Following myeloablative therapy, it is unknown to what extent age-dependent thymic involution limits the generation of new T cells with a diverse repertoire. Normal T-cell receptor gene rearrangement in T-cell progenitors results in the generation of T-cell receptor rearrangement excision circles (TRECs). In this study, a quantitative assay for TRECs was used to measure T-cell neogenesis in adult patients with leukemia who received myeloablative therapy followed by transplantation of allogeneic hematopoietic stem cells. Although phenotypically mature T cells had recovered by 1 to 2 months after bone marrow transplantation (BMT), TREC levels remained low for 3 months after BMT. T-cell neogenesis became evident by 6 months, and normal levels of adult thymic function were restored at 6 to 12 months after BMT. Subsequent leukemia relapse in some patients was associated with reduced TREC levels, but infusion of mature donor CD4+ T cells resulted in rapid restoration of thymic function. These studies demonstrate that T-cell neogenesis contributes to immune reconstitution in adult patients and suggest that thymic function can be manipulated in vivo.
Introduction
The generation of functionally diverse and mature T cells has been extensively studied in a variety of murine and human systems.1,2 Although many aspects of this complex process have not yet been defined, it is well established that the thymus is the primary anatomic site for T-cell neogenesis from undifferentiated hematopoietic progenitor cells. Within the thymus, hematopoietic progenitor cells that have been committed to the T-cell lineage undergo rapid proliferation and differentiation to mature T cells. In this process, T cells undergo both positive and negative selection, which results in the elimination of self-reactive cells.3 The vast majority of differentiating T cells undergo apoptotic death, but surviving cells are exported to peripheral blood and lymphoid organs.4-6 Despite the elimination of most differentiating T cells, mature T cells have an extensively diverse T-cell receptor (TCR) repertoire and are therefore able to respond to a wide array of internally and externally processed antigens.7
The process of T-cell generation from hematopoietic progenitors is not well defined in adults. Histologically, the thymus is largely replaced by fat during the second decade of life, yet measurements of naive T-cell numbers and clinical estimates of T-cell repertoire diversity show no evidence of a corresponding deficit.8,9 Similarly, molecular analysis of TCR-Vβ repertoire does not demonstrate a consistent age-dependent loss of T-cell repertoire. These findings suggest that the diversity of the TCR repertoire is maintained through adult life, either by expansion of naive T cells or by continued generation of new T cells. Although some evidence suggests that extrathymic sites are responsible for the production of new T cells in adults, other studies suggest that the thymus itself continues to be a major site for T-cell neogenesis.10 11
The process of αβ TCR generation creates a byproduct that can be used to measure thymic function. A mandatory early step in αβ T-cell neogenesis is the excision of the TCR-δ locus from within the TCR-α locus. This process of TCR-α gene rearrangement, which occurs in all T cells expressing conventional TCR-αβ receptors, results in the generation of an episomal DNA fragment termed a TCR rearrangement excision circle (TREC).12,13 TRECs become stable intracellular episomes, which are neither degraded nor replicated with cellular division. TRECs are found in almost all maturing T cells within the thymus and at very high levels in cord blood T cells.13 Relatively high numbers of TRECs are found in peripheral blood T cells in the first 2 decades of life, and TREC levels subsequently decline with increasing age.10 Levels of TRECs within a mature T-cell population decline with ongoing cell division, and as a result, TREC levels are higher in naive T cells.14 Taken together, these studies have shown that TRECs can be used as an accurate measure of thymic function and the general level of T-cell neogenesis in an individual.
In the present studies, we examined levels of TRECs in the peripheral blood of adult patients with chronic myelocytic leukemia who received myeloablative therapy followed by transplantation of hematopoietic stem cells from normal sibling donors. Prior to transplantation, hematopoietic stem cells were depleted of mature T cells by in vitro treatment of donor marrow with anti-CD6 monoclonal antibody. Thus, measurement of TRECs in these individuals represents the assessment of T-cell neogenesis in patients in whom endogenous T cells were ablated by the bone marrow transplantation (BMT)–preparative regimen, and the reconstitution of a broad repertoire is dependent primarily on the differentiation of T cells from normal undifferentiated hematopoietic progenitor cells. This experimental population thus provides a unique opportunity to examine T-cell neogenesis in adults and to identify clinical factors that influence the rapidity and extent of T-cell neogenesis in vivo.
Materials and methods
Cell preparation
Heparinized blood samples were obtained from normal controls and from patients at various times before and after allogeneic BMT. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll/Hypaque density gradient centrifugation, cryopreserved with 10% dimethyl sulfoxide and stored in vapor-phase liquid nitrogen until the time of analysis.
Genomic DNA extraction
Genomic DNA was extracted from 3 to 10 × 106PBMCs by means of the Wizard Genomic DNA Purification Kit (Promega, Madison, WI) according to the manufacturer's instructions. Prior to polymerase chain reaction (PCR) amplification, DNA concentration in all samples was determined by ultraviolet spectrophotometry and adjusted to a concentration of 0.03 μg/μL with sterile distilled water.
Real-time PCR detection and quantitation of TRECs
We developed a method to quantify signal-joint TRECs by means of real-time PCR (ABI, Foster City, CA). This method uses a fluoresceinated probe that hybridizes between the PCR primers. The PCR primer sequences were as follows: sense 5′-CGT GAG AAC GGT GAA TGA AGA GCA GAC A-3′, antisense 5′-CAT CCC TTT CAA CCA TGC TGA CAC CTC T-3′. The probe sequence was 5′-VIC-TTT TTG TAA AGG TGC CCA CTC CTG TGC ACG GTG A-TAMRA-3′.15 The VIC fluorophore emits at 340-nm wavelength once it is liberated from the proximity of the TAMRA quencher element by the action of the DNA polymerase. Each 50-μL reaction mixture contained .075 μg DNA and the following concentrations of other components: Taqman buffer A (ABI); 3 mM MgCl2; 300 nmol each primer; 100 nmol probe; 200 nmol deoxyadenosine triphosphate, deoxycytidine triphosphate, and deoxyguanosine triphosphate; 400 nmol deoxyuridine triphosphate (dUTP); 17 U uracil-N-glycosylase; and 2 U AmpliTaq Gold DNA polymerase (ABI). One cycle of denaturation (95°C for 10 minutes) was performed followed by 50 cycles of amplification (94°C for 30 seconds, 60°C for 30 seconds). PCR was performed in a spectrofluorometric thermal cycler (ABI Prism 7700 Sequence Detector System) that measures the independent fluorescent spectrum of each well in a 96-well plate while simultaneously carrying out the thermal cycling.
A series of standard dilutions of a plasmid containing the signal-joint breakpoint was used to quantitate TRECs in each patient and control DNA sample. By comparing the PCR cycle at which fluorescence was first significantly elevated above background (the CT or threshold cycle) in the patient sample with the standard curve of known concentrations of the plasmid, we could accurately measure the starting copy number of TRECs in the sample. Each patient and control DNA sample was run in duplicate on a 96-well plate along with the dilution series of the TREC plasmid as well as a dilution series of a glyceraldehyde phosphate dehydrogenase (GAPDH) plasmid. The same samples were also run in duplicate on the same plate with established primers and probes for GAPDH. The GAPDH copy number served as a control for both the quality and the amount of genomic DNA in the sample. To further validate the PCR methodology, quantitative TREC levels were determined for blood samples obtained from 43 normal controls ranging in age from birth to 92 years. TREC counts in these samples ranged from 0 to 105 TRECs per microgram DNA. The slope of the curve described by the plot of TREC count per microgram DNA versus age demonstrated the age-dependent decrease of TREC levels that has previously been described.10 13
Flow cytometry
PBMCs (0.5 to 1 × 106 cells) were incubated at 4°C for 30 minutes with murine monoclonal antibodies specific for CD3, CD4, CD8, and CD56 antigens conjugated to fluorescein or phycoerythrin (Coulter Immunology, Hialeah, FL). Antibodies were used at 1:100 dilution, and cells were washed with PBS followed by fixation with 2% paraformaldehyde. Immunophenotypic analysis of the stained and fixed cells was performed on a Coulter Epics XL flow cytometer (Beckman Coulter, Hialeah, FL).
Statistical analysis
TREC levels were determined at baseline prior to allogeneic marrow transplantation and at various times after transplantation. Values were also determined before and after donor lymphocyte infusion. Association with dichotomized clinical factors at a given time point was assessed by the Wilcoxon rank sum test and linear regression models. Changes in TREC counts over time were also assessed, both as actual counts and as recovery to normal values. Changes from baseline were assessed with the Wilcoxon signed-rank test. Nominal Pvalues are presented; there have been no corrections for multiple comparisons across time points.
Results
Patient characteristics
T-cell reconstitution was examined in 18 patients with chronic myelocytic leukemia (CML) who underwent allogeneic BMT from sibling donors. The clinical characteristics of these 18 patients are summarized in Table 1. The patients ranged in age from 24 to 57 years (median, 42 years). Sixteen patients were in stable phase CML at the time of the diagnosis and transplantation. Patient 1 was in blast crisis at the time of diagnosis and received high-dose cytarabine prior to transplantation. Patient 13 presented in accelerated-phase CML. Two patients had relapsed after a previous allogeneic BMT. All post-BMT samples from these 2 patients were taken after the second transplantation. The donors for 2 patients were partially HLA-mismatched; all other patients and donors were HLA-identical. The BMT myeloablative regimen consisted of cyclophosphamide (120 mg/kg) and total body irradiation (1200 to 1400 cGy), except for 2 patients who received busulfan (16 mg/kg) and cyclophosphamide (120 mg/kg). Five patients received splenic irradiation (750 cGy), and 2 patients received total lymphoid irradiation in addition to their myeloablative conditioning.
Patient characteristics
| Patient no. . | Patient sex/age (y) . | Donor sex/age (y) . | Stage of CML at Dx/BMT . | Time from Dx to BMT (mo) . | Treatment pre BMT* . | HLA match . | Ablative regimen . | Infused CD3+ cells per kg . | IL-2 post-BMT . | GVHD grade in first y . | Time to cytogenetic relapse (mo) . | Time from BMT to DLI (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/25 | F/22 | BC/S | 5 | HDAC, Hy | 4/6 | Cy/TBI | 1.40E+06 | N | 1 | — | — |
| 2 | F/40 | M/39 | S/S | 5 | Hy | 6/6 | Cy/TBI | 5.36E+06 | N | 2 | 24 | 27 |
| 3 | F/52 | F/53 | S/S | 57 | IFN-α | 6/6 | Cy/TBI | 8.00E+05 | N | 0 | 37 | 40 |
| 4 | M/43 | F/45 | S/S | 7 | Hy | 6/6 | Cy/TBI | 1.13E+06 | Y | 0 | 34 | 57 |
| 5 | F/50 | F/40 | S/S | 9 | Hy | 6/6 | Cy/TBI | 1.20E+06 | Y | 1 | 11 | 33 |
| 6 | F/41 | M/35 | S/S | 12 | Hy | 5/6 | Cy/TBI | 1.60E+05 | Y | 0 | — | — |
| 7 | F/39 | M/47 | S/S | 23 | Hy | 6/6 | Cy/TBI | 7.00E+05 | Y | 0 | 6 | 24 |
| 8 | M/47 | M/39 | S/S | 55 | BMT, Hy | 6/6 | Bu/Cy | 6.00E+05 | Y | 0 | 11 | 38 |
| 9 | F/51 | F/52 | S/S | 3 | Hy | 6/6 | Cy/TBI | 1.55E+06 | N | 0 | 12 | 27 |
| 10 | F/57 | F/58 | S/S | 3 | Hy | 6/6 | Cy/TBI | 1.10E+06 | N | 0 | 13 | 18 |
| 11 | F/50 | F/46 | S/S | 12 | Hy, IFN-α | 6/6 | Cy/TBI | 2.45E+06 | N | 1 | 23 | 27 |
| 12 | M/30 | M/32 | S/S | 61 | BMT, Hy | 6/6 | Bu/Cy | 5.00E+06 | Y | 1 | — | — |
| 13 | M/24 | M/26 | AP/S | 7 | Hy, IFN-α | 6/6 | Cy/TBI | 1.65E+06 | Y | 0 | 27 | 42 |
| 14 | M/31 | F/29 | S/S | 17 | Bu, Hy | 6/6 | Cy/TBI | 3.00E+05 | Y | 0 | — | — |
| 15 | F/32 | M/34 | S/S | 9 | Hy | 6/6 | Cy/TBI | 1.00E+06 | Y | 0 | — | — |
| 16 | M/49 | M/40 | S/S | 5 | Hy | 6/6 | Cy/TBI | 3.19E+05 | Y | 0 | 24 | 27 |
| 17 | M/44 | M/45 | S/S | 5 | Hy | 6/6 | Cy/TBI | 3.90E+06 | N | 0 | 12 | 56 |
| 18 | M/33 | F/29 | S/S | 6 | Hy | 6/6 | Cy/TBI | 7.66E+05 | Y | 1 | 23 | 25 |
| Patient no. . | Patient sex/age (y) . | Donor sex/age (y) . | Stage of CML at Dx/BMT . | Time from Dx to BMT (mo) . | Treatment pre BMT* . | HLA match . | Ablative regimen . | Infused CD3+ cells per kg . | IL-2 post-BMT . | GVHD grade in first y . | Time to cytogenetic relapse (mo) . | Time from BMT to DLI (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/25 | F/22 | BC/S | 5 | HDAC, Hy | 4/6 | Cy/TBI | 1.40E+06 | N | 1 | — | — |
| 2 | F/40 | M/39 | S/S | 5 | Hy | 6/6 | Cy/TBI | 5.36E+06 | N | 2 | 24 | 27 |
| 3 | F/52 | F/53 | S/S | 57 | IFN-α | 6/6 | Cy/TBI | 8.00E+05 | N | 0 | 37 | 40 |
| 4 | M/43 | F/45 | S/S | 7 | Hy | 6/6 | Cy/TBI | 1.13E+06 | Y | 0 | 34 | 57 |
| 5 | F/50 | F/40 | S/S | 9 | Hy | 6/6 | Cy/TBI | 1.20E+06 | Y | 1 | 11 | 33 |
| 6 | F/41 | M/35 | S/S | 12 | Hy | 5/6 | Cy/TBI | 1.60E+05 | Y | 0 | — | — |
| 7 | F/39 | M/47 | S/S | 23 | Hy | 6/6 | Cy/TBI | 7.00E+05 | Y | 0 | 6 | 24 |
| 8 | M/47 | M/39 | S/S | 55 | BMT, Hy | 6/6 | Bu/Cy | 6.00E+05 | Y | 0 | 11 | 38 |
| 9 | F/51 | F/52 | S/S | 3 | Hy | 6/6 | Cy/TBI | 1.55E+06 | N | 0 | 12 | 27 |
| 10 | F/57 | F/58 | S/S | 3 | Hy | 6/6 | Cy/TBI | 1.10E+06 | N | 0 | 13 | 18 |
| 11 | F/50 | F/46 | S/S | 12 | Hy, IFN-α | 6/6 | Cy/TBI | 2.45E+06 | N | 1 | 23 | 27 |
| 12 | M/30 | M/32 | S/S | 61 | BMT, Hy | 6/6 | Bu/Cy | 5.00E+06 | Y | 1 | — | — |
| 13 | M/24 | M/26 | AP/S | 7 | Hy, IFN-α | 6/6 | Cy/TBI | 1.65E+06 | Y | 0 | 27 | 42 |
| 14 | M/31 | F/29 | S/S | 17 | Bu, Hy | 6/6 | Cy/TBI | 3.00E+05 | Y | 0 | — | — |
| 15 | F/32 | M/34 | S/S | 9 | Hy | 6/6 | Cy/TBI | 1.00E+06 | Y | 0 | — | — |
| 16 | M/49 | M/40 | S/S | 5 | Hy | 6/6 | Cy/TBI | 3.19E+05 | Y | 0 | 24 | 27 |
| 17 | M/44 | M/45 | S/S | 5 | Hy | 6/6 | Cy/TBI | 3.90E+06 | N | 0 | 12 | 56 |
| 18 | M/33 | F/29 | S/S | 6 | Hy | 6/6 | Cy/TBI | 7.66E+05 | Y | 1 | 23 | 25 |
CML indicates chronic myelocytic leukemia; Dx, diagnosis; BMT, bone marrow transplantation; IL, interleukin; GVHD, graft-vs-host disease; DLI, donor lymphocyte infusion; BC, blast crisis; S, stable phase; AP, accelerated phase; HDAC, high-dose cytarabine; Hy, hydroxyurea; IFN-α, interferon α; Bu, busulfan; Cy, cyclophosphamide; and TBI, total body irradiation.
All patients received donor marrow that had been depleted of CD6+ T cells as previously described.16 As a result, the number of CD3+ T cells infused with donor marrow was low, with a median of 1.63 × 106CD3+ cells/kg (range, 0.16 to 5.3 × 106cells/kg). After transplantation, 11 patients received low-dose interleukin-2 (IL-2) (3 to 6 × 105U/m2 per day) for approximately 12 weeks beginning 3 months after BMT.17 Five patients developed grade 1 graft-vs-host disease (GVHD), and only 1 patient developed grade 2 GVHD after BMT. Thirteen patients demonstrated a cytogenetic relapse at a median of 23 months (range, 6-37 months) after BMT. The remaining 5 patients have been followed for 8 to 9 years and remain in cytogenetic remission. The 13 patients who relapsed all received donor lymphocyte infusions (DLIs) at a median of 16 months after relapse (range, 2-44 months).18
Changes in TREC counts after allogeneic BMT
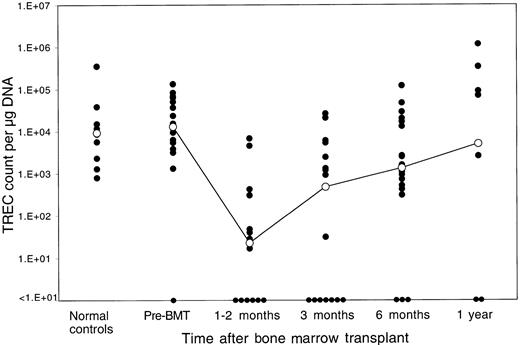
The median TREC count in PBMCs for the patients prior to BMT was 1.3 × 104 TRECs per microgram DNA. Only one patient had levels of TRECs prior to BMT that were below the limit of detection by our assay (fewer than 1 × 101 TRECs per microgram DNA). As shown in Figure 1, pre-BMT TREC values were similar to the TREC values of 10 normal controls aged 32 to 52 years (median, 9.3 × 103 TRECs per microgram DNA; P = .68 by Wilcoxon rank sum test). Marked decreases in TREC counts were noted soon after myeloablative therapy. TREC counts at 1 to 2 months after BMT (median, 1.7 × 101 TRECs per microgram DNA) (n = 14) were significantly lower than the pretransplant values (P = .0001 by Wilcoxon signed-rank test). Although TREC counts were higher at 3 months after BMT (median, 4.8 × 102 TRECs per microgram DNA) (n = 17), they remained significantly lower than baseline prior to BMT (P = .006 by signed-rank test). TREC counts increased further at 6 months (median, 1.35 × 103 TRECs per microgram DNA) (n = 18), indicating further improvement of thymic T-cell generation, and were no longer statistically different from pre-BMT values (P = .12 by signed-rank test). At 1 year after BMT, median TREC counts reached a level of 5.0 × 103 TRECs per microgram DNA (n = 10), which clearly indicated normal levels of adult thymic function (P = .65 by signed-rank test compared with pre-BMT values).
Reconstitution of TRECs in peripheral blood lymphocytes after allogeneic BMT.
TREC counts per microgram PBMC DNA were determined by quantitative PCR in samples obtained at various times after BMT. The closed circles represent individual patient samples. The open circles denote the median value for all samples at that time period. The column to the far left shows results for 10 normal controls of similar ages (32 to 52 years). The limit of detection for this assay is fewer than 10 TRECs per microgram DNA.
Reconstitution of TRECs in peripheral blood lymphocytes after allogeneic BMT.
TREC counts per microgram PBMC DNA were determined by quantitative PCR in samples obtained at various times after BMT. The closed circles represent individual patient samples. The open circles denote the median value for all samples at that time period. The column to the far left shows results for 10 normal controls of similar ages (32 to 52 years). The limit of detection for this assay is fewer than 10 TRECs per microgram DNA.
Several patients had no detectable TRECs in peripheral blood at various time points after allogeneic BMT. Each of these samples had been run in duplicate on the same PCR plate with another set of duplicates, which was amplified with GAPDH primers.19 Levels of GAPDH were within normal range for each of these samples, indicating that the value for TRECs in these samples was truly below the limit of detection for our assay (fewer than 1 × 101 TRECs per microgram DNA). Blood samples without detectable TRECs were most common in the first 3 months after BMT. Only 3 samples were TREC-negative at 6 months after BMT, and only 2 samples were TREC-negative 1 year after BMT. Samples without detectable TRECs were not always found in the same patient, and there were no patients in whom TRECs were not detectable at all time points.
We also examined whether reconstitution of TRECs in peripheral blood T cells was associated with a variety of clinical variables. The following clinical variables were examined: recipient age, recipient sex, matched versus mismatched sex, completeness of HLA matching, type of conditioning regimen, number of CD3+ T cells infused after T-cell depletion, presence of GVHD in the first year, administration of IL-2 after BMT, and relapse after BMT. There was no statistically significant correlation of either the rapidity or the magnitude of recovery of TRECs with any of these clinical variables although the absolute number of samples in each of these clinical categories was small. We also examined the possibility that TREC values reflected the total number of mature T cells in the blood samples that were analyzed. Flow cytometric analysis of PBMCs from these individuals indicated that the mean percentage of CD3+ cells was 37.2% at 1 to 2 months after BMT; 43.3% at 3 months; 42.9% at 6 months; and 49.8% at 1 year. These relatively small changes in the levels of mature T cells could not account for the changes in TREC levels during this period, and the inability to detect TRECs was not correlated with the percentage of CD3+ cells in the samples.
Reconstitution of TRECs after DLI
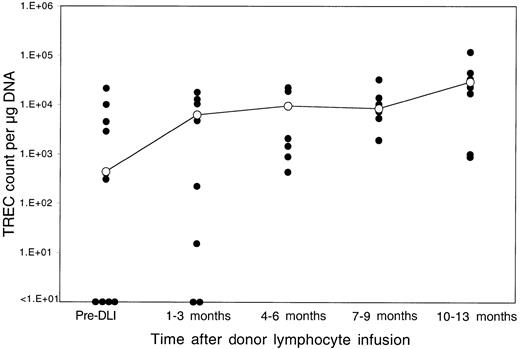
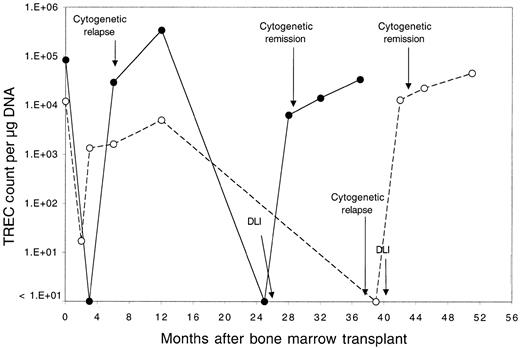
Thirteen patients relapsed after BMT and were enrolled in clinical trials evaluating the toxicity and efficacy of DLI as the primary therapy for recurrent disease.18 Donor lymphocytes were obtained by apheresis without additional growth factor stimulation from the same siblings that had previously donated marrow, and each lymphocyte infusion was depleted of CD8+ T cells by in vitro treatment with anti-CD8 monoclonal antibody and rabbit complement. Patients received defined doses of CD4+ donor T cells ranging between 0.3 and 1.5 × 108 CD4+cells/kg, and all patients achieved complete cytogenetic remission following DLI.18 Although almost all of these patients had recovered normal TREC levels by 1 year after BMT, TREC counts prior to DLI were again significantly low compared with counts in the same patients prior to BMT (P = .03 by Wilcoxon signed-rank test). As shown in Figure 2, TREC levels in 4 of the 13 patients were below the limits of detection in our assay. The sequence of TREC values for 2 of these patients is summarized in Figure 3. In the first 3 months after BMT, both of these patients had very low TREC values, which recovered to normal levels by 6 months after BMT. Normal TREC values were maintained for at least 1 year after BMT, but TRECs were no longer detectable prior to DLI. TREC values rapidly returned to normal levels in both patients soon after DLI, coincident with the restoration of complete donor hematopoiesis and achievement of complete cytogenetic remission. By 1 to 3 months post-DLI (Figure 2), only 2 patients still had no detectable TRECs, and TREC values were significantly increased compared with pre-DLI values. Compared with pre-BMT values, the TREC counts were no longer significantly different (P = .15 by Wilcoxon signed-rank test). By 4 to 6 months after DLI, all patients had detectable TREC levels, and normal levels of TREC persisted for at least 1 year of follow-up. At the remaining time points, TREC values were not significantly different from pre-BMT values (P = .65 at 6 months, P = .81 at 9 months, and P = .81 at 12 months by Wilcoxon signed-rank test).
Reconstitution of TRECs after DLI.
TREC counts per microgram PBMC DNA were determined by quantitative PCR in samples obtained at various times after DLI. The closed circles represent individual patient samples. The open circles denote the median value for all samples at that time period. The limit of detection for this assay is fewer than 10 TRECs per microgram DNA.
Reconstitution of TRECs after DLI.
TREC counts per microgram PBMC DNA were determined by quantitative PCR in samples obtained at various times after DLI. The closed circles represent individual patient samples. The open circles denote the median value for all samples at that time period. The limit of detection for this assay is fewer than 10 TRECs per microgram DNA.
Serial quantitation of TRECs in 2 selected patients.
Serial quantitation of TRECs in peripheral blood lymphocytes in 2 patients (●, patient 7; ○, patient 3) who underwent allogeneic BMT followed by infusion of CD4+ lymphocytes from the same donor for treatment of leukemia relapse. Times of cytogenetic relapse, CD4+ DLI, and cytogenetic remission are indicated by arrows.
Serial quantitation of TRECs in 2 selected patients.
Serial quantitation of TRECs in peripheral blood lymphocytes in 2 patients (●, patient 7; ○, patient 3) who underwent allogeneic BMT followed by infusion of CD4+ lymphocytes from the same donor for treatment of leukemia relapse. Times of cytogenetic relapse, CD4+ DLI, and cytogenetic remission are indicated by arrows.
Discussion
Following myeloablative therapy, successful engraftment of hematopoietic stem cells proceeds in a well-defined, sequential manner.20 Myeloid stem cells engraft within weeks of transplantation with reconstitution of granulocytes preceding recovery of platelets and red blood cells. Natural killer cells also reconstitute quickly and are the first lymphoid cells to engraft.21 T cells also begin to engraft quickly, but 6 to 9 months are often required before normal numbers of circulating CD3+ T cells are achieved.21 These CD3+ cells are predominately CD8+, and the number of circulating CD4+ cells often remains low for more than 1 year after BMT. Phenotypically naive T cells (CD4+CD45RA+) are also decreased, suggesting that the initial phase of T-cell engraftment is due primarily to the proliferation of mature T cells in the stem cell infusion rather than to the differentiation of new T cells from hematopoietic progenitors.22,23 B cells reconstitute more slowly than T cells, and 12 to 18 months are often required to achieve relatively normal numbers of CD20+ cells in peripheral blood.24
Despite reconstitution of substantial numbers of phenotypically mature T cells in peripheral blood within 3 months after transplantation, further characterization of these cells almost always reveals marked functional impairment. Administration of immune-suppressive medications and transplantation complications such as GVHD contribute to this functional deficiency in some patients, but marked defects in response to in vitro stimulation with mitogens, soluble antigens, and allo-antigens often persist for more than 1 year after transplantation in patients who are not receiving immune-suppressive agents.25-27 Although the causes of these functional defects have not been precisely defined, a variety of abnormalities in various signaling pathways have been described, and these abnormalities can persist for 1 to 2 years after transplantation.
The reconstitution of normal T-cell function is also dependent on the generation of large numbers of T cells with a diverse TCR repertoire. Previous studies have demonstrated that the TCR repertoire is markedly skewed in the first 3 to 6 months after transplantation, but the diversity of TCR repertoire gradually recovers to normal levels by 12 to 18 months after transplantation. Full reconstitution of TCR diversity appears to occur more slowly in patients with mixed hematopoietic chimerism, and recent studies suggest that slow recovery of TCR repertoire contributes significantly to immune deficiency after allogeneic BMT.22,23,28 Almost all aspects of T-cell reconstitution appear to occur more rapidly in children than in adults.29,30 This probably reflects the normal progressive involution of the thymus that occurs in adults and suggests that thymic processing also plays an important role in the reconstitution of T-cell immunity following allogeneic stem cell transplantation.1Immunosuppressive regimens have also been shown to inhibit normal maturation of thymocytes. FK50631 and cyclosporin31 32 have both been demonstrated to inhibit positive selection of double-positive thymocytes. Immunosuppressive regimens should thus lead to decreases in TREC levels, although this has not been directly examined.
Although the gradual reconstitution of a normally diverse TCR repertoire suggests that new T cells are being generated after BMT, it has not previously been possible to accurately measure the generation of new T cells from uncommitted hematopoietic progenitors in humans. To address this issue, we used a quantitative assay for TRECs to serially measure T-cell neogenesis in a unique and homogeneous population of adult patients. This population had received myeloablative therapy followed by infusion of hematopoietic stem cells from normal donors. Moreover, mature T cells had been depleted from the stem cell transplant prior to infusion, and patients did not receive immune-suppressive therapy that might have affected thymic function. This population therefore provided an ideal setting in which to examine T-cell neogenesis from normal hematopoietic stem cells in adults. Since TREC levels were very low in the first 3 months after transplantion, these studies provide evidence that the phenotypically mature T cells present in the circulation at this time are derived from the expansion of other mature T cells rather than from the differentiation of lymphoid progenitors. These observations are consistent with the previous demonstration of a very limited TCR repertoire in the early posttransplantation period.28,33 However, TREC levels begin to improve by 1 to 3 months after BMT and have returned to normal levels for adults by 6 to 12 months after transplantation. These results demonstrate that T-cell neogenesis from undifferentiated hematopoietic precursors contributes to the restoration of T-cell immunity after transplantation. These observations also provide evidence for the recovery of thymic function after ablative therapy and suggest that the thymus maintains the ability to facilitate the reconstitution of T-cell immunity despite involution in adult individuals. In comparison with prior studies, we found the kinetics of TREC recovery similar to what was observed in infants with severe combined immunodeficiency undergoing stem cell transplantation29 and in non–T-cell-depleted allogeneic transplants.34 In patients with multiple myeloma receiving autologous stem cells, TRECs have also been reported to recover to baseline levels 1 to 2 years after transplantation.35
Although TREC levels had returned to normal levels by 1 year after BMT, subsequent follow-up demonstrated that leukemia relapse was associated with loss of TRECs in peripheral blood. When considered in conjunction with the loss of TCR complexity that we have previously demonstrated in patients with relapsed CML after allogeneic BMT,36 these observations suggest that relapse of leukemia markedly affects the ability to maintain normal thymic function and normal levels of T-cell neogenesis. Alternatively, the expansion of limited populations of peripheral T cells would also produce a decrease in TRECs via dilution and would limit the TCR repertoire. The mechanism whereby leukemia cells affect T-cell neogenesis is not known, but recovery of TRECs was consistently observed after infusion of CD8-depleted donor T cells. Interestingly, the recovery of TRECs was associated with the elimination of leukemia cells and the restoration of normal donor hematopoiesis. Taken together, these observations provide evidence that T-cell neogenesis can be influenced by external factors and that quantitative assessment of TRECs may be a useful surrogate marker in the evaluation of future therapies designed to improve T-cell immunity.
Supported by National Institutes of Health (NIH) grants AI29530 and AI39966; C.J.W. is supported by a Howard Hughes Medical Institute Postdoctoral Research Fellowship and NIH grant K08 HL04293-01; R.J.S. is a Clinical Research Scholar of the Leukemia Society of America; E.P.A. is a Special Fellow of the Leukemia Society of America.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jerome Ritz, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: jerome_ritz@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal