It has been reported that mononuclear cells harvested from murine skeletal muscle are capable of hematopoietic reconstitution of lethally irradiated mice. First, the nature of the hematopoietic progenitors in the muscle of C57BL/6–Ly-5.1 mice was examined by means of methylcellulose culture. The types and incidences of colonies grown from muscle mononuclear cells were different from those cultured from bone marrow (BM) or peripheral blood mononuclear cells. The next step was to examine the origin of the hematopoietic progenitors and stem cells in the muscle with the use of Ly-5.2 mice that had been made chimeric by transplantation of Ly-5.1 BM cells. The percentages of Ly-5.1 cells cultured from the muscle of the chimeric mice correlated with those cultured from BM, indicating BM origin of hematopoietic progenitors in the muscle. Long-term hematopoietic engrafting cells in the muscle of the chimeric mice were also derived from BM. However, mobilization of progenitors into circulation by granulocyte colony-stimulating factor did not change the population of hematopoietic progenitors in the muscle. It is proposed that hematopoietic progenitors and stem cells in the muscle tissue are of BM origin but their transition from BM to muscle may be a slow process.
Introduction
It has been held for decades that, in organ systems with regenerative ability, populations of cells called stem cells exist and are able to self-renew and generate committed progenies. It has also been generally believed that stem cells possess organ/tissue specificity. However, this dogma was recently challenged by a number of reports that a variety of cells, including those in the hematopoietic, neuronal, and muscular organs, have the potential to differentiate into tissues other than those specified in their origins.1-13 For example, bone marrow (BM) cells have been shown to be capable of regenerating a number of mesenchymal tissues, such as bone, cartilage, and muscle1-7; neuronal cells have been reported to be capable of differentiation into hematopoietic cells8,9; and it was reported that BM cells are also capable of hepatocyte regeneration.10-12
Equally strikingly, it has recently been reported that mononuclear cells harvested from murine skeletal muscle are capable of hematopoietic reconstitution of lethally irradiated mice.7 13 These observations not only raised important questions about cell fate specification in developmental biology but also offered the potential of medical utility of the ectopic stem cells for the reconstitution of hematopoiesis. In this report, we have examined the nature and origin of the hematopoietic progenitors and stem cells in the muscle by using clonal cell culture and transplantation techniques.
Materials and methods
Cytokines
Recombinant human thrombopoietin (TPO) was provided by Kirin Brewery (Tokyo, Japan); and recombinant rat steel factor (SF), recombinant human erythropoietin (EPO), and granulocyte colony-stimulating factor (G-CSF; filgrastim) by Amgen (Thousand Oaks, CA). Recombinant human interleukin-11 (IL-11) was a gift from Genetics Institute (Cambridge, MA). Recombinant murine IL-3 and granulocyte/macrophage colony-stimulating factor (GM-CSF) were purchased from R&D Systems (Minneapolis, MN). The final concentrations of cytokines used in culture were as follows: TPO, 100 ng/mL; SF, 100 ng/mL; IL-11, 100 ng/mL; IL-3, 10 ng/mL; GM-CSF, 10 ng/mL; and EPO, 2000 mU/mL (2 U/mL).
Monoclonal antibodies
Fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)-conjugated A20 (anti–Ly-5.1; mouse immunoglobulin G2a [IgG2a]); FITC-conjugated 104 (anti–Ly-5.2; mouse IgG2a); PE-conjugated RB6-8C5 (anti–Gr-1; rat IgG2b); PE-conjugated RA3-6B2 (anti-CD45R/B220; rat IgG2a); and PE-conjugated 30-H12 (anti–Thy-1.2; rat IgG2b) were purchased from Pharmingen (San Diego, CA). PE-conjugated M1/70.15 (anti–Mac-1; rat IgG2b) was purchased from Caltag Laboratories (Burlingame, CA).
Mice
C57BL/6–Ly-5.1 mice and C57BL/6–Ly-5.2 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and Charles River Laboratories (Raleigh, NC), respectively. The Institutional Animal Care and Use Committee of the Department of Veterans Affairs Medical Center approved the animal studies. In order to study the origin of the hematopoietic cells in the muscle, we used Ly-5.2 mice that had been made chimeric by transplantation of BM cells from Ly-5.1 mice. As will be described in more detail later, we used 4 groups of chimeric mice that were survivors of studies described previously.14Four additional groups of chimeric mice were prepared specifically for the current study by transplanting unfractionated or CD34−BM cells of Ly-5.1 mice into Ly-5.2 mice after lethal (950-cGy) irradiation.
Cell preparation
Muscle mononuclear cells were prepared by means of minor modifications of the method described by Yablonka-Reuveni and Nameroff.15 This method has been used for isolation of the muscle mononuclear cells that were capable of hematopoietic reconstitution.13 Hind legs of 5- to 7-week-old Ly-5.1 mice or chimeric Ly-5.2 mice were separated just above the head of femurs following dislocation of hip joints. Muscles were then harvested on a dissecting microscope (Leica MZ6) (Heerburg, Switzerland), with care being used to avoid cutting bones. After removal of fat, epimysium, tendons, and internal connective tissues, the muscles were minced with scissors and incubated with constant agitation for 45 minutes at 37°C in 0.1% trypsin (Life Technologies, Grand Island, NY). Cells were released from the tissue fragments by vigorous trituration. Pooled cells were filtered through a 40-μm nylon mesh, washed, and resuspended in α-modification of Eagle medium (α-MEM) (ICN Biochemicals, Aurora, OH). The samples were further purified by discontinuous density-gradient centrifugation by means of Percoll (Amersham Pharmacia Biotech, Piscataway, NJ).15Here, the samples were centrifuged at 15 000g for 5 minutes at 8°C in a fixed angle rotor, and mononuclear cells were collected from the 20% to 60% interface.15 Peripheral blood (PB) was collected by cardiac puncture with the use of methoxyflurane anesthesia, and BM cells flushed from femora and tibiae were made into single cell suspension by repeated pipetting. Mononuclear cells were prepared from PB and BM cells by Percoll. For determining the origin of engrafting cells, we sorted the muscle mononuclear cells of chimeric mice using a FACSVantage (Becton Dickinson, San Jose, CA) fluorescence-activated cell sorter (FACS).
Clonal cell culture
Methylcellulose culture was performed in 35-mm Petri dishes (Becton Dickinson Labware, Lincoln Park, NJ). Unless otherwise specified, 1 mL culture mixture contained designated numbers of mononuclear cells, α-MEM, 1.2% 1500-cP methylcellulose (Shinetsu Chemical, Tokyo, Japan), 1% deionized fraction V bovine serum albumin (Intergen, Purchase, NY), 0.1 mM 2-mercaptoethanol (Sigma-Aldrich, St Louis, MO), 30% fetal calf serum (Intergen), and TPO, SF, EPO, IL-3, and IL-11. Dishes were incubated at 37°C in a humidified atmosphere with 5% CO2, 5% O2, and 90% N2. Erythroid colony-forming units (CFU-Es) were counted on day 2 of culture, and all other colonies consisting of 50 or more cells were scored on an inverted microscope after 8 days of culture. Specific cellular composition of the colonies was determined by means of cytospin preparations of individual colonies stained with May-Grünwald-Giemsa on days 8 to 14 of culture. Abbreviations of colony types are as follows: erythroid burst-forming units (BFU-Es); granulocyte and/or macrophage (GM) colonies; mixed colonies containing erythroid and myeloid cells and megakaryocytes (GEMM).16
Transplantation
The hematopoietic engrafting capability of muscle and BM cells of the chimeric mice were studied by injecting test cells into the tail vein of recipient mice after a single 950-cGy dose of total body irradiation of recipients using 4 × 106 V linear accelerator. To prevent posttransplantation death, designated types of radioprotective cells were also transplanted. Two or 6 months later, PB was obtained from the retro-orbital plexus of the recipients with the use of heparin-coated micropipettes (Drummond Scientific, Broomall, PA). Red blood cells were lysed by 0.15 M NH4Cl, and the samples were analyzed for Ly-5 expression on a FACSCalibur (Becton Dickinson). Cells in the T-cell, B-cell, granulocyte, and monocyte/macrophage lineages were analyzed by staining with PE-conjugated anti–Thy-1.2, anti-CD45R/B220, anti–Gr-1, and anti–Mac-1.
Progenitor mobilization by G-CSF
Ly-5.1 mice were injected at 12-hour intervals subcutaneously with 125 μg/kg human recombinant G-CSF in 0.1 mL phosphate-buffered saline (Life Technologies) for 5 consecutive days. Three hours after the last injection, PB and muscles were harvested. Mononuclear cells of PB and muscles were plated in methylcellulose in the presence of TPO, SF, EPO, IL-3, and GM-CSF. On day 8 of culture, colonies were scored. Mice that received no G-CSF treatment were used as controls.
Statistical analysis
Student t test was used to determine statistical significance. Correlation was determined by using Pearson correlation coefficient.
Results
Hematopoietic progenitors in the muscle
We examined the nature and the incidence of the progenitors in the muscle of Ly-5.1 mice by using methylcellulose culture and compared them with the progenitors in BM and PB. The results are presented in Table 1. For measurement of CFU-Es, we plated 5 × 104 muscle, BM, or PB mononuclear cells per dish. The incidence of day-2 CFU-Es of muscle was less than one fiftieth that of BM. PB had no CFU-Es even when 2 × 105cells were plated (data not shown). For analysis of day-8 colony-forming units in culture (CFU-Cs), we plated the numbers of cells that would yield 30 to 40 colonies per dish. The incidence of day-8 CFU-Cs in muscle was approximately one tenth that of BM and 10 times higher than PB. The incidences of BFU-Es and mast cell progenitors in the muscle, relative to total day-8 CFU-Cs, were much lower than those in PB.
Comparison of colony formation from different sources of hematopoietic progenitors
| Day-8 colonies . | Day-2 CFU-E . | |||||
|---|---|---|---|---|---|---|
| Sample . | BFU-E . | GM . | GEMM . | Mast . | Total . | |
| Muscle, (2 × 104/dish) | 1 ± 1 | 27 ± 3 | 2 ± 1 | 2 ± 1 | 34 ± 1 | 3 ± 1 |
| BM, (2 × 103/dish) | 3 ± 0 | 23 ± 2 | 2 ± 1 | 2 ± 1 | 30 ± 3 | 189 ± 12 |
| PB, (2 × 105/dish) | 8 ± 1 | 3 ± 1 | 2 ± 1 | 17 ± 1 | 32 ± 3 | 0 |
| Day-8 colonies . | Day-2 CFU-E . | |||||
|---|---|---|---|---|---|---|
| Sample . | BFU-E . | GM . | GEMM . | Mast . | Total . | |
| Muscle, (2 × 104/dish) | 1 ± 1 | 27 ± 3 | 2 ± 1 | 2 ± 1 | 34 ± 1 | 3 ± 1 |
| BM, (2 × 103/dish) | 3 ± 0 | 23 ± 2 | 2 ± 1 | 2 ± 1 | 30 ± 3 | 189 ± 12 |
| PB, (2 × 105/dish) | 8 ± 1 | 3 ± 1 | 2 ± 1 | 17 ± 1 | 32 ± 3 | 0 |
Mac-1+ cells were removed from the mononuclear cells by immunomagnetic separation17 to reduce the background monocyte/macrophage population. For day-8 colony formation, designated numbers of cells were plated in quadruplicate in the presence of thrombopoietin, steel factor, erythropoietin, IL-11, and IL-3. For CFU-E assay, 5 × 104 cells were plated per dish. Data represent the mean and SD of values obtained from 4 dishes. Two additional experiments showed similar results.
BFU-E indicates erythroid burst-forming units; GM, granulocyte and/or macrophage colonies; GEMM, mixed colonies containing erythroid and myeloid cells and megakaryocytes; CFU-E, erythroid colony-forming units; BM, bone marrow; PB, peripheral blood.
We then performed a time course study of colony formation from muscle, PB, and BM. On day 8, 10, 12, and 14 of culture, all colonies from quadruplicate cultures were individually lifted from methylcellulose culture, centrifuged to glass slides by means of a Cytospin 2 (Shandon Southern, Sewickley, PA), and stained with May-Grünwald-Giemsa for determination of cellular composition. Various types of single-lineage and multilineage colonies were seen. Predominant types of colonies are presented in Table2. Similarly to the multilineage colonies cultured from BM cells,16 a variety of lineage combinations were seen in the muscle-derived multilineage colonies, such as colonies containing neutrophils, macrophages, mast cells, erythrocytes, and megakaryocytes. Again, the relative incidence of muscle-derived erythroid bursts was very low. The number of colonies consisting of undifferentiated blast cells in cultures of muscle cells gradually increased during incubation. In replating studies, these blast cell colonies proved to be committed to B-cell lineage (data not shown). Together, these results indicated that the hematopoietic progenitors in the muscle constitute a distinct population of progenitors.
Time course comparison of colony formation from different sources of hematopoietic progenitors
| Colony . | Muscle, 1.0 × 104 per dish day of colony formation . | PB, 1.2 × 105 per dish day of colony formation . | BM, 1.2 × 103 per dish day of colony formation . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 . | 10 . | 12 . | 14 . | 8 . | 10 . | 12 . | 14 . | 8 . | 10 . | 12 . | 14 . | |
| E | 1 | 1 | 1 | 1 | 19 | 13 | 19 | 11 | 7 | 6 | 6 | 7 |
| n | 14 | 10 | 3 | 1 | 2 | 1 | 1 | 0 | 19 | 8 | 1 | 0 |
| m | 15 | 10 | 26 | 22 | 0 | 0 | 2 | 0 | 4 | 11 | 18 | 29 |
| n/m | 20 | 11 | 9 | 6 | 4 | 0 | 2 | 5 | 15 | 17 | 15 | 13 |
| E/M | 0 | 3 | 5 | 1 | 4 | 4 | 1 | 2 | 2 | 4 | 1 | 1 |
| mast | 4 | 5 | 4 | 3 | 26 | 27 | 11 | 13 | 4 | 3 | 5 | 3 |
| mast/Bl | 0 | 0 | 0 | 1 | 21 | 34 | 22 | 51 | 1 | 0 | 0 | 0 |
| Bl | 1 | 5 | 7 | 16 | 0 | 1 | 0 | 0 | 5 | 0 | 0 | 1 |
| Others | 5 | 3 | 2 | 6 | 9 | 10 | 7 | 6 | 8 | 7 | 12 | 8 |
| Total | 60 | 48 | 57 | 57 | 85 | 90 | 65 | 88 | 65 | 56 | 58 | 62 |
| Colony . | Muscle, 1.0 × 104 per dish day of colony formation . | PB, 1.2 × 105 per dish day of colony formation . | BM, 1.2 × 103 per dish day of colony formation . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 . | 10 . | 12 . | 14 . | 8 . | 10 . | 12 . | 14 . | 8 . | 10 . | 12 . | 14 . | |
| E | 1 | 1 | 1 | 1 | 19 | 13 | 19 | 11 | 7 | 6 | 6 | 7 |
| n | 14 | 10 | 3 | 1 | 2 | 1 | 1 | 0 | 19 | 8 | 1 | 0 |
| m | 15 | 10 | 26 | 22 | 0 | 0 | 2 | 0 | 4 | 11 | 18 | 29 |
| n/m | 20 | 11 | 9 | 6 | 4 | 0 | 2 | 5 | 15 | 17 | 15 | 13 |
| E/M | 0 | 3 | 5 | 1 | 4 | 4 | 1 | 2 | 2 | 4 | 1 | 1 |
| mast | 4 | 5 | 4 | 3 | 26 | 27 | 11 | 13 | 4 | 3 | 5 | 3 |
| mast/Bl | 0 | 0 | 0 | 1 | 21 | 34 | 22 | 51 | 1 | 0 | 0 | 0 |
| Bl | 1 | 5 | 7 | 16 | 0 | 1 | 0 | 0 | 5 | 0 | 0 | 1 |
| Others | 5 | 3 | 2 | 6 | 9 | 10 | 7 | 6 | 8 | 7 | 12 | 8 |
| Total | 60 | 48 | 57 | 57 | 85 | 90 | 65 | 88 | 65 | 56 | 58 | 62 |
Mac-1+ cells were removed from the mononuclear cells by immunomagnetic separation17 to reduce the background monocyte/macrophage population. Designated numbers of cells were plated in quadruplicate in the presence of thrombopoietin, steel factor, erythropoietin, IL-11, and IL-3. All colonies consisting of 50 or more cells were harvested and stained with May-Grünwald-Giemsa for lineage determination.
PB indicates peripheral blood; BM, bone marrow; E, erythrocyte; n, neutrophil; m, monocyte-macrophage; M, megakaryocyte; mast, mast cell-basophil; Bl, blast cell.
Origin of the hematopoietic progenitors and stem cells in the muscle
Next, we examined the origin of the progenitors and stem cells in muscle by using chimeric mice. The details of the conditions for creating the chimera for progenitor studies are presented in Table3. In experiments 1 through 5, muscle and BM cells of the chimeric mice were cultured in methylcellulose media for 8 days, and the cells in the colonies were individually picked, pooled, and analyzed for Ly-5 expression. Results of colony formation and analysis of the percentage of donor (Ly-5.1) cells in the colonies are also presented in Table 3. There was significant correlation between the percentage of Ly-5.1 cells in the pooled colonies from the muscle and from the BM (Pearson correlation coefficient, 0.961;P < .01). For example, in experiment 5, in which all mice revealed very high levels of donor (Ly-5.1) cells in PB, the cells cultured from both BM and muscles were derived completely from Ly-5.1 BM cells. The fact that we were able to analyze groups of mice with levels of chimerism between 25% and 99% ensured an accurate sampling of the population. These observations indicated that hematopoietic progenitors in the muscle are derived from BM.
Conditions for development of chimeric mice and colony formation from muscle of chimeric mice
| Experiment no. . | Chimeric mice . | Cell culture . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age of donor mice (week) . | Donor BM cells transplanted3-150 . | No. recipients . | Time of harvest after BMT (month) . | No. muscle cells harvested . | % Donor cells in recipients' PB . | Cells cultured . | Total CFU-Cs . | % Donor cells in pooled colonies3-151 . | |
| 1 | 10 | CD34− | 8 | 7 | 5.0 × 105 | 25 ± 20 | Muscle 4 × 104 | 51 | 6 |
| 2.0 × 105 | BM 4 × 104 | 150 | 26 | ||||||
| 2 | 2 | CD34+ | 3 | 11 | 1.1 × 105 | 52 ± 17 | Muscle 2 × 104 | 46 | 18 |
| 4.0 × 104 | BM 2 × 104 | 43 | 12 | ||||||
| 3 | 10 | CD34− | 10 | 3 | 2.8 × 105 | 38 ± 12 | Muscle 2 × 104 | 67 | 44 |
| 2.0 × 105 | BM 4 × 104 | 106 | 55 | ||||||
| 4 | 19 | CD34− | 5 | 12 | 8.0 × 105 | 37 ± 17 | Muscle 4 × 104 | 21 | 63 |
| 2.9 × 105 | BM 4 × 104 | 180 | 62 | ||||||
| 5 | 10 | Unfractionated | 7 | 3 | 6.1 × 105 | 94 ± 2 | Muscle 4 × 104 | 34 | 100 |
| 2.0 × 1063-152 | BM 6 × 1033-153 | 17 | 100 | ||||||
| 6 | 5 | CD34+ | 6 | 10 | 4.5 × 105 | 47 ± 10 | |||
| 5.0 × 104 | |||||||||
| 7 | 9 | Unfractionated | 10 | 6 | 1.7 × 106 | 99 ± 0 | |||
| 2.0 × 1063-152 | |||||||||
| 8 | 10 | Unfractionated | 8 | 10 | 1.2 × 106 | 99 ± 1 | |||
| 2.0 × 1063-152 | |||||||||
| Experiment no. . | Chimeric mice . | Cell culture . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age of donor mice (week) . | Donor BM cells transplanted3-150 . | No. recipients . | Time of harvest after BMT (month) . | No. muscle cells harvested . | % Donor cells in recipients' PB . | Cells cultured . | Total CFU-Cs . | % Donor cells in pooled colonies3-151 . | |
| 1 | 10 | CD34− | 8 | 7 | 5.0 × 105 | 25 ± 20 | Muscle 4 × 104 | 51 | 6 |
| 2.0 × 105 | BM 4 × 104 | 150 | 26 | ||||||
| 2 | 2 | CD34+ | 3 | 11 | 1.1 × 105 | 52 ± 17 | Muscle 2 × 104 | 46 | 18 |
| 4.0 × 104 | BM 2 × 104 | 43 | 12 | ||||||
| 3 | 10 | CD34− | 10 | 3 | 2.8 × 105 | 38 ± 12 | Muscle 2 × 104 | 67 | 44 |
| 2.0 × 105 | BM 4 × 104 | 106 | 55 | ||||||
| 4 | 19 | CD34− | 5 | 12 | 8.0 × 105 | 37 ± 17 | Muscle 4 × 104 | 21 | 63 |
| 2.9 × 105 | BM 4 × 104 | 180 | 62 | ||||||
| 5 | 10 | Unfractionated | 7 | 3 | 6.1 × 105 | 94 ± 2 | Muscle 4 × 104 | 34 | 100 |
| 2.0 × 1063-152 | BM 6 × 1033-153 | 17 | 100 | ||||||
| 6 | 5 | CD34+ | 6 | 10 | 4.5 × 105 | 47 ± 10 | |||
| 5.0 × 104 | |||||||||
| 7 | 9 | Unfractionated | 10 | 6 | 1.7 × 106 | 99 ± 0 | |||
| 2.0 × 1063-152 | |||||||||
| 8 | 10 | Unfractionated | 8 | 10 | 1.2 × 106 | 99 ± 1 | |||
| 2.0 × 1063-152 | |||||||||
After lethal irradiation of the recipients, designated numbers of donor (Ly-5.1) BM cells were injected into recipient (Ly-5.2) mice along with 2 × 105 unfractionated marrow cells from Ly-5.2 mice as radioprotective cells. In experiments 1 through 5, cells were harvested from the muscle and BM of chimeric mice 3 to 12 months after transplantation, and the indicated numbers of unfractionated cells were cultured in the presence of thrombopoietin, steel factor, erythropoietin, IL-11, and IL-3. On day 8 of culture, all hematopoietic colonies consisting of 50 or more cells were scored, pooled, and analyzed for Ly-5 expression. In experiments 6 through 8, the chimeric mice were used only for the secondary transplantation studies. In experiments 1, 2, 4, and 6, the chimeric mice were survivors of studies described previously.14
BM indicates bone marrow; BMT, bone marrow transplantation; PB, peripheral blood; CFU-Cs, colony-forming units in culture.
CD34+ and CD34− populations of BM mononuclear cells were prepared by fluorescence-activated cell sorting with fluorescein isothiocyanate–conjugated anti-CD34.14
Significant correlation between muscle and BM chimerism was observed (Pearson correlation coefficient, 0.961; P < .01).
Ly-5.1 cells were transplanted without Ly-5.2 radioprotective cells.
Mononuclear cells were used for culture.
We then tested the engrafting capabilities of the muscle cells of the chimeric mice by transplantation into secondary Ly-5.2 mice. In our preliminary studies, transplantation of 1 × 105 muscle mononuclear cells of healthy adult Ly-5.1 mice yielded low-level (about 1% or less) hematopoietic chimerism in primary recipients 6 months after transplantation (data not shown). Therefore, we transplanted more than 1 × 105 muscle cells of the chimeric mice in the secondary-transplantation studies. The cells harvested from the muscle or BM of chimeric mice (experiments 5 and 6 in Table 3) were injected into lethally irradiated Ly-5.2 mice along with radioprotective cells (Table 4). Six months later, PB was collected from the Ly-5.2 secondary-recipient mice, and the levels of engraftment were analyzed by flow cytometry. Although the levels of chimerism were low in the recipients of muscle cell transplantation, multilineage reconstitution by Ly-5.1 cells was clearly observed in both groups of recipients (Table 4, Figure1). These results indicated that some hematopoietic stem cells in the muscle are derived from BM.
Hematopoietic reconstitution by Ly-5.1 bone marrow and muscle cells of chimeric mice
| Experiment no.4-150 . | Cells transplanted . | Radioprotective Ly-5.2 BM cells . | No. secondary Ly-5.2 recipients . | % Ly-5.1 cells in secondary Ly-5.2 recipients' PB . | Multilineage reconstitution by Ly-5.1 cells, %4-151 . | ||
|---|---|---|---|---|---|---|---|
| G/M . | T . | B . | |||||
| 5 | Muscle 1.5 × 105 | Lin−c-kit+Sca-1+CD34+‡ | 2 | 7 | 26 | 53 | 6 |
| 2 × 103 | 3 | 10 | 75 | 16 | |||
| BM 1.0 × 106 | Lin−c-kit+Sca-1+CD34+‡ | 2 | 87 | 12 | 31 | 40 | |
| 2 × 103 | 81 | ND | ND | ND | |||
| 6 | Muscle 4.0 × 105 | Unfractionated 2 × 105 | 1 | 4 | 29 | 27 | 45 |
| BM 1.0 × 106 | Unfractionated 2 × 105 | 1 | 47 | 7 | 43 | 50 | |
| Experiment no.4-150 . | Cells transplanted . | Radioprotective Ly-5.2 BM cells . | No. secondary Ly-5.2 recipients . | % Ly-5.1 cells in secondary Ly-5.2 recipients' PB . | Multilineage reconstitution by Ly-5.1 cells, %4-151 . | ||
|---|---|---|---|---|---|---|---|
| G/M . | T . | B . | |||||
| 5 | Muscle 1.5 × 105 | Lin−c-kit+Sca-1+CD34+‡ | 2 | 7 | 26 | 53 | 6 |
| 2 × 103 | 3 | 10 | 75 | 16 | |||
| BM 1.0 × 106 | Lin−c-kit+Sca-1+CD34+‡ | 2 | 87 | 12 | 31 | 40 | |
| 2 × 103 | 81 | ND | ND | ND | |||
| 6 | Muscle 4.0 × 105 | Unfractionated 2 × 105 | 1 | 4 | 29 | 27 | 45 |
| BM 1.0 × 106 | Unfractionated 2 × 105 | 1 | 47 | 7 | 43 | 50 | |
Designated numbers of muscle-derived nucleated cells or lineage-negative (Lin−) BM cells17 of chimeric mice were transplanted into lethally irradiated secondary Ly-5.2 recipient mice along with radioprotective cells. At 6 months later, PB was collected from the secondary recipients, and the levels of engraftment were analyzed by flow cytometry. Nucleated PB cells were stained with anti-Ly-5.1 and either anti-Gr-1 and anti-Mac-1, anti-Thy-1.2, or anti-B220.
BM indicates bone marrow; PB, peripheral blood; G/M, granulocytes and/or monocytes; T, T lymphocytes; B, B lymphocytes; ND, not determined.
The experimental mice correspond to experiments 5 and 6 in Table 3.
The percentages of cells within each lineage that expressed Ly-5.1.
The cells were prepared as described previously.17
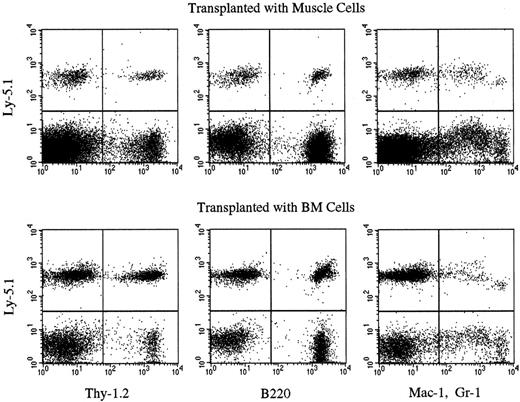
Multilineage hematopoietic reconstitution by Ly-5.1 muscle cells of chimeric mice.
The results of experiment 6 in Table 4 are shown. Nucleated PB cells of secondary Ly-5.2 recipients were analyzed by flow cytometry 6 months after secondary transplantation with BM or muscle cells of chimeric mice. Thy-1.2+ cells, B220+ cells, and Gr-1+ and/or Mac-1+ cells with the Ly-5.1 phenotype are seen in both recipients.
Multilineage hematopoietic reconstitution by Ly-5.1 muscle cells of chimeric mice.
The results of experiment 6 in Table 4 are shown. Nucleated PB cells of secondary Ly-5.2 recipients were analyzed by flow cytometry 6 months after secondary transplantation with BM or muscle cells of chimeric mice. Thy-1.2+ cells, B220+ cells, and Gr-1+ and/or Mac-1+ cells with the Ly-5.1 phenotype are seen in both recipients.
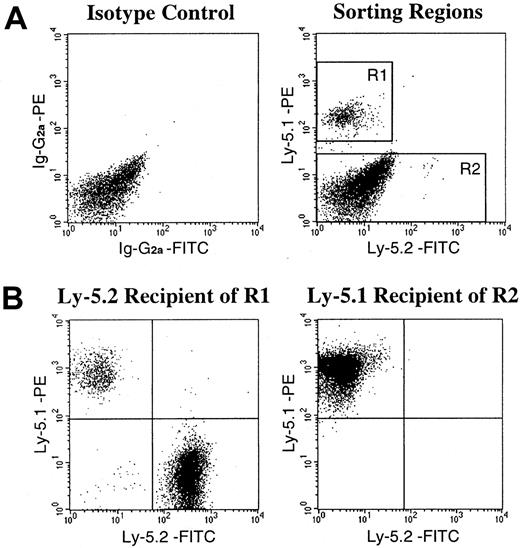
Recent reports from Gussoni et al7 and Jackson et al13 suggested the existence, in the muscle, of a Ly-5− population of stem cells that are capable not only of muscular regeneration but also of hematopoietic reconstitution. This bipotential population of stem cells in the muscle tissue of Ly-5.2 origin may have survived the irradiation and contributed to the high levels of engraftment by Ly-5.2 cells in the secondary transplantation. Therefore, in the next series of experiments, we created chimeric mice showing very high (99%) levels of hematopoietic chimerism (experiments 7 and 8 in Table 3), fractionated the muscle mononuclear cells, and analyzed the engrafting abilities of the fractionated cell populations. The sorting gates for 2 populations of muscle mononuclear cells of the chimeric mice are presented in Figure 2A. Reflecting the high levels of Ly-5.1 cells in PB, the majority of Ly-5+cells in the muscle were also of Ly-5.1 type. The R1 gate was used for isolation of Ly-5.1+ donor BM-derived cells. The Ly-5.1− R2 gate may have included Ly-5.1–derived cells that are not expressing the antigen and/or Ly-5.2–derived cells. After FACS sorting, the isolated 2 populations of muscle cells were injected into lethally irradiated Ly-5.2 or Ly-5.1 recipients, respectively, along with radioprotective BM cells from mice having the recipients' Ly-5 genotypes. Two months later, hematopoietic engraftment in recipients' PB was analyzed by flow cytometry (Table5, Figure 2B). The Ly-5.1 cells (R1) engrafted in the Ly-5.2 recipients. However, transplantation of Ly-5.1− muscle cells (R2) did not show hematopoietic engraftment by Ly-5.2 cells. These results indicated that all hematopoietic stem cells in the muscle of chimeric mice are of BM origin.
FACS sorting regions used for preparation of test cell populations from the muscle of chimeric mice and an example of analysis of hematopoietic engraftment by FACS-sorted cells.
(A) Ly-5.1+ cells and Ly-5.1− cells were separated from mononuclear cells of the muscle of chimeric mice by FACS analysis with the use of R1 and R2 gates, respectively, for secondary-transplantation studies. Dot plots obtained in experiment 8 in Table 5 are shown. (B) Analysis of engraftment 2 months after secondary transplantation. Ly-5.1+ (R1) cells engrafted in the Ly-5.2 recipient. Ly-5.1− (R2) cells transplanted into the Ly-5.1 recipient did not show hematopoietic engraftment by Ly-5.2 cells.
FACS sorting regions used for preparation of test cell populations from the muscle of chimeric mice and an example of analysis of hematopoietic engraftment by FACS-sorted cells.
(A) Ly-5.1+ cells and Ly-5.1− cells were separated from mononuclear cells of the muscle of chimeric mice by FACS analysis with the use of R1 and R2 gates, respectively, for secondary-transplantation studies. Dot plots obtained in experiment 8 in Table 5 are shown. (B) Analysis of engraftment 2 months after secondary transplantation. Ly-5.1+ (R1) cells engrafted in the Ly-5.2 recipient. Ly-5.1− (R2) cells transplanted into the Ly-5.1 recipient did not show hematopoietic engraftment by Ly-5.2 cells.
Hematopoietic reconstitution by Ly-5.1+ cells separated from the muscle cells of chimeric mice
| Experiment no.5-150 . | Cells transplanted5-151 . | Radioprotective cells5-152 . | Secondary recipients (no. recipients) . | % Chimerism in secondary recipients' PB . | Multilineage reconstitution by designated muscle cells5-153 . | ||
|---|---|---|---|---|---|---|---|
| G/M . | T . | B . | |||||
| 7 | Ly-5.1+ 1.0 × 105 | Ly-5.2 BM cells 2 × 105 | Ly-5.2 (2) | 1 | 24 | 89 | 8 |
| 1 | 21 | 64 | 14 | ||||
| Ly-5.1−5.0 × 105 | Ly-5.1 BM cells 2 × 105 | Ly-5.1 (2) | 0 | 0 | 0 | 0 | |
| 0 | ND | ND | ND | ||||
| 8 | Ly-5.1+ 8.0 × 104 | Ly-5.2 BM cells 2 × 105 | Ly-5.2 (2) | 9 | 18 | 86 | 3 |
| 5 | 18 | 87 | 5 | ||||
| Ly-5.1− 3.4 × 105 | Ly-5.1 BM cells 2 × 105 | Ly-5.1 (2) | 0 | 0 | 0 | 0 | |
| 0 | ND | ND | ND | ||||
| Experiment no.5-150 . | Cells transplanted5-151 . | Radioprotective cells5-152 . | Secondary recipients (no. recipients) . | % Chimerism in secondary recipients' PB . | Multilineage reconstitution by designated muscle cells5-153 . | ||
|---|---|---|---|---|---|---|---|
| G/M . | T . | B . | |||||
| 7 | Ly-5.1+ 1.0 × 105 | Ly-5.2 BM cells 2 × 105 | Ly-5.2 (2) | 1 | 24 | 89 | 8 |
| 1 | 21 | 64 | 14 | ||||
| Ly-5.1−5.0 × 105 | Ly-5.1 BM cells 2 × 105 | Ly-5.1 (2) | 0 | 0 | 0 | 0 | |
| 0 | ND | ND | ND | ||||
| 8 | Ly-5.1+ 8.0 × 104 | Ly-5.2 BM cells 2 × 105 | Ly-5.2 (2) | 9 | 18 | 86 | 3 |
| 5 | 18 | 87 | 5 | ||||
| Ly-5.1− 3.4 × 105 | Ly-5.1 BM cells 2 × 105 | Ly-5.1 (2) | 0 | 0 | 0 | 0 | |
| 0 | ND | ND | ND | ||||
Ly-5.1+ cells or Ly-5.1− cells separated from the muscle of chimeric mice were transplanted into lethally irradiated secondary recipient mice along with radioprotective cells. At 2 months later, PB was collected from the secondary recipients, and the levels of engraftment were analyzed by flow cytometry. Nucleated PB cells were stained with anti-Ly-5.1 or anti-Ly-5.2 and either anti-Gr-1 and anti-Mac-1, anti-Thy-1.2, or anti-B220.
PB indicates peripheral blood; G/M, granulocytes and/or monocytes; T, T lymphocytes; B, B lymphocytes; ND, not determined.
The experimental mice correspond to experiments 7 and 8 in Table 3.
The numbers of cells transplanted per mouse are in proportion to the numbers of Ly-5.1+ cells and Ly-5.1− cells in the mononuclear cells harvested from the muscle of chimeric mice.
Unfractionated BM cells were used as radioprotective cells.
Numbers indicate the percentages of cells within each lineage that expressed Ly-5.1 (for Ly-5.2 recipients) or Ly-5.2 (for Ly-5.1 recipients).
Effects of mobilization of hematopoietic progenitors
Our studies using chimeric mice suggested communication between the hematopoietic cell population in the muscle and BM. In the next experiment, we tested the effects of G-CSF–induced progenitor mobilization on the progenitors in the muscle. Administration of G-CSF caused more than 20-fold increases in the numbers of CFU-Cs, CFU-GMs, and CFU-GEMMs in PB (Table 6). However, the number and type of progenitors in muscle tissue were not affected by administration of G-CSF.
Effects of mobilization by granulocyte colony-stimulating factor on the prognitors in the muscle and peripheral blood
| Colony . | PB (per mL) . | Muscle (per 2 hind legs) . | ||
|---|---|---|---|---|
| G-CSF(−) . | G-CSF(+) . | G-CSF(−) . | G-CSF(+) . | |
| BFU-E | 18 ± 6 | 216 ± 1266-150 | 3 ± 2 | 2 ± 2 |
| GEMM | 4 ± 0 | 83 ± 336-151 | 1 ± 1 | 1 ± 1 |
| GM | 42 ± 8 | 1013 ± 2566-151 | 136 ± 9 | 138 ± 11 |
| Total | 74 ± 5 | 1594 ± 2366-151 | 158 ± 15 | 157 ± 11 |
| Colony . | PB (per mL) . | Muscle (per 2 hind legs) . | ||
|---|---|---|---|---|
| G-CSF(−) . | G-CSF(+) . | G-CSF(−) . | G-CSF(+) . | |
| BFU-E | 18 ± 6 | 216 ± 1266-150 | 3 ± 2 | 2 ± 2 |
| GEMM | 4 ± 0 | 83 ± 336-151 | 1 ± 1 | 1 ± 1 |
| GM | 42 ± 8 | 1013 ± 2566-151 | 136 ± 9 | 138 ± 11 |
| Total | 74 ± 5 | 1594 ± 2366-151 | 158 ± 15 | 157 ± 11 |
Peripheral blood and muscles were collected from 6 mice that received G-CSF treatment and 6 control mice without G-CSF treatment. After Percoll density-gradient separation, mononuclear cells were cultured in methylcellulose in the presence of thrombopoietin, steel factor, erythropoietin, IL-3, granulocyte/macrophage CSF. On day 8 of culture, colonies were counted. Data represent the mean and SD of values based on the results obtained from 4 dishes. Two additional experiments showed similar results.
PB indicates peripheral blood; G-CSF, granulocyte colony-stimulating factor; BFU-E, erythroid burst-forming units; GEMM, mixed colonies containing erythroid and myeloid cells and megakaryocytes; and GM, granulocyte and/or macrophage colonies.
P < .05, as compared with the control mice.
P < .01, as compared with the control mice.
Discussion
It was reported recently that murine skeletal muscles contain cells that are capable of significant hematopoietic reconstitution. Gussoni et al7 noted the presence in the muscle tissue of side population (SP) cells that had been reported to be highly enriched for hematopoietic stem cells.18 The muscle SP cells were capable not only of muscular regeneration but also of significant hematopoietic engraftment when transplanted into lethally irradiated syngeneic mice.7 Subsequently, Jackson et al13 reported that cultured mononuclear cells from muscle of adult mice are capable of hematopoietic reconstitution. They also attributed the hematopoietic engraftment to the muscle SP cells and speculated that skeletal muscle stem cells, ie, satellite cells, are responsible for the hematopoietic activity.
In this study, we attempted to characterize the hematopoietic progenitors and stem cells that are present in muscle tissue. Care was taken to harvest cells only from the muscle tissue and not to contaminate the samples with BM cells. We demonstrated that both the types and the relative incidences of the hematopoietic progenitors in muscle are different from those in PB or BM. However, methylcellulose culture studies of the muscle cells of chimeric mice revealed significant correlation between the incidences of donor origin progenitors in the muscle and the BM. We had a significant number of stable chimeric mice from other experiments that provided the opportunity to analyze various donor populations of stem cells. Whether we used CD34+, CD34−, or unfractionated donor cells, we were able to test the correlation of the hematopoietic potential between muscle and BM cells because this was analyzed in individual mice. This correlation indicated that hematopoietic progenitors in the muscle are of BM origin. Furthermore, the secondary-transplantation experiments with the chimeric mice demonstrated that hematopoietic stem cells in the muscle are also derived from BM. Since we gave lethal irradiation to create chimeric mice, we could not study the original population of hematopoietic cells in the muscle. However, our studies clearly documented communication of hematopoietic populations between BM and muscle tissue. Progenitor mobilization by G-CSF did not affect the hematopoietic progenitors in the muscle. Transition of the hematopoietic populations from BM to the muscle appears to be a slow process.
Following the initial identification of long-term hematopoietic engrafting cells in murine muscle,7,13 investigators in other laboratories19-21 also documented hematopoietic populations in the muscle. However, there are some discrepancies concerning the level of hematopoietic reconstitution. While Gussoni et al7 and Jackson et al13 revealed high levels of hematopoietic engraftment by the muscle cells, Farace et al19 and Bauermeister et al20 described lower engrafting potentials of the muscle cells. There are a number of technical differences in the preparation of the test cells. Although our studies indicated lower levels (1% to 9%) of engraftment, our analysis was based primarily on secondary transplantation of chimeric animals. Gussoni et al7 used muscle SP cells from young (3- to 5-week-old) mice. Jackson et al13 used muscle cells that were cultured for 5 days under conditions for satellite cell growth. Both groups suggested that the Ly-5− cells in the muscle SP cells are bipotential, having myogenic and hematopoietic capabilities.7,13 Subsequently, Howell et al21 also reported hematopoietic engraftment by Ly-5− muscle cells from neonatal (4- to 7-day-old) mice. We have observed that Ly-5− muscle cells derived from the chimeric mice do not possess hematopoietic capability. However, our results do not exclude the possibility of a BM-derived stem cell that has the potential for both hematopoietic and myogenic differentiation. Clonal studies are necessary to assess the true potentials of stem cells in muscle tissue.
The authors wish to thank Drs Fumihiko Ishikawa, Akaru Ishida, and Takao Deguchi for assistance in breeding of experimental mice; Dr Pamela N. Pharr, Anne G. Livingston, and Karen A. Rivers for assistance in preparation of this manuscript; Dr Haiqun Zeng for assistance in FACS sorting; and the staff of the Radiation Oncology Department of the Medical University of South Carolina for assistance in the irradiation of the mice.
Supported by grants PO1-CA78582 and RO1-DK54197 from the National Institutes of Health; the Office of Research and Development, Medical Research Services, Department of Veterans Affairs; The Japan Society for the Promotion of Science grant JSPS-RFTF97-I-00201; and Tokai University General Research Organization.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Makio Ogawa, Department of Veterans Affairs Medical Center, 109 Bee St, Charleston, SC 29401-5799; e-mail:ogawam@musc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal