Abstract
A nonmyeloablative conditioning regimen was investigated in 47 patients with hematological malignancy receiving allogeneic progenitor cells from matched, unrelated donors. The median patient age was 44 years. The majority of patients had high-risk features, including having failed a prior transplantation (29 individuals). Twenty of the transplants were mismatched for HLA class I and/or class II alleles. Recipient conditioning consisted of 20 mg CAMPATH-1H on days −8 to −4, 30 mg/m2fludarabine on days −7 to −3, and 140 mg/m2 melphalan on day −2. Graft-versus-host disease (GVHD) prophylaxis was with cyclosporine A alone. Primary graft failure occurred in only 2 of 44 evaluable patients (4.5%). Chimerism studies in 34 patients indicated that the majority (85.3%) attained initial full donor chimerism. Only 3 patients developed grade III to IV acute GVHD, and no patients have yet developed chronic extensive GVHD. The estimated probability of nonrelapse mortality at day 100 was 14.9% (95% confidence interval [CI], 4.7%-25.1%). With a median follow-up of 344 days (range, 79-830), overall and progression-free survivals at 1 year were 75.5% (95% CI, 62.8%-88.2%) and 61.5% (95% CI, 46.1%-76.8%), respectively. In summary, a nonmyeloablative regimen incorporating in vivo CAMPATH-1H is effective in promoting durable engraftment in most patients and in reducing the risk of severe GVHD following matched unrelated donor transplantation.
Introduction
Unrelated donor stem cell transplantation (SCT) using standard myeloablative preparative regimens is associated with a poorer outcome than HLA-identical sibling-donor SCT.1Higher rates of regimen-related toxicity, graft rejection, graft-versus-host disease (GVHD), and impaired immune reconstitution have limited the wide application of unrelated donor SCT in older patients or those with a poor performance status.2-6Although high-resolution HLA molecular typing7 and other strategies such as T-cell depletion8 have the potential to reduce these risks, few centers routinely offer this treatment to patients older than age 45 years. There is an urgent need for novel transplantation protocols that can extend the applicability of allogeneic SCT to the majority of patients who have no HLA-compatible related donor.
In an effort to reduce the transplantation-related mortality associated with allogeneic SCT, reduced-intensity regimens have been developed that have been designed to be immunosuppressive rather than myeloablative and that permit donor engraftment but limit systemic toxicity.9-12 There is a spectrum of hematopoietic toxicity associated with these nonmyeloablative regimens from minimally cytopenic regimens that use low-dose total body irradiation alone13 to regimens that combine fludarabine with melphalan or busulfan.10,12 While these studies have demonstrated impressive allogeneic engraftment with minimal nonhematological toxicity, there remains a significant risk of severe GVHD, particularly following unrelated donor SCTs. For example, in a recent study of nonmyeloablative SCT using unrelated donors, a purine analog and melphalan-containing regimen was associated with a 39% probability of severe grade III to IV acute GVHD, and death due to GVHD was observed in 27.5% of patients.12 The high risk of GVHD following nonmyeloablative unrelated donor SCT may thus offset any benefits in terms of reduced regimen-related toxicity.
In a previous report, we presented evidence that a fludarabine/melphalan–based preparative regimen, which incorporated in vivo CAMPATH-1H, was highly effective in preventing significant GVHD in patients following HLA-identical sibling-donor SCT.14 As part of this study, we included preliminary data suggesting that this approach was also feasible in a small number of patients receiving unrelated donor progenitor cells. We have extended these observations in 47 patients and demonstrate that this protocol is highly effective in limiting the risk of acute and chronic GVHD following unrelated donor SCT, even following transplantations that involve significant HLA class I or class II disparity.
Patients, materials, and methods
Eligibility criteria
Patients with hematological malignancies were enrolled at 6 centers in the United Kingdom. At each participating site, the study design was approved by the ethical committee. All patients gave written informed consent to participate. Patients with hematological malignancies were eligible for this study if they had no HLA-identical sibling donor and a relative contraindication to conventional myeloablative unrelated-donor SCT. Relative contraindications were nonstandard-risk disease (with standard risk defined as acute leukemia in first remission or chronic myeloid leukemia in first chronic phase15), age older than 45 years, previous transplantation, extensive prior therapy (more than 3 cycles), coexisting significant medical problems, or poor performance status. Patients were excluded if life expectancy was less than 8 weeks, left ventricular ejection fraction was less than 40%, creatinine clearance was less than 30 mL/min, bilirubin was greater than 34 μM, or liver transaminases were greater than 3× the upper limit of normal. Unrelated donor selection was performed according to published criteria16 and involved serological typing for HLA-A and HLA-B antigens and molecular typing for HLA-C, DRB1, and DQB1 (n = 13) or full molecular typing for HLA-A, HLA-B, HLA-C, DRB1, and DQB1 (n = 34). Unrelated donors gave written consent through the current accepted standards and procedures of the relevant registry.
Patient characteristics
Detailed characteristics are shown in Table1. Forty-seven consecutive patients who were enrolled into the study from October 1998 to December 2000 are reported. Of these patients, 8 were included in a previous report and are now included here with extended follow-up. Twenty-seven were male and 20 were female. Age range at the time of transplantation was 18 to 62 years (median, 44). The diagnoses were multiple myeloma (n = 11); high-grade non-Hodgkin lymphoma (NHL) (n = 8); low-grade NHL (n = 7); chronic myeloid leukemia (CML) in first chronic phase (n = 5); Hodgkin disease (n = 5); acute myeloid leukemia (AML) in complete remission (CR) 2 (n = 5); secondary AML (n = 1); acute lymphoblastic leukemia CR 2 (n = 2); T-prolymphocytic lymphoma (n = 1); plasma cell leukemia (n = 1); and chronic myelomonocytic leukemia (n = 1). Twenty-nine patients (61.7%) had received previous autografts, and only 5 patients had standard-risk disease. Median time from diagnosis to transplantation was 29 months (range, 4-189 months). Twenty-six transplantations were performed in which either the donor or the recipient was cytomegalovirus (CMV) seropositive. Twenty of the transplantations were mismatched for HLA class I and/or class II alleles (details given in Table 1). All mismatches were at the allele level except for 3 patients (patients 30, 35, and 43) with class I mismatches detected by serological typing. Of 13 patients with at least one class I mismatch, 12 had bidirectional mismatches and 1 had a unidirectional host-versus-graft mismatch. Of 11 patients with class II mismatches, 10 had bidirectional mismatches and 1 had a unidirectional host-versus-graft mismatch.
Patient and disease characteristics
| Patient no. . | Age (y) . | Sex . | Disease . | Status . | Previous autograft . | Time to transplantation (mo)* . | Stem cell source . | HLA mismatch . |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | HGNHL | PR | Yes | 73 | BM | 1 Ag C† |
| 2 | 38 | M | HGNHL | PR | Yes | 17 | BM | — |
| 3 | 18 | M | ALL | CR 2 | Yes | 29 | BM | — |
| 4 | 30 | M | AML | CR 2 | Yes | 23 | BM | —† |
| 5 | 44 | F | AML | CR 2 | Yes | 14 | BM | — |
| 6 | 30 | F | HD | Refractory | Yes | 189 | BM | — |
| 7 | 35 | M | PCL | CR 1 | No | 9 | BM | 1 Ag DQB1 |
| 8 | 22 | F | HD | PR | Yes | 32 | BM | — |
| 9 | 53 | F | CML | CP1 | No | 34 | BM | 1 Ag C† |
| 10 | 42 | F | MM | PR | No | 8 | BM | 2 Ag C, 1 Ag DQB1 |
| 11 | 30 | F | 2° AML | PR | Yes | 46 | BM | — |
| 12 | 47 | M | MM | PR | No | 9 | BM | 1 Ag DQB1 |
| 13 | 31 | M | HD | PR | Yes | 65 | BM | —† |
| 14 | 20 | M | HGNHL | CR 2 | Yes | 44 | BM | —† |
| 15 | 27 | F | ALL | CR 2 | Yes | 17 | BM | — |
| 16 | 27 | M | LGNHL | PR | No | 8 | BM | — |
| 17 | 35 | M | LGNHL | PR | Yes | 65 | BM | 1 Ag DRB1 |
| 18 | 34 | M | MM | PR | No | 6 | BM | — |
| 19 | 40 | M | LGNHL | PR | Yes | 133 | BM | — |
| 20 | 44 | F | MM | Refractory | Yes | 4 | BM | — |
| 21 | 18 | F | HGNHL | PR | Yes | 26 | BM | 1 Ag DQB1 |
| 22 | 44 | F | HGNHL | PR | Yes | 51 | BM | — |
| 23 | 36 | M | CML | CP1 | No | 51 | BM | — |
| 24 | 31 | F | HD | PR | Yes | 56 | BM | — |
| 25 | 39 | F | AML | CR 2 | No | 18 | BM | 1 Ag C† |
| 26 | 47 | M | MM | PR | No | 7 | BM | 1 Ag C |
| 27 | 44 | M | MM | Refractory | Yes | 23 | BM | 1 Ag DQB1† |
| 28 | 50 | F | T-PLL | PR | No | 10 | BM | — |
| 29 | 54 | M | AML | CR 2 | No | 24 | BM | — |
| 30 | 62 | M | CMML | PR | No | 13 | BM | 1 Ag A† |
| 31 | 52 | M | MM | PR | No | 51 | BM | 1 Ag C |
| 32 | 44 | F | HGNHL | PR | Yes | 29 | BM | — |
| 33 | 52 | M | MCL | PR | Yes | 76 | BM | — |
| 34 | 29 | F | LGNHL | CR 3 | Yes | 66 | BM | — |
| 35 | 40 | F | AML | CR 2 | Yes | 26 | BM | 1 Ag A† |
| 36 | 56 | F | MCL | PR | No | 49 | BM | 1 Ag DQB1 |
| 37 | 47 | M | MM | PR | No | 6 | BM | — |
| 38 | 44 | F | MM | Refractory | Yes | 15 | BM | 1 Ag C |
| 39 | 55 | M | HGNHL | PR | Yes | 20 | BM | — |
| 40 | 44 | M | MCL | PR | No | 61 | BM | — |
| 41 | 46 | M | HGNHL | CR 2 | Yes | 44 | BM | 1 Ag C, 1 Ag DQB1‡ |
| 42 | 50 | M | CML | CP1 | No | 21 | BM | 1 Ag DRB1 |
| 43 | 41 | M | CML | CP1 | Yes | 43 | BM | 1 Ag B, 1 Ag DQB1† |
| 44 | 49 | M | MM | PR | Yes | 70 | PBSCs | 1 Ag C, 1 Ag DQB1† |
| 45 | 48 | F | CML | CP1 | No | 21 | BM | 1 Ag C† |
| 46 | 38 | M | HD | PR | Yes | 83 | BM | — |
| 47 | 47 | F | MM | PR | Yes | 36 | BM | † |
| Patient no. . | Age (y) . | Sex . | Disease . | Status . | Previous autograft . | Time to transplantation (mo)* . | Stem cell source . | HLA mismatch . |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | HGNHL | PR | Yes | 73 | BM | 1 Ag C† |
| 2 | 38 | M | HGNHL | PR | Yes | 17 | BM | — |
| 3 | 18 | M | ALL | CR 2 | Yes | 29 | BM | — |
| 4 | 30 | M | AML | CR 2 | Yes | 23 | BM | —† |
| 5 | 44 | F | AML | CR 2 | Yes | 14 | BM | — |
| 6 | 30 | F | HD | Refractory | Yes | 189 | BM | — |
| 7 | 35 | M | PCL | CR 1 | No | 9 | BM | 1 Ag DQB1 |
| 8 | 22 | F | HD | PR | Yes | 32 | BM | — |
| 9 | 53 | F | CML | CP1 | No | 34 | BM | 1 Ag C† |
| 10 | 42 | F | MM | PR | No | 8 | BM | 2 Ag C, 1 Ag DQB1 |
| 11 | 30 | F | 2° AML | PR | Yes | 46 | BM | — |
| 12 | 47 | M | MM | PR | No | 9 | BM | 1 Ag DQB1 |
| 13 | 31 | M | HD | PR | Yes | 65 | BM | —† |
| 14 | 20 | M | HGNHL | CR 2 | Yes | 44 | BM | —† |
| 15 | 27 | F | ALL | CR 2 | Yes | 17 | BM | — |
| 16 | 27 | M | LGNHL | PR | No | 8 | BM | — |
| 17 | 35 | M | LGNHL | PR | Yes | 65 | BM | 1 Ag DRB1 |
| 18 | 34 | M | MM | PR | No | 6 | BM | — |
| 19 | 40 | M | LGNHL | PR | Yes | 133 | BM | — |
| 20 | 44 | F | MM | Refractory | Yes | 4 | BM | — |
| 21 | 18 | F | HGNHL | PR | Yes | 26 | BM | 1 Ag DQB1 |
| 22 | 44 | F | HGNHL | PR | Yes | 51 | BM | — |
| 23 | 36 | M | CML | CP1 | No | 51 | BM | — |
| 24 | 31 | F | HD | PR | Yes | 56 | BM | — |
| 25 | 39 | F | AML | CR 2 | No | 18 | BM | 1 Ag C† |
| 26 | 47 | M | MM | PR | No | 7 | BM | 1 Ag C |
| 27 | 44 | M | MM | Refractory | Yes | 23 | BM | 1 Ag DQB1† |
| 28 | 50 | F | T-PLL | PR | No | 10 | BM | — |
| 29 | 54 | M | AML | CR 2 | No | 24 | BM | — |
| 30 | 62 | M | CMML | PR | No | 13 | BM | 1 Ag A† |
| 31 | 52 | M | MM | PR | No | 51 | BM | 1 Ag C |
| 32 | 44 | F | HGNHL | PR | Yes | 29 | BM | — |
| 33 | 52 | M | MCL | PR | Yes | 76 | BM | — |
| 34 | 29 | F | LGNHL | CR 3 | Yes | 66 | BM | — |
| 35 | 40 | F | AML | CR 2 | Yes | 26 | BM | 1 Ag A† |
| 36 | 56 | F | MCL | PR | No | 49 | BM | 1 Ag DQB1 |
| 37 | 47 | M | MM | PR | No | 6 | BM | — |
| 38 | 44 | F | MM | Refractory | Yes | 15 | BM | 1 Ag C |
| 39 | 55 | M | HGNHL | PR | Yes | 20 | BM | — |
| 40 | 44 | M | MCL | PR | No | 61 | BM | — |
| 41 | 46 | M | HGNHL | CR 2 | Yes | 44 | BM | 1 Ag C, 1 Ag DQB1‡ |
| 42 | 50 | M | CML | CP1 | No | 21 | BM | 1 Ag DRB1 |
| 43 | 41 | M | CML | CP1 | Yes | 43 | BM | 1 Ag B, 1 Ag DQB1† |
| 44 | 49 | M | MM | PR | Yes | 70 | PBSCs | 1 Ag C, 1 Ag DQB1† |
| 45 | 48 | F | CML | CP1 | No | 21 | BM | 1 Ag C† |
| 46 | 38 | M | HD | PR | Yes | 83 | BM | — |
| 47 | 47 | F | MM | PR | Yes | 36 | BM | † |
HGNHL indicates high-grade NHL; ALL, acute lymphoblastic leukemia; HD, Hodgkin disease; PCL, plasma cell leukemia; MM, multiple myeloma; LGNHL, low-grade NHL; T-PLL, T-prolymphocytic lymphoma; CMML, chronic myelomonocytic leukemia; MCL, mantle cell lymphoma; PR, partial remission; CP, chronic phase; BM, bone marrow; Ag, antigen.
Time from diagnosis.
Serological typing for HLA-A and HLA-B antigens.
HLA disparity unidirectional in host-versus-graft direction.
Conditioning regimen
Treatment consisted of 20 mg/d CAMPATH-1H, a humanized monoclonal antibody, by intravenous infusion over 8 hours on days −8 to −4; 30 mg/m2 fludarabine intravenous infusion over 30 minutes on days −7 to −3; and 140 mg/m2 melphalan intravenous infusion over 30 minutes on day −2. CAMPATH-1H is a humanized immunoglobulin G1 monoclonal antibody against the CD52 antigen.17 18
Stem cell and bone marrow collection
Bone marrow from 46 donors was collected on day 0 under general anesthesia by means of conventional techniques. The median mononuclear cell dose of bone marrow was 3.5 × 108 mononuclear cells per kilogram (range, 1.8-6.9). A single donor received 10 μg/kg granulocyte colony-stimulating factor subcutaneously once daily on day −4 to day 0. Leukapheresis was performed on day 0 by means of conventional techniques for peripheral blood stem cell (PBSC) collection. Unmanipulated mobilized peripheral blood or bone marrow was infused through central venous catheters on day 0.
Supportive care
Patients were managed in reverse isolation in conventional or laminar airflow rooms. All patients received prophylaxis with cotrimoxazole or pentamidine against Pneumocystis cariniiinfection. Acyclovir and fluconazole or itraconazole prophylaxis was routinely used. Blood products were irradiated to 25 Gy. Red cell and platelet transfusions were given to maintain hemoglobin at greater than 90 g/L (9 g/dL) and platelet count at greater than 10 to 15 × 109/L. The CMV-seronegative patients received only CMV-negative blood products; seropositive patients received CMV-unscreened blood products. Febrile neutropenic patients received broad-spectrum intravenous antibiotics according to their respective hospital's policy for the management of neutropenic sepsis. CMV-seropositive patients were monitored weekly from transplantation until at least day 120 by qualitative polymerase chain reaction (PCR) of CMV DNA from peripheral blood. Preemptive ganciclovir therapy (5 mg/kg twice daily intravenously, or adjusted according to renal function) was given following 2 consecutive positive PCR results and continued according to local institutional protocols.
GVHD prophylaxis and grading
GVHD prophylaxis consisted of 3 mg/kg cyclosporine A starting on day −1. Intravenous cyclosporine was switched to an oral dose as soon as the patient could tolerate medications by mouth and was continued for 3 months.
Donor leukocyte infusions
Patients who relapsed or who showed evidence of disease progression were candidates for donor leukocyte infusions. More recently, some patients with persistent disease or mixed hematopoietic chimerism at 6 months after transplantation were also candidates for preemptive donor leukocyte infusion (DLI) with an escalated-dose regimen starting at 1 × 106CD3+ T cells per kilogram with dose escalation at 3-month intervals.
Follow-up
Patients had regular follow-ups at 3-month intervals after transplantation to assess disease response and remission status. These evaluations varied depending on the underlying diagnosis but included bone marrow aspirates/biopsies, cytogenetic or molecular evaluations, computed tomography scans, measurement of paraprotein levels, and skeletal surveys.
Chimerism analysis
Chimerism studies were performed by means of fluorescent in situ hybridization for X and Y chromosomes or by microsatellite PCR. DNA was prepared from pretransplantation recipient blood and donor blood. Following transplantation, either unfractionated buffy coat or granulocyte, T-cell and B-cell preparations were obtained from peripheral blood as previously described.19 PCRs of highly polymorphic short tandem repeat units were performed as previously described in detail.14
Study end points
Data were analyzed as of March 31, 2001. Major study end points were engraftment, transplantation-related mortality, and the incidence/severity of GVHD. Patients who survived 100 days or longer were evaluable for chronic GVHD. Acute and chronic GVHD were graded according to the consensus criteria.20 DLI patients were evaluable for GVHD if they survived for at least 100 days following the first infusion or developed clinical signs of GVHD before that time. Secondary end points included overall survival and progression-free survival. CRs and partial responses were determined by standard disease-specific criteria as defined by the International Bone Marrow Transplant Registry. Toxicity was graded according to standard criteria.21 An infective complication was defined as any infection that occurred after day 21 and required continued or new hospital admission/referral. CMV reactivation was defined as 2 consecutive peripheral blood PCR positive results. CMV disease was diagnosed on the basis of an inflammatory process that was due to CMV confirmed by the presence of typical cytopathic and immunofluorescent features in histological preparations or positive detection of early antigen fluorescent foci and/or CMV culture from relevant material such as washings from bronchoalveolar lavage. Neutrophil recovery was defined as a neutrophil count exceeding 0.5 × 109/L for at least 3 consecutive days. Platelet recovery was defined as an untransfused platelet count greater than 20 × 109/L for 7 consecutive days.
Statistical methods
Actuarial curves were estimated according to the Kaplan-Meier method, and the significance of differences between the curves was estimated by the log-rank test. Overall survival was measured from transplantation until death from any cause. Patients still alive at the time of the analysis were censored at the last follow-up date. Progression-free survival was measured from transplantation until progression or death from any cause. Patients still alive at the time of the analysis were censored at the last follow-up date. Transplantation-related mortality was determined from the date of transplantation until death related to transplantation. Patients who died from other causes were censored at the time of death.
Results
Toxicity
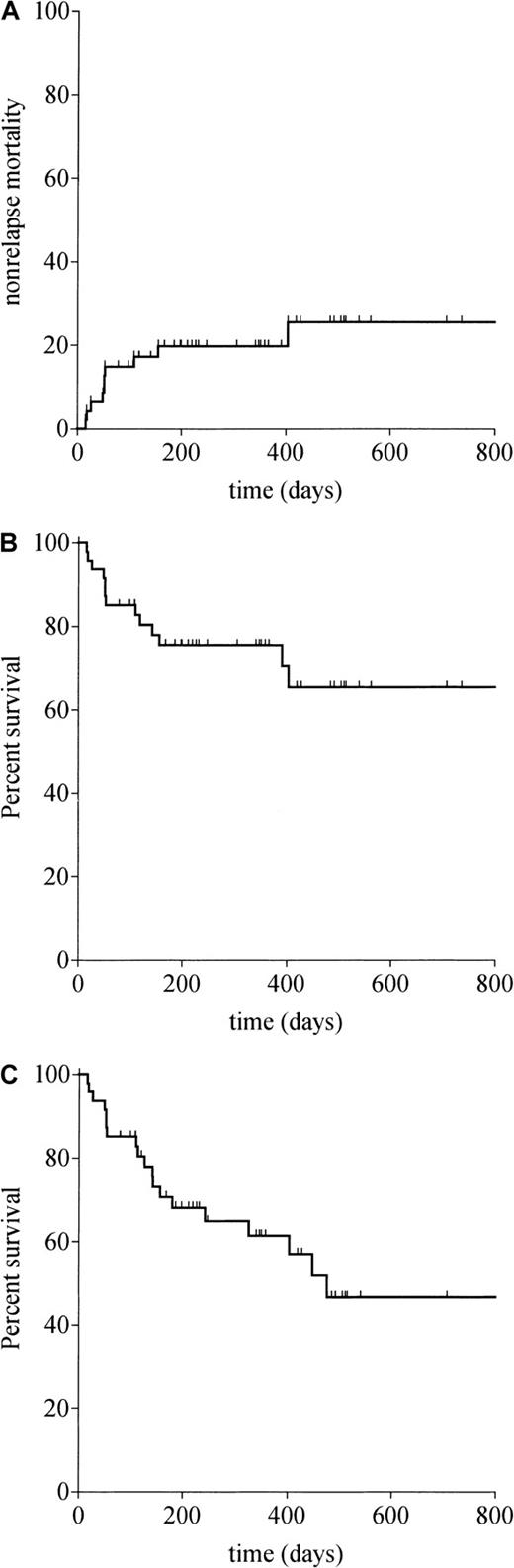
All patients were assessable for toxicity. Causes of death from all causes are listed in Table2. Infections were the most common causes of death (n = 5), with single cases of invasive fungal infection, CMV pneumonitis, parainfluenza III, Epstein Barr virus (EBV)–related lymphoproliferative disorder, and cerebral toxoplasmosis. Other procedure-related deaths were secondary to idiopathic pneumonitis (n = 2), multiorgan failure (n = 2), and perforated duodenal ulcer (n = 1). There were no cases of veno-occlusive disease. No patients developed grade 3 or 4 mucositis. The estimated probability of nonrelapse mortality at day 100 was 14.9% (95% CI, 4.7%-25.1%) and at 1 year was 19.8% (95% CI, 8.2%-31.5%) as shown in Figure1A.
Outcomes
| Patient no. . | Neutrophils > 0.5 × 109/L . | Platelets > 20 × 109/L . | Acute GVHD . | Chronic GVHD . | Follow-up (days) . | Current disease status . | Cause of death . |
|---|---|---|---|---|---|---|---|
| 1 | 13 | — | 0 | NE | 26 | Dead | IP |
| 2 | 10 | 15 | 0 | 0 | 830 | CR | |
| 3 | 18 | 30 | 0 | 0 | 801 | CR | |
| 4 | 11 | 10 | I | L | 736 | Relapse | |
| 5 | 14 | 18 | 0 | 0 | 708 | CR | |
| 6 | 14 | 19 | 0 | L | 391 | Dead | Prog |
| 7 | 14 | 18 | III | 0 | 142 | Dead | Prog |
| 8 | 12 | 12 | 0 | 0 | 563 | Relapse | |
| 9 | 15 | 10 | I | L | 540 | CR | |
| 10 | 10 | 13 | II | 0 | 511 | PR* | |
| 11 | 17 | 15 | 0 | 0 | 515 | CR | |
| 12 | 12 | 14 | 0 | 0 | 505 | PR* | |
| 13 | 10 | 29 | 0 | 0 | 492 | CR | |
| 14 | 14 | 17 | 0 | 0 | 485 | CR | |
| 15 | 12 | — | 0 | NE | 18 | Dead | Multiorgan failure |
| 16 | 12 | 16 | I | 0 | 404 | Dead | Perforated DU |
| 17 | 12 | 16 | 0 | 0 | 428 | CR | |
| 18 | 10 | 9 | 0 | 0 | 420 | PR* | |
| 19 | 10 | 14 | 0 | NE | 16 | Dead | IFI |
| 20 | 11 | 14 | 0 | 0 | 366 | Prog | |
| 21 | 14 | 20 | 0 | NE | 49 | Dead | PFIII |
| 22 | 13 | 18 | II | 0 | 156 | Dead | EBV LPD |
| 23 | 11 | 12 | II | 0 | 359 | CR | |
| 24 | 10 | 12 | I | 0 | 351 | CR | |
| 25 | 15 | 12 | I | 0 | 347 | CR | |
| 26 | 13 | 13 | II | 0 | 341 | PR* | |
| 27 | 15 | — | I | 0 | 110 | Dead | Cerebral toxoplasmosis |
| 28 | 13 | 18 | 0 | 0 | 305 | Relapse | |
| 29 | 11 | 19 | 0 | NE | 52 | Dead | Multiorgan failure |
| 30 | † | † | 0 | 0 | 248 | CR | |
| 31 | 12 | 21 | 0 | 0 | 227 | PR | |
| 32 | 15 | 13 | 0 | 0 | 119 | Dead | Prog |
| 33 | 15 | 15 | 0 | 0 | 220 | PR | |
| 34 | 13 | 20 | III | 0 | 212 | CR | |
| 35 | 17 | 23 | 0 | 0 | 232 | CR | |
| 36 | 15 | — | 0 | NE | 52 | Dead | IP |
| 37 | 13 | 19 | I | 0 | 199 | Prog | |
| 38 | 18 | 23 | 0 | 0 | 199 | Prog | |
| 39 | 10 | 17 | 0 | 0 | 198 | CR | |
| 40 | † | † | 0 | 0 | 186 | CR | |
| 41 | 11 | 15 | 0 | NE | 53 | Dead | CMV Pn |
| 42 | 16 | 51 | II | 0 | 168 | CR | |
| 43 | 17 | 18 | 0 | 0 | 120 | CR | |
| 44 | 14 | — | II | 0 | 109 | PR | |
| 45 | 15 | 20 | 0 | 0 | 108 | CR | |
| 46 | 13 | 20 | III | NE | 99 | NE | |
| 47 | 16 | 11 | II | NE | 79 | NE |
| Patient no. . | Neutrophils > 0.5 × 109/L . | Platelets > 20 × 109/L . | Acute GVHD . | Chronic GVHD . | Follow-up (days) . | Current disease status . | Cause of death . |
|---|---|---|---|---|---|---|---|
| 1 | 13 | — | 0 | NE | 26 | Dead | IP |
| 2 | 10 | 15 | 0 | 0 | 830 | CR | |
| 3 | 18 | 30 | 0 | 0 | 801 | CR | |
| 4 | 11 | 10 | I | L | 736 | Relapse | |
| 5 | 14 | 18 | 0 | 0 | 708 | CR | |
| 6 | 14 | 19 | 0 | L | 391 | Dead | Prog |
| 7 | 14 | 18 | III | 0 | 142 | Dead | Prog |
| 8 | 12 | 12 | 0 | 0 | 563 | Relapse | |
| 9 | 15 | 10 | I | L | 540 | CR | |
| 10 | 10 | 13 | II | 0 | 511 | PR* | |
| 11 | 17 | 15 | 0 | 0 | 515 | CR | |
| 12 | 12 | 14 | 0 | 0 | 505 | PR* | |
| 13 | 10 | 29 | 0 | 0 | 492 | CR | |
| 14 | 14 | 17 | 0 | 0 | 485 | CR | |
| 15 | 12 | — | 0 | NE | 18 | Dead | Multiorgan failure |
| 16 | 12 | 16 | I | 0 | 404 | Dead | Perforated DU |
| 17 | 12 | 16 | 0 | 0 | 428 | CR | |
| 18 | 10 | 9 | 0 | 0 | 420 | PR* | |
| 19 | 10 | 14 | 0 | NE | 16 | Dead | IFI |
| 20 | 11 | 14 | 0 | 0 | 366 | Prog | |
| 21 | 14 | 20 | 0 | NE | 49 | Dead | PFIII |
| 22 | 13 | 18 | II | 0 | 156 | Dead | EBV LPD |
| 23 | 11 | 12 | II | 0 | 359 | CR | |
| 24 | 10 | 12 | I | 0 | 351 | CR | |
| 25 | 15 | 12 | I | 0 | 347 | CR | |
| 26 | 13 | 13 | II | 0 | 341 | PR* | |
| 27 | 15 | — | I | 0 | 110 | Dead | Cerebral toxoplasmosis |
| 28 | 13 | 18 | 0 | 0 | 305 | Relapse | |
| 29 | 11 | 19 | 0 | NE | 52 | Dead | Multiorgan failure |
| 30 | † | † | 0 | 0 | 248 | CR | |
| 31 | 12 | 21 | 0 | 0 | 227 | PR | |
| 32 | 15 | 13 | 0 | 0 | 119 | Dead | Prog |
| 33 | 15 | 15 | 0 | 0 | 220 | PR | |
| 34 | 13 | 20 | III | 0 | 212 | CR | |
| 35 | 17 | 23 | 0 | 0 | 232 | CR | |
| 36 | 15 | — | 0 | NE | 52 | Dead | IP |
| 37 | 13 | 19 | I | 0 | 199 | Prog | |
| 38 | 18 | 23 | 0 | 0 | 199 | Prog | |
| 39 | 10 | 17 | 0 | 0 | 198 | CR | |
| 40 | † | † | 0 | 0 | 186 | CR | |
| 41 | 11 | 15 | 0 | NE | 53 | Dead | CMV Pn |
| 42 | 16 | 51 | II | 0 | 168 | CR | |
| 43 | 17 | 18 | 0 | 0 | 120 | CR | |
| 44 | 14 | — | II | 0 | 109 | PR | |
| 45 | 15 | 20 | 0 | 0 | 108 | CR | |
| 46 | 13 | 20 | III | NE | 99 | NE | |
| 47 | 16 | 11 | II | NE | 79 | NE |
NE indicates not evaluated; IP, idiopathic pneumonitis; Prog, disease progression; DU, duodenal ulcer; IFI, invasive fungal infection; PFIII, parainfluenza III; EBV LPD, EBV-related lymphoproliferative disorder; CMV Pn, CMV pneumonitis; for other abbreviations, see Table 1.
Further significant disease response after transplantation.
Primary graft failure.
Survival probabilities according to Kaplan-Meier curves.
(A) Nonrelapse mortality. (B) Overall survival. (C) Progression-free survival.
Survival probabilities according to Kaplan-Meier curves.
(A) Nonrelapse mortality. (B) Overall survival. (C) Progression-free survival.
Engraftment
Of the 47 patients, 45 (95.7%) had sustained neutrophil recovery exceeding 0.5 × 109/L for 3 consecutive days with a median time to engraftment of 13 days (range, 10-18), and 40 patients (85.1%) achieved platelet-transfusion independence (nontransfused platelet count exceeding 20 × 109/L for 7 consecutive days) with a median time to platelet recovery of 16.5 days (range, 9-51). Two of 44 patients (4.5%) who survived to day 28 never had neutrophil recovery following the initial stem cell infusion and had primary graft failure (Table 2). In both cases, bone marrow examination revealed the absence of hematopoietic activity although chimerism studies were not performed. The first patient (patient 30) went on to receive a further dose of PBSCs from the same donor on day 56 following further conditioning with antilymphocyte globulin, methylprednisolone, and cyclophosphamide. Subsequently, neutrophil recovery occurred on day 72, and platelet recovery on day 149 following the date of the first stem cell infusion. The second patient (patient 40) received autologous PBSCs on day 28, which resulted in regeneration of neutrophil counts on day 10 but, to date, no platelet engraftment. Five patients had sustained neutrophil recovery but failed to maintain a nontransfused platelet count greater than 20 × 109/L. One of these patients (patient 44) developed thrombotic thrombocytopenic purpura in the early posttransplantation period. The remaining 4 patients had sustained neutrophil engraftment but remained platelet transfusion–dependent until their deaths within the first 16 weeks secondary to transplantation-related complications. One patient with CML (patient 42) developed secondary graft failure 5 months after transplantation.
Chimerism
Chimerism studies were performed in 34 of 44 evaluable patients at a median time of 1 month (range, 1-7 months). Initial full-donor chimerism was observed in 29 of 34 patients (85.3%), including 12 of 15 patients where lineage-specific chimerism data were available. Five patients failed to establish full-donor chimerism. One patient with primary graft failure who received autologous stem cell infusion had full host chimerism at 41 days (patient 40), and a second patient with secondary graft failure had mixed chimerism evident at 5 months after transplantation (patient 42). Of the remaining 3 patients, 2 (patients 12 and 17) had T-lineage mixed chimerism and converted to full donor chimerism after DLI. The last patient had mixed chimerism in T, B, and myeloid cells.
Of 22 patients with initial full donor chimerism who had serial estimations performed, 17 maintained full donor chimerism over a median of 5 months (range, 1-21 months). In the early posttransplantation period, 5 patients developed mixed chimerism that involved either the T lineage alone (n = 3); T and B lineage (n = 1); or T, B, and myeloid lineage (n = 1).
Graft-versus-host disease
As shown in Table 2, 10 patients (21.3%) developed grade II to IV acute GVHD after transplantation, and of these, only 3 patients (6.4%) had severe grade III to IV GVHD (both grade III). Of 38 evaluable patients, only 3 have developed limited chronic GVHD, and no patients have developed extensive chronic GVHD. No increase in the incidence of GVHD was evident in patients who received HLA-mismatched (an antigen match lower than 10) as compared with HLA-identical (10 of 10 antigens matching) transplantations. As shown in Table3, of 6 patients who received DLI doses, 3 developed grade II to IV acute GVHD; this was grade II in 2 patients (given 1 × 106 CD3+ cells per kilogram at 6 and 7 months after transplantation, respectively) and grade III in 1 patient (given 10 × 106 CD3+ cells per kilogram at 5 months after transplantation). No patients have died as a consequence of GVHD.
Donor leucocyte infusions
| Patient no. . | Indication . | DLI dose3-150 (mo after transplantation) . | Response . | GVHD . |
|---|---|---|---|---|
| 12 | Persistent disease/ mixed chimerism | 1 (7+) 3 (14+) | > 50% response/ full donor chimerism | II |
| 16 | Immune reconstitution | 1 (8+) | Increase in CD4+ T-cells | 0 |
| 17 | Mixed chimerism | 1 (9+) | Full donor chimerism | I |
| 18 | Persistent disease | 1 (6+) | > 50% response | 0 |
| 3 (9+) | ||||
| 10 (12+) | ||||
| 20 | Progression | 10 (5+) | No response | III |
| 37 | Persistent disease | 1 (6+) | NE | II |
| Patient no. . | Indication . | DLI dose3-150 (mo after transplantation) . | Response . | GVHD . |
|---|---|---|---|---|
| 12 | Persistent disease/ mixed chimerism | 1 (7+) 3 (14+) | > 50% response/ full donor chimerism | II |
| 16 | Immune reconstitution | 1 (8+) | Increase in CD4+ T-cells | 0 |
| 17 | Mixed chimerism | 1 (9+) | Full donor chimerism | I |
| 18 | Persistent disease | 1 (6+) | > 50% response | 0 |
| 3 (9+) | ||||
| 10 (12+) | ||||
| 20 | Progression | 10 (5+) | No response | III |
| 37 | Persistent disease | 1 (6+) | NE | II |
NE indicates not evaluated.
CD3+ T-cell dose administered × 106/kg.
Disease response and relapses
None of the 4 patients with refractory disease showed a response to the transplantation. Of 32 patients showing a partial response to chemotherapy at the time of transplantation, 16 patients attained CR, and 4 patients obtained further significant (greater than 50%) disease reductions as defined by International Bone Marrow Transplant Registry criteria (Table 2). There were no differences in the frequency of GVHD in patients showing responses and those who did not.
All 5 patients with CML, none of whom have received DLI, have entered cytogenetic remission, and all are negative for bcr-abl transcripts by reverse-transcription PCR. Of 11 patients with multiple myeloma, 4 obtained further significant disease responses following transplantation; 3 patients have progressed; 2 patients have stable disease; 1 patient has yet to be evaluated; and the remaining patient died of procedure-related complications. Of 7 patients with low-grade NHL, CRs have been obtained in 4 patients; 1 patient has had a partial response; and 2 patients have died of procedure-related complications. Of 8 patients with high-grade NHL, CRs were obtained in 2 patients; a further patient remains in CR; 1 patient died of disease progression; and the remainder died of transplantation-related complications. Of 5 patients with Hodgkin disease, CRs were induced in 3 patients (although 1 of them subsequently relapsed at day +476 and is currently awaiting DLI); 1 patient died of disease progression; and the final patient has yet to be evaluated. Of 8 patients with acute leukemia, 4 patients remain in CR; 1 patient has relapsed; and the remaining patients died of procedure-related complications.
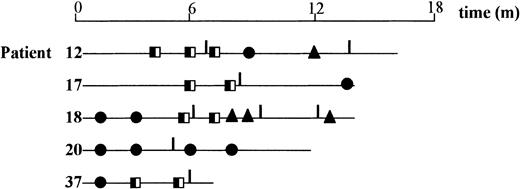
Six patients have received DLI to eradicate persistent disease (n = 3), as treatment for progression (n = 1), to correct mixed chimerism (n = 1), or to aid immune reconstitution because of disseminated mycobacterial infection (n = 1) (Figure2, Table 3). Three patients (patients 12, 18, and 37) with multiple myeloma and persistent stable disease at 6 months received DLI according to an escalated-dose regimen, and 2 showed significant (greater than 50%) subsequent disease responses, with the remaining patient awaiting further evaluation. One of the above patients converted from mixed to full donor chimerism at the time of disease response (patient 12). Another patient with early progression of refractory myeloma at 4 months after transplantation failed to respond to DLI (patient 20). A fifth patient (patient 17) with low-grade NHL that had entered CR following the transplantation but who had mixed chimerism converted to full donor chimerism following DLI. Finally, patient 16, who had a peripheral blood CD4+T-cell count of 40/μL at 7 months after transplantation, showed a rapid, sustained increase in CD4+ numbers after DLI (250 CD4+ T-cells per microliter at 1 month following lymphocyte infusion).
Chimerism studies in recipients of DLIs.
Chimerism studies were performed on T-cell, B-cell, or granulocytic subfractions or unfractionated PBMCs in 5 of 6 patients receiving DLI. The follow-up period (in months) for each patient is represented by a line, and sampling points are represented by the symbols on each line. ●, full donor in all lineages; ╡, mixed chimerism in one or more lineages; ▴, full donor, not lineage specific; ┃, DLI.
Chimerism studies in recipients of DLIs.
Chimerism studies were performed on T-cell, B-cell, or granulocytic subfractions or unfractionated PBMCs in 5 of 6 patients receiving DLI. The follow-up period (in months) for each patient is represented by a line, and sampling points are represented by the symbols on each line. ●, full donor in all lineages; ╡, mixed chimerism in one or more lineages; ▴, full donor, not lineage specific; ┃, DLI.
Infections
Serious infective complications (defined as any infection occurring after day 21 that required continued or new hospital admission/referral) occurred in 28 of 45 evaluable patients (62.2%). CMV viremia necessitating preemptive antiviral therapy occurred in 22 patients. There was one death due to CMV pneumonitis, but no other patients developed CMV disease. Other infective complications were bacterial (n = 6), parainfluenza III/respiratory syncytial virus (n = 6), adenovirus (n = 2), other viral infections (n = 4), invasive pulmonary aspergillosis (n = 2), EBV-related posttransplantation lymphoproliferative disorder (n = 1), mycobacterial infection (n = 1), and cerebral toxoplasmosis (n = 1).
Survival analyses
The median follow-up of the patients is 344 days (range, 79-830). The Kaplan-Meier estimated probabilities of progression-free survival and overall survival for all 47 patients are shown in Figure 1B-C. Overall and progression-free survivals at 1 year were 75.5% (95% CI, 62.8%-88.2%) and 61.5% (95% CI, 46.1%-76.8%), respectively. Overall 1-year survival was 80.4% (95% CI, 65.0%-95.8%) in recipients of HLA-matched grafts and 68.1% (95% CI, 46.8%-89.4%) in recipients of HLA-mismatched grafts (P = .60).
Discussion
We have demonstrated that severe GVHD following unrelated donor nonmyeloablative SCT can be effectively prevented by the incorporation of in vivo CAMPATH-1H into the preparative regimen. This approach secured high rates of donor engraftment and a low rate of regimen-related toxicity when one considers the high risk profile of many of the recipients. Although the long-term efficacy of this therapeutic approach remains to be verified, our preparative regimen induced CRs in 50% of patients with chemosensitive disease at the time of transplantation.
Previously published results of sibling donor SCT using other nonmyeloablative conditioning regimens have shown a 38% to 60% incidence of grade II to IV acute GVHD, which is the primary cause of death in some patients.9-12 The recently reported experience of 40 unrelated donor SCTs on a purine analog/melphalan protocol observed high rates of severe GVHD, with 1 in 4 patients dying as a direct result of GVHD.12 In contrast, our study demonstrates a low incidence of GVHD despite a significant HLA disparity in many of the transplantations. Only 3 patients (6.4%) had grade III to IV acute GVHD, and only 7 patients (14.9%) developed grade II acute GVHD. No patients have yet developed chronic extensive GVHD, and no patients have died as a consequence of GVHD. Although there may have been differences between the patients in the M. D. Anderson study12 and our own, the major differences between the protocols was our in vivo use of the humanized monoclonal antibody CAMPATH-1H as part of the conditioning regimen. This was administered to the patients on days −8 to −4 prior to transplantation. As CAMPATH-1H has, under these conditions, a half-life of 15 to 21 days in humans,22 there would be significant circulating antibody when the unmanipulated donor PBSCs or bone marrow was infused into the recipient, which may have resulted in a degree of in vivo T-cell depletion. It is also possible that depletion of other cells (such as dendritic cells) expressing CD52 by CAMPATH-1H could be relevant to the reduced incidence of GVHD.23
This regimen is effective in promoting high rates (greater than 90%) donor progenitor cell engraftment, including 13 patients receiving grafts mismatched for class I loci (HLA-A or HLA-B in 3 patients and HLA-C in 10 patients), which have been implicated in higher rates of graft rejection.7,24 In contrast to our previous report in which the majority of recipients received PBSCs,14 all but one of the patients in this study received bone marrow, although this had little impact either on the rates of engraftment or on the duration of neutropenia or platelet-transfusion dependence. The majority of patients (85.3%) receiving unrelated donor progenitor cells attained initial full donor chimerism. Although mixed chimerism may be associated with a higher risk of relapse following standard myeloablative SCT,19 25 the significance of mixed chimerism after nonmyeloablative SCTs remains to be determined. Thus, a key question for further investigation is whether patients who fail to achieve full donor chimerism or who subsequently develop mixed chimerism after nonmyeloablative SCT require DLI in order to reduce the risk of relapse.
As anticipated, there was a higher risk of procedure-related complications with the use of this protocol in unrelated donors as compared with HLA-identical donors.1 Although direct regimen-related toxicity was low in this high-risk cohort of patients, the most common problem was defective immune reconstitution, which resulted in significant infections in 62.2% of patients and was directly responsible for death in 5 patients. T-cell subset analysis suggested slow recovery of the CD4+ subset even 9 months following transplantation (data not shown). It is difficult to gauge whether immune reconstitution following our protocol is more of a problem than following other nonmyeloablative protocols since any negative effects in the latter may be masked by a greater number of noninfective deaths owing to regimen-related toxicity or a higher incidence of GVHD requiring more in the way of immunosuppressive treatment. Since initial immune reconstitution in adults is dependent primarily upon thymus-independent expansion of peripheral memory T cells from the graft,26 a reduction in their number as a result of in vivo CAMPATH-1H may have resulted in the loss of certain pathogen-specific immune responses.27 Although thymic output of naive T cells may eventually increase the T-cell repertoire, its contribution is age dependent and is likely to be delayed in adult patients.28,29 It is possible that thymic function is further compromised in the unrelated donor setting, thus exacerbating the immunoincompetence of the recipient.30
High-dose melphalan can induce durable remissions in patients with hematological malignancies with little nonhematopoietic toxicity31 and may act synergistically with purine analogs, which inhibit DNA repair following alkylator-induced damage.32 The combination of the 2 agents in this preparative regimen appeared to be effective in achieving initial tumor control in a significant proportion of patients with chemosensitive disease prior to transplantation, although patients with refractory disease failed to show any significant response. Long-term disease control may be dependent upon the induction of a graft-versus-tumor response, particularly in patients with CML or the lymphoproliferative disorders.33-35 A key issue to be addressed is whether the significant reduction in the risk of GVHD will also lead to a reduction in a graft-versus-tumor response and a corresponding increase in the risk of relapse. The precise role and optimal timing of DLI following this transplantation protocol and in the unrelated donor setting remain to be determined and did not form a primary end point of this study. The risk of GVHD with the use of this approach is currently unknown and will require greater numbers of patients with extended follow-up before definitive conclusions regarding safety or efficacy can be made.
In summary, our results show that in the unrelated donor setting, a nonmyeloablative regimen incorporating in vivo CAMPATH-1H facilitates high rates of allogeneic progenitor cell engraftment with a low incidence of GVHD and relatively low procedure-related mortality. It is a feasible approach in high-risk patients, including those who have failed previous autografts. The long-term antitumor activity of this regimen remains unknown, although if it is used in combination with the prophylactic or preemptive use of DLI, prolonged remissions might be obtained in some types of hematological malignancies.
We thank the staff of the Therapeutic Antibody Centre, University of Oxford, United Kingdom, for their contributions to the production of CAMPATH-1H antibody.
Supported by the United Kingdom Medical Research Council, Leukosite Inc, and the E. P. Abraham's Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen Mackinnon, Department of Haematology, University College Hospital, 98 Chenies Mews, London WC1E 6HX, United Kingdom; e-mail: s.mackinnon@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal