Abstract
The signal transducer and activator of transcription 3 (Stat3), a member of the Stat family of proteins, is commonly activated by thrombopoietic cytokines including thrombopoietin (TPO), interleukin (IL)-6, and interleukin-11. This finding strongly suggested that Stat3 has an important role in megakaryopoiesis and thrombopoiesis. To clarify the functional role of Stat3 in in vivo megakaryopoiesis and thrombopoiesis, we generated transgenic mice overexpressing a dominant-negative Stat3, Stat3F, to suppress the function of endogenous Stat3. To accomplish the selective expression of Stat3F in megakaryocytic lineage cells, we used the regulatory gene region of GATA-1 transcription factor selectively expressed in megakaryocytic and erythroid lineage cells. Two independent transgenic (Tg) mice lines were established. It was confirmed by Western blotting analysis that Stat3F proteins were highly expressed in the platelets from the Tg mice. In addition, it was found that Stat3 activation induced by TPO stimulation was drastically suppressed in these Tg mice compared with littermates. These findings indicate that Stat3F works well in the Tg mice. Platelet counts were within the normal range in steady-state conditions and were recovered normally from transient thrombocytopenia induced by antiplatelet serum injection. Interestingly, the platelet recovery from myelosuppression after 5-fluorouracil treatment was significantly delayed in the Tg mice. Collectively, our results strongly suggest that Stat3 plays an important role in the early stage of megakaryopoiesis, presumably through the expansion of megakaryocytic progenitor cells.
Introduction
Megakaryopoiesis and thrombopoiesis are skillfully orchestrated with various thrombopoietic cytokines, including interleukin (IL)-3, stem cell factor (SCF), IL-6, IL-11, and thrombopoietin (TPO).1-5 In the early stage of megakaryocytic development, these cytokines support the growth of immature megakaryocytic progenitor cells as megakaryocyte colony-stimulating factors. Subsequently, some of these cytokines also promote megakaryocytic differentiation and platelet production as megakaryocyte potentiator or thrombopoiesis-stimulating factors.
The action of cytokines is mediated by the activation of their cognate receptors and downstream signal transduction pathways, including protein kinase C, phospholipase C-γ1, phosphatidylinositol 3-kinase–Akt, RAS-mitogen-activated protein kinase (MAPK), and Janus tyrosine kinase-signal transducer and activator of transcription (Stat) activation pathways.6 Recent studies revealed that these signal transduction pathways are commonly activated by several cytokines in normal megakaryocytes and play important roles in the regulation of megakaryopoiesis.7-13 For example, the constitutive activation of MAPK is required for the maturation of megakaryocytes with an increase of ploidy.7 Protein kinase C-α is involved in proplatelet formation.8 However, the precise function of Stat proteins in megakaryopoiesis remains to be elucidated. Among the Stat proteins, normal megakaryocytes predominantly express Stat3.14 In addition, thrombopoietic cytokines appear to activate Stat3. For example, TPO and IL-11 activate Stat3 and Stat5, and Stat3, respectively, in normal megakaryocytes,14 15 suggesting that Stat3 has an important role in megakaryopoiesis and thrombopoiesis.
Although Stat 3 knockout mice have already been established, these mice showed embryonic lethality at E6.5 days before the onset of hematopoiesis.16 Therefore, an alternative approach is required for examining the Stat3 function in in vivo megakaryopoiesis and thrombopoiesis.17,18 To overcome this problem, we used transgenic (Tg) constructs in which the expression of a dominant-negative form of Stat3, Stat3F, is controlled by the GATA-1 regulatory gene elements, because GATA-1 expression is restricted to megakaryocytes and erythroid cells.19 20 In this study, we established the Tg mice selectively expressing Stat3F in megakaryocytic lineage cells. Using these Tg mice, we examined the in vivo function of Stat3 in megakaryocytic development, platelet production, and platelet function. Our results suggest that Stat3 activation may be required for promoting the expansion of megakaryocytic progenitor cells.
Materials and methods
Generation of transgenic mice
A cDNA of HA-tagged Stat3F was cloned into an expression cassette downstream of the IE3.9int GATA-1 promoter.21Constructs were isolated by SalI digestion from the vector sequence and were injected into fertilized BDF-1 eggs using standard procedures. Tg mice were identified by polymerase chain reaction (PCR) methods using primer pairs 5′-ATGTACCCATACGACGTCCC-3′ and 5′-GACATTGGACTCTTGCAGGAAT-3′ to amplify the HA tag–containing sequence in the transgene.
Preparation of platelets
For the preparation of platelets, mice were anesthetized with ether, and blood was drawn from the superior vena cava into 40 mM D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK; Sigma, St Louis, MO) and was gently mixed. Platelet-rich plasma (PRP) was prepared by centrifuging whole blood at 200g for 20 minutes and aspirating PRP. PRP was incubated with aspirin (2 mM) for 30 minutes at room temperature. To prepare washed platelets, prostaglandin E1 (1 mM) was added from a stock solution in absolute ethanol (1 mM). PRP was spun at 800g to form a soft platelet pellet. The pellet was resuspended in 1 mL modified HEPES–Tyrode buffer (129 mM NaCl, 8.9 mM NaHCO3, 0.8 mM KH2PO4, 0.8 mM MgCl2, 5.6 mM dextrose, and 10 mM HEPES, pH 7.4) also containing apyrase (2 U/mL) and was washed twice with PBS. Platelets were resuspended in the same buffer at a concentration of 3 to 9 × 108 cells/mL with apyrase (2 U/mL) containing 1 mM CaCl2 at 37°C.
Measurement of platelet aggregation
Platelet aggregation using PPACK (final concentration, 40 mM)-PRP was measured using an aggregometer (Hema Tracer model 601; Niko Bio Science, Tokyo, Japan) with continuous stirring (1000 rpm), as previously described.22
Real-time quantitative PCR
Real-time quantitative PCR was performed according to the standard methods using ABI prism 770 with SYBR Green PCR and RT-PCR reagents (Applied Biosystems, Foster City, CA). For the internal control, we used GAPDH forward and reverse primers included in the GAPDH Control Reagents Kit (Applied Biosystems).
Reverse transcription–PCR
Total cellular RNA was extracted from the spleens of 4-month-old mice using the RNeasy Midi Kit (QIAGEN, Valencia, CA) and was subjected to reverse transcription (RT)-PCR using the mRNA Selective PCR Kit (Takara Shuzo, Shiga, Japan). Primers used for RT-PCR were the same as those used in the screening.
Western blot analysis
Washed platelets were prepared from Tg mice and control mice as previously described.23 After incubation with or without TPO for 10 minutes, platelets were lysed for immunoprecipitation using sufficient amounts of anti-Stat3 antibodies to immunoprecipitate endogenous Stat3. Immunoprecipitated proteins were analyzed on 7.5% polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. Blots were incubated with the appropriate concentrations of primary antibodies, including antiphosphotyrosine monoclonal antibody PY20 (Transduction Laboratories, Lexington, KY) and an antibody against Stat3, and then were visualized using the enhanced chemiluminescence detection kit (ECL Western Blotting Analysis System; Amersham Pharmacia Biotech, Buckinghamshire, England).
Hematologic analysis
Orbital plexus blood was collected from the anesthetized mice, and peripheral blood white blood cell count, hematocrit, concentration of hemoglobin, and platelet counts were determined using Sysmex F820 (Sysmex GmbH Europe, Hamburg, Germany).
In vitro colony assay
For megakaryocyte colony-forming unit (CFU-Meg) assay, bone marrow cells were harvested from mice by flushing the femoral contents with α-modified minimum essential medium. Marrow cells (1 × 105) were cultured with MegaCult-C media (StemCell Technologies, Vancouver, Canada) in the presence of 10 ng/mL recombinant mouse IL-3 (Kirin Brewery), 20 ng/mL recombinant human IL-6 (Kirin Brewery), and 50 ng/mL recombinant human TPO (Kirin Brewery) according to the instruction manual. After culturing for 7 days, the colonies formed were stained with acetylcholine esterase (AChE) according to the method described by Dr C. W. Jackson.24 CFU-Meg–derived colonies were defined as colonies with at least 3 megakaryocytes.25
Histologic analysis
Samples of the femur and spleen were removed from normal and Tg mice after they were killed. Samples were fixed in formalin and embedded in paraffin, cut into 4-μM sections, and stained with hematoxylin–eosin.
Administration of thrombopoietin
TPO (30 μg/kg; PEG-rHUMGDF) was injected intraperitoneally once daily into Stat3F Tg mice and normal littermates for 5 days, according to the methods described elsewhere.26 After TPO treatment, platelet numbers were determined at the indicated time points.
5-Fluorouracil administration
After intravenous injection of a single dose of 5-fluorouracil (5-FU; 150 μg/kg) to the wild-type littermates and the Stat3F Tg mice, blood analysis was sequentially performed as described above at days 0, 5, 10, 15, and 20.
Antiplatelet serum administration
One hundred microliters of a 1:20 dilution of rabbit antimouse platelet serum (Inter-Cell Technologies, Hopewell, NJ) was injected intraperitoneally into the wild-type and Stat3F Tg mice. The number of platelets was counted at the indicated time points.
Establishment of Stat3F-expressing UT-7/TPO cells
UT-7/TPO cells were grown in liquid culture with Iscoves modified Dulbecco medium containing 10% fetal calf serum and 10 ng/mL TPO.11 Stat3F cDNA was introduced into the UT-7/TPO cells by lipofection, and cell lines expressing Stat3F were established as previously described.
Luciferase assay
Luciferase assay was performed with the reporter construct that contains 4 copies of the acute-phase response element (APRE), which is transactivated by Stat3, in front of the minimal jun-B promoter linked to the luciferase gene (4XAPREluc).
Results
Stat3F works as a dominant-negative mutant in thrombopoietin signaling
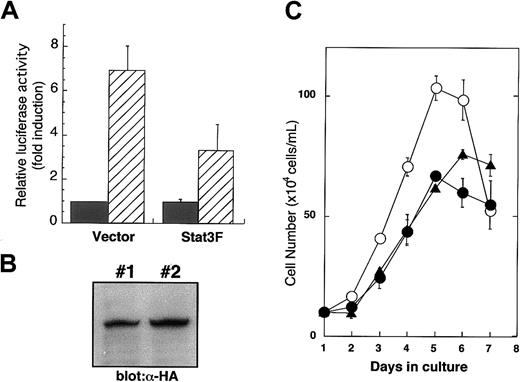
In our previous study, we demonstrated that Stat3F, in which tyrosine residue 705 was replaced by phenylalanine, works as a dominant-negative regulator in IL-6 and granulocyte macrophage–colony-stimulating factor signaling.27 28 To confirm whether this mutant can inhibit Stat3 function in TPO signaling, we introduced the reporter construct containing Stat3-responsive elements with or without Stat3F cDNA into UT-7/TPO cells and then performed a luciferase assay. As shown in Figure1A, Stat3F significantly inhibited the reporter activation in response to TPO. Furthermore, the UT-7/TPO cells expressing Stat3F showed a clearly reduced-growth response to TPO. These results support the notion that Stat3F works as a dominant-negative regulator in TPO signaling.
Stat3F works as a dominant-negative mutant in TPO signaling.
(A) After 24-hour starvation, UT-7/TPO cells were transfected with luciferase reporter plasmid 4XAPREluc and pCAGGS vector or pCAGGS-Stat3F vector. After 12-hour culture, the cells were treated with TPO (hatched bar) or fetal calf serum alone (black bar) for 6 hours and then were harvested for luciferase assay. Values were normalized to transfection efficiency and represent the means of 3 independent experiments. (B) pCAGGS-Stat3F plasmids were introduced into UT-7/TPO cells, and 2 independent transfectants were established. Overexpression of Stat3F was confirmed by Western blot analysis using anti–HA-tag antibody. (C) Parental UT-7/TPO cells (○) and 2 lines of Stat3F expressing UT-TPO cells (clone 1, ●; clone 2, ▴) were cultured with 10 ng/mL TPO for the indicated periods. Cell numbers and viability were assessed by trypan blue dye exclusion.
Stat3F works as a dominant-negative mutant in TPO signaling.
(A) After 24-hour starvation, UT-7/TPO cells were transfected with luciferase reporter plasmid 4XAPREluc and pCAGGS vector or pCAGGS-Stat3F vector. After 12-hour culture, the cells were treated with TPO (hatched bar) or fetal calf serum alone (black bar) for 6 hours and then were harvested for luciferase assay. Values were normalized to transfection efficiency and represent the means of 3 independent experiments. (B) pCAGGS-Stat3F plasmids were introduced into UT-7/TPO cells, and 2 independent transfectants were established. Overexpression of Stat3F was confirmed by Western blot analysis using anti–HA-tag antibody. (C) Parental UT-7/TPO cells (○) and 2 lines of Stat3F expressing UT-TPO cells (clone 1, ●; clone 2, ▴) were cultured with 10 ng/mL TPO for the indicated periods. Cell numbers and viability were assessed by trypan blue dye exclusion.
Generation of transgenic mice selectively expressing Stat3F in megakaryocytic lineage cells
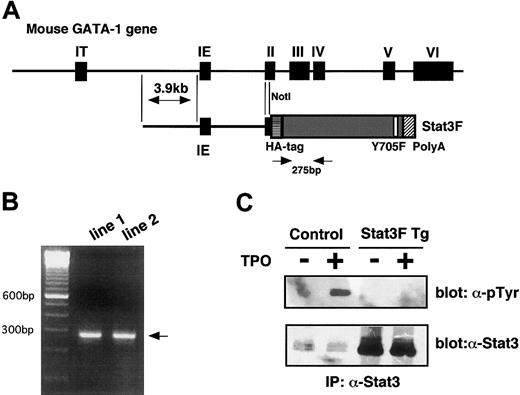
To investigate the function of Stat3 in in vivo megakaryopoiesis, we generated mice selectively bearing the dominant-negative form of Stat3 in the megakaryocytic and erythroid cells. Previous studies demonstrated that this GATA-1 gene regulatory region is required and sufficient for lineage-specific expression of GATA-1 in primitive and definitive erythropoiesis.21,29 It was also reported that Tg mice generated by the introduction of the green fluorescent protein (GFP) gene under the control of this GATA-1 gene regulatory cassette, named IE3.9int, showed selective expression of GFP in the erythroid and the megakaryocytic lineage cells.21 Based on these observations, we inserted Stat3F into the IE3.9int cassette (Figure2A) and introduced the Stat3F transgene into fertilized eggs and generated 2 independent Tg mice lines. Real-time quantitative PCR revealed that each Tg line contained an equal number of transgenes (data not shown). The expression of the transgene was monitored by mRNA-selective RT-PCR methods (Figure 2B). In addition, we confirmed by Western blotting that Stat3F proteins were overexpressed in platelets derived from the Tg mice (Figure 2C). To confirm whether Stat3F actually inhibited in vivo Stat3 activation, we examined whether Stat3 is tyrosine phosphorylated by TPO stimulation in the platelets of the Tg mice. As shown in Figure 2C, TPO rapidly induced the tyrosine phosphorylation of Stat3 in the normal platelets but not in the platelets from the Tg mice. These results indicated that Stat3F works well in the Tg mice. In contrast, the expression and activation of Stat5 were not affected in the Stat3F Tg mice (data not shown).
Establishment of Stat3F Tg mice.
(A) Schematic structure of the GATA-1 gene regulatory region (IE3.9int) cassette. This cassette contains 3.9-kb pairs of sequences 5′ to the IE exon, the IE exon itself, the first intron, and part of the second exon of the mouse GATA-1 gene. Stat3cDNA was linked to this cassette. (B) mRNAs were isolated from the spleen of the Tg mice, and the expression of Stat3F was verified by RT-PCR. Positions of the primer pairs are shown in Figure 2A. (C) High expression and dominant-negative regulation of Stat3F in the platelets from the Tg mice. Platelets were isolated from the Tg mice, and the expression of Stat3F was confirmed by Western blotting (lower panel). Stat3F worked as a dominant-negative regulator in TPO-induced tyrosine phosphorylation of Stat3 (upper panel).
Establishment of Stat3F Tg mice.
(A) Schematic structure of the GATA-1 gene regulatory region (IE3.9int) cassette. This cassette contains 3.9-kb pairs of sequences 5′ to the IE exon, the IE exon itself, the first intron, and part of the second exon of the mouse GATA-1 gene. Stat3cDNA was linked to this cassette. (B) mRNAs were isolated from the spleen of the Tg mice, and the expression of Stat3F was verified by RT-PCR. Positions of the primer pairs are shown in Figure 2A. (C) High expression and dominant-negative regulation of Stat3F in the platelets from the Tg mice. Platelets were isolated from the Tg mice, and the expression of Stat3F was confirmed by Western blotting (lower panel). Stat3F worked as a dominant-negative regulator in TPO-induced tyrosine phosphorylation of Stat3 (upper panel).
Stat3 is dispensable for steady-state megakaryopoiesis
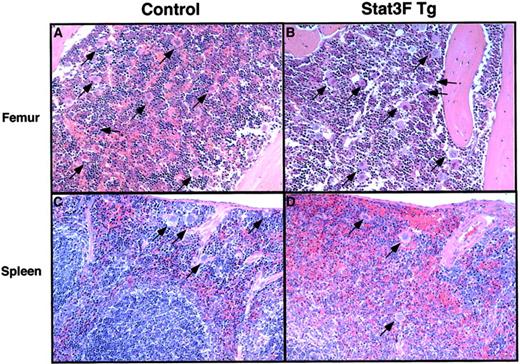
We initially analyzed the peripheral blood cell counts of the Tg mice. As summarized in Table 1, we did not find any differences in the peripheral blood cell counts, including platelet number, between the Tg mice and normal littermates. The size of the platelets in the Tg mice was also not affected. Histologic examination demonstrated that the numbers of megakaryocytes in bone marrow and spleen were similar in both groups of mice (Figure3). In addition, megakaryocytes of the Tg mice did not show any significant changes in nuclear morphology or lobulation. These results indicated that the activation of Stat3 in megakaryocytic cells was dispensable for steady-state megakaryopoiesis.
Peripheral blood analysis
| Hematologic parameter . | Wild type (n = 8) . | Stat3F Tg (n = 8) . |
|---|---|---|
| Platelets (× 106/mL) | 987 ± 117 | 944 ± 144 |
| WBC count (× 106/mL) | 8.39 ± 2.12 | 7.43 ± 1.68 |
| Erythrocytes (× 107/mL) | 1057.8 ± 35.5 | 1036.3 ± 76.7 |
| Hemoglobin (g/dL) | 16.3 ± 0.7 | 15.7 ± 1.1 |
| Hematocrit (%) | 55.7 ± 2.4 | 54.5 ± 4.0 |
| Mean corpuscular volume (fL) | 52.7 ± 2.8 | 52.5 ± 1.2 |
| Mean corpuscular hemoglobin (pg) | 15.1 ± 0.3 | 15.2 ± 0.6 |
| Mean corpuscular hemoglobin concentration (g/dL) | 28.8 ± 0.3 | 28.8 ± 0.6 |
| Mean platelet volume (fL) | 6.5 ± 0.6 | 6.7 ± 0.7 |
| Hematologic parameter . | Wild type (n = 8) . | Stat3F Tg (n = 8) . |
|---|---|---|
| Platelets (× 106/mL) | 987 ± 117 | 944 ± 144 |
| WBC count (× 106/mL) | 8.39 ± 2.12 | 7.43 ± 1.68 |
| Erythrocytes (× 107/mL) | 1057.8 ± 35.5 | 1036.3 ± 76.7 |
| Hemoglobin (g/dL) | 16.3 ± 0.7 | 15.7 ± 1.1 |
| Hematocrit (%) | 55.7 ± 2.4 | 54.5 ± 4.0 |
| Mean corpuscular volume (fL) | 52.7 ± 2.8 | 52.5 ± 1.2 |
| Mean corpuscular hemoglobin (pg) | 15.1 ± 0.3 | 15.2 ± 0.6 |
| Mean corpuscular hemoglobin concentration (g/dL) | 28.8 ± 0.3 | 28.8 ± 0.6 |
| Mean platelet volume (fL) | 6.5 ± 0.6 | 6.7 ± 0.7 |
Pathologic analysis of bone marrow and spleen in the Tg mice.
Hematoxylin–eosin-stained sections from the femurs of a wild-type littermate (A) and Stat3F Tg mice (B) and from the spleens of a wild-type littermate (C) and the Tg mice (D). Arrows show mature megakaryocytes (original magnification, ×100).
Pathologic analysis of bone marrow and spleen in the Tg mice.
Hematoxylin–eosin-stained sections from the femurs of a wild-type littermate (A) and Stat3F Tg mice (B) and from the spleens of a wild-type littermate (C) and the Tg mice (D). Arrows show mature megakaryocytes (original magnification, ×100).
Numbers of CFU-Meg colonies in transgenic mice and normal littermates
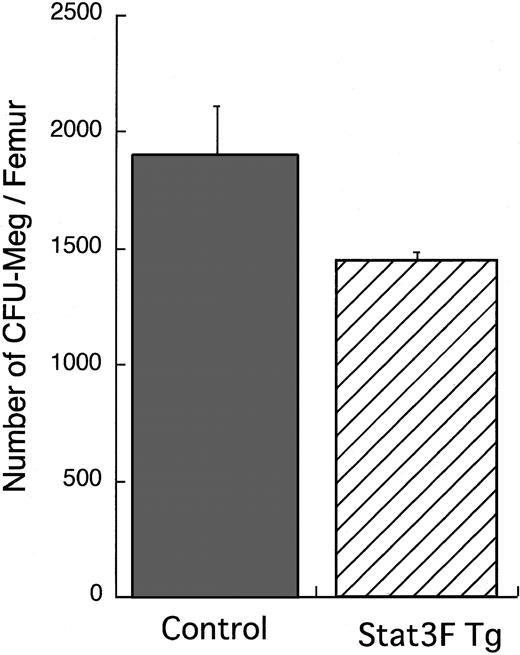
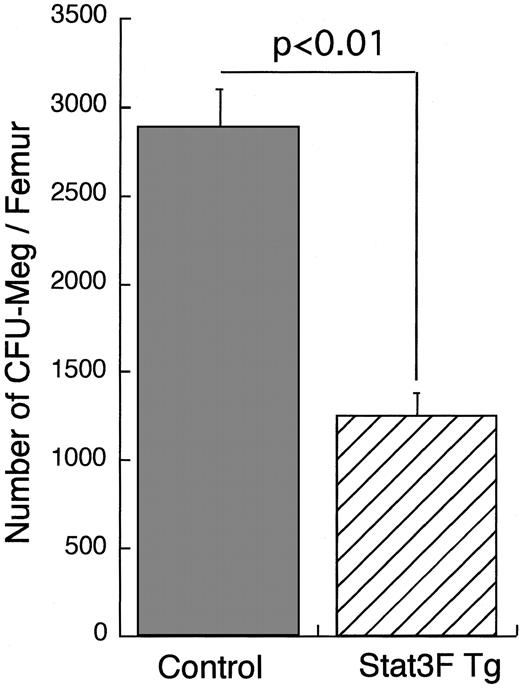
We analyzed the number of hematopoietic progenitors by in vitro clonal culture with bone marrow cells (Figure4). The number of CFU-Meg per femur in the Tg mice was slightly smaller than that in the wild-type littermates, though there was no significant difference between the 2 groups (P = .11). Moreover, we compared the sizes of CFU-Meg in the 2 groups. The proportion of larger colonies containing more than 20 cells was significantly decreased in cultures from the Stat3F Tg mice than in those from the wild-type littermates (Table2; P < .05). These results suggest that the growth activity of megakaryocytic progenitors was reduced in the Stat3F Tg mice.
Megakaryocytic progenitor assay.
Bone marrow cells (1 × 105) were cultured with MegaCult-C media in the presence of 10 ng/mL IL-3, 20 ng/mL IL-6, and 50 ng/mL TPO according to the instruction manual. After 7-day culture, the colonies formed were stained with AChE. Results represent the mean (±SD) number of CFU-Meg colonies per femur in 2 independent experiments.
Megakaryocytic progenitor assay.
Bone marrow cells (1 × 105) were cultured with MegaCult-C media in the presence of 10 ng/mL IL-3, 20 ng/mL IL-6, and 50 ng/mL TPO according to the instruction manual. After 7-day culture, the colonies formed were stained with AChE. Results represent the mean (±SD) number of CFU-Meg colonies per femur in 2 independent experiments.
Differences in CFU-Meg colony size between control and Tg mice
| Colony size (no. megakaryocytes) . | Control . | Stat3F Tg . |
|---|---|---|
| 3 to 5 | 31.4 ± 3.3 | 33.6 ± 2.6 |
| 6 to 19 | 48.9 ± 2.0 | 57.3 ± 1.1 |
| 20 to 50 | 15.9 ± 2.5 | 8.9 ± 1.1* |
| > 50 | 3.8 ± 2.7 | 0.3 ± 0.4* |
| Colony size (no. megakaryocytes) . | Control . | Stat3F Tg . |
|---|---|---|
| 3 to 5 | 31.4 ± 3.3 | 33.6 ± 2.6 |
| 6 to 19 | 48.9 ± 2.0 | 57.3 ± 1.1 |
| 20 to 50 | 15.9 ± 2.5 | 8.9 ± 1.1* |
| > 50 | 3.8 ± 2.7 | 0.3 ± 0.4* |
Sizes of the CFU-Meg colonies were estimated according to the number of cells included in an individual colony (3-5, 6-19, 20-50, and > 50 megakaryocytes). Results represent the mean (± SD) of CFU-Meg colonies in 2 independent experiments.
P< .05.
Normal response to thrombopoietin in transgenic mice
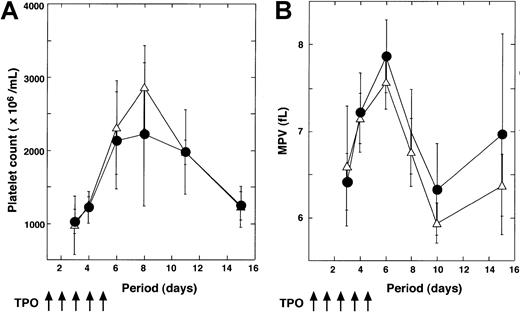
To confirm that the inhibition of Stat3 signaling affects TPO-induced platelet production, we injected TPO into normal (n = 6) and Tg (n = 6) mice for 5 days and then analyzed the platelet counts. As shown in Figure 5A, platelet numbers were rapidly increased after TPO injection in both groups, and the kinetics of platelet number were almost equal in both groups. We also monitored the changes in mean platelet volume (MPV). Treatment with TPO induced transient elevations of MPV level in control and Tg mice (Figure 5B).
Response of Tg mice to PEG-MGDF treatment.
Tg mice (n = 6) and wild-type littermates (n = 6) were treated with TPO for the indicated periods. Platelet count (A) and mean platelet volume (B) are represented (●, wild-type mice; ▵, Tg mice).
Response of Tg mice to PEG-MGDF treatment.
Tg mice (n = 6) and wild-type littermates (n = 6) were treated with TPO for the indicated periods. Platelet count (A) and mean platelet volume (B) are represented (●, wild-type mice; ▵, Tg mice).
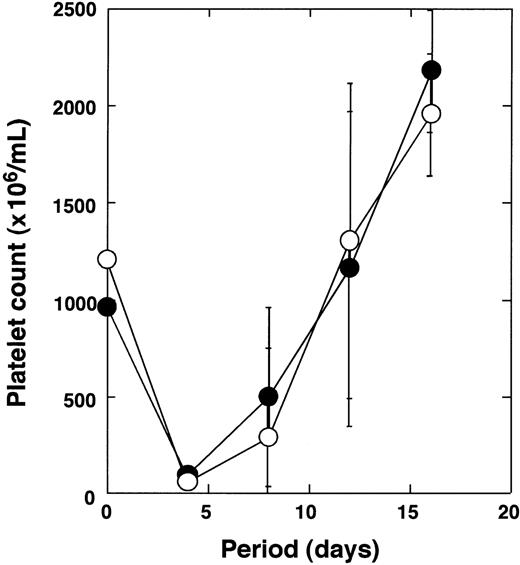
Normal platelet recovery from antiplatelet serum-induced thrombocytopenia
Next, we analyzed the recovery of platelets after antiplatelet serum-induced thrombocytopenia. It was hypothesized that the recovery from thrombocytopenia induced by such stress mainly depends on platelet production from existing megakaryocytes.30 31Anti-GpIIb/IIIa antibodies were administrated to the Tg (n = 3) mice and normal (n = 3) littermates. Platelet numbers were monitored at the indicated time points (Figure 6). Tg mice and control mice showed severe thrombocytopenia 4 days after administration. Reduction rates of the platelets were almost the same in both groups. After nadir, the platelet counts recovered rapidly in both groups. There was no significant difference in the recovery kinetics of platelet number between the Tg mice and the normal littermates.
Recovery of platelet number after antiplatelet serum injection.
Tg mice (n = 3) and wild-type littermates (n = 3) were treated with antiplatelet serum as described in “Materials and methods,” and changes in platelet number were monitored (●, wild-type mice; ○, Tg mice).
Recovery of platelet number after antiplatelet serum injection.
Tg mice (n = 3) and wild-type littermates (n = 3) were treated with antiplatelet serum as described in “Materials and methods,” and changes in platelet number were monitored (●, wild-type mice; ○, Tg mice).
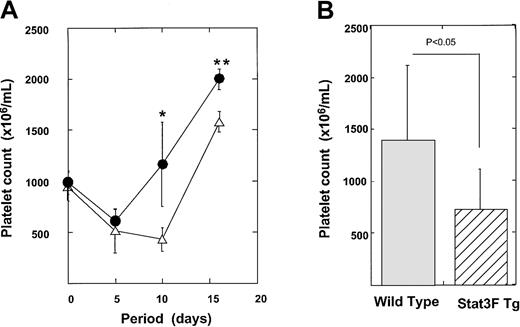
Delayed recovery of platelet number after myelosuppression in Stat3F transgenic mice
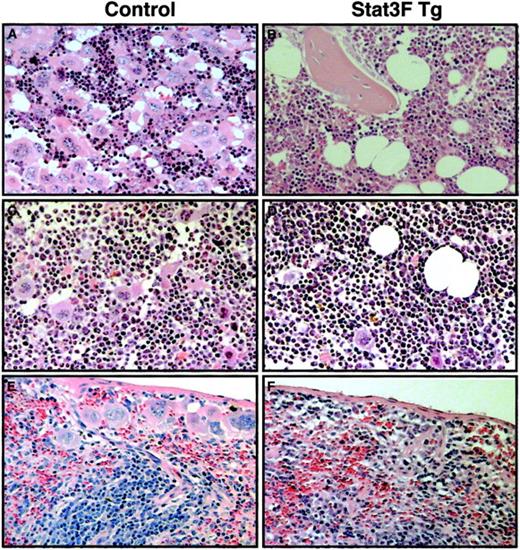
We also analyzed the recovery of platelet number after myelosuppression induced by 5-FU treatment. 5-FU selectively kills cycling progenitor cells, including megakaryocytic progenitor cells.31 32 Therefore, this model allows us to examine the role of Stat3 in the development of megakaryocytic progenitor cells at less mature stages. 5-FU (150 μg/kg) was administrated to 6 Tg mice and 7 control mice. Platelet counts were monitored for a 20-day recovery period (Figure 7A). Tg and control mice showed a 50% reduction in platelet count by day 5. In the control mice, the platelet number had already returned to the pretreated levels at day 10. In contrast, Stat3F Tg mice exhibited a more pronounced nadir and slower recovery from thrombocytopenia. On day 10, the average platelet count was 1162 ± 412.0 × 106/mL in the wild-type littermates, whereas it was 429.0 ± 118.0 × 106/mL in the Tg mice (P < .005). We performed 5-FU treatment experiments 3 times independently; the platelet numbers of these mice at day 10 after 5-FU treatment are summarized in Figure 7B (Tg mice, n = 12; wild-type mice, n = 14). The number of platelets was significantly lower in the Tg mice group than in the wild-type group (728.1 ± 385.3 × 106/mL vs 1366.1 ± 746 × 106/mL; P < .05). After 5-FU injection some Tg mice died, presumably from bleeding. Sixty percent (9 of 15) of the Stat3F Tg mice survived, whereas 100% (15 of 15) of the wild-type mice survived 20 days after the injection of 5-FU (P < .05). To elucidate whether the slower recovery of platelet number in the Tg mice was caused by the delayed megakaryocyte development, we performed histopathologic analysis. On day 7 after 5-FU injection, numerous megakaryocytes were detected in the bone marrow and spleen of the normal littermates (Figure8). In contrast, the numbers of megakaryocytes in the bone marrow and spleen of the Tg mice were clearly reduced (Figure 8). On day 10, the number of megakaryocytes was slightly increased in the bone marrow and spleen of the Tg mice. However, the number of megakaryocytes was still lower than in the control. We also found a significant reduction in the number of CFU-Meg per femur in the Tg mice on day 7 after 5-FU treatment (Figure9). These results strongly suggested that Stat3 is involved in megakaryopoiesis at an early stage.
Recovery of platelet number after 5-FU injection.
(A) Tg mice (n = 7) and normal littermates mice (n = 6) were injected with 150 μg/kg 5-FU. Platelet counts were checked at the indicated time points (●, wild-type mice; ▵, Tg mice). *P < .005; **P < .01. (B) Platelet numbers of Tg mice (n = 12) and wild-type mice (n = 14) at day 10 after 5-FU injection.
Recovery of platelet number after 5-FU injection.
(A) Tg mice (n = 7) and normal littermates mice (n = 6) were injected with 150 μg/kg 5-FU. Platelet counts were checked at the indicated time points (●, wild-type mice; ▵, Tg mice). *P < .005; **P < .01. (B) Platelet numbers of Tg mice (n = 12) and wild-type mice (n = 14) at day 10 after 5-FU injection.
Histopathologic analysis of bone marrow and spleen of Tg mice after 5-FU injection.
Hematoxylin–eosin-stained sections from the femurs of a wild-type littermate (A, C) and Stat3F Tg mice (B, D). Sections were prepared at day 7 (A, B) or day 10 (C, D) after 5-FU injection. Histologic analysis of the spleens from wild-type (E) and Tg mice (F) was also performed at day 10 after 5-FU injection. Original magnification, ×100
Histopathologic analysis of bone marrow and spleen of Tg mice after 5-FU injection.
Hematoxylin–eosin-stained sections from the femurs of a wild-type littermate (A, C) and Stat3F Tg mice (B, D). Sections were prepared at day 7 (A, B) or day 10 (C, D) after 5-FU injection. Histologic analysis of the spleens from wild-type (E) and Tg mice (F) was also performed at day 10 after 5-FU injection. Original magnification, ×100
Delayed recovery of CFU-Meg from myelosuppression in Tg mice.
On day 7 after 5-FU injection, control mice (n = 3) and Stat3F Tg mice (n = 2) were killed. Isolated bone marrow cells were cultured with MegaCult-C media in the presence of 10 ng/mL IL-3, 20 ng/mL IL-6, and 50 ng/mL TPO. After 7-day culture, the colonies formed were stained with AChE. Results represent the mean number (±SD) of CFU-Meg colonies per femur.
Delayed recovery of CFU-Meg from myelosuppression in Tg mice.
On day 7 after 5-FU injection, control mice (n = 3) and Stat3F Tg mice (n = 2) were killed. Isolated bone marrow cells were cultured with MegaCult-C media in the presence of 10 ng/mL IL-3, 20 ng/mL IL-6, and 50 ng/mL TPO. After 7-day culture, the colonies formed were stained with AChE. Results represent the mean number (±SD) of CFU-Meg colonies per femur.
Platelet function is intact in Stat3F Tg mice
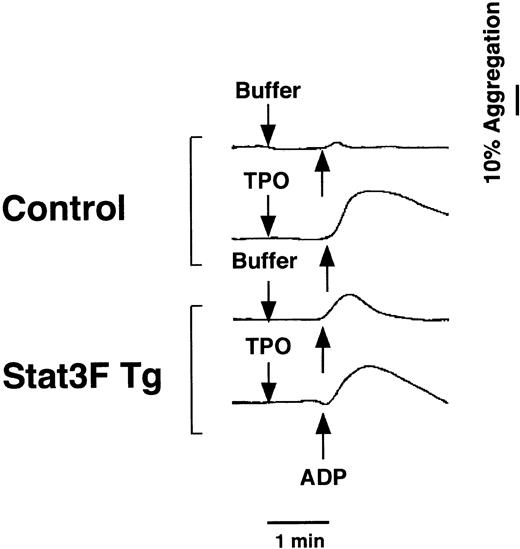
Previous reports demonstrate that TPO primes platelet aggregation induced by multiple agents and that TPO activates Stat proteins in platelets. Based on these findings, we examined whether the inhibition of Stat3 affects platelet function in the Tg mice. As shown in Figure10, the addition of TPO equally enhanced the adenosine diphosphate (ADP)-induced aggregation of platelets in the Tg mice and control mice. This result definitely indicated that Stat3 is not involved in the priming effect of TPO on ADP-induced platelet aggregation.
Effects of TPO on ADP-induced platelet aggregation in Tg mice.
Hirudin PRP was incubated with TPO (100 ng/mL) or autologous platelet-poor plasma for 5 minutes. ADP (2 μM) was added at the time indicated by the arrow, and the platelets were incubated with continuous stirring (1000 rpm) at 37°C for the measurement of aggregation.
Effects of TPO on ADP-induced platelet aggregation in Tg mice.
Hirudin PRP was incubated with TPO (100 ng/mL) or autologous platelet-poor plasma for 5 minutes. ADP (2 μM) was added at the time indicated by the arrow, and the platelets were incubated with continuous stirring (1000 rpm) at 37°C for the measurement of aggregation.
Discussion
One of the best ways to elucidate the physiological in vivo function of Stat3 is the establishment of Stat3 gene-targeting animals. However, because of embryonic lethality,16 Stat3-deficient mice do not provide information concerning Stat3 function in in vivo megakaryopoiesis and thrombopoiesis. To overcome this problem, we established Tg mice selectively expressing a dominant-negative form of Stat3 in megakaryocytic lineage cells. Because GATA-1 is highly expressed in erythroid and megakaryocytic lineage cells,19,20 we used Tg constructs in which the expression of Stat3F is controlled by the GATA-1 regulatory gene elements. Previous studies demonstrated that the GATA-1 regulatory region, including 3.9 kb upstream of the IE exon and the first intron, are required and sufficient for lineage-specific GATA-1 expression.21,29 Tg mice with constructs in which GFP cDNA was linked to these GATA-1 regulatory elements showed the expression of GFP in erythroid and megakaryocytic lineage cells.21 In our experiments, we confirmed that Stat3F was actually expressed in the platelets of the Tg mice. Furthermore, TPO-induced Stat3 activation was highly blocked in the Tg mice. Thus, the Tg mice allowed us to analyze the function of Stat3 in in vivo megakaryopoiesis and thrombopoiesis.
We initially analyzed the effect of Stat3 on steady-state megakaryopoiesis. There was no significant difference in the platelet count of peripheral blood between the Tg mice and the control mice. Furthermore, many megakaryocytes were observed in the bone marrow and spleen in the Tg mice, as in the control mice. To examine the effect of thrombopoietin on platelet production in the Tg mice, we administered TPO to the mice once a day for 5 days and analyzed the platelet production. As shown in Figure 5, the platelet counts were transiently increased in response to TPO not only in the control mice but also in the Tg mice, indicating that TPO did not affect platelet production in the Tg mice. Because enhanced platelet production after TPO injection is dependent on the maturation of pre-existing megakaryocytes,33 our data strongly suggest that Stat3 is not involved in the maturation of megakaryocytes. In addition, we found a transient increase in MPV after TPO injection in the Tg mice, as in the control mice. Given that a transient elevation of MPV level after treatment with TPO was observed as a result of the accelerated formation of demarcation membranes and the shedding of larger fragments from megakaryocytes,34 our observation indicates that TPO signals are normally transduced into the nucleus in Tg mice. Luoh et al35 previously reported that mice having the truncated form of TPO receptor produced platelets within the normal range in steady-state conditions, regardless of impaired activation of signal transduction molecules, including Stat3. Taken together with our observation, Stat3 may not be required for the maturation of megakaryocytes and platelet production in steady-state conditions.
To further understand the in vivo function of Stat3 in megakaryopoiesis and thrombopoiesis, we analyzed the kinetics of recovery time of platelet production after induced thrombocytopenia. We adopted 2 methods; one was antiplatelet serum administration, and the other was 5-FU treatment. The administration of antiplatelet serum can induce a rapid decrease in platelet number without damaging the megakaryocytes and immature megakaryocytic progenitors.30,31 In this method, the recovery of platelet count is mainly dependent on the platelet production from mature megakaryocytes and the development of megakaryocytic progenitor cells. Thus, this method allows us to clarify the functional role of Stat3 in the late stage of in vivo megakaryopoiesis and platelet production. In contrast, 5-FU kills immature megakaryocytic progenitors and megakaryocytes in endomitosis.31,32 Therefore, this method allows us to examine the functional role of Stat3 in the early stage of megakaryocytic development. In the Tg mice, the recovery of platelet counts after antiplatelet serum-induced thrombocytopenia was not affected. In contrast, after 5-FU administration, we found a significant delay in platelet recovery in the Tg mice. These results led us to the hypothesis that Stat3 works mainly on the early stage of megakaryopoiesis and regulates the growth of megakaryocytic progenitor cells. Considering the previous reports that Stat3 is essential for the maintenance of embryonic stem cell pluripotency,36-38Stat3 may function as an effector of expansion of the megakaryocytic progenitor cells.
Previous studies indicate that TPO activates Stat3 and Stat5 in normal platelets.23 In addition, pretreatment of platelets with TPO enhances ADP-induced platelet aggregation.22 These findings led us to examine the platelet function of Tg mice. Although Stat3F proteins were abundantly expressed in the platelets of the Tg mice, platelet function was almost normal. There are 2 possible explanations for this discrepancy. One is that the Stat3 proteins in the platelets are just remnants of those in the megakaryocytes and do not have any function in the platelets. This notion is supported by evidence that platelets have no nuclei. The other possibility is that another signal transduction molecule rescued the function of Stat3. Considering that TPO activates Stat5 and Stat3 in normal megakaryocytes and platelets,14 Stat5 may be one of the candidates for compensating the function of Stat3 in ADP-induced platelet aggregation. However, because the expression and activation levels of Stat5 were not enhanced in the platelets of the Tg mice as they were in the control mice, it is unlikely that Stat5 rescued the function of Stat3. Therefore, taken together with the report by Yang et al39 that activation of Stat3 is insufficient for the priming effects of TPO on platelet aggregation, it is likely that Stat3 is dispensable for platelet function. In addition, the unprimed response to ADP (ie, in the absence of TPO) was greater in the Stat3F Tg mice than in the control mice (Figure 10). Although this was repeatable in 2 independent experiments, the detailed mechanism remains to be resolved.
Although we did not identify the target genes for Stat3 in this study, one of the candidates of Stat3 may be c-myc gene. Several lines of evidence support this notion. First, the promoter region of the c-myc gene contains Stat3-binding sites, and IL-6 enhances c-myc gene promoter activity throughout Stat3 activation.40 Second, overexpression of the c-myc gene in megakaryocytic progenitors causes an increase in immature megakaryocytes.41 Third, we found that TPO-induced c-myc gene expression is dependent on Stat3 activation (unpublished data, March 4, 2002). Thus, c-myc may play an important role in cell expansion as a downstream molecule of Stat3,42 though this notion is speculative.
In this study, we did not examine the expression of Stat3F in erythroid lineage cells. However, because the IE3.9int GATA-1 promoter works on megakaryocytic lineage cells and on erythroid lineage cells, it is strongly suggested that Stat3F is expressed in erythroid lineage cells. If so, given that the colony number of mature erythroid progenitor colony-forming units (CFU-E) was almost the same in the Tg mice and the control mice (data not shown), it is unlikely that Stat3 functions on late erythropoiesis. This result is consistent with previous findings that a dominant-negative Stat3 did not inhibit the erythroid colony formation from CFU-E in fetal liver cells.43Interestingly, the number of immature erythroid progenitor blast-forming units was smaller in the Tg mice (data not shown), suggesting the possibility that Stat3 plays some role in early erythropoiesis. Unexpectedly, however, there was no significant difference between the Tg mice and the control mice in the recovery of hemoglobin level after 5-FU treatment (data not shown). Therefore, whether Stat3 is involved in erythropoiesis is still open to question.
Although Stat3F completely inhibited tyrosine phosphorylation of endogenous Stat3 in the platelets of Stat3F Tg mice (Figure 2C), approximately half the Stat3 activity was still detected after transfection of UT-7/TPO cells with Stat3F (Figure 1A). Thus, there was a discrepancy in the Stat3F-induced inhibition of tyrosine phosphorylation and transcriptional activity of endogenous Stat3. Although we did not assess the DNA-binding activity of endogenous Stat3 in platelets of the Tg mice, one possibility is that the changes in phosphorylation were more pronounced than the changes in transcriptional activity of Stat3. This hypothesis may be supported by the previous report by Kaptein et al.44 Alternatively, the discrepancy may be attributed to the different cells used in these experiments.
In conclusion, our observations showed that Stat3 is dispensable for steady-state megakaryopoiesis, maturation of megakaryocytes, and platelet function. However, our results suggest that Stat3 is required for effective expansion of megakaryocytic progenitor cells in the early stage of megakaryopoiesis.
Supported by Grants-in-Aid for Cancer Research and Scientific Research from the Ministry of Education, Science and Culture of Japan and by a grant from the Yamanouchi Foundation for Research on Metabolic Disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Norio Komatsu, Division of Hematology, Department of Medicine, Jichi Medical School, Minamikawachi-machi, Kawachi-gun, Tochigi-ken 329-0498, Japan; e- mail: nkomatsu@ms.jichi.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal