Several patients with clinical features of chronic myeloid leukemia (CML) have fusion of the TEL (ETV6) gene on 12p13 with ABL on 9q34 and express a chimeric Tel-Abl protein that contains the same portion of the Abl tyrosine kinase fused to Tel, an Ets family transcription factor, rather than Bcr. In a murine retroviral bone marrow transduction-transplantation model, a Tel (exon 1-5)–Abl fusion protein induced 2 distinct illnesses: a CML-like myeloproliferative disease very similar to that induced by Bcr-Abl but with increased latency and a novel syndrome characterized by small-bowel myeloid cell infiltration and necrosis, increased circulating endotoxin and tumor necrosis factor α levels, and fulminant hepatic and renal failure. Induction of both diseases required the Tel pointed homology oligomerization domain and Abl tyrosine kinase activity. Myeloid cells from mice with both diseases expressed Tel-Abl protein. CML-like disease induced by Tel-Abl and Bcr-Abl was polyclonal and originated from cells with multilineage (myeloid, erythroid, and B- and T-lymphoid) repopulating ability and the capacity to generate day-12 spleen colonies in secondary transplantations. In contrast to findings with Bcr-Abl, however, neither Tel-Abl–induced disease could be adoptively transferred to irradiated secondary recipient syngeneic mice. These results show that Tel-Abl has leukemogenic properties from distinct from those of Bcr-Abl and may act in a different bone marrow progenitor.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disease characterized by excessive clonal production of maturing myeloid cells.1 The direct cause of CML is the product of the t(9;22) Philadelphia chromosome translocation, theBCR-ABL oncogene, and its corresponding protein, the Bcr-Abl tyrosine kinase. Retroviral transduction of the BCR-ABL gene into mouse bone marrow cells, followed by transplantation into irradiated syngeneic mice, induces CML-like myeloproliferative leukemia in all recipients within 4 weeks after transplantation.2-4Mice with BCR-ABL–induced CML-like disease have massive polyclonal expansion of maturing myeloid cells, principally neutrophils, which express Bcr-Abl protein and infiltrate the spleen, liver, and lungs. Although neutrophils are the predominant hematopoietic lineage overproduced in murine CML-like disease, macrophages, erythroid progenitors, B lymphocytes, and sometimes T lymphocytes from diseased mice carry the same spectrum ofBCR-ABL proviral clones as the granulocytes, thereby demonstrating that the cells initiating the CML-like leukemia are early multipotential progenitors.2
The CML-like disease is efficiently transferred by transplantation of bone marrow or spleen cells from a primary mouse to irradiated secondary recipients.2-4 Interestingly, of the many different BCR-ABL–transduced clones that contribute to the leukemia in the primary mouse, only a small subset are capable of generating day-12 spleen colonies and of engrafting and inducing CML-like disease in secondary recipients,2,4 suggesting that the cells initiating CML-like disease are heterogeneous for self-renewal. Murine CML-like leukemia is an accurate and faithful model of human CML that has been proved to be useful for analysis of the molecular pathophysiologic characteristics of the human disease.2,5 6
The TEL gene (also known as ETS–variant gene 6 [ETV6]), located on chromosome 12p13, was originally identified at the breakpoint of a (5;12) translocation in a patient with chronic myelomonocytic leukemia, where it was fused to the platelet-derived growth factor β (PDGF-βR) receptor gene.7TEL encodes a ubiquitously expressed 452–amino acid protein with 2 regions of homology to the Ets family of transcription factors: the pointed (PNT) homology domain and the DNA-binding domain. Subsequently, TEL was recognized to be rearranged in many different chromosomal translocations in human leukemia, most frequently by means of t(12;21) in childhood pre-B acute lymphoblastic leukemia, in which TEL is fused to theAML1 gene.8,9TEL has been discovered to be fused to ABL in 6 patients with leukemia. Three of these patients had acute leukemia involving different lineages, including adult B-lymphoblastic leukemia (B-ALL),10 acute undifferentiated myeloid leukemia,11 and childhood T-lymphoblastic leukemia (T-ALL).12 Interestingly, the other 3 patients had myeloproliferative disease diagnosed as atypical13 or typical12,14 CML. Fusion ofTEL and ABL in human acute or chronic leukemia appears to be a rare event,15,16 which may reflect the opposite orientation of the TEL and ABL genes in relation to the centromeres of chromosomes 9 and 12,12 so that a fusion between the 2 genes that does not cause an unbalanced chromosome loss requires a complex rearrangement of at least 3 steps. Indeed, all the patients with TEL-ABL in whom studies at the cytogenetic and molecular levels have been conducted were found to have complex translocations or cryptic insertions.12 14
The TEL-ABL fusion oncogenes produce 2 different Tel-Abl proteins; in the patients with B-ALL and atypical CML, the first 4 exons of TEL were found to be fused to ABL exon 2, whereas the other 4 patients had TEL exons 1 to 5 fused to ABL exon 2. The resulting chimeric proteins contain Tel amino acids 1 to 154 and 1 to 336, respectively, fused to the same 1104 carboxy (COOH)-terminal amino acids of c-Abl found in the Bcr-Abl fusion proteins. The Tel-Abl fusion proteins show increased tyrosine kinase activity in vitro10 and are constitutively phosphorylated on tyrosine in vivo.11,17 Although Tel is a predominantly nuclear protein,7 Tel-Abl is localized to the cytoplasm and F-actin cytoskeleton,11,17 similar to Bcr-Abl. An amino (NH2)-terminal region of Tel between amino acids 60 and 120 shares homology with the Ets proteins ETS-1, FLI-1, PNTP2, and Yan and has been referred to as the PNT domain. In Tel, the PNT domain mediates oligomerization11,18 and is required for activation of the Tel-PDGFβR,18,19Tel-Jak2,20 and Tel-Abl11 fusion tyrosine kinases. The fact that Bcr contains a coiled-coil oligomerization domain that is also required for activation of Bcr-Abl kinase activity and transformation21 has led to the suggestion that oligomerization of Abl may be the critical event in the pathogenesis of these leukemias and that other functions of the NH2-terminal Abl fusion partner may be unimportant. Consistent with this idea, Tel-Abl has been shown to transform Rat-1 fibroblasts,11 primary bone marrow B-lymphoid cells,11 and cytokine-dependent Ba-F3 hematopoietic cells11,17 in vitro in a manner indistinguishable from that of Bcr-Abl. Furthermore, Tel-Abl and Bcr-Abl activate similar intracellular signaling pathways in cultured hematopoietic cells.22 23
We are interested in Tel-Abl not because of the clinical importance of Tel-Abl–associated human leukemia but because careful comparative analysis of signaling and leukemogenesis by Tel-Abl may help illuminate the pathophysiologic mechanism of Bcr-Abl–induced CML. Studies of Tel-Abl transformation in vitro failed to identify any significant differences between Tel-Abl and Bcr-Abl.22,23 However, studies of Bcr-Abl signaling mutants demonstrated that transformation studies in cultured cells do not correlate well with leukemogenesis in vivo.5 6 In the study described here, we directly compared leukemogenesis by Tel-Abl and by Bcr-Abl in mice and demonstrated that Tel-Abl induces distinct hematologic diseases that differ both in phenotype and ability to be transplanted.
Materials and methods
DNA constructs
All ABL oncogenes were introduced into the retroviral vector murine stem cell virus (MSCV) neo. TheTEL-ABL complementary DNA (B form, containing TELexons 1-5 fused to ABL exon 211) was transferred from the pSRαTKneo vector as an EcoRI fragment. The ΔPNT deletion mutation was described previously.11The TEL-ABL K581R mutant was generated by transferring a 1720–base-pair KpnI-SgrAI fragment from theABL portion of the kinase-inactive BCR-ABL mutant K1172R (a gift of Dr R. Ren, Brandeis University, Waltham, MA) toTEL-ABL.
In vitro kinase assay
Tel-Abl, Bcr-Abl, and c-Abl proteins were expressed by transient transfection of 293 cells with the MSCVneo vectors. Cells were labeled with sulfur 35 (35S)–l-methionine before harvesting, and the relative abundance of Abl protein in each extract was determined by a preliminary anti-Abl immunoprecipitation and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Volumes of extracts with equivalent amounts of Abl protein were immunoprecipitated with anti-Abl antiserum and subjected to an immune complex kinase assay using a glutathione-S-transferase (GST)–Crk substrate as described previously.6 The amount of 35S-Abl and phosphorus 32 (32P)–Crk incorporation was measured by using phosphorimager analysis (Storm 850; Molecular Dynamics, Sunnyvale, CA), and the relative Abl protein levels, which were within 10% of each other, were corrected for methionine content and used to normalize 32P incorporation to calculate relative kinase activity.
Bone marrow transduction and transplantation
Generation and titering of retroviral stocks was done as described previously;2 all stocks had titers of 3 to 5 × 106 neomycin-resistant colony-forming units (CFUs)/mL, and yielded equivalent proviral copy numbers, as assessed by Southern blotting on transduction of National Institutes of Health (NIH) 3T3 cells or primary bone marrow. Balb/c mice (Taconic Farms, Germantown, MD) were used for all experiments. Bone marrow from male donors treated with 5-fluorouracil (200 mg/kg) was transduced and 5 × 105 cells were transplanted into lethally irradiated (900 cGy) female recipients as described previously;2 in some experiments, recipients received only 450 cGy of irradiation. For secondary transplantation, a 1:1 mixture of 3 to 6 × 106marrow and spleen cells was injected intravenously into pairs of lethally irradiated (900 cGy) female Balb/c secondary recipient mice. The viability of all cells used for secondary transplantation was confirmed by trypan blue staining to be greater than 90%. For isolation of day-12 spleen colonies, pairs of lethally irradiated recipient mice were injected with 1 × 105, 3 × 105, and 1 × 106 bone marrow cells, spleens were isolated 12 days after injection, and macroscopic colonies were dissected out. Colonies that were not visibly red were assessed by cytospin and Wright-Giemsa staining to confirm that they were composed of mature myeloid cells.
Southern blotting and lineage analysis
Purification of neutrophils, spleen erythroid progenitors, and spleen or lymph node B lymphocytes employed lineage-specific monoclonal antibodies and immunomagnetic beads (Miltenyi Biotec, Germany) as described previously,2 except in some cases in which microbeads directly conjugated with TER119 antibodies were used for isolation of erythroid cells. Peritoneal or bone marrow macrophages were isolated by adherence and by culture in medium containing macrophage colony-stimulating factor. The purity of the selected populations was assessed by Wright-Giemsa staining of cytospin preparations and, in some cases, by fluorescence-activated cell-sorter scanning, and only samples with at least 80% purity were used for Southern blot analysis. Genomic DNA was prepared, digested withBglII, and hybridized with radioactive probes from the neomycin-resistance gene to detect distinct proviral integration events. It was then stripped and reprobed with a human ABLprobe that detects a common 2.2-kb fragment from all integrants and allows determination of total proviral content of each sample, as described previously.2
Western blotting
For assessment of in vivo kinase activity, NIH 3T3 cells were transduced with retrovirus expressing p210 BCR-ABL, TEL-ABL,TEL-ABL ΔPNT, TEL-ABL K581R, or empty MSCVneo vector. All stocks had equivalent titer in fibroblasts, based on G418 resistance. Transduced cells were selected in 1.0 mg/mL G418 for 48 hours, starved for 4 hours in Dulbecco modified Eagle medium with 0.5% bovine serum albumin, and then lysed in radioimmunoprecipitation assay buffer. Extracts from peripheral blood (PB) leukocytes or spleen cells (predominantly maturing myeloid cells) were prepared by direct boiling as described previously2 and clarified by centrifugation at 100 000g for 15 minutes. Equivalent amounts of protein (verified by Ponceau-S staining) were fractionated by 5% to 20% gradient SDS-PAGE, transferred to nitrocellulose membranes, and blotted with anti-Abl (3F12, a gift of Dr R. Salgia, Dana-Farber Cancer Institute, Boston, MA) and antiphosphotyrosine (4G10; Upstate Biotechnology, Charlottesville, VA) monoclonal antibodies.
Results
Tel-Abl has increased in vivo and in vitro tyrosine kinase activity relative to p210 Bcr-Abl
Previous studies demonstrated that Tel-Abl had tyrosine kinase activity in vitro that was similar to that of Bcr-Abl10and was constitutively tyrosine phosphorylated in fibroblasts11 and hematopoietic cells,17,22whereas mutants of Tel-Abl lacking the NH2-terminal PNT homology oligomerization domain lacked phosphotyrosine in vivo.11 To assess quantitatively the kinase activity of Tel-Abl relative to Bcr-Abl, we compared Tel-Abl (containing Tel amino acids 1-336 fused to Abl), a deletion mutant of Tel-Abl (ΔPNT) lacking most of the PNT domain, and a catalytically inactive mutant Tel-Abl protein (K581R) with p210 Bcr-Abl (Figure1) in an immune complex kinase assay. Tel-Abl reproducibly had more kinase activity for a GST-Crk substrate than did p210 Bcr-Abl (Figure 2A), whereas the Tel-Abl ΔPNT mutant had significantly lower activity but still more than c-Abl. Immunoprecipitates of the Tel-Abl K581R protein had very low kinase activity that likely originated from endogenous c-Abl. When these fusion kinases were stably expressed in NIH 3T3 fibroblasts by retroviral transduction, cells expressing Tel-Abl had slightly higher levels of tyrosine phosphoproteins than cells expressing p210 Bcr-Abl, whereas cells expressing Tel-Abl ΔPNT had lower but significantly higher levels than cells expressing Tel-Abl K581R or nontransduced cells (Figure 2B). These results show that Tel-Abl has as much or higher tyrosine kinase activity than p210 Bcr-Abl in vivo and in vitro that depends partly on the PNT oligomerization domain.
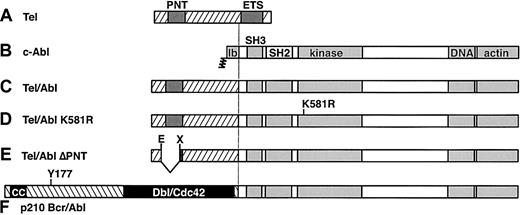
Structure of Tel, Abl, and chimeric Tel-Abl and Bcr-Abl proteins.
(A) Full-length Tel (ETV6) protein of 452 amino acids, with positions of the PNT homology oligomerization domain and ETS DNA-binding domain indicated by dark shaded boxes. (B) Full-length type Ib c-Abl protein of 1142 amino acids, with NH2-terminal myristoylation site, SH3 and SH2 domains, tyrosine kinase catalytic domain, and COOH-terminal DNA- and actin-binding domains indicated by lightly shaded boxes. (C) Tel-Abl fusion protein, consisting of sequences fromTEL exons 1 to 5 (amino acids 1-336) fused to the COOH-terminal 1104 amino acids of Abl. (D) Tel-Abl K581R mutant, containing an inactivating point mutation in the Abl catalytic domain of lysine 581 to arginine. (E) Tel-Abl ΔPNT, with an inframe deletion of 76 amino acids between Eco47III and XmnI sites11 resulting in loss of most of the PNT domain. (F) The p210 Bcr-Abl fusion protein, consisting of Bcr amino acids 1 to 927 fused to the COOH-terminal 1104 amino acids of Abl, with the NH2-terminal coiled-coil (CC) domain and region of homology to Dbl-Cdc42 designated by black boxes and the location of the tyrosine 177 Grb2 binding site indicated.
Structure of Tel, Abl, and chimeric Tel-Abl and Bcr-Abl proteins.
(A) Full-length Tel (ETV6) protein of 452 amino acids, with positions of the PNT homology oligomerization domain and ETS DNA-binding domain indicated by dark shaded boxes. (B) Full-length type Ib c-Abl protein of 1142 amino acids, with NH2-terminal myristoylation site, SH3 and SH2 domains, tyrosine kinase catalytic domain, and COOH-terminal DNA- and actin-binding domains indicated by lightly shaded boxes. (C) Tel-Abl fusion protein, consisting of sequences fromTEL exons 1 to 5 (amino acids 1-336) fused to the COOH-terminal 1104 amino acids of Abl. (D) Tel-Abl K581R mutant, containing an inactivating point mutation in the Abl catalytic domain of lysine 581 to arginine. (E) Tel-Abl ΔPNT, with an inframe deletion of 76 amino acids between Eco47III and XmnI sites11 resulting in loss of most of the PNT domain. (F) The p210 Bcr-Abl fusion protein, consisting of Bcr amino acids 1 to 927 fused to the COOH-terminal 1104 amino acids of Abl, with the NH2-terminal coiled-coil (CC) domain and region of homology to Dbl-Cdc42 designated by black boxes and the location of the tyrosine 177 Grb2 binding site indicated.
Tel-Abl has increased in vitro and in vivo tyrosine kinase activity relative to p210 Bcr-Abl.
(A) In vitro kinase assay. The c-Abl, p210 Bcr-Abl, and Tel-Abl wild-type (WT; with Tel amino acids 1-336 fused to Abl), ΔPNT, and K581R (KR) proteins were labeled in vivo with35S–l-methionine, immunoprecipitated, and incubated with γ-32P–adenosine triphosphate and GST-Crk substrate. The top panel is the 35S label, indicating relative levels of expression of the different Abl proteins; the lower panel is the 32P label, showing levels of autophosphorylation of Abl proteins and transphosphorylation of the GST-Crk substrate. The Crk kinase activity of the different Abl proteins relative to c-Abl after correction for levels of expression is shown at the top. (B) In vivo kinase activity. NIH 3T3 fibroblasts were transduced with empty MSCVneo virus or MSCV virus containing p210 BCR-ABL or TEL-ABL WT, ΔPNT, or KR, and selected for G418 resistance. Lysates were analyzed by Western blotting with anti-Abl (top panel) and antiphosphotyrosine (bottom panel) antibodies.
Tel-Abl has increased in vitro and in vivo tyrosine kinase activity relative to p210 Bcr-Abl.
(A) In vitro kinase assay. The c-Abl, p210 Bcr-Abl, and Tel-Abl wild-type (WT; with Tel amino acids 1-336 fused to Abl), ΔPNT, and K581R (KR) proteins were labeled in vivo with35S–l-methionine, immunoprecipitated, and incubated with γ-32P–adenosine triphosphate and GST-Crk substrate. The top panel is the 35S label, indicating relative levels of expression of the different Abl proteins; the lower panel is the 32P label, showing levels of autophosphorylation of Abl proteins and transphosphorylation of the GST-Crk substrate. The Crk kinase activity of the different Abl proteins relative to c-Abl after correction for levels of expression is shown at the top. (B) In vivo kinase activity. NIH 3T3 fibroblasts were transduced with empty MSCVneo virus or MSCV virus containing p210 BCR-ABL or TEL-ABL WT, ΔPNT, or KR, and selected for G418 resistance. Lysates were analyzed by Western blotting with anti-Abl (top panel) and antiphosphotyrosine (bottom panel) antibodies.
TEL-ABL induces CML-like myeloproliferative disease and a novel disease of the small bowel in mice
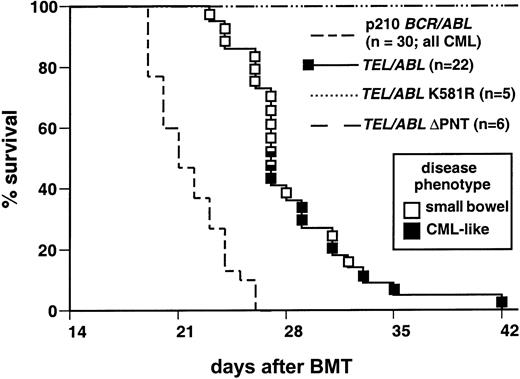
We tested the ability of the TEL-ABL oncogenes to induce leukemia in mice in the retroviral bone marrow transduction-transplantation model system.2 Bone marrow from donors pretreated with 5-flurouracil was transduced with retrovirus expressing either p210 BCR-ABL,TEL-ABL (containing the first 5 exons of TELfused to ABL exon 2), the kinase-inactiveTEL-ABL mutant (K581R), or the TEL-ABL ΔPNT mutant. The ΔPNT deletion mutation was previously shown to abolish transformation of fibroblasts and primary bone marrow B-lymphoid cells by TEL-ABL.11 All recipients ofTEL-ABL–transduced bone marrow died within 42 days after transplantation, 1 to 2 weeks later than recipients of p210BCR-ABL–transduced marrow (Figure3). This difference in survival was highly significant (P < .001 on Mantel-Cox test). No recipients of marrow transduced with either TEL-ABL K581R orTEL-ABL ΔPNT had onset of disease during this period; these mice had normal peripheral blood (PB) counts at 42 and 86 days after transplantation (data not shown) and remained healthy at 6 months after transplantation. These results show that both the Abl tyrosine kinase activity and the Tel PNT oligomerization domain are required for induction of fatal hematologic disease by TEL-ABL.
Recipients of TEL-ABL–transduced bone marrow have increased survival compared with those givenBCR-ABL marrow.
Kaplan-Meier–style survival curve for recipients of bone marrow from donors treated with 5-flurouracil, with the marrow transduced with MSCVneo retrovirus expressing TEL-ABL (solid line) or p210 BCR-ABL (broken line). The number of recipients in each arm is shown in parentheses. The curve forBCR-ABL is a composite of data from previously described mice5 and additional mice given transplants concurrently with the TEL-ABL recipients. CML-like disease developed in all recipients in this arm. The square symbols indicate individualTEL-ABL recipient mice, with the disease phenotype of each designated by the shading (solid black indicates CML-like disease; and open indicates small-bowel necrosis with acute fatty liver). Mice with prominent histopathological features of both disease processes are indicated by multiply shaded symbols. The TEL-ABL curve is a composite of 3 independent transplantation experiments that yielded identical results. The difference in survival between recipients ofBCR-ABL–transduced and TEL-ABL–transduced marrow was significant (P < .0001 by Mantel-Cox test). All recipients of marrow transduced with TEL-ABL, ΔPNT, or K581R (large dashed or dotted lines) remained healthy.
Recipients of TEL-ABL–transduced bone marrow have increased survival compared with those givenBCR-ABL marrow.
Kaplan-Meier–style survival curve for recipients of bone marrow from donors treated with 5-flurouracil, with the marrow transduced with MSCVneo retrovirus expressing TEL-ABL (solid line) or p210 BCR-ABL (broken line). The number of recipients in each arm is shown in parentheses. The curve forBCR-ABL is a composite of data from previously described mice5 and additional mice given transplants concurrently with the TEL-ABL recipients. CML-like disease developed in all recipients in this arm. The square symbols indicate individualTEL-ABL recipient mice, with the disease phenotype of each designated by the shading (solid black indicates CML-like disease; and open indicates small-bowel necrosis with acute fatty liver). Mice with prominent histopathological features of both disease processes are indicated by multiply shaded symbols. The TEL-ABL curve is a composite of 3 independent transplantation experiments that yielded identical results. The difference in survival between recipients ofBCR-ABL–transduced and TEL-ABL–transduced marrow was significant (P < .0001 by Mantel-Cox test). All recipients of marrow transduced with TEL-ABL, ΔPNT, or K581R (large dashed or dotted lines) remained healthy.
Two different fatal outcomes were observed in recipients ofTEL-ABL–transduced marrow. In some recipients (7 of 22), a progressive myeloproliferative disease developed that was very similar to the disease induced by p210 BCR-ABL (Figure 3, black squares). This disease mimicked the distinctive features of human CML: leukocytosis with maturing neutrophils, splenomegaly, hepatomegaly, and myeloid cell infiltration into peripheral tissues, including the spleen, liver, and lungs (Table 1).
Clinicopathologic characteristics of mice with chronic myeloid leukemia–like disease or small-bowel syndrome
| ABL oncogene . | Disease . | PB WBC (× 109/L) . | Spleen wt (g) . | Fatty liver . | Pulmonary hemorrhage . | Liver EMH . |
|---|---|---|---|---|---|---|
| p210BCR-ABL | CML-like (n = 10)* | 295 ± 59 | 0.92 ± 0.06 | − | + | + |
| TEL-ABL | CML-like (n = 11)* | 254 ± 47 | 0.74 ± 0.05 | − | + | + |
| TEL-ABL | SBS (n = 26)* | 56 ± 16 | 0.31 ± 0.02 | + | − | − |
| ABL oncogene . | Disease . | PB WBC (× 109/L) . | Spleen wt (g) . | Fatty liver . | Pulmonary hemorrhage . | Liver EMH . |
|---|---|---|---|---|---|---|
| p210BCR-ABL | CML-like (n = 10)* | 295 ± 59 | 0.92 ± 0.06 | − | + | + |
| TEL-ABL | CML-like (n = 11)* | 254 ± 47 | 0.74 ± 0.05 | − | + | + |
| TEL-ABL | SBS (n = 26)* | 56 ± 16 | 0.31 ± 0.02 | + | − | − |
Plus-minus values are means ± SE.
PB indicates peripheral blood; WBC, white blood cell count, and EMH, extramedullary hematopoiesis.
Number of diseased mice analyzed; the data forTEL-ABL-induced CML-like disease and SBS include recipients from additional transplantation experiments that yielded results similar to those in shown in Figure 3.
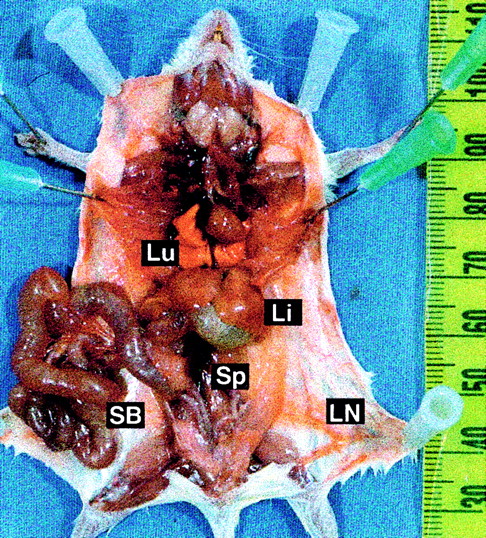
In most mice (13 of 22) given transplants ofTEL-ABL–transduced marrow, a distinct fatal illness developed (Figure 3, open squares). At necropsy, these mice were found to have enlarged, reddened, and distended small intestine; a pale liver; and increased prominence of the vasculature (Figure4). In contrast to mice with CML-like disease, these mice had only slightly elevated PB leukocyte counts and their spleens were minimally enlarged (Figure 4 and Table 1). We named this distinct disease process small-bowel syndrome (SBS). Mice in which SBS developed tended to die earlier, between 23 and 28 days after transplantation, than mice with CML-like disease. Some animals (2 of 22) that were premorbid slightly later clearly died of SBS but had evidence of CML-like disease, with marked splenomegaly and leukocytosis (Figure 3, mixed symbols). This suggests that the 2 diseases can occur independently and that mice that survive the rapid onset of SBS may subsequently have CML-like disease.
Distinctive gross pathological features of TEL-ABL–induced SBS.
Prosected necropsy specimen from a mouse with SBS, showing an enlarged and distended small bowel (SB), pale liver (Li), and increased vascular prominence. The specimen has a lack of lung hemorrhages (Lu), a normal thymus and lymph nodes (LN), and minimal splenomegaly (Sp).
Distinctive gross pathological features of TEL-ABL–induced SBS.
Prosected necropsy specimen from a mouse with SBS, showing an enlarged and distended small bowel (SB), pale liver (Li), and increased vascular prominence. The specimen has a lack of lung hemorrhages (Lu), a normal thymus and lymph nodes (LN), and minimal splenomegaly (Sp).
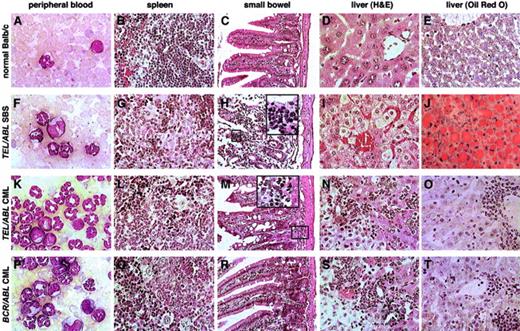
Histopathological analysis of the mice with SBS (Figure5) showed extensive myeloid cell infiltration of villi in the small bowel, with prominent villous necrosis (Figure 5H). Myeloid cells were also found adjacent to Peyer patches, which are lymphoid nodules normally present in the gut. Some sections showed evidence of transmural necrosis and possible perforation. Livers from these mice had a distinctive histopathological feature: a striking microvesicular change in the cytoplasm of hepatocytes (Figure 5I), an appearance that can be caused by an accumulation of either glycogen or neutral lipids such as triglycerides. To distinguish between these, liver sections were stained with periodic acid–Schiff (PAS), which stains glycogen, and with oil red O (ORO), which stains neutral lipids. Livers from mice with SBS were stained strongly by ORO (Figure 5J) but not by PAS (data not shown). In contrast, livers from healthy mice or mice with CML-like disease induced by BCR-ABL or TEL-ABL showed only minimal ORO staining (Figure 5O,T), demonstrating that this fatty change was unique to SBS.
Comparative histopathological findings in TEL-ABL–induced andBCR-ABL–induced hematologic diseases.
Photomicrographs of Wright-Giemsa–stained PB (panels A, F, K, and P); hematoxylin-eosin–stained spleen (panels B, G, L, and Q), small bowel (panels C, H, M, and R), and hematoxylin-eosin–stained liver (panels D, I, N, and S); and ORO-stained liver (panels E, J, O, and T) from mice with TEL-ABL–induced SBS (panels F-J),TEL-ABL–induced CML-like disease (panels K-O), andBCR-ABL–induced CML-like disease (panels P-T). A Balb/c mouse killed 5 weeks after transplantation with untransduced bone marrow was used as the control (panels A-E). Magnifications are ×750 for PB, ×100 for small bowel, and ×200 for liver and spleen. Although mice with TEL-ABL–induced CML-like disease occasionally had infiltration of the bowel submucosa with neutrophils (panel M, ×500 insert), neutrophil infiltration of villi was found only in mice with SBS (panel H, ×500 insert).
Comparative histopathological findings in TEL-ABL–induced andBCR-ABL–induced hematologic diseases.
Photomicrographs of Wright-Giemsa–stained PB (panels A, F, K, and P); hematoxylin-eosin–stained spleen (panels B, G, L, and Q), small bowel (panels C, H, M, and R), and hematoxylin-eosin–stained liver (panels D, I, N, and S); and ORO-stained liver (panels E, J, O, and T) from mice with TEL-ABL–induced SBS (panels F-J),TEL-ABL–induced CML-like disease (panels K-O), andBCR-ABL–induced CML-like disease (panels P-T). A Balb/c mouse killed 5 weeks after transplantation with untransduced bone marrow was used as the control (panels A-E). Magnifications are ×750 for PB, ×100 for small bowel, and ×200 for liver and spleen. Although mice with TEL-ABL–induced CML-like disease occasionally had infiltration of the bowel submucosa with neutrophils (panel M, ×500 insert), neutrophil infiltration of villi was found only in mice with SBS (panel H, ×500 insert).
Livers from mice with SBS also showed marked hepatocyte degeneration suggestive of apoptosis (Figure 5I) but lacked the myeloid and erythroid cell infiltrates and periportal macrophage aggregates that were uniform features of CML-like disease (Figure 5N,S). Spleens from mice with SBS had partial disruption of follicular architecture with mild infiltration of neutrophils and erythroid cells (Figure 5G). Kidneys from these animals had evidence of acute tubular necrosis, with degeneration of proximal tubular cells and proteinaceous material in the tubular lumen (data not shown). Finally, lungs from mice with SBS had minimal focal myeloid infiltrates without hemorrhages (data not shown). In contrast, mice with CML-like disease had extensive pulmonary myeloid cell infiltration and hemorrhages that caused morbidity or death.2
Endotoxemia is a possible pathophysiologic mechanism ofTEL-ABL–induced SBS
The histopathological findings in mice with SBS, including fatty liver and acute tubular necrosis in the kidneys, were suggestive of endotoxin-induced injury. A likely source of endotoxin is the small bowel, in which necrosis of villi might lead to increased vascular permeability and endotoxemia from gut bacteria. The pathophysiologic response to endotoxin challenge in mice, which has been studied extensively,24 is followed by an elevation in levels of circulating tumor necrosis factor α (TNFα) within 2 hours and an elevation in interleukin 1β levels within 6 hours. Liver damage, including acute fatty liver and hepatocyte apoptosis, is induced directly by TNFα.25 Secondary effects include hypotension and renal acute tubular necrosis.
To investigate the hypothesis that endotoxin was involved, serum from premorbid mice with TEL-ABL–induced SBS and CML-like disease was assayed for endotoxin and TNFα. Serum chemistry testing of these mice was also performed. Mice with SBS had variable but highly significant elevations in levels of circulating endotoxin, with one animal showing an increase of more than 100 fold, whereas mice with either TEL-ABL– or BCR-ABL–induced CML-like disease had only slightly elevated levels (Table2). Most mice with SBS also had a prominent increase in circulating TNFα, with lesser increases found in a few mice with TEL-ABL– and BCR-ABL–induced CML-like disease. In addition, mice with SBS had marked elevations in levels of liver transaminases, severe hypoglycemia, and azotemia (elevated blood urea nitrogen level), consistent with fulminant hepatic and renal failure (Table 2). Mice with CML-like disease induced by either TEL-ABL or BCR-ABL had moderate hypoglycemia and increased plasma aspartate aminotransferase levels, possibly as a consequence of extensive hepatic neutrophil infiltration. Overall, analysis of serum from mice with TEL-ABL–induced SBS supported the hypothesis that endotoxemia and increased levels of circulating TNFα developed in these animals and they died of acute hepatic and renal failure. The variation in circulating levels of endotoxin in mice with SBS may have reflected its transient presence (due to its rapid clearance),24 but we did not have sequential samples from enough individual mice to confirm this hypothesis.
Endotoxin, TNFα, and serum chemistry findings in mice with TEL-ABL– and BCR-ABL–induced diseases
| Disease . | Endotoxin (EU/mL) . | TNFα (ng/L) . | Glucose (mmol/L) . | BUN (mmol/L) . | AST (U/L) . | ALT (U/L) . |
|---|---|---|---|---|---|---|
| Healthy Balb/c mouse (n = 2*) | 1.2 ± 0.05 | 0 | 9.55 ± 0.03 | 5 ± 0.7 | 11 ± 2 | 12 ± 5 |
| BCR-ABL CML (n = 2*) | 3.6 | 38 ± 29 | 1.22 | 6.1 | 25 | 6 |
| TEL-ABL SBS (n = 5*) | 50.9 ± 44.7 | 214 ± 108 | < 0.11 | 45.7 ± 5 | 146 ± 38 | 69 ± 22 |
| TEL-ABL CML (n = 4*) | 5.0 ± 1.9 | 36 ± 31 | 2.28 ± 2.05 | 11.1 ± 2.5 | 22 ± 3 | 4 ± 0.4 |
| Disease . | Endotoxin (EU/mL) . | TNFα (ng/L) . | Glucose (mmol/L) . | BUN (mmol/L) . | AST (U/L) . | ALT (U/L) . |
|---|---|---|---|---|---|---|
| Healthy Balb/c mouse (n = 2*) | 1.2 ± 0.05 | 0 | 9.55 ± 0.03 | 5 ± 0.7 | 11 ± 2 | 12 ± 5 |
| BCR-ABL CML (n = 2*) | 3.6 | 38 ± 29 | 1.22 | 6.1 | 25 | 6 |
| TEL-ABL SBS (n = 5*) | 50.9 ± 44.7 | 214 ± 108 | < 0.11 | 45.7 ± 5 | 146 ± 38 | 69 ± 22 |
| TEL-ABL CML (n = 4*) | 5.0 ± 1.9 | 36 ± 31 | 2.28 ± 2.05 | 11.1 ± 2.5 | 22 ± 3 | 4 ± 0.4 |
Values are means ± SE.
TNFα indicates tumor necrosis factor α; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CML, chronic myeloid leukemia; and SBS, small-bowel syndrome.
Number of mice analyzed.
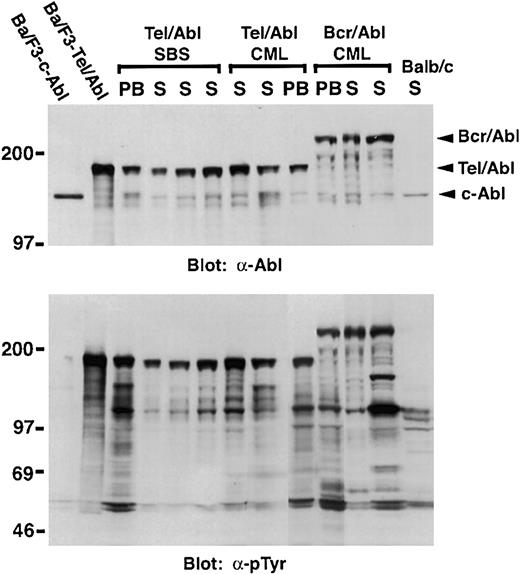
Myeloid cells from mice with TEL-ABL–induced SBS and CML-like disease express Tel-Abl protein
PB myeloid cells and spleen cells from mice withTEL-ABL–induced CML-like disease expressed the Tel-Abl fusion protein at levels several-fold greater than did endogenous c-Abl (Figure 6). Importantly, both splenic and PB myeloid cells from mice with TEL-ABL–induced SBS also expressed Tel-Abl protein at similar levels. Because of the difficulty in recovering sufficient numbers of neutrophils from diseased intestine and the lack of a reporter gene such are green fluorescent protein or β-galactosidase in our viral vector, direct demonstration of the presence of Tel-Abl in myeloid cells infiltrating the small bowel in these mice was not possible. However, because of the expression of Tel-Abl in circulating neutrophils, it is very likely that Tel-Abl was also expressed in the myeloid cells infiltrating the intestinal villi in mice with SBS.
Myeloid cells from mice with SBS and CML-like leukemia express Tel-Abl protein.
Protein lysates from PB leukocytes and spleen cells (S) from mice withTEL-ABL–induced SBS, TEL-ABL–induced CML-like disease, and p210 BCR-ABL–induced CML-like disease were analyzed by Western blotting with anti-Abl (top panel) and antiphosphotyrosine (bottom panel) antibodies. Extracts from splenocytes from a normal Balb/c mouse (Balb/c) and from Ba-F3 cells expressing c-Abl or Tel-Abl were included as controls. The figure shows a composite of 2 blotting studies done simultaneously under identical conditions.
Myeloid cells from mice with SBS and CML-like leukemia express Tel-Abl protein.
Protein lysates from PB leukocytes and spleen cells (S) from mice withTEL-ABL–induced SBS, TEL-ABL–induced CML-like disease, and p210 BCR-ABL–induced CML-like disease were analyzed by Western blotting with anti-Abl (top panel) and antiphosphotyrosine (bottom panel) antibodies. Extracts from splenocytes from a normal Balb/c mouse (Balb/c) and from Ba-F3 cells expressing c-Abl or Tel-Abl were included as controls. The figure shows a composite of 2 blotting studies done simultaneously under identical conditions.
The TEL-ABL–transduced bone marrow cell that initiates CML-like leukemia is an early multipotential progenitor
Analysis of genomic DNA from spleen cells from mice given transplants of TEL-ABL–transduced bone marrow demonstrated that many distinct proviral clones contributed to the SBS and CML-like diseases (Figure 7A). Polyclonal disease is also characteristic of BCR-ABL–induced CML-like leukemia,2 and this suggests that TEL-ABL is both necessary and sufficient to induce the diseases observed in mice. In BCR-ABL–induced CML-like disease, the same spectrum of proviral clones is found in neutrophils, macrophages, erythroid cells, B-lymphoid cells, and sometimes T lymphocytes, demonstrating that the cells initiating the myeloproliferative disease have multilineage repopulating ability.2 To characterize the cells initiating TEL-ABL–induced myeloproliferative disease in this study, we purified cells of different hematopoietic lineages from selected mice. Genomic DNA from PB neutrophils, peritoneal macrophages, splenic erythroid progenitors, splenic B lymphocytes, thymocytes, and peripheral node lymphocytes from a mouse withTEL-ABL–induced CML-like disease carried the same spectrum of proviral clones, at about one proviral copy per cell, whereas splenic erythroid progenitors and B lymphocytes from another mouse shared the same proviral clones as the spleen tissue and PB neutrophils (Figure 7B). Similar patterns were observed in 4 other mice (data not shown). These results show that the cells initiating CML-like leukemia after transduction with TEL-ABL can repopulate multiple myeloid and lymphoid lineages in transplant recipients.
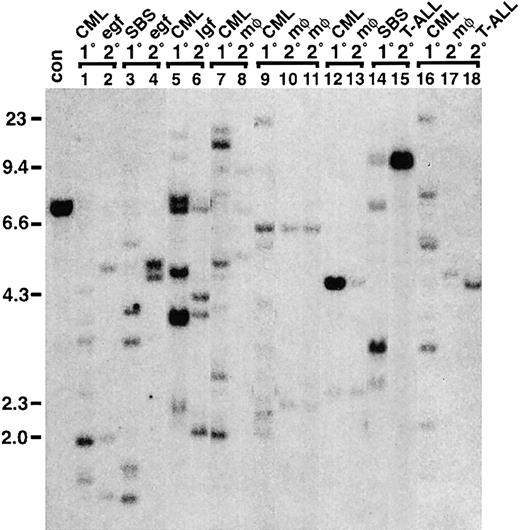
TEL-ABL–induced myeloproliferative disease is polyclonal and originates from multipotential progenitor cells.
(A) Multiple clones contribute to TEL-ABL–induced SBS and CML-like disease. Genomic DNA isolated from spleens of animals withTEL-ABL–induced SBS (lanes c-l), mixed SBS and CML-like disease (mix; lanes m and n), and CML-like disease (lanes o-v) was digested with BglII and analyzed by Southern blotting with a probe from the neomycin-resistance gene that yields a distinct band for each individual proviral integrant. For comparison, DNA results in spleens of 2 mice with p210 BCR-ABL–induced CML-like disease given transplants contemporaneously with the TEL-ABLmice are included (B/A; lanes a and b). Genomic DNA from a cell line containing a single provirus (con) is shown at the extreme left, and the positions of DNA size markers are indicated. The blot was stripped and reprobed with an ABL probe that detects a common 2.2-kb fragment from all proviruses (PV) as well as the endogenous murine c-abl gene, thereby permitting quantitation of the proviral copy number per diploid genome2 (bottom panels). (B) The cell initiatingTEL-ABL–induced CML-like disease is a multipotential progenitor. Hematopoietic cells from different lineages were isolated, and genomic DNA was isolated and analyzed by Southern blotting with a neomycin gene probe as described in the legend for panel A. The same spectrum of proviral clones was observed in spleen cells, PB leukocytes (principally maturing neutrophils), peritoneal macrophages, splenic erythroid cells (spl. T119+), splenic B-lymphoid cells (spl. B220+), thymocytes, and peripheral node lymphocytes (LN) from one mouse with TEL-ABL–induced CML-like disease (left panel), whereas spleen cells, PB leukocytes, erythroid progenitors, and B-lymphoid progenitors shared the same proviral clones in a second mouse (right panel), thus demonstrating that these clones repopulate multiple hematopoietic lineages in the recipients. The blots were stripped and reprobed with an ABL probe to determine the proviral copy number, as described in the legend for panel A, and this demonstrated the presence of provirus at similar levels in all populations. (C) Cells initiating TEL-ABL–induced myeloproliferative disease include progenitors that can generate day-12 spleen colonies in secondary recipients. Day-12 spleen colonies were isolated from secondary recipients of bone marrow from 2 different donor mice (top and bottom panels) with TEL-ABL–induced CML-like disease, genomic DNA isolated, and Southern blotting was done as described in the legend for panel A. Both primary mice contained multiple TEL-ABL–transduced clones that were capable of generating day-12 spleen colonies (lanes 1-18 of each blot). However, not every clone in the primary mice (represented by spleen DNA [left]) contained CFU-S12. Lane 18 (top panel) is likely a mixture of 2 different colonies, whereas lane 17 (top) and lane 10 (bottom) likely represent CFU-S12 containing 2 distinct proviruses.
TEL-ABL–induced myeloproliferative disease is polyclonal and originates from multipotential progenitor cells.
(A) Multiple clones contribute to TEL-ABL–induced SBS and CML-like disease. Genomic DNA isolated from spleens of animals withTEL-ABL–induced SBS (lanes c-l), mixed SBS and CML-like disease (mix; lanes m and n), and CML-like disease (lanes o-v) was digested with BglII and analyzed by Southern blotting with a probe from the neomycin-resistance gene that yields a distinct band for each individual proviral integrant. For comparison, DNA results in spleens of 2 mice with p210 BCR-ABL–induced CML-like disease given transplants contemporaneously with the TEL-ABLmice are included (B/A; lanes a and b). Genomic DNA from a cell line containing a single provirus (con) is shown at the extreme left, and the positions of DNA size markers are indicated. The blot was stripped and reprobed with an ABL probe that detects a common 2.2-kb fragment from all proviruses (PV) as well as the endogenous murine c-abl gene, thereby permitting quantitation of the proviral copy number per diploid genome2 (bottom panels). (B) The cell initiatingTEL-ABL–induced CML-like disease is a multipotential progenitor. Hematopoietic cells from different lineages were isolated, and genomic DNA was isolated and analyzed by Southern blotting with a neomycin gene probe as described in the legend for panel A. The same spectrum of proviral clones was observed in spleen cells, PB leukocytes (principally maturing neutrophils), peritoneal macrophages, splenic erythroid cells (spl. T119+), splenic B-lymphoid cells (spl. B220+), thymocytes, and peripheral node lymphocytes (LN) from one mouse with TEL-ABL–induced CML-like disease (left panel), whereas spleen cells, PB leukocytes, erythroid progenitors, and B-lymphoid progenitors shared the same proviral clones in a second mouse (right panel), thus demonstrating that these clones repopulate multiple hematopoietic lineages in the recipients. The blots were stripped and reprobed with an ABL probe to determine the proviral copy number, as described in the legend for panel A, and this demonstrated the presence of provirus at similar levels in all populations. (C) Cells initiating TEL-ABL–induced myeloproliferative disease include progenitors that can generate day-12 spleen colonies in secondary recipients. Day-12 spleen colonies were isolated from secondary recipients of bone marrow from 2 different donor mice (top and bottom panels) with TEL-ABL–induced CML-like disease, genomic DNA isolated, and Southern blotting was done as described in the legend for panel A. Both primary mice contained multiple TEL-ABL–transduced clones that were capable of generating day-12 spleen colonies (lanes 1-18 of each blot). However, not every clone in the primary mice (represented by spleen DNA [left]) contained CFU-S12. Lane 18 (top panel) is likely a mixture of 2 different colonies, whereas lane 17 (top) and lane 10 (bottom) likely represent CFU-S12 containing 2 distinct proviruses.
To characterize further the cells initiatingTEL-ABL–induced CML-like disease, we tested the ability of these cells to generate day-12 spleen colonies in a secondary transplant. Such colonies are clonal and are initiated by an early multipotential myeloid progenitor cell, day-12 spleen colony-forming unit (CFU-S12). Previous studies demonstrated that a subset of BCR-ABL–transduced clones contributing to the leukemia in a primary animal with CML-like disease generate day-12 spleen colonies in secondary recipients.2 We observed a similar phenomenon with TEL-ABL–induced CML-like disease (Figure7C). Most day-12 spleen colonies in secondary recipients of bone marrow from 2 different primary mice with TEL-ABL–induced myeloproliferative disease contained TEL-ABL provirus. Interestingly, as with BCR-ABL, only a minor subset of the clones contributing to the leukemia in the primary animal generated day-12 spleen colonies, and these were often not the most abundant clones in the primary mouse. These results indicate that only some of the cells initiating TEL-ABL–induced CML-like disease are early hematopoietic progenitors that contain or can give rise to CFU-S12.
TEL-ABL–induced SBS and CML-like disease cannot be transplanted to secondary recipients
We attempted to adoptively transferTEL-ABL–induced hematologic diseases by transplanting large amounts of bone marrow and spleen from diseased primary mice into lethally irradiated syngeneic Balb/c secondary recipient mice. Cells from 17 primary mice (5 with SBS, 10 with CML-like disease, and 2 with mixed SBS and CML-like disease) were transplanted into pairs of recipients. None of the recipients developed either SBS or myeloproliferative disease; instead, several different outcomes were observed (Table 3). The most frequent outcome of secondary transplantation was failure to engraft, with the recipients dying between 11 and 23 days after transplantation with pancytopenia, hypocellular bone marrow, and often with signs of disseminated bacterial infection. Two other mice appeared to have delayed graft failure, with pancytopenia developing 84 and 99 days after transplantation.
Fate of secondary transplant recipients from primary mice with TEL-ABL–induced small-bowel syndrome and chronic myeloid leukemia–like disease
| Primary disease . | Secondary disease . | |||||
|---|---|---|---|---|---|---|
| Early graft failure . | Late graft failure . | T-cell lymphoma . | Macrophage tumors . | Healthy . | Not analyzed . | |
| SBS (n = 10) | 3 (15) | — | 2 (153) | 2 (175) | 2 (293) | 1 (225) |
| Mixed SBS-CML (n = 4) | 4 (17) | — | — | — | — | — |
| CML (n = 20) | 4 (17) | 2 (91) | 4 (193) | 5 (95) | 2 (272) | 3 (250) |
| Primary disease . | Secondary disease . | |||||
|---|---|---|---|---|---|---|
| Early graft failure . | Late graft failure . | T-cell lymphoma . | Macrophage tumors . | Healthy . | Not analyzed . | |
| SBS (n = 10) | 3 (15) | — | 2 (153) | 2 (175) | 2 (293) | 1 (225) |
| Mixed SBS-CML (n = 4) | 4 (17) | — | — | — | — | — |
| CML (n = 20) | 4 (17) | 2 (91) | 4 (193) | 5 (95) | 2 (272) | 3 (250) |
Values are numbers of secondary recipient mice and, in parentheses, the mean number of days of survival after transplantation.
In 11 secondary recipients, hematologic malignant diseases developed after long latent periods (85-194 days). In 6 mice, T-cell leukemia-lymphoma developed, characterized by thymic and abdominal tumors of Thy-1–positive lymphoblasts. Five mice had onset of a histiocytic malignant disease of macrophage-monocyte origin that was characterized by tumors in the liver, mesentery, bone marrow, myocardium, and lungs and frequently accompanied by hemiparesis. Both these malignant diseases are observed in recipients ofBCR-ABL–transduced marrow under certain circumstances; similar T lymphomas develop in recipients of marrow transduced with a mutant of BCR-ABL (Y177F) that is deficient in binding to the Grb2 adapter molecule,5 whereas monocyte-macrophage tumors unaccompanied by myeloproliferative disease can occur in recipients of BCR-ABL–transduced marrow from donors not treated with 5-fluororuracil.2 Finally, 4 mice remained healthy and were killed 270 to 300 days after transplantation; their blood counts were normal and they had no evidence of disease. In contrast to these findings, BCR-ABL–induced CML-like disease was successfully transplanted to secondary recipient mice in contemporaneous experiments more than 80% of the time by using identical techniques (data not shown).
We analyzed genomic DNA from tissues of different secondary recipients for the presence of TEL-ABL provirus (Figure8). Significant levels of provirus-positive cells were found in hematopoietic tissues of mice with graft failure, suggesting that TEL-ABL–transduced cells from the primary mouse were engrafted in these recipients but lacked radioprotective and long-term repopulating activity. T lymphomas and macrophage tumors that developed in secondary recipients also uniformly contained TEL-ABL provirus. In some cases, the T lymphomas and macrophage tumors shared proviral integration sites with myeloid cells from the donor (Figure 8), suggesting that these diseases arose from the clones that were responsible for the myeloproliferative disease in the primary mouse. In other cases, the macrophage tumors and T lymphomas arose from novel clones not highly represented in the donor.
Secondary transplant recipients in which graft failure or late-onset hematologic malignant disease develops have provirally marked clones from the primary animal.
Bone marrow and spleen cells from primary mice (1°) withTEL-ABL–induced SBS or CML-like disease (CML) were transplanted into lethally irradiated secondary recipients (2°). Genomic DNA from spleens of 2 secondary recipient mice that died with early graft failure (egf) and pancytopenia between 14 and 18 days after transplantation (lanes 1 and 2 and lanes 3 and 4) and from a recipient with late graft failure (lgf) that had pancytopenia and hypocellular bone marrow at 99 days after transplantation (lanes 5 and 6) contained provirally marked cells that shared some clones with myeloid cells from the primary mouse. Spleen DNA from several secondary recipient mice in which macrophage tumors developed (mφ) after long latent periods also shared proviral clones with myeloid cells from the donor (lanes 9, 10, and 11 and lanes 12 and 13), as did thymus DNA from a secondary recipient in which T-ALL developed (lanes 14 and 15). However, some macrophage tumors and cases of T-ALL that arose in secondary recipients were derived from proviral clones that were not represented at significant levels in the myeloid cells of the donor (lanes 16, 17, and 18).
Secondary transplant recipients in which graft failure or late-onset hematologic malignant disease develops have provirally marked clones from the primary animal.
Bone marrow and spleen cells from primary mice (1°) withTEL-ABL–induced SBS or CML-like disease (CML) were transplanted into lethally irradiated secondary recipients (2°). Genomic DNA from spleens of 2 secondary recipient mice that died with early graft failure (egf) and pancytopenia between 14 and 18 days after transplantation (lanes 1 and 2 and lanes 3 and 4) and from a recipient with late graft failure (lgf) that had pancytopenia and hypocellular bone marrow at 99 days after transplantation (lanes 5 and 6) contained provirally marked cells that shared some clones with myeloid cells from the primary mouse. Spleen DNA from several secondary recipient mice in which macrophage tumors developed (mφ) after long latent periods also shared proviral clones with myeloid cells from the donor (lanes 9, 10, and 11 and lanes 12 and 13), as did thymus DNA from a secondary recipient in which T-ALL developed (lanes 14 and 15). However, some macrophage tumors and cases of T-ALL that arose in secondary recipients were derived from proviral clones that were not represented at significant levels in the myeloid cells of the donor (lanes 16, 17, and 18).
Discussion
Although Tel-Abl and Bcr-Abl have essentially identical transforming properties in cultured cells, a direct comparison of the leukemogenic properties of the 2 kinases in mice revealed important differences between them. In some recipients ofTEL-ABL– transduced marrow, a CML-like myeloproliferative disease developed that was very similar to that induced by BCR-ABL, but the latency of the disease was significantly delayed. This was not a consequence of a lower or weaker intrinsic tyrosine kinase activity of Tel-Abl because direct comparison of in vitro and in vivo kinase activity reproducibly found Tel-Abl to be significantly more active than p210 Bcr-Abl (Figure 2). Tel-Abl and Bcr-Abl may differ in their ability to phosphorylate particular substrates, particularly those that might be recruited by specific interactions with the Tel or Bcr polypeptides. However, 2 studies failed to detect significant differences in tyrosine-phosphorylated proteins in cultured cells expressing the 2 kinases.22 23
Alternatively, other functions of the Abl fusion partner in addition to oligomerization may influence disease latency. Oligomerization of c-Abl by fusion of just the Bcr coiled-coil domain induces myeloproliferative disease in mice only after a long latent period,26suggesting that other Bcr domains contribute to the efficiency of disease induction. One critical function is direct binding of the Grb2 adapter protein by Bcr, which is required for induction of fatal myeloproliferative disease in mice by p210 Bcr-Abl.5 26However, the Tel-Abl fusion protein studied here also directly binds Grb2, and a site-specific mutation that blocks Grb2 binding attenuates induction of the CML-like disease by Tel-Abl (R.P.M. and R.A.V., unpublished data, January 2002). Additional studies are necessary to define the biochemical basis of the differences in leukemogenic activity of Tel-Abl and Bcr-Abl.
Our results confirm that Tel-Abl is the direct cause of the myeloproliferative disease in patients with typical and atypical CML and TEL-ABL fusion genes. Clinical studies have suggested that the reciprocal ABL-TEL fusion gene is not present in most patients with leukemia and TEL-ABL,12perhaps because of the complex translocations involved, whereas loss of the second TEL allele, a frequent finding inTEL-AML1–induced B-ALL,27 does not occur inTEL-ABL–associated disease.12,28 Our studies demonstrated directly that the leukemogenic action ofTEL-ABL is independent of ABL-TEL expression or loss of TEL function. Because the kinase activity of Tel-Abl is inhibited29 by the Abl kinase inhibitor STI-571 (imatinib mesylate [Gleevec; Novartis Pharmaceuticals, East Hanover, NJ]), this drug should be of value in treatingTEL-ABL–associated myeloproliferative disease.
Most recipients of TEL-ABL–transduced marrow in this study died of a distinct illness characterized by extensive infiltration of small-bowel villi and submucosa with neutrophils, accompanied by hepatic and renal failure. The clinical picture was suggestive of endotoxin-mediated injury, and this idea was supported by the findings of an analysis of circulating endotoxin and TNFα levels in these mice. Only 3 patients with TEL-ABL–associated myeloproliferative disease have been described, but interestingly, one of them had concurrent ulcerative colitis.12 The SBS is unique to Tel-Abl and has not been observed in more than 100 recipients of BCR-ABL–transduced marrow. It is possible that Tel-Abl expression alters the adhesive or trafficking properties of neutrophils to make them home to small bowel; this might be confirmed by comparative studies of mesenteric homing of Tel-Abl– and Bcr-Abl–expressing neutrophils in an intravital microscopy model.
Some additional factor, however, must account for the shift in migration of Tel-Abl–expressing neutrophils from small bowel early after transplantation to spleen, liver, and lungs later on. A plausible candidate is altered expression of chemokine or adhesion molecule in the gut resulting from ionizing radiation used to prepare the recipients, which persists for 1 to 2 weeks and then resolves. The same frequency of SBS was observed in mouse recipients ofTEL-ABL–transduced marrow that received only 450 cGy total-body irradiation as conditioning and in those given larger doses (data not shown), but it would be interesting to test the efficiency of induction of SBS with further deceases in radiation dose. An alternative possibility is that TNFα expression might be induced directly by TEL-ABL in myeloid cells, leading to hypotension and intestinal necrosis with subsequent infiltration of neutrophils. Further studies of TEL-ABL–induced SBS are warranted because it may be clinically relevant in patients withTEL-ABL–associated leukemia, particularly those with bowel injury from chemoradiotherapy.
The most dramatic difference between TEL-ABL–induced andBCR-ABL–induced myeloproliferative diseases was the absolute failure of the former to be adoptively transplanted into secondary recipients. Thirty-four lethally irradiated secondary recipient mice given transplants of large doses of viable marrow and spleen cells from primary mice with TEL-ABL–induced SBS or CML-like disease failed to have development of either illness. In contrast, in contemporaneous experiments withBCR-ABL–induced CML-like disease, the myeloproliferative disease could be adoptively transferred, with use of identical techniques, more than 80% of the time. The failure of transplantability of TEL-ABL–induced leukemia therefore represents a very striking biologic difference. It is possible that more efficient TEL-ABL transduction or transplantation of larger numbers of cells might permit adoptive transfer ofTEL-ABL–induced myeloproliferative disease, but the fact that CFU-S12 cells were effectively transduced and transplanted to secondary recipients suggests that this would not be the case.
Its lack of transplantability does not necessarily imply that theTEL-ABL–induced myeloproliferative disease is not a malignant disease. The use of transplantability as a criterion for malignancy originated from studies of solid tumors, in which acquisition of the ability to proliferate autonomously in a secondary host is an important functional test for distinguishing hyperplasia and benign tumors from true neoplastic lesions. Transplantability is less relevant to leukemias, which can kill the host without spreading outside the bone marrow and blood system. Retroviral gene-marking studies in patients given autografts30 found that at least some cases of BCR-ABL–induced CML are transplantable into irradiated syngeneic recipients (ie, the patients themselves), as is the case with murine CML-like disease induced by BCR-ABL. However, it is not known whether human TEL-ABL–associated myeloproliferative disease can likewise be transplanted.
What is the explanation for the lack of transplantability ofTEL-ABL–induced myeloproliferative disease? It can be assumed that the BCR-ABL and TEL-ABL retroviruses transduce an identical spectrum of hematopoietic progenitors in the marrow of donors given 5-fluorouracil, including hematopoietic stem cells. In both cases, the cells initiating the CML-like leukemia can repopulate multiple hematopoietic lineages in the primary recipient. However, the cells initiating myeloproliferative disease in the primary recipient of TEL-ABL–transduced marrow lack self-renewal capacity, as measured by secondary transplantation. Only a subset of the many BCR-ABL–positive clones contributing to the expansion of myeloid cells in a primary animal can generate day-12 spleen colonies and engraft and induce disease in secondary recipients,2 thus suggesting that the cells initiatingBCR-ABL–induced CML-like disease are also heterogeneous for self-renewal.
Because of these observations, we initially anticipated that cells initiating TEL-ABL–induced myeloproliferative disease would lack the ability to generate day-12 spleen colonies in secondary recipients. Surprisingly, a subset of TEL-ABL–transduced clones from primary mice with CML-like leukemia was found to generate day-12 spleen colonies efficiently, but the same bone marrow used in larger doses was unable to reconstitute the myeloproliferative disease in other recipients. Most of these recipients died of early graft failure but had provirally marked cells in the spleen between 2 and 3 weeks after transplantation, a finding that constitutes direct evidence of a lack of self-renewal of these TEL-ABL–transduced progenitors. Some recipients had engraftment, survived lethal irradiation, and showed no evidence of myeloproliferative disease, but T lymphoma or histiocytic tumors developed after several months. Some of these tumors shared proviral clones with myeloid cells from the primary animal, confirming the multipotential nature of the cells initiating the myeloproliferative disease and representing disease progression to acute leukemia. It is curious that aTEL-ABL–marked clone can be transferred successfully to a secondary recipient and induce T lymphoma or histocytic tumors without causing CML-like disease, but a similar phenomenon was observed in some secondary transplants with BCR-ABL–induced CML-like disease31 and may represent transfer of transformed acute leukemia cells present at low numbers in the primary animal.
There are at least 2 possible mechanisms that might account for the lack of transplantation of TEL-ABL–induced myeloproliferative diseases. The first is that the bone marrow target cell that initiates CML-like disease on transduction withTEL-ABL is a progenitor—similar to those described in normal mouse bone marrow32—that has limited short-term repopulating activity in a primary transplant but cannot repopulate a secondary recipient. If this model is accurate, our results suggest that loss of the self-renewal potential of hematopoietic stem cells occurs before the decline in CFU-S12 activity and thatTEL-ABL acts within this compartment. Alternatively, expression of Tel-Abl in a hematopoietic stem/progenitor cell may directly inhibit self-renewal. These possibilities can be distinguished only by isolating different stem/progenitor cells from bone marrow before TEL-ABL transduction and transplantation and by employing conditional expression or function of TEL-ABL. In either case, it is possible that the block in transplantability reflects defective homing of TEL-ABL–expressing stem/progenitor cells to bone marrow compared with spleen. Interestingly, the myeloproliferative disease induced in mice by theTEL-PDGFβR fusion oncogene is also very difficult to adoptively transfer to secondary recipients.33
Defining the mechanistic basis of the difference in secondary transplantation of tyrosine kinase–induced murine myeloproliferative diseases will be valuable for understanding the pathophysiologic characteristics of human myeloproliferative and myelodysplastic syndromes. In addition, our results may have clinical implications, since human TEL-ABL–induced myeloproliferative disease might be treatable by autologous marrow transplantation without the need for graft purging.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-12-0244.
Supported in part by National Institutes of Health grants CA90576 (R.A.V.), CA09595 (R.P.M.), DK50654, and CA66996 (D.G.G), and grants from the Leukemia and Lymphoma Society. D.G.G. is an Associate Investigator of the Howard Hughes Medical Institute. R.A.V. is a Scholar of the Leukemia and Lymphoma Society and the Carl and Margaret Walter Scholar in Blood Research at Harvard Medical School.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard A. Van Etten, The Center for Blood Research, 200 Longwood Ave, Boston, MA 02115; e-mail:vanetten@cbr.med.harvard.edu.


![Fig. 7. TEL-ABL–induced myeloproliferative disease is polyclonal and originates from multipotential progenitor cells. / (A) Multiple clones contribute to TEL-ABL–induced SBS and CML-like disease. Genomic DNA isolated from spleens of animals withTEL-ABL–induced SBS (lanes c-l), mixed SBS and CML-like disease (mix; lanes m and n), and CML-like disease (lanes o-v) was digested with BglII and analyzed by Southern blotting with a probe from the neomycin-resistance gene that yields a distinct band for each individual proviral integrant. For comparison, DNA results in spleens of 2 mice with p210 BCR-ABL–induced CML-like disease given transplants contemporaneously with the TEL-ABLmice are included (B/A; lanes a and b). Genomic DNA from a cell line containing a single provirus (con) is shown at the extreme left, and the positions of DNA size markers are indicated. The blot was stripped and reprobed with an ABL probe that detects a common 2.2-kb fragment from all proviruses (PV) as well as the endogenous murine c-abl gene, thereby permitting quantitation of the proviral copy number per diploid genome2 (bottom panels). (B) The cell initiatingTEL-ABL–induced CML-like disease is a multipotential progenitor. Hematopoietic cells from different lineages were isolated, and genomic DNA was isolated and analyzed by Southern blotting with a neomycin gene probe as described in the legend for panel A. The same spectrum of proviral clones was observed in spleen cells, PB leukocytes (principally maturing neutrophils), peritoneal macrophages, splenic erythroid cells (spl. T119+), splenic B-lymphoid cells (spl. B220+), thymocytes, and peripheral node lymphocytes (LN) from one mouse with TEL-ABL–induced CML-like disease (left panel), whereas spleen cells, PB leukocytes, erythroid progenitors, and B-lymphoid progenitors shared the same proviral clones in a second mouse (right panel), thus demonstrating that these clones repopulate multiple hematopoietic lineages in the recipients. The blots were stripped and reprobed with an ABL probe to determine the proviral copy number, as described in the legend for panel A, and this demonstrated the presence of provirus at similar levels in all populations. (C) Cells initiating TEL-ABL–induced myeloproliferative disease include progenitors that can generate day-12 spleen colonies in secondary recipients. Day-12 spleen colonies were isolated from secondary recipients of bone marrow from 2 different donor mice (top and bottom panels) with TEL-ABL–induced CML-like disease, genomic DNA isolated, and Southern blotting was done as described in the legend for panel A. Both primary mice contained multiple TEL-ABL–transduced clones that were capable of generating day-12 spleen colonies (lanes 1-18 of each blot). However, not every clone in the primary mice (represented by spleen DNA [left]) contained CFU-S12. Lane 18 (top panel) is likely a mixture of 2 different colonies, whereas lane 17 (top) and lane 10 (bottom) likely represent CFU-S12 containing 2 distinct proviruses.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/12/10.1182_blood-2001-12-0244/4/m_h81222720007.jpeg?Expires=1769139080&Signature=mT~JkahljtfHTts7wrCP~wdhlmcihskxp9HXjfelKbD6rUM5N~DL4LRh5Lx8beJYHVKMvkfQCJgG6BBKaRt~VbU9CPgX0xjyykKAP4xf2qHSTNY5if-~8rgP7ZBLtWJ5OsOncpWLItPZJ0q1iSmCQWkCTzkxpR2f8k5ebB5Mav0kDYPhStURwaU0XMhEHCueiWmJs7IRMKuYvOmwHrA87KZAIMnK4U2l6rfCllZkmXrH-w2o1Sa3j9QoIQVo~5jLHJgTUXPOgFzglsQImDN0OUtqF4fuoPkGV~jcFM9VLoa41Jjc5KFq0fhLHs9nDPb3a5KhlP0er0qHdm2xETzzgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal