Abstract
EphB4 (HTK) and its ligand, ephrinB2, are critical for angiogenesis and result in fatal abnormalities of capillary formation in null mice. EphB4 was originally identified in human bone marrow CD34+cells by us and has since been reported to be expressed in erythroid progenitors, whereas the ligand ephrinB2 is expressed in bone marrow stromal cells. Reasoning that the developmental relationship between angiogenesis and hematopoiesis implies common regulatory molecules, we assessed whether EphB4 signaling influences the function and phenotype of primitive human hematopoietic cells. Ectopically expressed EphB4 in cell lines of restricted differentiation potential promoted megakaryocytic differentiation, but not granulocytic or monocytic differentiation. Primary cord blood CD34+ cells transduced with EphB4 resulted in the elevated expression of megakaryocytic and erythroid specific markers, consistent with EphB4 selectively enhancing some lineage-committed progenitors. In less mature cells, EphB4 depleted primitive cells, as measured by long-term culture-initiating cells or CD34+CD38− cell numbers, and increased progenitor cells of multiple cell types. Effects of ectopic EphB4 expression could be abrogated by either targeted mutations of select tyrosine residues or by the tyrosine kinase inhibitor, genistein. These data indicate that EphB4 accelerates the differentiation of primitive cells in a nonlineage-restricted manner but alters only select progenitor populations, influencing lineages linked by common ancestry with endothelial cells. EphB4 enforces preferential megakaryocytic and erythroid differentiation and may be a molecular bridge between angiogenesis and hematopoiesis.
Introduction
Hematopoietic stem cells (HSCs) have the capacity to self-renew and to differentiate along a number of pathways, thereby generating all blood cells. Understanding the molecular mechanisms that regulate the formation, growth, and differentiation of HSCs has become increasingly complex.1 The bone marrow (BM) is a diverse environment that contains a variety of different cell types and extracellular matrix molecules. The BM microenvironment produces a wide range of stimuli to different hematopoietic cells, providing combinatorial relationships that result in the finely tuned hematopoietic system. How factors (in either membrane-bound or soluble form) produced by BM stromal cells regulate the balance of self-renewal and differentiation of specific blood cell lineages remains a major question.
Eph receptor tyrosine kinases and their ligands, ephrins, play important roles in various processes during embryonic development, including the targeting behavior of migratory neurons, vascular cell assembly, and angiogenesis.2 Fourteen Eph receptors have been catalogued into EphA or EphB subclasses based on their affinity for ligands. Eight ephrins have been identified to date. They are membrane proteins of either glycerophosphatidylinsitol (GPI)–linked (ephrinA) or transmembrane (ephrinB).3 Rather than long-range communication, signaling from Eph receptors and their ligands is restricted to sites of direct cell-cell contact and is capable of inducing reciprocal bidirectional events between interacting cells.4 Some Eph and ephrin molecules have been found to be expressed in the hematopoietic system. EphA3 (Hek) was originally cloned from a pre–B-cell leukemia and is expressed in some T-cell lines.5 EphA1 (Esk), EphA2 (Eck), and EphB2 (Hek5) were reported to be expressed in thymus.6-8 EphA4 (Hek8) and EphA7 (Hek11) appear to be expressed in human fetal bone marrow pro-B cells.9
The receptor tyrosine kinase EphB4 (HTK) and its cognate ligand, ephrinB2 (HTKL) are widely expressed in fetal and adult tissues.10,11 Unlike most of the Eph subfamily members, EphB4 does not appear to be expressed in the central nervous system. Recent studies have shown that EphB4 is specifically expressed at the venous endothelium, whereas ephrinB2 is specifically and reciprocally expressed on arterial endothelial cells at the earliest stages of vascular development.12 Mice lacking either EphB4 or ephrinB2 display identical defects in angiogenesis by arteries and veins in the capillary networks of the head and yolk sac.12,13 This is of particular interest given the mounting data that hematopoiesis is closely associated with angiogenesis and that primordial cells of these tissues share a common precursor, the hemangioblast.14 15 The mechanism regulating mesodermal commitment to these lineages and the relationship of these lineages to one another remains poorly understood.
We previously reported that EphB4 is expressed in primary CD34+ hematopoietic progenitors and other myeloid cells.10 Others have noted that EphB4 is expressed in a subset of monocytes and that EphB4 expression is up-regulated in cord blood cells by induction with stem cell factor SCF.16 Most EphB4+ cells in bone marrow are immature cells expressing c-kit but not lineage-specific markers (CD3, CD14, CD19, CD20, and CD33). EphB4 expression is up-regulated on immature erythroid cells during erythroid differentiation of bone marrow CD34+cells.17 The EphB4 ligand, ephrinB2, is expressed in stromal cells of human bone marrow, but not in bone marrow mononuclear cells.17 EphB4 and ephrinB2 are coexpressed in the yolk sac,18 the first site of hematopoiesis and vascular development during embryogenesis. Reciprocal distribution of EphB4-ephrinB2 within hematopoietic organs suggests that their signaling may participate in the regulation of hematopoiesis. This may extend to malignant hematopoiesis. Most Eph receptors do not exert pronounced mitogenic or transforming activities.19However, EphB4 and ephrinB2 are coexpressed in most of the known leukemia–lymphoma cell lines.20
Here we describe the direct impact of EphB4 on hematopoiesis by the transduction of primary cord blood CD34+ cells with a retroviral vector expressing human EphB4 and green fluorescence protein (GFP). We demonstrate that activation by ectopic expression of human EphB4 promotes megakaryocytic and erythroid differentiation and accelerates transition of primitive cells from a stem cell to a lineage-restricted progenitor phenotype. These data, combined with earlier reports that EphB4 influences blood vessel formation, suggest that EphB4 may provide a shared regulatory molecule between hematopoiesis and angiogenesis.
Materials and methods
Cell cultures
Cord blood samples were obtained from the Pediatric Research Institute (University of St Louis, MO), according to the guidelines established by the Human Investigation Committee. Mononuclear cells were separated by Ficoll-Hypaque (Pharmacia-Biotech, Uppsala, Sweden) density gradient centrifugation, and CD34+ cells were purified by an immunomagnetic separation system (MiniMACS; Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. The percentages of CD34+ cells were generally from 95% to 98%. CD34+ progenitors were cultured in Iscoves modified Dulbecco medium (IMDM) (Mediatech, Herndon, VA) containing 10% fetal bovine serum (FCS; Sigma) supplemented with SCF (50 ng/mL), thrombopoietin (TPO; 25 ng/mL), and Flt-3 ligand (FL; 50 ng/mL) (R&D Systems, Minneapolis, MN) for 12 hours before transduction. Human megakaryoblastic cell lines CMK, CMY, and HEL21-23were cultured in RPMI 1640 medium (RPMI 1640; Mediatech) containing 10% FCS. Murine hematopoietic progenitor cell line 32D was maintained in RPMI 1640 containing 10% FCS and interleukin-3 (IL-3) (5 ng/mL; R&D Systems) or 5% WEHI-conditioned medium.24 The tyrosine kinase inhibitor genistein (100 μM) or the protein kinase C inhibitor PD98059 (50 μM) was used for some experiments. Myeloid cell line HL-60 was maintained in RPMI 1640 containing 10% FCS.
Plasmid construction and gene expression
Human EphB4 cDNA10 was subcloned intoEcoRI site of either MSCV-GFP or pcDNA3 to generate MSCV-EphB4 or pcEphB4, respectively. For retrovirus transduction, MSCV-GFP vectors were cotransfected into 293T cells using a calcium phosphate precipitation method with pKat (kindly provided by M. Finer, Cell Genesys, Foster City, CA), an amphotropic packaging plasmid, and pCMV-VSV-G, a plasmid encoding the vesicular stomatitis virus G-glycoprotein (kindly provided by T. Friedmann, University of San Diego, CA). Supernatants containing pseudo-typed retrovirus were collected at 48 hours and were used to transduce primary CD34+ cells and megakaryoblastic cell lines CMK and CMY. Cells in suspension were transduced by the same volume of retroviral supernatant with polybrene (final concentration, 5 μg/mL; Sigma) in a Retronectin (Takara Shuzo, Kyoto, Japan)-coated, 48-well plate centrifuged at 1200g for 30 minutes, cultured at 37°C and 5% CO2 for an additional 8 hours, washed, and resuspended in fresh medium. Second and third infections were conducted on the following days using an identical procedure. Two days after the last infection, GFP-positive cells were sorted by fluorescence-activated cell sorting (FACS Vantage; Becton Dickinson, San Jose, CA) and used in subsequent experiments. 32D cells were stably transfected by electroporation as previously described.25 Forty-eight hours after electroporation, the cells were cultured for 3 weeks in medium containing 400 μg/mL G418 (Gibco BRL, Rockville, MD) and were subcloned by limiting dilution.
Flow cytometry
Lineage markers were examined in MSCV-EphB4–transduced CD34+ cord blood cells using flow cytometry (FACScalibur; Becton Dickinson). Cells were cultured in IMDM containing 10% FCS supplemented with SCF (50 ng/mL), IL-3 (10 ng/mL), and IL-6 (10 ng/mL) or with SCF (50 ng/mL), TPO (25 ng/mL), and Flt-3 (50 ng/mL) (R&D Systems). After 7 to 10 days, cells were harvested and stained with the following monoclonal antibodies to analyze differentiation: phycoerythrin (PE)–conjugated mAbs against CD41, glycophorin A, and CD34; and allophycocyanin (APC)–conjugated mAbs against CD14, CD33, and CD38 (mAbs; Becton Dickinson). Dead cells were gated out by 7-amino actinomycin D (7AAD) staining. Cells were analyzed by a flow cytometer using CellQuest software (FACScalibur; Becton Dickinson). Human anti-Eph B4 monoclonal antibody IC2-2C2 (kindly provided by Genentech, South San Francisco, CA) was fluorescein isothiocyanate (FITC) conjugated by using conjugation kit (Prozyme, San Leandro, CA), according to the manufacturer's instructions. Annexin-V FITC antibody was used for apoptotic analysis.
The expression of functional EphB4 in MSCV-EphB4–transduced cells was examined by flow cytometry using APC-conjugated ephrin-B2 (HTK ligand). Plasmid encoding ephrinB2-Fc (kindly provided by H. C. Aasheim, Norwegian Radium Hospital, Oslo, Norway) was transfected into COS cells, and EphrinB2-Fc fusion protein was purified, as previously described.26 APC conjugation of ephrin-B2 was performed by using the Phycolink Allophycocyanin Conjugation Kit (Prozyme), according to the manufacturer's instructions.
Ploidy evaluation
Megakaryoblastic cell lines (CMK and CMY) transduced by MSCV-EphB4 or MSCV were cultured in RPMI 1640 containing 10% fetal bovine serum. On day 3, cells were resuspended in buffer containing 50 μg/mL propidium iodide, 0.2% Triton X-100, and 30 μg/mL RNase (Sigma). Flow cytometry was performed (FACScalibur; Becton Dickinson), and ploidy values were determined by plotting the propidium iodide fluorescence of the cells using a semilogarithmic scale.
Colony-forming assay
Three hundred transduced CD34+ cells were plated in triplicate 24-well plates with 1% methylcellulose in IMDM containing 30% fetal bovine serum, 1% bovine serum albumin, 0.1 mM 2-mercaptoethanol, and the following recombinant human cytokines: 50 ng/mL SCF, 20 ng/mL IL-3, 20 ng/mL IL-6, 3 U/mL erythropoietin, 20 ng/mL granulocyte monocyte–colony-stimulating factor, and 20 ng/mL granulocyte–colony-stimulating factor) (MethoCult; StemCell Technologies, Vancouver, BC, Canada). After 10 days at 37°C and 5% CO2 in a humidified incubator, erythroid burst-forming units (BFU-E), granulocyte, monocyte–colony-forming units (CFU-GM), and mixed-lineage CFU-GEMM (granulocyte, erythroid, monocyte, megakaryocyte) colonies were counted from each of the plates, and averages were determined for each individual. CFU-Meg colonies were determined separately using the MegaCult System (StemCell Technologies). Transduced CD34+ cells were cultured on collagen-based slide chambers in the presence of recombinant human TPO, IL-3, and IL-6, at a density of 2500 cells per chamber. After 14 days, the chamber slides were dehydrated and stained for GpIIb/IIIa according to the manufacturer's guidelines. All colony numbers were quantified using an inverted-phase light microscope.
Long-term culture with limiting dilution
Long-term culture-initiating cell (LTC-IC) cultures were assessed according to described methods.27 Sorted cells were plated on irradiated (15 Gy) primary human bone marrow stromal feeder layers and were cultured in human long-term bone marrow culture media (StemCell Technologies) containing hydrocortisone (10−6 M). Cultures were initiated in limiting dilution from 37 to 600 cells per well in 96-well plates. Wells were maintained at 33°C, 5% CO2, and were fed weekly by half-medium change. Blast colonies were scored after 6 weeks by plating in methylcellulose assay (see above), and 12 wells per cell concentration were tested. The absolute number of LTC-ICs was calculated by Poisson statistics.
Results
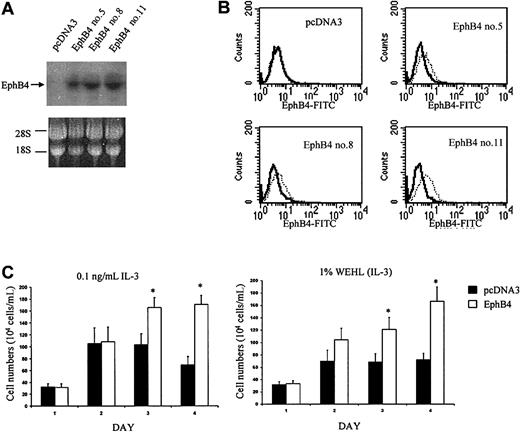
EphB4 reduces the requirement of IL-3 and increases the growth rate in 32D cells
We sought to determine whether EphB4 signaling had an effect on hematopoietic proliferation by using the primary cell line, 32D, as a model. These cells are well-characterized diploid murine hematopoietic progenitor cells that have an absolute requirement for IL-3 or 5% WEHI-conditioned medium (WEHI CM) for growth.24 An effect of EphB4 on proliferation would be expected to alter the requirements for IL-3 or WEHI CM on 32D cell growth. Because of the low transduction efficiency of 32D cells by retroviral vector (data not shown) and the need for highly uniform test populations, we used a pcDNA3-based vector to generate 32D/EphB4 stable cell lines. Limiting dilution was used to generate EphB4-positive subclones. Overexpression of receptor tyrosine kinases (RTKs) is a well-defined method of assessing their function because physical association induces cross-phosphorylation and activation.28 29 The expression of EphB4 was verified by Northern blot analysis, and FACS analysis was performed to ensure EphB4 cell surface expression (Figure 1A-B). Three different EphB4-positive sublines of 32D cells transfected with pcDNA3 were used for the proliferation assay to avoid cell line–specific artifacts. EphB4-positive and control cells were cultured in the presence of different WEHI CM concentrations. Cells overexpressing EphB4 demonstrated sustained growth kinetics with reduced concentrations of IL-3 or WEHI CM (Figure 1C). In the absence of IL-3 or WEHI CM (0%), no cells consistently grew (data not shown). In the presence of 5 ng/mL IL-3 or 5% WEHI CM, the proliferation rates for 32DEphB4 and for the control cells were the same (data not shown). However, when the cells were cultured in the presence of 0.1 ng/mL IL-3 or 1% WEHI CM, 32D EphB4 cells exhibited a higher proliferation rate than control cells (Figure 1C). Overexpression of EphB4 in 32D cells significantly lowered the IL-3 requirement for growth, suggesting a role of EphB4 in cell proliferation.
Proliferative effects of EphB4 on 32 cells.
(A) Expression of EphB4 in 3 sublines of 32D, EphB4 no. 5, EphB4 no. 8, and EphB4 no. 11, confirmed by Northern blot analysis. (B) EphB4 expression was analyzed by FACS using anti-EphB4 FITC-conjugated monoclonal antibody (right, dotted line). (C) Cell proliferation assay. Three 32D EphB4-positive sublines were cultured in the presence of 0.1 ng/mL IL-3 or 1% WEHI CM. Triplicate cultures of each subline were set at each condition at 10 × 104 cells/mL in a 24-well plate. Viable cells were counted and presented as the mean of 3 sublines. Similar results were obtained in 3 experiments. *P < .01.
Proliferative effects of EphB4 on 32 cells.
(A) Expression of EphB4 in 3 sublines of 32D, EphB4 no. 5, EphB4 no. 8, and EphB4 no. 11, confirmed by Northern blot analysis. (B) EphB4 expression was analyzed by FACS using anti-EphB4 FITC-conjugated monoclonal antibody (right, dotted line). (C) Cell proliferation assay. Three 32D EphB4-positive sublines were cultured in the presence of 0.1 ng/mL IL-3 or 1% WEHI CM. Triplicate cultures of each subline were set at each condition at 10 × 104 cells/mL in a 24-well plate. Viable cells were counted and presented as the mean of 3 sublines. Similar results were obtained in 3 experiments. *P < .01.
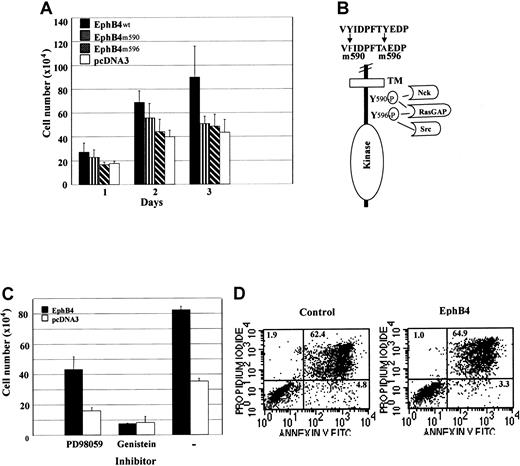
EphB4 overexpression results in EphB4 activation
To evaluate whether the effects of EphB4 overexpression were caused by activation, we altered critical features of RTKs. Eph receptors share common features of RTKs, one of which is that receptor activation is dependent on receptor autophosphorylation.4,30 Tyrosine phosphorylation of the receptor creates docking sites for signaling molecules that bind phosphotyrosine residues with their SH2 domain.31 To investigate EphB4 signal transduction, we generated 2 point mutations (Y590 and Y596) of tyrosine residues in the juxtamembrane region of EphB4 (Figure 2B) by using site-directed mutagenesis. These 2 tyrosine residues, highly conserved in all Eph receptors, have been identified as major autophosphorylation sites.32-34 One or both of these sites can be recognized by the SH2 domain proteins RasGAP, Src, Fyn, and the adapter protein Nck.32-35 We stably transfected these mutants into 32D cells and assessed their functional effect. As predicted, mutations of either Y590 or Y596 abrogated the effect of EphB4 overexpression (Figure 2A). The mutants were inactive compared with the wild-type receptor and were no different than the control vector. These results indicate that the overexpression of wild-type EphB4 results in activation of the receptor, requiring autophosphorylation at the SH2 docking motif. Dependence on the tyrosine kinase function of EphB4 for the effects of overexpression was further documented by treatment with the tyrosine kinase inhibitor, genistein (Figure 2C). In contrast to the inhibition noted with genistein treatment, the protein kinase C inhibitor (PD98059) had no effect, confirming the critical role of tyrosine kinase function in the cellular effects of EphB4 overexpression. To test whether overexpression of EphB4 in 32D cells can prolong the survival of 32D cells, anti–annexin V antibody was used for FACS analysis of apoptosis. As Figure 2D indicates, 32D cell growth enhanced by EphB4 is apoptosis independent.
Mutation of EphB4 juxtamembrane tyrosine residues or tyrosine kinase inhibition abolishes the cell growth effect of transduced EphB4.
(A) Wild-type EphB4 (EphB4wt), mutants EphB4 (EphB4m590 and EphB4m596), and pcDNA3 (vector) were stably transfected in 32D cells and selected for G418-resistance for 3 weeks. Cell proliferation assay was performed in the presence of 0.1 ng/mL IL-3. Data represent the mean ± SD of 3 experiments (each in duplicate). (B) Schematic representation of mutations inEphB4 intracellular domain. (C). EphB4-expressing and control 32D (pcDNA3) cells were incubated in 0.1 ng/mL IL-3 with 50 μM PD98059 (protein kinase C inhibitor) or 100 μM genistein (tyrosine kinase inhibitor) for 48 hours. Data represent the mean ± SD for 3 experiments (each in duplicate). (D) Apoptosis analysis. EphB4-expressing and control 32D (pcDNA3) cells were incubated in 0.1 ng/mL IL-3 for 3 days. Cells were stained with Annexin V-FITC antibodies and analyzed by FACS.
Mutation of EphB4 juxtamembrane tyrosine residues or tyrosine kinase inhibition abolishes the cell growth effect of transduced EphB4.
(A) Wild-type EphB4 (EphB4wt), mutants EphB4 (EphB4m590 and EphB4m596), and pcDNA3 (vector) were stably transfected in 32D cells and selected for G418-resistance for 3 weeks. Cell proliferation assay was performed in the presence of 0.1 ng/mL IL-3. Data represent the mean ± SD of 3 experiments (each in duplicate). (B) Schematic representation of mutations inEphB4 intracellular domain. (C). EphB4-expressing and control 32D (pcDNA3) cells were incubated in 0.1 ng/mL IL-3 with 50 μM PD98059 (protein kinase C inhibitor) or 100 μM genistein (tyrosine kinase inhibitor) for 48 hours. Data represent the mean ± SD for 3 experiments (each in duplicate). (D) Apoptosis analysis. EphB4-expressing and control 32D (pcDNA3) cells were incubated in 0.1 ng/mL IL-3 for 3 days. Cells were stained with Annexin V-FITC antibodies and analyzed by FACS.
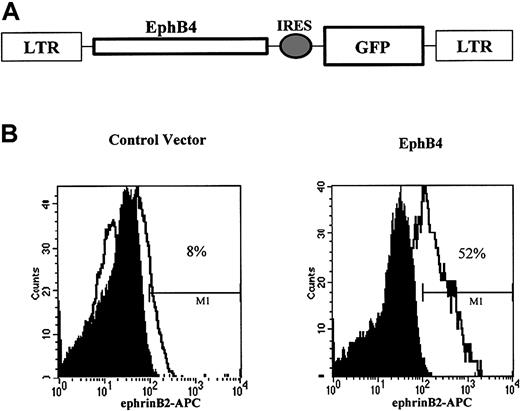
Efficient expression of EphB4 in primary CD34+ cells with retroviral gene transfer
To investigate the role of EphB4 in hematopoietic differentiation, we used the retroviral vector MSCV-GFP to express EphB4 in umbilical cord blood (UCB) CD34+ cells. The retroviral vector MSCV is known to drive high expression levels of transgene cDNA in hematopoietic progenitor cells.36 MSCV-GFP has an internal ribosomal entry sequence that permits the expression of cDNAs of interest and a marker protein, GFP, from a single bicistronic mRNA. The construct MSCV-EphB4 expresses both EphB4 from the LTR promoter and GFP from a downstream internal ribosomal entry sequence (Figure3A). We used stem cell–rich umbilical cord blood37,38 and transduced the CD34+fraction with MSCV-EphB4 or control vector MSCV-GFP. The EphB4 ligand, ephrinB2, conjugated to APC was used for flow cytometric analysis to assess the functional expression of EphB4. We observed that approximately 52% of GFP+ CD34+ cells transduced with MSCV-EphB4 were able to bind to ephrinB2, compared with 8% of GFP expressing MSCV–GFP-transduced controls (Figure 3B). The low level of binding to control cells may reflect endogenous EphB4 expression previously observed.10,17 Alternatively, ephrinB2 has been reported to bind the receptors EphB2 and EphB3,39 though expression of these receptors has not been detected in CD34+ cells.
Cell surface expression of EphB4 in UBC CD34+ cells by retroviral transduction.
(A) Structure of MSCV-EphB4 retrovirus EphB4 carrying the cDNA for EphB4 and GFP. (B) Flow cytometric analysis (FACS) of MSCV-EphB4 expression. UBC CD34+ cells were purified by an immunomagnetic separation system and transduced with MSCV-EphB4 (right) or MSCV-GFP vector (left). EphB4 ligand, ephrinB2, was conjugated with APC and was used to detect the cell surface expression of EphB4 receptor by FACS for ephrinB2 binding on CD34+GFP+ cells.
Cell surface expression of EphB4 in UBC CD34+ cells by retroviral transduction.
(A) Structure of MSCV-EphB4 retrovirus EphB4 carrying the cDNA for EphB4 and GFP. (B) Flow cytometric analysis (FACS) of MSCV-EphB4 expression. UBC CD34+ cells were purified by an immunomagnetic separation system and transduced with MSCV-EphB4 (right) or MSCV-GFP vector (left). EphB4 ligand, ephrinB2, was conjugated with APC and was used to detect the cell surface expression of EphB4 receptor by FACS for ephrinB2 binding on CD34+GFP+ cells.
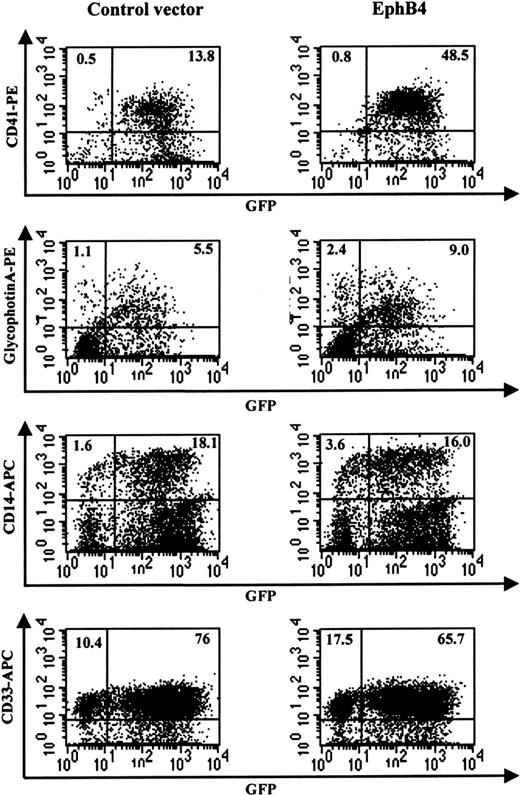
Primary CD34+ cells transduced with EphB4 increase expression of megakaryocytic and erythroid lineage markers
We evaluated the effect of the overexpression of EphB4 on differentiation using liquid culture of cord blood CD34+cells. After transduction with MSCV-EphB4, CD34+GFP+ cells were sorted and cultured in the presence of SCF, IL-3, and IL-6. The effects of EphB4 overexpression were assessed by measuring the expression of a megakaryocytic lineage cell surface marker, CD41, an erythroid cell surface marker, glycophorin A, a monocytic marker, CD14, and the myeloid marker, CD33. Our result indicated that, 7 days after transduction (Figure4), the expression of CD41 was significantly increased in MSCV-EphB4–transduced CD34+cells, compared with MSCV-GFP–transduced cells (mean = 20.7 vs 8.6;P = .019; n = 6). Expression of glycophorin A in MSCV-EphB4–transduced CD34+ cells was also significantly increased, compared with MSCV-GFP–transduced cells (mean = 16.2 vs 5.6; P = .038; n = 5). In contrast, overexpression of EphB4 in CD34+ cells had no significant effect on the expression of either CD14 (mean = 24.2 vs 25.0; P = .22) or CD33 (90.0 vs 92.8; P = .21). Similar results were observed when transduced CD34+GFP+ cells were cultured in the presence of SCF, TPO, and Flt-3 ligand (Table1). These results suggest that EphB4 acts specifically on restricted lineages and may affect a common precursor for the megakaryocytic and erythroid lineages, sparing the granulocyte–monocyte progenitor. Alternatively, EphB4 may selectively enhance differentiation of more primitive cells into megakaryocyte–erythroid precursors.
Enhancement of megakaryocytic and erythrocytic differentiation in CD34+ cells by EphB4.
CD34+ cells were transduced with MSCV-GFP (left) or MSCV-EphB4 (right). GFP+ cells were then sorted and cultured in the presence of SCF, IL-3, and IL-6 for 7 days. Two-color FACS was performed for the expression of CD41-phycoerythrin and CD14-APC or the expression of glycophorin A-PF (gpA-PE) and CD33-APC. Data shown are from 1 representative experiment of 5 or 6 experiments with data summarized in the text.
Enhancement of megakaryocytic and erythrocytic differentiation in CD34+ cells by EphB4.
CD34+ cells were transduced with MSCV-GFP (left) or MSCV-EphB4 (right). GFP+ cells were then sorted and cultured in the presence of SCF, IL-3, and IL-6 for 7 days. Two-color FACS was performed for the expression of CD41-phycoerythrin and CD14-APC or the expression of glycophorin A-PF (gpA-PE) and CD33-APC. Data shown are from 1 representative experiment of 5 or 6 experiments with data summarized in the text.
Effect of EphB4 on primary human CD34+ cells cultured with SCF, TPO, and FL
| . | CD41 . | GlycophrinA . | CD14 . | CD33 . | ||||
|---|---|---|---|---|---|---|---|---|
| Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | |
| Sample no. | ||||||||
| 1 | 4.1 | 13.8 | 3.8 | 11.1 | — | — | — | — |
| 2 | 2.9 | 8.4 | 2.0 | 10.5 | 27.2 | 40.3 | 65.4 | 67.4 |
| 3 | 6.8 | 15.2 | 2.8 | 4.6 | 41.0 | 33.9 | 97.4 | 97.8 |
| 4 | 30.2 | 59.8 | 18.8 | 31.7 | 54.5 | 44.7 | 97.2 | 98.9 |
| 5 | 24.4 | 35.4 | 10.2 | 25.2 | 43.5 | 44.6 | 89.5 | 98.1 |
| Mean | 13.7 | 26.5 | 7.5 | 16.6 | 41.6 | 40.9 | 87.4 | 90.6 |
| P | .020 | .008 | .131 | .092 | ||||
| . | CD41 . | GlycophrinA . | CD14 . | CD33 . | ||||
|---|---|---|---|---|---|---|---|---|
| Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | |
| Sample no. | ||||||||
| 1 | 4.1 | 13.8 | 3.8 | 11.1 | — | — | — | — |
| 2 | 2.9 | 8.4 | 2.0 | 10.5 | 27.2 | 40.3 | 65.4 | 67.4 |
| 3 | 6.8 | 15.2 | 2.8 | 4.6 | 41.0 | 33.9 | 97.4 | 97.8 |
| 4 | 30.2 | 59.8 | 18.8 | 31.7 | 54.5 | 44.7 | 97.2 | 98.9 |
| 5 | 24.4 | 35.4 | 10.2 | 25.2 | 43.5 | 44.6 | 89.5 | 98.1 |
| Mean | 13.7 | 26.5 | 7.5 | 16.6 | 41.6 | 40.9 | 87.4 | 90.6 |
| P | .020 | .008 | .131 | .092 | ||||
CD34+ cells were transduced with MSCV-GFP (vector) or MSCV-EphB4 (EphB4). GFP+ cells were sorted and cultured in the presence of SCF (50 ng/mL), TPO (25 ng/mL), and flt3 ligand (FL) (50 ng/mL) for 7 days. P values were calculated using Wilcoxon matched-pairs signed-rank test.
Ectopically expressed EphB4 in cell lines promoted megakaryocytic differentiation but not granulocytic or monocytic differentiation
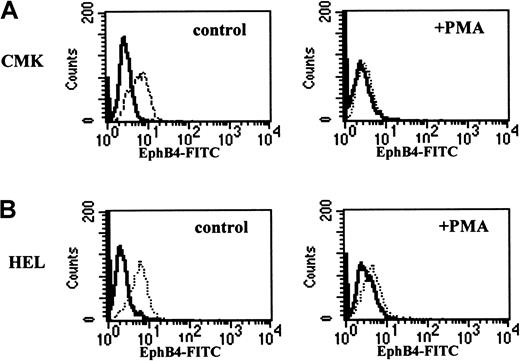
To address the above 2 possibilities, we evaluated the impact of EphB4 on the differentiation of cell lines with known restricted potential. It has been reported that during erythroid differentiation, EphB4 expression is up-regulated on immature erythroid cells that correspond to BFU-E and CFU-E and down-regulated on erythroblasts.17 CMK and HEL are human megakaryoblast cell lines that have been shown to undergo megakaryocytic differentiation in response to the phorbol ester, PMA.21,23 FACS analysis using anti-EphB4 antibody revealed that EphB4 was expressed on CMK (Figure 5A) and HEL (Figure 5B), and the expression of EphB4 was down-regulated during terminal megakaryocytic differentiation in the presence of PMA. These results are consistent with the down-regulation of EphB4 mRNA shown previously by others using Northern blot analysis during megakaryocytic differentiation.16 To determine whether the overexpression of EphB4 alters megakaryocytic differentiation, EphB4 was transduced into CMK cells using the MSCV retrovirus. We observed that forced expression of EphB4 in CMK cells enhanced the expression of the megakaryocytic marker, CD41, at early time points (Figure6A). In the absence of PMA, CD41 expression was increased from 7% to 15% (n = 3; P = .025) by overexpression of EphB4. After a 24-hour induction by PMA, CD41 expression was distinct with 60% in MSCV-GFP CMK cells, compared with 74% in MSCV-EphB4 CMK cells (n = 3;P = .031). After 48 hours of PMA induction, CD41 expression reached the maximum level for both EphB4-transduced cells and controls (data not shown). CD41 is perhaps the earliest megakaryocyte marker and is expressed on the bipotential erythroid–megakaryocytic progenitor and the committed megakaryocyte progenitor.40,41 Because megakaryocytes have a unique identifying feature of endomitosis to form polyploid nuclei, we used ploidy analysis to verify megakaryocytic differentiation. Ploidy of CMK or CMY megakaryocytic cell lines was induced by the overexpression of EphB4 compared with vector controls (Figure 6B). These results indicate that EphB4 enhances early megakaryocytic differentiation in a committed cell type. In contrast, the myeloid cell line, HL-60, which is capable of granulocytic and monocytic differentiation,42yielded different results. Overexpression of EphB4 in HL-60 did not affect either granulocytic or monocytic differentiation as evaluated by CD11b expression in the presence of the all-trans retinoic acid or in the presence of PMA, respectively (Figure 6C). Therefore, to the extent that these cell lines represent lineage-restricted progenitors, EphB4 expression selectively affects those progenitors committed to a megakaryocytic–erythroid outcome.
Down-regulation of EhpB4 expression on megakaryoblast CMK and HEL cells during terminal megakaryocytic differentiation.
CMK (A) and HEL (B) cells were cultured in the absence (left) or the presence of 100 nM PMA (right) for 2 days. EphB4 expression was analyzed by FACS using anti-EphB4, FITC-conjugated monoclonal antibody (right, dotted line).
Down-regulation of EhpB4 expression on megakaryoblast CMK and HEL cells during terminal megakaryocytic differentiation.
CMK (A) and HEL (B) cells were cultured in the absence (left) or the presence of 100 nM PMA (right) for 2 days. EphB4 expression was analyzed by FACS using anti-EphB4, FITC-conjugated monoclonal antibody (right, dotted line).
Effects of EphB4 overexpression on cell lines.
(A) CMK cells were transduced with MSCV-GFP (left) or MSCV-EphB4 (right). GFP+ cells were sorted and cultured in the absence or the presence of 100 nM PMA for 24 hours. Expression of the megakaryocytic marker CD41 was evaluated by FACS using anti-CD41 PE-conjugated monoclonal antibody. Data from 3 independent experiments are summarized in the text. (B) Ploidy analysis of CMK and CMY. CMK or CMY cells transduced by MSCV-GFP (left) or MSCV-EphB4 (right) were sorted and cultured for 3 days. Cells were fixed and stained with 50 μg/mL propidium iodide. Ploidy values were determined by plotting propidium iodide fluorescence of the cells using a semilogarithmic scale. (C) HL-60 cells transduced with MSCV-GFP (left) or MSCV-EphB4 (right). GFP+ cells were sorted and cultured in the presence of 2 μM all-trans retinoic acid for 5 days (upper) or in the presence of 4 nM PMA for 3 days (lower). Other time point data were not shown here. Expression of the granulocytic and monocytic marker CD11b was evaluated by FACS using anti-CD11b PE-conjugated monoclonal antibody. Data presented are from a representative experiment of 3 performed for panels A, B, and C.
Effects of EphB4 overexpression on cell lines.
(A) CMK cells were transduced with MSCV-GFP (left) or MSCV-EphB4 (right). GFP+ cells were sorted and cultured in the absence or the presence of 100 nM PMA for 24 hours. Expression of the megakaryocytic marker CD41 was evaluated by FACS using anti-CD41 PE-conjugated monoclonal antibody. Data from 3 independent experiments are summarized in the text. (B) Ploidy analysis of CMK and CMY. CMK or CMY cells transduced by MSCV-GFP (left) or MSCV-EphB4 (right) were sorted and cultured for 3 days. Cells were fixed and stained with 50 μg/mL propidium iodide. Ploidy values were determined by plotting propidium iodide fluorescence of the cells using a semilogarithmic scale. (C) HL-60 cells transduced with MSCV-GFP (left) or MSCV-EphB4 (right). GFP+ cells were sorted and cultured in the presence of 2 μM all-trans retinoic acid for 5 days (upper) or in the presence of 4 nM PMA for 3 days (lower). Other time point data were not shown here. Expression of the granulocytic and monocytic marker CD11b was evaluated by FACS using anti-CD11b PE-conjugated monoclonal antibody. Data presented are from a representative experiment of 3 performed for panels A, B, and C.
EphB4 promotes hematopoietic colony-forming capacity
We next assessed whether EphB4 might influence committed progenitors versus more primitive multipotent cells by quantitating specific colony types in methylcellulose colony-forming assays. After transduction of UCB CD34+ cells with MSCV-EphB4 or MSCV-GFP, GFP+ cells were sorted, plated in methylcellulose, and scored for total colony-forming cells (CFCs). CD34+ cells transduced with MSCV-EphB4 gave rise to a higher number of CFCs than CD34+ cells transduced with MSCV-GFP (Table 2; P = .007; n = 5). CFU-Meg was also found to be higher from CD34+cells transduced with EphB4 than from the control (Table 2;P = .045; n = 4). Within CFCs, both CFU-GM and BFU-E/CFU-E were higher in MSCV-EphB4–transduced cells than MSCV-GFP cells. Of note, colony size was not appreciably affected. These results suggest that EphB4 can promote the differentiation of primitive cells into multiple types of lineage-committed progenitors. In contrast, the liquid culture and cell line data suggest that EphB4 may play a more selective role in the further differentiation of the megakaryocytic and erythroid committed progenitor pools.
Effects of EphB4 on colony-forming ability of CD34+ cells
| Sample no. . | CFU-GM . | BFU/CFU-E . | CFU-GEMM . | Total CFCs . | CFU-Meg . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | |
| 1 | 82 | 111 | 29 | 43 | 5 | 7 | 114 | 161 | 38.0 | 48.0 |
| 2 | 102 | 128 | 25 | 32 | 5 | 4 | 132 | 164 | — | — |
| 3 | 13 | 30 | 14 | 12 | 3 | 3 | 30 | 45 | 9.6 | 14.4 |
| 4 | 96 | 109 | 23 | 47 | 9 | 17 | 128 | 171 | 18.4 | 32.4 |
| 5 | 21 | 27 | 12 | 17 | 10 | 11 | 43 | 55 | 12.8 | 13.2 |
| Mean | 63 | 81 | 20.6 | 30 | 6.4 | 8.4 | 89.4 | 119.2 | 19.7 | 27.0 |
| P | .006 | .047 | .137 | .007 | .045 | |||||
| Sample no. . | CFU-GM . | BFU/CFU-E . | CFU-GEMM . | Total CFCs . | CFU-Meg . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | Vector . | EphB4 . | |
| 1 | 82 | 111 | 29 | 43 | 5 | 7 | 114 | 161 | 38.0 | 48.0 |
| 2 | 102 | 128 | 25 | 32 | 5 | 4 | 132 | 164 | — | — |
| 3 | 13 | 30 | 14 | 12 | 3 | 3 | 30 | 45 | 9.6 | 14.4 |
| 4 | 96 | 109 | 23 | 47 | 9 | 17 | 128 | 171 | 18.4 | 32.4 |
| 5 | 21 | 27 | 12 | 17 | 10 | 11 | 43 | 55 | 12.8 | 13.2 |
| Mean | 63 | 81 | 20.6 | 30 | 6.4 | 8.4 | 89.4 | 119.2 | 19.7 | 27.0 |
| P | .006 | .047 | .137 | .007 | .045 | |||||
Three hundred transduced CD34+/GFP+ cells were plated in triplicate 24-well plates with 1% methylcellulose containing SCF, IL-3, IL-6, erythropoietin, GM-CSF, and G-CSF for total CFCs. Colonies were counted from each of the plates, and averages were determined for each individual. CFU-Meg colonies were determined separately using collagen-based slide chambers in the presence of TPO, IL-3, and IL-6, at a density of 2500 cells per chamber. All colony numbers were quantified using an inverted-phase light microscope.P values were calculated using Wilcoxon matched-pairs signed-rank test.
EphB4 may augment exit from the primitive hematopoietic cell pool
To further assess the impact of EphB4 on the primitive cell compartment, limiting-dilution LTC-IC assays were performed as a surrogate measure of stem cell numbers. We observed a decreased number of LTC-IC from CD34+ cells transduced with EphB4 compared with the vector control (P = .023; n = 3) (Figure7A). To address this issue, we analyzed cells in liquid culture for the relative preservation of a primitive immunophenotype. We found that CD34+CD38−cells were decreased in CD34+ cells transduced with EphB4 compared with vector control (P = .03; n = 5) (Figure7B). Taken together, these results are consistent with EphB4 influencing primitive cells, reducing their proportion and absolute number. The mechanism of this effect may be through increased cell death or through enhanced differentiation, driving cells from the primitive pool. We did not observe a significantly increased fraction of cells staining for annexin-V, thereby arguing for enhanced differentiation rather than cell death accounting for the effect of EphB4.
Effects of EphB4 overexpression on primitive cell compartment.
(A) LTC-IC assays were performed at week 6 from CD34+ cells transduced with MSCV-EphB4 or control. Data presented are from a representative experiment of 3 performed. (B) Reduction of CD34+CD38− population in liquid culture by the transduction of EphB4. CD34+ cells were transduced with MSCV-GFP or MSCV-EphB4. GFP+ cells were cultured for 7 days. Two-color FACS was performed for the expression of CD34-PE and CD38-APC. Data shown represent the average of 5 experiments. Error bars represent standard deviation (P = .003).
Effects of EphB4 overexpression on primitive cell compartment.
(A) LTC-IC assays were performed at week 6 from CD34+ cells transduced with MSCV-EphB4 or control. Data presented are from a representative experiment of 3 performed. (B) Reduction of CD34+CD38− population in liquid culture by the transduction of EphB4. CD34+ cells were transduced with MSCV-GFP or MSCV-EphB4. GFP+ cells were cultured for 7 days. Two-color FACS was performed for the expression of CD34-PE and CD38-APC. Data shown represent the average of 5 experiments. Error bars represent standard deviation (P = .003).
Discussion
The data presented here indicate that EphB4 is capable of altering hematopoiesis at several levels. It appears to enhance the differentiation of primitive cells, driving cells into the lineage-committed progenitor compartment. Cells in the committed progenitor cell pool are induced to undergo differentiation restricted to the megakaryocyte–erythroid lineages. A bimodal effect has been noted in other RTKs known to modulate hematopoiesis, such as c-kit and flt-3, with their predominately prosurvival effect on primitive cells and augmentation of proliferation in more mature progenitor cells. However, the Eph family is generally not associated with hematopoietic function, and identification of a role for EphB4 does open the possibility of a broader participation of this large subfamily of RTKs in hematopoiesis. Characteristically, Eph family members act in microenvironmental interactions because the ligands are cell bound and bidirectional signaling occurs in ligand- and receptor-expressing cells. In that Eph RTKs act locally, further definition of this subfamily in hematopoiesis may be informative in mapping the bone marrow space. Fine detailing of receptor and ligand expression may be of interest, in particular to define whether these receptors participate in the clustering of certain hematopoietic elements in the bone marrow.
During embryogenesis, hematopoiesis is closely associated with angiogenesis. The hemangioblast is the common precursor of endothelial and hematopoietic cells from mesoderm in the developing embryo, where the blood islands of yolk sac consist of a central core of primitive erythroid cells surrounded by vascular endothelial cells.14,43 Primitive erythropoiesis and the vascular system must develop in close temporal and geographic order to establish the embryo's oxygen-delivery system during organogenesis. Molecular and cellular events involved in hemangioblast differentiation remain unclear. A number of receptor tyrosine kinases have been suggested to participate in angiogenesis and hematopoiesis, including flk-1 (the receptor for vascular endothelial growth factor [VEGF]) and tie-2 (the receptor for angiopoietin-1).44-49 Indirect evidence has suggested that an association of blood and vessel formation persists in the adult. For example, a recent study demonstrated that erythroid cells produce the angiogenic factors VEGF-A and PIGF.50 Vascular endothelial cells have been reported to support the proliferation and differentiation of megakaryocytes by secretion of cytokines, including IL-6 and TPO.51Conversely, megakaryocytes and platelets have been demonstrated to release VEGF and angiopoietins,52-54 factors essential for vasculogenesis and angiogenesis, respectively. The link of erythropoiesis and megakaryocytopoiesis with angiogenesis has, therefore, been made with factors that are released from cells and can act at a distance. Here, we present evidence that a locally acting member of the RTK subfamily strongly associated with angiogenesis can also regulate distinct steps in the hematopoietic cascade, augmenting the differentiation of cells into erythroid and megakaryocytic elements. This critical mediator of vessel formation is capable of influencing adult hematopoiesis to selectively enhance mature blood elements most closely linked to the repair and essential function of the vasculature. As such, it may be viewed as a molecular regulator spanning the processes of hematopoiesis and angiogenesis in local contexts.
We thank D. Dombkowski for his expert help in cell sorting and in guidance with flow cytometry analyses. We also thank Dr T. Cheng for helpful suggestions and discussions and Dr K. Cohen for careful reading of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David T. Scadden, Experimental Hematology, AIDS Research Center and MGH Cancer Center, Massachusetts General Hospital, Harvard Medical School, 149 13th St, Room 5212D, Boston, MA 02129; e-mail: scadden.david@mgh.harvard.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal