraftment-promoting capacity of anti-CD154 mAb. These data have important ramifications for the design of clinical nonmyeloablative regimens based on anti-CD154 mAb administration.
Introduction
Traditionally, allogeneic bone marrow transplantation (BMT) has required substantial cytoreductive treatment of recipients with irradiation or cytotoxic drugs to achieve high levels of stable donor engraftment. The nonspecific, multiorgan systemic toxicity associated with this conditioning and high incidence of complications after BMT have limited the practical clinical applicability of allogeneic BMT. A nonmyeloablative, nontoxic conditioning regime that would result in durable donor chimerism in the recipient would make BMT a more attractive candidate in the treatment of certain hematologic disorders, including autoimmune disease, and for the induction of tolerance for transplantation of solid organs. Additionally, a nonmyeloablative conditioning regime would increase the number of potential transplantation candidates to include those who are ineligible under current protocols because of age restrictions, hepatic or renal dysfunction, active infection, or history of failed previous transplantation.1 2
In murine preclinical studies, strategies to induce chimerism with minimal conditioning have included various combinations of the use of high-dosage bone marrow cells with sublethal total body irradiation (TBI), depleting anti-CD4 and anti-CD8 monoclonal antibody (mAb), thymic irradiation, anti-CD154 (anti-CD40L) mAb, and CTLA4Ig, a fusion protein that blocks CD28/CD152:B7 interactions.3-6Engraftment in the complete absence of cytoreductive conditioning has been achieved in mice by repetitive infusions of donor bone marrow and anti-CD154 mAb7 or by high numbers of bone marrow cells, anti-CD154 mAb, and CTLA4Ig.8
Anti-CD154 mAb administered as a single agent has been shown to inhibit graft-versus-host disease (GVHD) induction and to facilitate solid organ graft acceptance in rodents and nonhuman primates.9-12 The mechanism(s) responsible for the surprising efficacy of anti-CD154 mAb in facilitating tolerance induction and alloengraftment under nonmyeloablative conditions is unknown.
In this study, we explored the host cell requirements for inducing alloengraftment and tolerance under nonmyeloablative conditioning. We found that anti-CD154 mAb induced stable macrochimerism in most recipients conditioned with as little as 50 cGy TBI receiving a single infusion of a relatively modest dosage of 40 million (approximately 2 × 109/kg) allogeneic bone marrow cells. The engraftment promotion effect was highly dependent on the bone marrow cell dosage because engraftment was greatly reduced when the bone marrow dosage was reduced from 40 million to 20 million cells. Given along with 200 cGy TBI, anti-CD154 mAb provided alloengraftment effects equivalent to 450 to 500 cGy TBI. Engraftment promotion by anti-CD154 mAb was superior to in vivo T-cell depletion, which led to only transient engraftment. The administration of anti-B7 mAbs did not result in the promotion of alloengraftment. The engraftment promotion capacity of anti-CD154 mAb required balanced CD28:B7 and CD152:B7 interactions, suggesting that successful combined therapeutic targeting of the CD28/CD152:B7 pathway with the CD154:CD40 pathway for engraftment promotion may be clinically challenging to achieve. Depletion of CD8+ T cells in combination with anti-CD154 mAb did not increase engraftment in comparison with anti-CD154 mAb alone, indicating that anti-CD154 mAb can be effective in inducing donor tolerance even in the presence of alloreactive CD8+cells. Interestingly, anti-CD154 mAb increased alloengraftment in a strain combination in which CD8+ cells, but not CD4+ cells, mediate rejection. Host CD4+ cells were required for the induction of donor tolerance by anti-CD154 mAb because depletion, but not coating, of CD4+ T cells abrogated engraftment induced by anti-CD154 mAb. The absence of alloengraftment in recipients deficient in CD154, conditioned with 200 cGy TBI, suggest that engraftment promotion by anti-CD154 mAb may not simply be the result of a blockade of the CD154:CD40 costimulatory pathway. Taken together, these data are consistent with the induction of a CD4+ regulatory cell by anti-CD154 mAb. These data have important clinical considerations for the development of nonmyeloablative transplantation protocols.
Materials and methods
Mice
C57BL/6 (H2b, CD45.2) (termed B6), congeneic CD45.1 B6, BALB/c (H2d), and C3H/HeN (H2k) mice were purchased from the National Institutes of Health (Bethesda, MD). B6 CD28 deletional (termed CD28−/−) and B6.CH-2bm1 (B6 class I mutants, H2b, CD45.2) (termed bm1) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6 and BALB/c CD154 deletional mice (termed CD154−/−), both back-crossed 10 generations onto their respective backgrounds, were bred at the University of Minnesota. Female mice were used at 8 to 12 weeks of age and housed in a specific pathogen-free facility in micro-isolator cages.
Bone marrow transplantation
Mice were subjected to total body irradiation (TBI) by x-ray (39 cGy/min) at the indicated dosage on day −1. On day 0, 40 × 106 whole bone marrow cells (unless otherwise indicated) from BALB/c or B6 CD45.1 donor mice were injected intravenously by lateral tail vein injection into irradiated B6 or bm1 recipients. Survival was monitored daily, and mice were weighed twice weekly for the first month after transplantation, then once weekly thereafter. Anti-CD154 mAb (hybridoma MR1, hamster immunoglobulin G [IgG]) was obtained by culturing the hybridoma in 10% fetal bovine serum–Dulbecco minimum essential medium in a hollow fiber bioreactor (AccucystJr; Cellex Biosciences, Minneapolis, MN). Supernatant was purified by ammonium sulfate precipitation. Anti-CD154 mAb or irrelevant hamster IgG (hIgG) (Rockland, Gilbertsville, PA) was administered intraperitoneally at a dosage of 200 μg daily from days −1 through +5, then twice weekly until day 14 after BMT. An abbreviated schedule of 200 μg daily on days −1, 0, and 1 was used in some experiments as indicated. Anti-CD154 injections were initiated on day −1 to ensure mAbs would be circulating at the time of BM infusion. Where indicated, depleting anti-CD4 (hybridoma GK1.5, rat IgG2), anti-CD8 (hybridoma 2.43, rat IgG2b), or both was administered intraperitoneally at a dosage of 400 μg on days −2, 1, 5, and 9. Nondepleting anti-CD4 (hybridoma YTS. 177.9.6.1, rat IgG), was administered intraperitoneally at a dosage of 1 mg on days −1, 1, 3, 5, 8, and 11, a dosage and a schedule that have been shown to induce skin graft tolerance in CD4+ cells.13Anti-CD28 (hybridoma 37.51, hamster IgG) was administered intraperitoneally at a dosage of 100 μg every other day from day −1 through day 14, a dosage and a schedule that have been found to prevent lethal GVHD.14 Anti-CD80 (anti–B7-1) (hybridoma 16-10A1, hamster IgG) and anti-CD86 (anti–B7-2) (hybridoma GL1, rat IgG) were administered intraperitoneally at a dosage of 250 μg on days −1, 0, and 1, then 125 μg 3 times/wk through day 14, a dosage and a schedule that we have found inhibits lethal GVHD.15 Anti-CD152 (hybridoma UC10-4F10-11, hamster IgG) was administered intraperitoneally at a dosage of 400 μg on days −1 through 5, then 200 μg 3 times/wk through day 14, a dosage and a schedule that we have found inhibits engraftment under increased conditioning.16 Antibodies other than anti-CD154 were obtained by ascites collection in nude mice and were purified by ammonium sulfate precipitation.
Flow cytometric analysis
Peripheral blood leukocytes (PBLs) were obtained by retro-orbital venipuncture and harvested over a Ficoll gradient. Single cell suspensions were made of bone marrow cells, thymi, and spleens, where indicated. Cells were stained with fluorochrome-conjugated Abs (anti-CD8, anti-CD4, anti–MAC-1, anti-CD19, anti-CD45.1, anti-CD45.2, anti-H2d, and anti-H2b) purchased from PharMingen (San Diego, CA). The irrelevant control used was 3A1E (human CD7). Stained cells were analyzed using CellQuest software on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Skin grafting
Full-thickness skin grafts (approximately 1 cm2) obtained from BALB/c and C3H/HeN donor mice were transplanted on the lateral thorax of recipient mice and secured with a bandage for 7 days. Grafts were monitored daily for the first 3 weeks and weekly thereafter. Rejection was defined as the complete loss of viable donor graft tissue.
Statistics
Group comparisons were made by Student t test.P ≤ .05 was considered significant.
Results
Anti-CD154 mAb results in the engraftment of a single dose of allogeneic bone marrow in more than 90% of recipients undergoing a nonmyeloablative conditioning regime of 200 cGy TBI
B6 mice were conditioned with 200 cGy TBI and administered a single dose of 40 × 106 (∼2 × 109/kg) BALB/c allogeneic bone marrow cells and either irrelevant hamster IgG or anti-CD154 mAb. Survival rates were greater than 99%, with no morbidity at any time after transplantation in rejected control mice or anti-CD154–treated engrafted chimeric mice. Neither group of mice experienced weight loss after transplantation (data not shown). Mice conditioned with 200 cGy TBI experienced relatively modest and transient lymphopenia. Total spleen counts on day 7 after irradiation were 21% those of nonirradiated controls and rebounded to 59% of nonirradiated controls by day 14. Absolute splenic T-cell numbers were 26% and 34% those of nonirradiated controls on days 7 and 14, respectively. Thymus counts were 55% and 49% in irradiated mice 7 and 14 days, respectively, after irradiation compared to those in nonirradiated controls. Irradiated mice experienced 5% or less reduction in bone marrow counts on either day 7 or day 14 after TBI (n = 5 per group, data not shown).
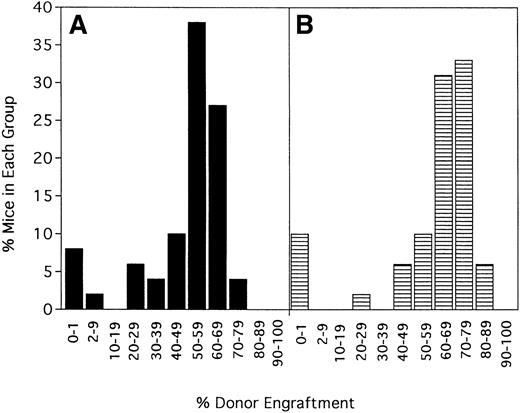
Irrelevant hamster IgG–treated controls and anti-CD154–treated mice were evaluated for percentage donor chimerism at 6 weeks after transplantation by PBL phenotyping (Figure1). In contrast to 0 of 100 hIgG-treated controls in a total of 10 experiments, 91% (90 of 99) of anti-CD154–treated recipients had levels of donor engraftment ranging from 2% to 75% (Figure 1 and data not shown). Including the nonengrafted mice, the 99 anti-CD154–treated mice had a mean donor engraftment level of 48% ± 19%. Moreover, 60% of anti-CD154–treated mice had donor engraftment levels of 50% to 69%.
Anti-CD154 mAb administration results in donor chimerism of allogeneic bone marrow in more than 90% of recipients receiving a nonmyeloablative conditioning regime of 200 cGy TBI.
B6 mice were irradiated on day −1 and infused with 40 × 106 BALB/c bone marrow cells on day 0. Irrelevant hIgG or anti-CD154 mAb was administered from day 1 through day 14 after BMT. PBLs were typed for percentage donor-host chimerism at 6 weeks after BMT. Ten mice per group underwent transplantation. The graph represents a pool of 10 experiments. No hIgG-treated mice had any level of detectable donor chimerism (not shown). On the x-axis are ranges of percentage donor engraftment. On the y-axis are percentages of mice in each donor percentile. The total number of mice in each percentage donor range is shown above the column. The average percentage donor engraftment for all 99 mice was 48% ± 19%.
Anti-CD154 mAb administration results in donor chimerism of allogeneic bone marrow in more than 90% of recipients receiving a nonmyeloablative conditioning regime of 200 cGy TBI.
B6 mice were irradiated on day −1 and infused with 40 × 106 BALB/c bone marrow cells on day 0. Irrelevant hIgG or anti-CD154 mAb was administered from day 1 through day 14 after BMT. PBLs were typed for percentage donor-host chimerism at 6 weeks after BMT. Ten mice per group underwent transplantation. The graph represents a pool of 10 experiments. No hIgG-treated mice had any level of detectable donor chimerism (not shown). On the x-axis are ranges of percentage donor engraftment. On the y-axis are percentages of mice in each donor percentile. The total number of mice in each percentage donor range is shown above the column. The average percentage donor engraftment for all 99 mice was 48% ± 19%.
Engraftment promotion by anti-CD154 mAb is long-term and multilineage and involves specific host-donor tolerance
To determine the stability of engraftment, 5 separate experiments (n = 48 mice) were evaluated at 6 weeks and again at 100 to 139 days after transplantation (Figure 2). The average donor engraftment was 49% ± 20% at 6 weeks after transplantation and was modestly higher at 59% ± 23% at 100 to 139 days after transplantation (P = .022). Mice followed up for more than 1 year had stable chimerism, with no mice losing their grafts (data not shown).
Engraftment is durable and long term.
Forty-eight mice from 5 of the experiments shown in Figure 1 were phenotyped a second time for percentage donor-host chimerism at 100 to 139 days after BMT. On the x-axis are ranges of percentage donor engraftment. On the y-axis are percentages of mice in each of those donor percentiles. (A) Chimerism results at 6 weeks after BMT (X = 49 ± 20%). (B) Results at the second chimerism evaluation more than 100 days after BMT (X = 59 ± 23%).
Engraftment is durable and long term.
Forty-eight mice from 5 of the experiments shown in Figure 1 were phenotyped a second time for percentage donor-host chimerism at 100 to 139 days after BMT. On the x-axis are ranges of percentage donor engraftment. On the y-axis are percentages of mice in each of those donor percentiles. (A) Chimerism results at 6 weeks after BMT (X = 49 ± 20%). (B) Results at the second chimerism evaluation more than 100 days after BMT (X = 59 ± 23%).
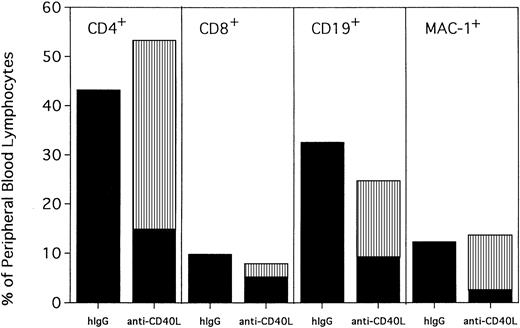
PBLs from hIgG-treated rejected control mice and anti-CD154–treated mice were phenotyped for percentages of T cells, B cells, and myeloid cells at 120 days after transplantation (Figure3). Anti-CD154–treated mice had a modest but statistically higher proportion of CD4+ T cells (53% vs 43%; P = .0004) and a lower proportion of CD19+ B cells (25% vs 32%; P = .0002) and CD8+ T cells (8% vs 10%; P = .0109) than hIgG-treated rejected control mice. The proportion of myeloid cells was similar between the 2 groups (P = .2794). Interestingly, although CD4+ T cells, B cells, and myeloid cells from anti-CD154–treated mice were mostly donor in origin (74%, 64%, and 83%, respectively), only 37% of the CD8+ cells were of donor origin.
Anti-CD154–facilitated alloengraftment is multilineage.
Twenty mice from 2 representative experiments shown in Figure 1 were phenotyped at 120 days after BMT for donor-host origin of CD4+ and CD8+ T cells, CD19+ B cells, and MAC-1+ myeloid cells. On the x-axis are shown the host and donor proportions of each of the lineages. ▪ indicates the proportion of each lineage of host origin; ▥, the proportion of each lineage that is of donor origin. On the y-axis is shown the percentage of PBLs of each lineage. Irrelevant hIgG–treated mice had no detectable donor chimerism and thus are composed entirely of host-type cells. Note that most CD4+ T cells, CD19+ B cells, and MAC-1+ myeloid cells in anti-CD154–treated mice are of donor origin. In contrast, most of the CD8+ T cells are of host origin.
Anti-CD154–facilitated alloengraftment is multilineage.
Twenty mice from 2 representative experiments shown in Figure 1 were phenotyped at 120 days after BMT for donor-host origin of CD4+ and CD8+ T cells, CD19+ B cells, and MAC-1+ myeloid cells. On the x-axis are shown the host and donor proportions of each of the lineages. ▪ indicates the proportion of each lineage of host origin; ▥, the proportion of each lineage that is of donor origin. On the y-axis is shown the percentage of PBLs of each lineage. Irrelevant hIgG–treated mice had no detectable donor chimerism and thus are composed entirely of host-type cells. Note that most CD4+ T cells, CD19+ B cells, and MAC-1+ myeloid cells in anti-CD154–treated mice are of donor origin. In contrast, most of the CD8+ T cells are of host origin.
To demonstrate that donor BM stem cells had engrafted, BM was obtained from anti-CD154 mAb–treated mixed chimeras and adoptively transferred into lethally irradiated B6 recipients. PBL phenotyping at 3 months after BMT revealed that these secondary recipients had high levels of BALB/c donor chimerism, indicating that allogeneic stem cell engraftment had occurred in the initial mixed chimeras (data not shown).
Anti-CD154–treated, engrafted, long-term chimeras (5 months after BMT) accepted donor type skin grafts for more than 6 months, in contrast to hIgG-treated, nonengrafted control mice that rejected donor skin grafts in 16 days (Table 1). All mice in both groups promptly rejected third-party skin grafts, indicating donor-specific tolerance in anti-CD154–treated chimeras.
Anti-CD154 monoclonal antibody promotes donor-specific tolerance
| Group . | Average time to rejection of skin grafts (d) . | |
|---|---|---|
| BALB/c . | C3H/HeN . | |
| B6 nontransplantation controls | 19.8 ± 1.9* | 17.2 ± 3.5 |
| hIgG-treated rejected mice | 15.8 ± 2.3 | 15.8 ± 2.3 |
| Anti-CD154–treated chimeric mice | Accept > 6 mo† | 15.4 ± 2.6 |
| Group . | Average time to rejection of skin grafts (d) . | |
|---|---|---|
| BALB/c . | C3H/HeN . | |
| B6 nontransplantation controls | 19.8 ± 1.9* | 17.2 ± 3.5 |
| hIgG-treated rejected mice | 15.8 ± 2.3 | 15.8 ± 2.3 |
| Anti-CD154–treated chimeric mice | Accept > 6 mo† | 15.4 ± 2.6 |
B6 mice were irradiated with 200 cGy TBI on day −1 and infused with 40 × 106 BALB/c BM on day 0. Irrelevant hamster IgG or anti-CD154 mAb was administered from day −1 through day 14 after BMT. PBLs were typed for percentage donor-host at 6 weeks after BMT. Five months after BMT, donor-type (BALB/c) or third-party (C3H/HeN) skin grafts were placed on B6 nontransplantation control mice, hIgG-treated rejected mice, and anti-CD154–treated chimeric mice (n = 5 per group). Shown is average time to rejection of grafts in days ± 1 SD. Anti-CD154–treated chimeric mice maintained their BALB/c skin grafts for more than 6 months.
hIgG indicates hamster immunoglobulin G; TBI, total body irradiation; BMT, bone marrow transplantation; mAb, monoclonal antibody; PBL, peripheral blood leukocyte.
P = .017
P < .001 compared with hIgG-treated rejected mice.
Anti-CD154 mAb combined with 200 cGy TBI results in equivalent engraftment as 450 to 500 cGy TBI
Previous engraftment studies by our group have indicated that 500 to 550 cGy TBI is required to achieve donor engraftment in mice receiving one infusion of 10 to 20 × 106 BM cells in the absence of any graft-promoting agent in the strain combination used for these studies. Because anti-CD154 mAb resulted in significant donor chimerism in most recipients at 200 cGy TBI, we wanted to determine how much the TBI could be reduced while still achieving donor chimerism in most recipients. We also wanted to relate the engraftment promotion capacity of anti-CD154 mAb to the level of TBI it could replace. In the absence of anti-CD154 treatment, no mice had detectable levels of donor chimerism at 300, 350, or 400 cGy TBI (Figure4). At 450 cGy TBI, 2 of 10 mice had evidence of donor chimerism (group average, 17% ± 37%). Increasing TBI to 500 cGy resulted in 7 of 10 mice with donor chimerism (group average, 69% ± 47%). All mice receiving 550 and 600 cGy TBI were 94% to 100% donor. In contrast, anti-CD154 promoted donor engraftment in 80% of recipients conditioned with just 50 cGy TBI (group average, 10% ± 6%; range, 0%-18%) (Figure 4 and data not shown). Increasing conditioning to 100 cGy TBI resulted in 79% of anti-CD154 mAb–treated recipients with donor chimerism (group average, 24% ± 14%; range, 0%-39%). Anti-CD154 did not promote significant macrochimerism in the absence of TBI in mice receiving a single infusion of 40 × 106 BM cells (Figure 4). Alloengraftment promotion by anti-CD154 mAb was highly dependent on the BM cell dose because only 2 of 10 mice conditioned with 200 cGy TBI and receiving anti-CD154 mAb had evidence of donor chimerism when the BM dose was reduced by half, to 20 × 106 cells (approximately 109/kg) (data not shown).
Anti-CD154 mAb administration results in donor chimerism in 80% of mice conditioned with as little as 50 cGy.
Anti-CD154 mAb–facilitated engraftment results in engraftments equivalent to 450 to 500 cGy TBI. B6 mice were conditioned with the indicated cGy TBI (shown on the x-axis) on day −1 and were administered 40 × 106 BALB/c bone marrow cells on day 0. Mice were untreated (▪) or administered anti-CD154 mAb (▥). PBLs were phenotyped at 6 weeks after BMT for percentage donor-host chimerism. On the y-axis are the percentages of mice with donor chimerism. The average percentage of donor chimerism for the entire group ± 1 SD is shown above each column. n = 10 per group for control mice and for anti-CD154–treated mice with 0 cGy TBI. n = 20, a pool of 2 experiments for mice receiving anti-CD154 conditioned with 50 cGy TBI. n = 38, a pool of 4 experiments for mice receiving anti-CD154 conditioned with 100 cGy TBI. n = 99, a pool of 10 experiments as shown in Figure 1 for mice receiving anti-CD154 conditioned with 200 cGy TBI.
Anti-CD154 mAb administration results in donor chimerism in 80% of mice conditioned with as little as 50 cGy.
Anti-CD154 mAb–facilitated engraftment results in engraftments equivalent to 450 to 500 cGy TBI. B6 mice were conditioned with the indicated cGy TBI (shown on the x-axis) on day −1 and were administered 40 × 106 BALB/c bone marrow cells on day 0. Mice were untreated (▪) or administered anti-CD154 mAb (▥). PBLs were phenotyped at 6 weeks after BMT for percentage donor-host chimerism. On the y-axis are the percentages of mice with donor chimerism. The average percentage of donor chimerism for the entire group ± 1 SD is shown above each column. n = 10 per group for control mice and for anti-CD154–treated mice with 0 cGy TBI. n = 20, a pool of 2 experiments for mice receiving anti-CD154 conditioned with 50 cGy TBI. n = 38, a pool of 4 experiments for mice receiving anti-CD154 conditioned with 100 cGy TBI. n = 99, a pool of 10 experiments as shown in Figure 1 for mice receiving anti-CD154 conditioned with 200 cGy TBI.
Anti-B7 mAbs alone do not promote alloengraftment but can enhance engraftment promotion by anti-CD154 mAb under certain conditions
Given that the CD28:B7 costimulatory pathway has a well-recognized role in alloresponses, we investigated whether blockade with anti-CD80 and anti-CD86 mAbs would also promote alloengraftment under similar transplantation conditions. In contrast to anti-CD154, anti-B7 mAbs alone did not result in any donor chimerism at either 100 or 200 cGy TBI (Tables 2,3). However, at 100 cGy TBI, the addition of anti-B7 mAbs to anti-CD154 resulted in an increase in average donor chimerism from 32% to 51% (P = .01) (Table2). A second, more abbreviated schedule of anti-CD154 mAb was also examined in which anti-CD154 mAb was administered only on days −1, 0, and 1 to recipients conditioned with 100 cGy TBI. Chimerism was lower, albeit not statistically different in recipients receiving the more abbreviated schedule of anti-CD154 mAb than in those receiving anti-CD154 mAb for 2 weeks after BMT (20% ± 16% vs 32% ± 20%;P = .176) (Table 2). Under this more abbreviated anti-CD154 mAb administration schedule, anti-B7 mAbs increased engraftment when combined with anti-CD154 mAb compared with anti-CD154 mAb alone (51% ± 5% vs 20% ± 16%; P < .001). Mice receiving 100 cGy TBI and 3 days of anti-CD154 and anti-B7 mAbs had increased chimerism levels compared with mice receiving a more prolonged schedule of anti-CD154 as a single agent for 2 weeks after transplantation (51% ± 5% vs 32% ± 20%;P = .009) (Table 2). In contrast, under increased conditioning of 200 cGy TBI, the chimerism level with anti-CD154 mAb alone was 67%, and the addition of anti-B7 mAbs did not further increase engraftment (Table 3).
Although anti-B7 monoclonal antibodies alone do not promote alloengraftment, they enhance engraftment promotion by anti-CD154 monoclonal antibody
| TBI (cGy) . | mAb . | No. chimeric . | % Donor . |
|---|---|---|---|
| 100 | Hamster IgG | 0/10 | 0 ± 0 |
| 100 | Anti-CD154* | 7/9 | 32 ± 20‡ |
| 100 | Anti-CD80, anti-CD86* | 0/10 | 0 ± 0 |
| 100 | Anti-CD154, anti-CD80, anti-CD86* | 10/10 | 51 ± 4‡,2-153 |
| 100 | Anti-CD154† | 7/10 | 20 ± 16‡ |
| 100 | Anti-CD154, anti-CD80, anti-CD86† | 10/10 | 51 ± 5‡,2-153 |
| TBI (cGy) . | mAb . | No. chimeric . | % Donor . |
|---|---|---|---|
| 100 | Hamster IgG | 0/10 | 0 ± 0 |
| 100 | Anti-CD154* | 7/9 | 32 ± 20‡ |
| 100 | Anti-CD80, anti-CD86* | 0/10 | 0 ± 0 |
| 100 | Anti-CD154, anti-CD80, anti-CD86* | 10/10 | 51 ± 4‡,2-153 |
| 100 | Anti-CD154† | 7/10 | 20 ± 16‡ |
| 100 | Anti-CD154, anti-CD80, anti-CD86† | 10/10 | 51 ± 5‡,2-153 |
B6 mice were irradiated with 100 cGy TBI on day −1 and infused with 40 × 106 BALB/c BM on day 0.
For abbreviations, see Table 1.
Antibodies were administered from day −1 through day 14.
A second schedule of antibodies was examined whereby antibodies were administered only on days −1, 0, and 1 after BMT. PBLs were typed for percentage donor-host chimerism on 120 day after BMT. Chimeric is defined as more than 3% donor PBLs. Percentage donor is average percentage donor cells of all mice in the group ± 1 SD.
P < .001 compared to hamster IgG.
P = .01 compared to anti-CD154 alone.P = .176 for anti-CD154 administered for 2 weeks after BMT versus days −1, 0, and 1.
Engraftment promotion by anti-CD154 monoclonal antibody is abrogated by anti-CD152 monoclonal antibody
| TBI (cGy) . | mAb . | No. chimeric . | % Donor . |
|---|---|---|---|
| 200 | Hamster IgG | 0/10 | 0 ± 0 |
| 200 | Anti-CD154 | 8/9 | 67 ± 273-150 |
| 200 | Anti-CD80, anti-CD86 | 0/10 | 0 ± 0 |
| 200 | Anti-CD154, anti-CD80, CD86 | 9/9 | 75 ± 83-150 |
| 200 | Anti-CD154, anti-CD152 | 0/10 | 0 ± 03-151 |
| TBI (cGy) . | mAb . | No. chimeric . | % Donor . |
|---|---|---|---|
| 200 | Hamster IgG | 0/10 | 0 ± 0 |
| 200 | Anti-CD154 | 8/9 | 67 ± 273-150 |
| 200 | Anti-CD80, anti-CD86 | 0/10 | 0 ± 0 |
| 200 | Anti-CD154, anti-CD80, CD86 | 9/9 | 75 ± 83-150 |
| 200 | Anti-CD154, anti-CD152 | 0/10 | 0 ± 03-151 |
B6 mice were irradiated with 200 cGy TBI on day −1 and infused with 40 × 106 BALB/c BM on day 0. Antibodies were administered from day −1 through day 14 after BMT. PBLs were typed for percentage donor-host chimerism at 139 days after BMT. Chimeric is defined as more than 3% donor PBL. Percentage donor is defined as average percentage donor cells of all mice in the group ± 1 SD.
For abbreviations, see Table 1.
P < .001 compared with hamster IgG.
P < .001 compared with anti-CD154 alone.
Anti-B7 mAbs can block the inhibitory CD152:B7 pathway and the stimulatory CD28:B7 pathway. We have previously shown that in vivo blockade of the CD152:B7 pathway with anti-CD152 mAb resulted in increased graft rejection.16 To determine whether the efficacy of anti-CD154 mAb in facilitating alloengraftment would be impeded by blocking CD152:B7 interactions, anti-CD152 mAb was administered in conjunction with anti-CD154 mAb. Anti-CD152 mAb completely abrogated engraftment promotion by anti-CD154 mAb (Table 3). Anti-CD28 mAb, administered as a single agent to selectively block CD28:B7 interactions, did not result in donor chimerism. Moreover, in contrast to the beneficial effect of combining anti-B7 and anti-CD154 mAbs, anti-CD28 mAb, like anti-CD152, abrogated engraftment promotion by anti-CD154 mAb (Table 4, experiments 1 and 2). Additionally, irradiated B6 CD28−/− mice, which are known to have blunted in vivo alloresponses,16-18 were resistant to engraftment promotion by anti-CD154 mAb (Table 4, experiment 3). Collectively, these data indicate that engraftment promotion conferred by anti-CD154 mAb likely requires balanced CD28:B7 and CD152:B7 interactions.
Anti-CD154 monoclonal antibody promotion of engraftment is CD28 dependent
| Expt . | TBI . | Recipient . | mAb . | No. chimeric . | % Donor . |
|---|---|---|---|---|---|
| 1 | 200 | +/+ | Hamster IgG | 0/10 | 0 ± 0 |
| 1 | 200 | +/+ | Anti-CD154 | 9/10 | 52 ± 224-150 |
| 1 | 200 | +/+ | Anti-CD28 | 0/10 | 0 ± 0 |
| 2 | 100 | +/+ | None | 0/10 | 0 ± 0 |
| 2 | 100 | +/+ | Anti-CD154 | 4/9 | 14 ± 174-150 |
| 2 | 100 | +/+ | Anti-CD154 + | 0/10 | 0.5 ± 14-151 |
| anti-CD28 | |||||
| 3 | 200 | +/+ | Hamster IgG | 0/10 | 0 ± 0 |
| 3 | 200 | +/+ | Anti-CD154 | 8/9 | 54 ± 214-150 |
| 3 | 200 | CD28−/− | Hamster IgG | 0/10 | 0 ± 0 |
| 3 | 200 | CD28−/− | Anti-CD154 | 0/10 | 0 ± 0 |
| Expt . | TBI . | Recipient . | mAb . | No. chimeric . | % Donor . |
|---|---|---|---|---|---|
| 1 | 200 | +/+ | Hamster IgG | 0/10 | 0 ± 0 |
| 1 | 200 | +/+ | Anti-CD154 | 9/10 | 52 ± 224-150 |
| 1 | 200 | +/+ | Anti-CD28 | 0/10 | 0 ± 0 |
| 2 | 100 | +/+ | None | 0/10 | 0 ± 0 |
| 2 | 100 | +/+ | Anti-CD154 | 4/9 | 14 ± 174-150 |
| 2 | 100 | +/+ | Anti-CD154 + | 0/10 | 0.5 ± 14-151 |
| anti-CD28 | |||||
| 3 | 200 | +/+ | Hamster IgG | 0/10 | 0 ± 0 |
| 3 | 200 | +/+ | Anti-CD154 | 8/9 | 54 ± 214-150 |
| 3 | 200 | CD28−/− | Hamster IgG | 0/10 | 0 ± 0 |
| 3 | 200 | CD28−/− | Anti-CD154 | 0/10 | 0 ± 0 |
B6 CD28+/+ (termed +/+) or CD28−/− mice were irradiated with 100 or 200 cGy TBI as indicated on day −1 and infused with 40 × 106 BALB/c BM on day 0. Antibodies were administered from day −1 through day 14. PBLs were typed for percentage donor-host at 6 weeks after BMT. Chimeric is defined as more than 3% donor PBLs. Percentage donor is defined as average percentage donor cells of all mice in the group ± 1 SD.
For abbreviations, see Table 1.
P ≤ .01 compared to hamster IgG.
P = .01 compared to anti-CD154 alone.
Host CD4+CD154+ cells are required for engraftment promotion by anti-CD154 mAb
Rejection in this strain combination can be mediated by both CD4+ and CD8+ T cells (B.R.B., unpublished data, 1996). Because CD154 is expressed primarily on activated CD4+ T cells and anti-CD154 mAb infusion has been shown to inhibit CD4+ but not CD8+ T-cell–mediated GVHD responses,11 the effect of CD4+ or CD8+ subset depletion on engraftment promotion by anti-CD154 mAb was studied. If host CD8+ T cells escape inhibition by anti-CD154 mAb, the depletion of CD8+ T cells should enhance the engraftment-promoting effects of anti-CD154 mAb administration. As expected, all recipients of anti-CD154 mAb conditioned with 200 cGy TBI engrafted, with an average engraftment of 57% donor cells at 44 days and 68% at 199 days, whereas no irrelevant mAb–treated controls had evidence of donor chimerism (Table5). Neither CD4+ nor CD8+ T-cell depletion alone led to donor chimerism in any recipients (Tables 5, 6). In contrast to the stable engraftment seen with the administration of anti-CD154 mAb, depletion of both CD4+ and CD8+ T cells resulted in only transient engraftment. Although all T-cell–depleted mice had donor chimerism ranging from 4% to 69% on day 44 (average, 31% ± 25%), all mice lost their graft by day 119 (Table 5). Interestingly, although CD8+ cells can mediate rejection and would not be expected to be directly affected by anti-CD154 mAb, there was no benefit in codepleting CD8+ cells in conjunction with anti-CD154 mAb compared to anti-CD154 mAb alone (68% ± 8% vs 72% ± 9% on day 119; P = .339; Table 5). Natural killer (NK) cells can mediate rejection in this strain combination under conditions of low BM cell number. However, there was no benefit in codepleting NK cells in conjunction with anti-CD154 mAb compared to anti-CD154 mAb alone at a dose of 40 × 106 BM cells (44% ± 33% vs 50% ± 31% on day 120; P = .723; n = 10 per group; data not shown). Significantly, the administration of a depleting anti-CD4 mAb in conjunction with anti-CD154 mAb completely abrogated engraftment in 18 of 20 recipients (pool of Tables 5 and 6). In contrast, the administration of a nondepleting anti-CD4 mAb in conjunction with anti-CD154 mAb did not abrogate engraftment compared to anti-CD154 alone (49% ± 28% vs 62% ± 6%; P = .160; Table6). Collectively, these data indicate a requirement for the presence of CD4+ cells for engraftment promotion by anti-CD154 mAb and suggest anti-CD154 mAb can be effective in inducing tolerance to donor BM grafts even in the presence of alloreactive CD8+ or NK cells.
Host CD4+ cells are required for engraftment promotion by anti-CD154 monoclonal antibody
| mAb . | No. chimeric (day 44) . | % donor (day 44) . | No. chimeric (day 119) . | % donor (day 119) . |
|---|---|---|---|---|
| None | 0/10 | 0 | 0/10 | 0 |
| Anti-CD154 | 10/10 | 57 ± 75-150 | 10/10 | 68 ± 65-150 |
| Depleting anti-CD4 | 0/9 | 05-151 | ND | ND |
| Depleting anti-CD8 | 0/10 | 05-151 | ND | ND |
| Depleting anti-CD4 + anti-CD8 | 10/10 | 31 ± 255-150,5-151 | 0/10 | 1 ± 15-150,5-151 |
| Anti-CD154 + depleting anti-CD4 | 2/10 | 8 ± 195-151 | 2/10 | 11 ± 235-151 |
| Anti-CD154 + depleting anti-CD8 | 10/10 | 61 ± 75-150 | 10/10 | 72 ± 95-150 |
| mAb . | No. chimeric (day 44) . | % donor (day 44) . | No. chimeric (day 119) . | % donor (day 119) . |
|---|---|---|---|---|
| None | 0/10 | 0 | 0/10 | 0 |
| Anti-CD154 | 10/10 | 57 ± 75-150 | 10/10 | 68 ± 65-150 |
| Depleting anti-CD4 | 0/9 | 05-151 | ND | ND |
| Depleting anti-CD8 | 0/10 | 05-151 | ND | ND |
| Depleting anti-CD4 + anti-CD8 | 10/10 | 31 ± 255-150,5-151 | 0/10 | 1 ± 15-150,5-151 |
| Anti-CD154 + depleting anti-CD4 | 2/10 | 8 ± 195-151 | 2/10 | 11 ± 235-151 |
| Anti-CD154 + depleting anti-CD8 | 10/10 | 61 ± 75-150 | 10/10 | 72 ± 95-150 |
B6 mice were irradiated with 200 cGy TBI on day − 1 and infused with 40 × 106 BALB/c BM on day 0. Anti-CD154 mAb was administered daily from day − 1 through day 5, then twice a week until day 14 at 200 μg/dose. Four hundred micrograms depleting anti-CD4, anti-CD8, or both was given on days − 2, 1, 5, and 9. PBLs were typed for percentage donor-host chimerism on days 44 and 119. n = 10 per group. Chimeric indicates number of mice with more than 3% donor cells. Shown is average percentage donor ± 1 SD of all mice in group.
ND indicates not done; for other abbreviations, see Table 1.
P ≤ .002 compared to untreated controls.
P ≤ .003 compared to mice receiving only anti-CD154 mAb.
Host CD4+ cells are required for engraftment promotion by anti-CD154 monoclonal antibody
| mAb . | No. chimeric . | % Donor . |
|---|---|---|
| None | 0/10 | 0 |
| Anti-CD154 | 0/10 | 62 ± 66-150 |
| Anti-CD154 + depleting anti-CD4 | 0/10 | 06-151 |
| Nondepleting anti-CD4 | 0/10 | 06-151 |
| Anti-CD154 + nondepleting anti-CD4 | 9/10 | 49 ± 286-150 |
| mAb . | No. chimeric . | % Donor . |
|---|---|---|
| None | 0/10 | 0 |
| Anti-CD154 | 0/10 | 62 ± 66-150 |
| Anti-CD154 + depleting anti-CD4 | 0/10 | 06-151 |
| Nondepleting anti-CD4 | 0/10 | 06-151 |
| Anti-CD154 + nondepleting anti-CD4 | 9/10 | 49 ± 286-150 |
B6 mice were irradiated with 200 cGy TBI on day −1 and infused with 40 × 106 BALB/c BM on day 0. Anti-CD154 mAb was administered daily from day −1 through day 5 daily, then twice a week until day 14 at 200 μg/dose. Four hundred micrograms depleting anti-CD4 was given on days −2, 1, 5, and 9, and 1 mg nondepleting anti-CD4 was given on days −1, 1, 3, 5, 8, and 11. PBLs were typed for percentage donor-host chimerism at 6 weeks after BMT. Ten mice per group underwent transplantation. No. chimeric indicates number of mice with more than 3% donor cells. Shown is average percentage donor ± 1 SD of all mice in group.
For abbreviations, see Table 1.
P ≤ .002 compared with untreated controls.
P = .160, anti-CD154 administered alone versus in combination with nondepleting anti-CD4.
To further determine whether anti-CD154 mAb could inhibit CD8+ T-cell–mediated donor BM rejection, we used a system in which B6 donor and host are disparate only at major histocompatibility complex class I loci (donor B6-CD45.1 BM into bm1-CD45.2 recipients). In this strain combination, CD8+T-cell depletion augments alloengraftment, whereas neither CD4+ nor NK cell depletion has any effect.19Anti-CD154 mAb significantly increased B6 donor chimerism levels in 50 cGy-irradiated bm1 recipients compared with irrelevant mAb–treated control (29% ± 11% vs 1% ± 3%; P < .001; n = 10 per group; data not shown). Because anti-CD154 mAb infusion has no beneficial effect in preventing GVHD induced by the infusion of purified CD8+ T cells in this strain combination, these data are most consistent with an effect of anti-CD154 mAb on nonalloreactive CD4+ T cells that influence CD8+ T cell alloreactivity in vivo.
If simple blockade of the CD154:CD40 pathway was sufficient to induce donor tolerance, CD154−/− mice should be tolerant of donor bone marrow grafts. Experiments revealed that CD154−/− mice were not tolerant of donor grafts, and, as expected, anti-CD154 mAb-treatment of these recipients did not result in the promotion of alloengraftment (Table7). Anti-CD154 mAb did promote the engraftment of donor BALB/c CD154−/− BM in wild-type B6 recipients, indicating that engraftment promotion by anti-CD154 mAb was due to targeting of host CD154-expressing cells (data not shown). In addition, anti-CD154 did promote the engraftment of rigorously T-cell–depleted bone marrow, providing further evidence that donor CD154+ T cells were not essential for engraftment promotion by anti-CD154 mAb (data not shown). Together these data indicate that it is specifically host CD4+CD154+ rather than donor CD4+CD154+ cells that are required for the host-donor tolerance induced by anti-CD154 mAb. Furthermore, these data may suggest that rather than promoting alloengraftment by simple costimulatory blockade of the CD154:CD40 pathway, anti-CD154 mAb may be delivering a negative signal to a host CD4+CD154+ T cell to actively promote the development of specific donor tolerance.
Host CD15+ cells are required for engraftment promotion by anti-CD154 monoclonal antibody
| Recipient . | mAb . | No. chimeric (day 119) . | % Donor . |
|---|---|---|---|
| +/+ | hIgG | 0/10 | 0 |
| +/+ | Anti-CD154 | 7/10 | 26 ± 227-150 |
| CD154−/− | hIgG | 0/10 | 0 |
| CD154−/− | Anti-CD154 | 0/10 | 0 |
| Recipient . | mAb . | No. chimeric (day 119) . | % Donor . |
|---|---|---|---|
| +/+ | hIgG | 0/10 | 0 |
| +/+ | Anti-CD154 | 7/10 | 26 ± 227-150 |
| CD154−/− | hIgG | 0/10 | 0 |
| CD154−/− | Anti-CD154 | 0/10 | 0 |
B6 CD154+/+ (termed +/+) or CD154−/−mice were irradiated with 200 cGy TBI on day −1 and infused with 40 × 106 BALB/c BM on day 0. Irrelevant or anti-CD154 mAb was administered daily from day −1 through day +5 daily, then 2 ×/week until day 14 after BMT at 200 μg/dose. 10 mice/group received transplants. PBLs were typed for percentage donor-host at 6 weeks after BMT. No. chimeric indicates number of mice having >3% donor cells. Shown is average percentage donor ± 1 SD.
For abbreviations, see Table 1.
P < 0.001 as compared with hIgG-treated controls.
Discussion
In this study anti-CD154 mAb induced donor engraftment in more than 90% of recipients conditioned with 200 cGy TBI and given a single infusion of bone marrow cells (approximately 2 × 109/kg). Donor engraftment was long-term and multilineage. Significant levels of donor chimerism were present in mice receiving as little as 50 cGy TBI. Engraftment promotion by anti-CD154 mAb was superior to global in vivo T-cell depletion, which led to only transient engraftment. In contrast to anti-CD154 mAb, anti-B7 mAbs did not promote alloengraftment. Host CD4+CD154+ cells were required for the engraftment promotion capacity of anti-CD154 mAb. Host CD28:B7 interactions were also necessary for the graft-promoting effect of anti-CD154 mAb. Selective blockade of CD152:B7 interactions by anti-CD152 mAb prohibited the engraftment-promoting effects of anti-CD154 mAb. The depletion of potentially alloreactive CD8+ T or NK cells, in combination with anti-CD154, did not increase alloengraftment compared with anti-CD154 alone. Engraftment promotion may be caused by active signaling of the T cell by the mAb rather than a simple blockade of the CD154:CD40 costimulatory pathway.
The high level of mixed chimerism was not associated with any apparent morbidity either during or after BMT. There was no clinical evidence of acute or chronic GVHD at any time after BMT. Three months after transplantation, weights exceeded pretransplantation weights by more than 30% (data not shown). Engrafted anti-CD154–treated chimeras examined 5 months after transplantation had similar thymic, splenic, and bone marrow counts compared with those of rejected hIgG-treated control mice. There was no evidence of thymic atrophy, peripheral B-cell lymphopenia, T-cell lymphopenia, or increased number of splenic myeloid cells as would be expected with chronic or ongoing acute GVHD (data not shown). Anti-CD154–treated mixed chimeras accepted donor-type skin grafts long-term and promptly rejected third-party skin grafts indicative of donor-specific tolerance.
In contrast to data by others, we were unable to achieve engraftment promotion with anti-CD154 mAb in the absence of TBI. However, Wekerle and Sykes8 used a 5-fold higher BM dose (approximately 1010/kg) than we did, along with a single dose of anti-CD154 mAb on day 0 and CTLA4Ig on day 2, and they obtained donor levels of approximately 15%. Durham et al7 used a conventional BM dose but with repetitive infusions of BM totaling 160 × 106 cells (approximately 8 × 109/kg) and anti-CD154 mAb over 3 months and achieved donor levels of 6% to 12%. In that study, prolonged repetitive infusions were required for engraftment because mice receiving infusions of BM and mAb in just the first week did not have long-term detectable engraftment. Higher numbers of stem cells found in very high donor cell number or repetitive donor marrow infusions may be able to circumvent the requirement for any host stem cell injury induced by TBI. In comparison to these studies, we achieved 10%, 24%, and 48% average donor engraftment in mice conditioned with 50, 100, and 200 cGy TBI, respectively, with a single infusion of a moderate, clinically relevant BM dose and a 2-week course of anti-CD154 mAb as a single agent (Figure 4). The dose of 40 × 106 BM cells used for our studies may be within the range achievable clinically by the combined infusion of BM plus granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells. The critical relation between bone marrow cell dose, host stem cell injury, and alloengraftment is further illustrated by our studies that show that reducing the BM cell dose by 50% to 20 × 106 cells (109/kg) resulted in a substantial loss of engraftment. Tomita and Sykes20 found that myelosuppressive conditioning was required to obtain durable engraftment of moderate cell doses of syngeneic bone marrow and that host stem cells sustained significant injury at 150 to 300 cGy TBI. Bachar-Lustig et al21demonstrated, in an allogeneic murine BMT model, that escalation of BM doses by 4- to 5-fold led to full donor type chimerism in sublethally irradiated recipients.
Although robust donor tolerance has been induced in the absence of cytoreductive host treatment, the level of donor T-cell engraftment has been reported to be low. Wekerle et al8 found that only approximately 2% to 3% of T cells was of donor origin in mice receiving a high dose of allogeneic BM cells, anti-CD154 mAb, and CTLA4Ig. We found that though mice receiving anti-CD154 mAb conditioned with 50 cGy TBI did have long-term, multilineage engraftment, less than 1% of their T cells was of donor origin (data not shown). Increasing conditioning to 100 cGy or 200 cGy TBI resulted in dramatically increasing donor T-cell representation to 23% or 44%, respectively (Figure 3 and data not shown). These data likely are clinically significant. This current approach using a single dose of BM and anti-CD154 mAb with very low nontoxic doses of irradiation may translate more easily into human studies than those approaches using high-dose BM with 2 immunologic agents or requiring repetitive BM infusions. Low donor T-cell engraftment achieved in the absence of TBI may be adequate for the induction of tolerance for the purpose of solid organ transplantation. However, other patients, especially those with malignancies or those who may have defects in immune surveillance mechanisms may benefit from increased levels of donor T-cell chimerism achieved by low doses of TBI by providing an antitumor or an antiviral effect.
Our studies indicate that 200 cGy TBI was associated with partial and transient peripheral T-cell depletion. Even though reductions in the host T-cell content are necessary to achieve donor engraftment in our model, a depleting anti-CD4 mAb completely abrogated donor tolerance induced by anti-CD154 mAb indicating a requirement for the presence of some CD4+ cells for engraftment promotion by anti-CD154 mAb. The administration of a nondepleting CD4 mAb did not abrogate donor tolerance induced by anti-CD154 mAb. Additionally, in vivo T-cell depletion with anti-CD4 and anti-CD8 mAbs, known to deplete more than 95% of peripheral T cells,22 led to only transient engraftment. Although partial T-cell reduction may be necessary for engraftment by this strategy, the finding that rigorous in vivo pan-T-cell depletion is counterproductive to achieving alloengraftment under nonmyeloablative conditions has important clinical considerations. Our data suggest that clinical protocols involving T-cell elimination or global and total immunosuppression of the recipient may not benefit from the inclusion of anti-CD154 mAb. The preservation of host CD4+ T cells not only provides a specific and necessary target for tolerance induction by anti-CD154 mAb, it also may allow the host to maintain other potential beneficial T cells such as those capable of mediating anti-infectious and perhaps antitumor effects.
Although CD8+ T cells can participate in BM graft rejection in this model and CD154 is not an important costimulatory molecule for CD8+ T cells, we did not find an increase in donor engraftment when CD8+ cells were depleted in conjunction with anti-CD154 mAb. This would appear discordant with data by several groups that have demonstrated that anti-CD154 mAb administration failed to prevent CD8+ T-cell–mediated rejection of major histocompatibility complex-disparate organ allografts.23-25 In these instances, donor BM was not infused, and tolerance-induction strategies were geared toward inhibiting or regulating host T-cell responses against solid organ grafts implanted at the time of immunosuppressive therapy. For example, Marshall and Waldmann26 found that both CD4+and CD8+ T-cell subsets had to be targeted by nondepleting antibodies to induce skin graft tolerance in mice previously primed to multiple minor histocompatability antigens. Ablation of CD4+ T cells abolished tolerance, whereas blocking anti-CD4 mAbs did not.26 However, some CD8+ T-cell responses have been demonstrated to be highly dependent on CD4+ T cells. For example, we have previously reported that the expansion of alloreactive CD8+ T cells could be blocked by the administration of anti-CD154 mAb.27 Our data in the class I–only disparate system may suggest that anti-CD154 mAb may induce CD4+ regulatory cells that inhibit CD8+T-cell expansion and function. However, we cannot exclude the possibility that anti-CD154 mAb had a direct effect on CD8+T-cell alloresponses or inhibited CD4+ T-cell help. Although in the present study we did not find a benefit to CD8 depletion in conjunction with anti-CD154 mAb, it is possible that we might have uncovered an effect of CD8 depletion under different TBI or BM cell doses. However, our data suggest that anti-CD154 mAb administration can be effective in inducing donor tolerance in the presence of CD8+ alloreactive cells.
The administration of anti-CD80 and anti-CD86 mAbs did not result in engraftment promotion in recipients conditioned with 200 cGy TBI. Further, we found that anti-B7 mAbs did not increase alloengraftment in the same strain combination using a substantially higher level of conditioning of 600 cGy TBI and 107 T-cell–depleted allogeneic bone marrow cells (B.R.B., unpublished data, 1997). However, anti-B7 mAbs in conjunction with anti-CD154 mAb increased engraftment as compared with anti-CD154 mAb alone at 100 cGy TBI, suggesting that there may be a benefit in targeting both pathways under circumstances of lower conditioning or using a shorter schedule of mAb administration (Tables 2, 4). The additive benefit of targeting both pathways may be modest because a prolonged schedule of the combined administration of anti-CD154 and anti-B7 mAbs did not result in any engraftment in recipients given 40 × 106 BM cells and no TBI (data not shown). The therapeutic targeting of the CD28/CD152:B7 pathway is potentially complicated by the fact that CD80 and CD86 are ligands for both the costimulatory counter-receptor, CD28, and for the inhibitory counter-receptor, CD152. The preservation of host CD152:B7 interactions may be desirable and perhaps necessary for allowing donor tolerance to occur. This is supported by our data in which the administration of anti-CD152 mAb abrogated engraftment promotion by anti-CD154 mAb, which is consistent with data by several investigators who found that the induction of T-cell tolerance in vivo required CD152 engagement.28-31 Administration of anti-CD28 mAb would potentially preserve CD152:B7 interactions but may deliver a strong positive signal to the T cell that may then preclude the induction of tolerance. Alternatively, CD28 modulation may occur that may be functionally different from B7 blockade achieved with anti-B7 mAb infusion. CD28:B7 interactions may benefit tolerance-inducing strategies by reducing the number of potentially alloreactive T cells by priming these cells for apoptosis. For example, Wells and Turka32 have demonstrated a requirement for T-cell apoptosis in the induction of peripheral tolerance. Our data that anti-CD28 mAb prevents engraftment promotion by anti-CD154 mAb does not distinguish the possibilities. Future studies with CD28F(ab') may be helpful in resolving the issue. Interestingly, despite the fact that T cells from CD28−/− mice have impaired alloresponses as measured by GVHD induction,16-18 CD28−/−mice were not tolerant of donor grafts and, further, were resistant to induction of donor tolerance by anti-CD154 mAb (Table 3). There are at least 3 possible explanations for these data that are not necessarily mutually exclusive: (1) T cells may require some signaling through CD28 for the induction of peripheral tolerance, perhaps to prime host alloreactive T cells for apoptosis. (2) Host CD28−/− T cells may fail to become activated enough in this model to sufficiently up-regulate CD154 on their surfaces. (3) Alternatively, our data may be owing to the fact that CD28−/− mice are severely deficient in CD4+CD25+ cells,33 an immunoregulatory cell population well recognized for its role in in vivo homeostasis and for the induction and maintenance of peripheral tolerance in vivo.34-37 We are investigating whether host CD4+CD25+ cells play a role in anti-CD154 mAb-induced tolerance.
Because CD154−/− were not tolerant of donor grafts, we hypothesize that anti-CD154 mAb may be capable of directly delivering a signal to the T cell, as has been shown for the human anti-CD154 mAb, hu5C8. Blair et al38 found that hu5C8 induced the production of immunomodulatory cytokines and apoptosis of allograft-specific CD4+ T cells. This signaling could result in the generation of CD4+ regulatory cells that have been implicated in the induction of infectious tolerance and linked suppression seen with anti-CD154 mAb administration.24 The data suggest that engraftment promotion by anti-CD154 mAb may not simply be the result of blockade of the CD154:CD40 costimulatory pathway in this experimental setting. If so, different anti-CD154 mAbs that bind to distinct epitopes may have a substantially different capacity to promote alloengraftment. This cannot be tested in our model because only one murine anti-CD154 mAb is available.
An important issue for the studies presented in this paper is that only nonsensitized recipients were analyzed. Our preliminary studies indicate that the infusion of a single dose of donor splenocytes into recipients 10 days before transplantation completely abrogated the engraftment-promoting capacity of anti-CD154 mAb given beginning on day −1, suggesting that anti-CD154 may not facilitate alloengraftment in primed individuals (P.A.T., unpublished data, 2000). These data have important clinical implications for the development of clinical protocols for nonmyeloablative allogeneic bone marrow transplantation.
Supported by National Institutes of Health grants RO1 AI 34495, RO1 HL 63452, and PO1 AI-35225 (B.R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce R. Blazar, University of Minnesota, MMC 109, 420 Delaware St SE, Minneapolis, MN 55455; e-mail:blaza001@tc.umn.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal