Key Points
Pharmacokinetic analysis of a novel oral ATO formulation in patients with APL shows bioequivalence with the IV formulation.
No unanticipated toxicity signals were evident during short-term exposure to the oral formulation.
Visual Abstract
The prognosis for patients with acute promyelocytic leukemia (APL) has improved dramatically since the introduction of all-trans retinoic acid and IV arsenic trioxide (ATO). However, ATO administration requires daily infusions over several months, representing an onerous burden for hospitals and patients. We evaluated the bioavailability of a novel encapsulated oral ATO formulation in patients with APL in first complete remission during standard-of-care consolidation. After a pilot study exploring the likely oral dose requirement, total arsenic pharmacokinetics were evaluated in 20 patients after both IV and oral ATO 0.15 mg/kg per day, with exposure to oral ATO restricted to the first week in 2 of 4 ATO cycles. The primary end point was bioequivalence of area under the curve from 0 to 24 hours (AUC0-24), with bioequivalence of maximum concentration achieved (Cmax) as the key secondary end point. The 90% confidence intervals (CIs) around point estimates of the geometric means of the oral-to-IV ratios for AUC0-24 and Cmax were compared with bioequivalence limits specified by the European Medicines Agency (0.80-1.25). The estimated oral-to-IV ratios and 90% CIs for AUC0-24 in whole blood and plasma were 0.993 (0.954-1.034) and 1.030 (0.977-1.087) respectively; data for Cmax also satisfied bioequivalence requirements. Exploratory studies of arsenic species in plasma showed bioequivalence for AUC0-24 with As(III) (oral-to-IV ratio, 0.966 [0.879-1.063]). The adverse event profiles of oral and IV ATO were comparable for cycles commencing with the IV and oral formulations. In conclusion, this novel oral ATO formulation is bioequivalent with IV ATO and offers a convenient alternative for patients with APL. This trial was registered at www.anzctr.org.au as #ACTRN12616001022459.
Introduction
Acute promyelocytic leukemia (APL) is a unique subtype of acute myeloid leukemia (AML) characterized by a high early death (ED) rate due to severe coagulopathy1; a reciprocal translocation of chromosomes 15 and 17 resulting in rearrangement of the PML and RARA genes2-4; a differentiation block at the promyelocyte stage; and striking sensitivity to all-trans retinoic acid5 (ATRA) and inorganic arsenicals, primarily arsenic trioxide6 (ATO) and tetraarsenic tetrasulfide.7 A white blood cell (WBC) count of >10 × 109/L at diagnosis has been used to stratify patients into high-risk (HR) vs standard-risk (SR; WBC count of ≤10 × 109/L) groups based on the probability of ED and relapse.8,9
The superiority of ATRA and ATO compared with ATRA and chemotherapy has been demonstrated conclusively for patients with SR disease,10 and is now widely accepted as the standard of care.9,11 For SR patients, ATRA and IV ATO are given during induction without chemotherapy (apart from hydroxyurea to control ATRA- and ATO-induced hyperleukocytosis) until hematologic complete remission (CR) is achieved. Consolidation typically involves 4 cycles of ATO together with 7 cycles of ATRA, and maintenance is not required. Acknowledgement of the benefit of ATO-based therapy for HR patients has occurred more slowly,9 but although ATRA and intensified chemotherapy protocols have improved outcomes for patients with HR disease, this has come at the cost of increased toxicity, particularly deaths in remission.12-14 Accordingly, protocols that include limited chemotherapy or gemtuzumab ozogamicin in combination with ATRA and ATO are emerging as the treatment of choice for patients who present with HR disease.11,15-19
A major limitation involving ATO therapy is the requirement for 2-hour IV infusions, 5 to 7 days per week, extending over several months. This imposes a significant burden on institutional resources, and is a major inconvenience for patients, particularly those who do not live within close proximity to oncology services able to infuse ATO. Delivery by oral administration circumvents many of these problems, but the availability of oral arsenic has primarily been restricted to mainland China and Hong Kong. The safety and efficacy of tetraarsenic tetrasulfide administered orally in the form of Realgar-Indigo Naturalis Formula is well documented in studies from China,7,20 and a liquid oral formulation of ATO has been available in Hong Kong for many years.21,22 However, neither of these agents nor any other oral arsenic formulation has been approved for the treatment of APL by regulatory agencies in the United States (US Food and Drug Administration), Europe (European Medicines Agency [EMA]), the United Kingdom (Medicines and Healthcare Products Regulatory Agency), or Australia (Therapeutic Goods Administration). The pharmacokinetic (PK) characteristics of an oral formulation of ATO (ORH-2014, subsequently known as SY-2101) have been studied in patients with refractory hematologic malignancies other than APL in the United States, but the dose recommended for further clinical investigation was only tested in 3 patients.23 We therefore chose to evaluate the bioavailability of a novel oral formulation of ATO produced by Eupharma, the research arm of Phebra Pty Ltd (Lane Cove West, NSW, Australia) in patients with SR and HR APL. To assess the novel formulation in its intended target population, while simultaneously minimizing the risk of treatment failure in the event of suboptimal bioavailability for an intrinsically curable leukemia, PK sampling was embedded within a standard-of-care consolidation protocol with exposure to oral ATO restricted to the first week in 2 of the 4 ATO cycles.
Methods
Patients
The study was open to previously untreated patients with PML::RARA-positive APL who had achieved hematologic complete remission (CR) or CR with incomplete count recovery after an ATO-based risk-stratified induction protocol (ATRA and IV ATO as per the chemotherapy-free arm of the GIMEMA-AMLSG-SAL APL0406 study10 for SR patients; and ATRA, ATO, and idarubicin as per the Australasian Leukaemia and Lymphoma Group [ALLG] APML4 study24 for HR patients). Eligibility also required age of ≥18 years; Eastern Cooperative Oncology Group performance status score of 0 to 3; serum creatinine of ≤200 μmol/L; serum bilirubin of ≤50 μmol/L; electrocardiogram showing sinus rhythm with QTc (Framingham) of <450 milliseconds; and absence of a previous history of serious cardiac, pulmonary, hepatic, or renal disease. All patients gave written informed consent before study entry.
Study design
The ALLG APML5 study (www.anzctr.org.au identifier: ACTRN12616001022459) was a cooperative trial group study led by the ALLG. It involved a phase 1, 2-part, 2-sequence, 4-period, bioavailability study embedded within a standard-of-care consolidation regimen. The study was conducted in accordance with Australian National Health and Medical Research Council guidelines, the International Conference on Harmonization E6 R2 guideline for good clinical practice, and the Declaration of Helsinki. Each participating site obtained human research and ethics committee approval. Accrual to part 1 occurred from June 2017 to July 2018, and accrual to part 2 from January 2019 to May 2020. Registered patients (supplemental Figure 1) were consolidated with 7 cycles of ATRA 45 mg/m2 per day in 2 divided doses (7 d/wk for 2 weeks, with 2 weeks between cycles), and 4 cycles of IV ATO 0.15 mg/kg per day by 2-hour infusion (5 d/wk for 4 weeks, with 4 weeks between cycles).
In part 1 (supplemental Figure 2), oral ATO 0.15 mg/kg per day ×5 days was substituted for IV ATO in week 1 of ATO cycle 2. In week 1 of ATO cycle 4, oral ATO was again substituted for IV ATO with allowance for patient-specific dose adjustment based on the relative bioavailability determined in ATO cycles 1 and 2. Target accrual for part 1 was 8 patients, with replacement permitted if required.
The aggregate PK data generated in part 1 were then used to designate a dose of oral ATO to be examined in all patients accrued to part 2 of the study. The target accrual for part 2 was 20 patients, with replacements permitted if insufficient PK data were obtained (see supplemental Data for the details of the PK requirements). The number chosen for part 2 was based on the EMA Guideline on the investigation of bioequivalence (Revision 1), which specifies that the minimum requirement for a bioequivalence study is not less than 12 evaluable subjects.25 To confirm oral bioequivalence, PK sampling was again performed in week 1 of each ATO cycle, but patients were randomized in part 2 to the IV or oral route in week 1 of cycles 1 and 3; the alternate route was used in week 1 of cycles 2 and 4 (supplemental Figure 3). The protocol specified that oral arsenic capsules should be taken 1 hour before food on an empty stomach, and the consumption of seafood should be avoided for 3 days before and during the first week of each cycle of arsenic (for both the oral and IV formulations).
Arsenic quantitation
Total elemental arsenic concentrations in whole blood (WB) and plasma (PL) were quantitated by inductively coupled PL mass spectrometry at the National Measurement Institute, West Lindfield, New South Wales, Australia; and the data were converted to micromole ATO per liter. For each cycle, baseline correction was applied by subtracting the day-1 predose total arsenic level in WB or PL from all subsequent values obtained within that cycle. The concentrations of arsenic species (arsenite [As(III)], arsenate [As(V)], monomethylarsonic acid [MMA], and dimethylarsinic acid [DMA]) in PL were quantified by Frontage Laboratories, Exton, PA, and expressed as micromole arsenic per liter.
Pharmacokinetics
PK sampling was performed on day 1 and day 4 in week 1 of each ATO cycle at the following time points: before dosing, and then at 0.5, 1, 2, 4, 6 to 8, and 24 hours after either oral dosing or the start of IV ATO. The area under the curve from 0 to 24 hours (AUC0-24; expressed as micromole x hour per liter) and maximum concentration achieved (Cmax; expressed as micromole per liter) were determined using noncompartmental methods (model 200-202) and a linear-trapezoidal approach. PK analyses were conducted using Phoenix WinNonlin version 8.3 or higher.
Statistical methodology
A linear mixed effects model was fitted, using the method of restricted maximum likelihood (REML) procedure in Genstat Release 21, VSN International), to each of the loge-transformed PK parameters (AUC0-24 and Cmax) derived for each observation day for each patient. Included in the model were (1) terms for formulations (IV or oral), periods (1, 2, 3, and 4), days within period (first or fourth day), and their interactions, all regarded as fixed effects; and (2) terms for patients, cycles within patients, and assessment days within cycles, all regarded as random effects. All other statistical analyses used SAS version 9.4.
A point estimate and the associated 90% confidence interval (CI) for the difference between the predicted PK means of the oral and IV formulations was calculated. This difference was back-transformed to obtain the point estimate and the associated 90% CI for the ratio of the geometric means of each PK parameter on the untransformed scale. The 90% CIs for the ratios of the geometric means were then compared with conventional EMA bioequivalence limits (0.80-1.25), a procedure known as the “two one-sided tests” (TOST) for equivalence.25
Results
Part 1: total arsenic
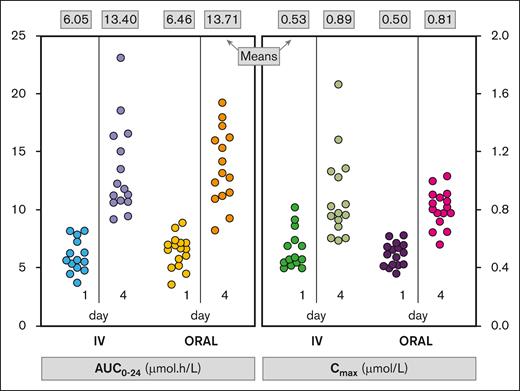
A total of 61 PK profiles were available from 9 patients (4 males, 5 females) with a median age of 52 years (range, 20-66). One patient was withdrawn during ATO cycle 1 because of grade 3 peripheral neuropathy attributed to IV ATO received during induction and the first consolidation cycle; the patient was never exposed to oral ATO. Both formulations were associated with significant increases from day 1 to day 4 in WB AUC0-24 and WB Cmax (Figure 1; P < .001 for each PK parameter), and similar increases were observed for PL AUC0-24 and PL Cmax (P < .001). For example, average WB AUC0-24 increased by 121.6% from day 1 to day 4 with the IV formulation, and by 112.2% with the oral formulation. The average WB Cmax increased by 67.3% from day 1 to day 4 with the IV formulation, and by 64.2% with the oral formulation. The increases in AUC0-24 and Cmax are indicative of short-term arsenic accumulation within each cycle, but there was no accumulation between sequential cycles.
Individual patient data for AUC0-24 and Cmax in WB by formulation and day (part 1). The mean of values in each column is shown at the top of the figure.
Individual patient data for AUC0-24 and Cmax in WB by formulation and day (part 1). The mean of values in each column is shown at the top of the figure.
Preliminary analysis of individual patient data (n = 6) from ATO cycle 1 and cycle 2 indicated that the oral and IV formulations were associated with comparable bioavailability. The average and range of the ratio of AUC0-24 and Cmax on day 1 of cycle 2 (oral ATO) to the corresponding result observed in cycle 1 (IV ATO) are shown in supplemental Table 1. More variability was seen in PL than in WB, but based on these data, no patient-specific dose adjustments were made for the dose of oral ATO in week 1 of cycle 4. The decision about oral ATO dosing for week 1 of ATO cycle 4 was made by a trial management committee comprising a pharmacologist, a statistician, 2 trial-associated clinicians, and an independent clinician.
After completion of part 1, aggregate data showed there were no significant PK differences between the oral and IV formulations, because estimates of the geometric mean of the oral-to-IV ratio for each PK parameter closely approximated unity, and the 90% CI for each PK parameter fell within the bioequivalence limits (Table 1). Although exposure to oral ATO was restricted to 10 days, when compared with cycles that commenced with IV ATO, cycles that commenced with oral ATO were not associated with an increase in the frequencies of grade 3/4 adverse events (AEs; supplemental Table 2), including gastrointestinal (GI) toxicity or QTc prolongation, and no oral ATO dose adjustments for toxicity were required. Only 1 patient experienced QTc prolongation (maximal grade 2) on multiple occasions in cycles that began with both IV and oral ATO.
Part 2: total arsenic bioavailability
Because the aggregate PK data from part 1 indicated that an oral ATO dose of 0.15 mg/kg per day was likely to be bioequivalent to IV ATO, that dose was formally evaluated in part 2. Twenty-two patients (the full analysis set) were accrued, but 2 were unevaluable due to the unavailability of sufficient PK samples. The PK analysis set (PKAS) therefore comprised 11 patients randomized to the IV-oral-IV-oral sequence, and 9 patients randomized to the alternate oral-IV-oral-IV sequence (supplemental Figure 3). A modified PKAS (mPKAS) was also studied; this differed from the PKAS by extension of the window for a valid predose PK sample from 1 hour to 3 hours. Overall, 116 of a theoretical 160 PK profiles (20 patients; 4 cycles; days 1 and 4) were included in the PKAS, and 145 profiles were included in the mPKAS. The safety set was planned to consist of all patients in the full analysis set who received at least 1 dose of ATO in consolidation, but 1 of 2 patients with insufficient PK data also had no postbaseline safety assessment and was consequently excluded from the safety set analysis (n = 21). The baseline demographics and the disposition of patients in part 2 are summarized in Tables 2 and 3, respectively.
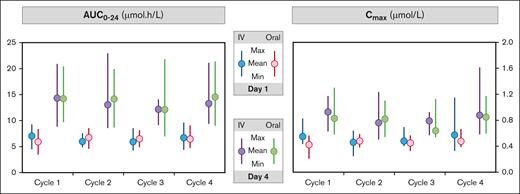
A significant day effect was again observed in part 2 in both the PKAS and mPKAS cohorts, with higher day-4 mean values for all PK parameters compared with the corresponding means for day 1 (P < .001 for WB AUC0-24, PL AUC0-24, WB Cmax, and PL Cmax). PKAS data for WB AUC0-24 and Cmax are shown in Figure 2.
Mean and range of AUC0-24 and Cmax data in WB by cycle, day and formulation in the PKAS (part 2). Max, maximum; Min, minimum.
Mean and range of AUC0-24 and Cmax data in WB by cycle, day and formulation in the PKAS (part 2). Max, maximum; Min, minimum.
Equivalence of the oral and IV formulations with regards to AUC0-24 and Cmax in WB and PL was demonstrated in both the PKAS and mPKAS by the TOST procedure (Table 4; supplemental Figure 4). The estimates of the ratio of the geometric means of each PK parameter closely approximated unity, and the upper and lower limits of the 90% CI fell within the bioequivalence limits specified by the EMA (0.80-1.25).25
To check for possible differences in variability between formulations, the residuals from fitting the full model were split by formulation, and the variances of these 2 groups of residuals were compared using Bartlett's test for homogeneity. There were no significant departures from homogeneity for each of the 4 PK parameters in the 2 analysis sets (supplemental Table 3).
Part 2: Adverse events
Observation periods for AEs extended from the time of the first dose of ATO in each cycle of consolidation until the earlier of the commencement of the next cycle or 28 days after the last dose of ATO in that cycle. Summary tables for worst grades of AEs (Common Terminology Criteria for Adverse Events, version 4.03) that started during observation periods in which the IV or oral formulation was administered in the first week, and occurring in >5% of patients, are presented in supplemental Tables 4 and 5, respectively. For ATO cycles commencing with the IV formulation, no grade 4/5 AEs were reported, and the most common AEs were headache (28.6% of patients) and nausea (23.8% of patients), predominantly grade 1. For cycles commencing with the oral formulation, 1 grade 4 and no grade 5 AEs were reported, and the most common AEs were headache (52.4% of patients) and nausea (38.1% of patients), also predominantly grade 1. The difference in the incidence of headache and nausea between ATO cycles commencing with the IV and oral formulations was not statistically significant (P = .208 and P = .506, respectively [Fisher's exact test]). Despite twice-weekly electrocardiographic assessment during each week of ATO therapy, there were no reports of QTc prolongation in part 2.
GI toxicity is summarized in Table 5, showing worst grade by formulation given in the first week of the consolidation cycles. In the 84 cycles (21 patients, 4 cycles each), no GI AEs were reported in 73% of cycles, grade 1 events were reported in 19%, and grade 2 in 7%. One grade 4 GI AE (1%) was reported in a cycle that commenced with the oral formulation but there was no significant association of worst grade with formulation (P = .482 for the exact χ2 test for an association; P = .239 for the approximate type-III fixed effects F test). The patient with grade 4 GI toxicity had a history of diverticulitis and became symptomatic just before the commencement of oral ATO at the start of cycle 2. Symptoms progressed during cycle 2, and imaging demonstrated a perforated sigmoid diverticulum with a pelvic and paracolic abscess adherent to the bladder, which required sigmoid colectomy and end-colostomy. The past history and the timing of onset of symptoms does not suggest that oral ATO was the predominant factor in this patient’s grade 4 toxicity.
In further exploratory analyses restricted to nausea and vomiting AEs, no events were reported in 88% of the cycles that commenced with the IV formulation and 71% of the cycles that commenced with the oral formulation. Grade 1 events were reported in 7% and 21% of the cycles commencing with the IV and oral formulations, respectively; this apparent association of incidence and severity with formulation delivered in the first week of a cycle was not statistically significant (P = .133 exact χ2 test; P = .072 type-III F test).
Arsenic speciation
The mechanism of the anti-APL effect of ATO has been extensively studied, and its primary mechanism of action is mediated by nuclear body reformation and PML::RARα SUMOylation, polyubiquitination, and proteosomal degradation.26-28 Bercier et al have shown that PML nuclear body reformation involves a gel-like transition resulting from the binding of As(III) in an arsenic-binding pocket formed by 3 C213 residues of PML B-box 2 trimers.29 In contrast, MMA, an intermediate metabolite of As(III), is unable to induce this gel-like transition nor does it induce cellular differentiation or PML::RARα degradation.29,30
PK analysis of arsenic metabolites was performed on a subset of heparinized PL samples that had been stored at −70°C. However, a significant proportion of As(V), MMA, and DMA values were missing. The predominant reasons included many As(V) values were below the limit of quantitation, the presence of plateaued MMA and DMA concentrations that interfered with linear-trapezoidal calculations for AUC0-24, and many MMA day-1 profiles lacked sufficient concentration-time samples for calculation of AUC0-24 and Cmax. In contrast, the proportion of missing values for As(III) did not exceed 3%. Accordingly, only the As(III) data were deemed sufficiently robust to assess bioequivalence. The estimated ratio (oral-to-IV) and 90% CI of the geometric means in PKAS and mPKAS profiles for As(III) are shown in supplemental Table 6. Bioequivalence with respect to As(III) AUC0-24 was demonstrated in both the PKAS and mPKAS, but was not demonstrated for As(III) Cmax because the lower limit of the 90% CI fell just outside the 0.8 to 1.25 bioequivalence limits.
Follow-up
Although not designed as an efficacy study, the protocol recommended PML::RARA molecular monitoring for a minimum of 2 years after completion of consolidation. The median follow-up from registration on the study of all 31 patients in parts 1 and 2 using the reverse Kaplan-Meier method was 40.6 months. The patient who was withdrawn from part 1 of the study because of peripheral neuropathy was treated with additional ATRA and idarubicin for consolidation followed by 2 years of triple maintenance with ATRA, methotrexate, and 6-mercaptopurine. The patient subsequently developed therapy-related myelodysplasia, which progressed to PML::RARA-negative AML, to which the patient succumbed. One patient with HR disease in part 2 of the study relapsed 4 months after completion of consolidation.
Discussion
Although originally regarded as the most aggressive form of AML when it was first recognized as a discrete subtype by Hillestad,31 APL has emerged as a clinically distinct and highly curable entity.32 Chemotherapy-free protocols based on ATRA and arsenic are now standard-of-care for patients with SR APL,10,11,16 and combinations of arsenic, ATRA, and limited anthracycline or gemtuzumab ozogamicin are also emerging as the treatment of choice for patients with HR APL.11,15,16,18 Preliminary data from the European intergroup randomized APOLLO study (ClinicalTrials.gov identifier: NCT0268840)17 support the use of arsenic during induction and consolidation for HR APL, and the Children’s Oncology Group AAML1331 study also provides evidence that arsenic-based therapy enables a reduction in exposure to anthracyclines and intrathecal chemotherapy without loss of efficacy for both SR and HR pediatric patients.19
The major challenges that remain in the management of APL include (1) the continuing problem of ED because of thrombohemorrhagic complications in the real-world context,33 (2) the excessive burden on clinical resources imposed by the administration of multiple cycles of IV ATO extending over several months,34 and (3) the delivery of optimal therapy in low- and middle-income countries.35 Although substitution of oral for IV arsenic is unlikely to significantly reduce the ED rate,7 oral arsenic offers major advantages with regards to safety profile, quality of life, and the cost of delivering therapy,34,36,37 and has the potential to improve the overall availability of optimal therapy.
Oral formulations of inorganic arsenic have been part of standard care in mainland China and Hong Kong for at least 10 years but, to date, have not been available elsewhere. The PKs of ORH-2014, a lyophilized form of ATO as As4O6, were evaluated in patients with advanced hematologic malignancies (excluding APL), and compared with historical PK data for intravenously administered ATO.23 Three patients received ORH-2014 5 mg/d, 6 patients received 10 mg/d, and 3 patients received 15 mg/d. At a dose of 10 mg/d, Cmax and AUC0-24 for ORH-2014 increased progressively from day 1 to day 22, whereas both parameters plateaued by day 15 when 15 mg/d was administered. The Cmax at day 15 was 114 ng/mL (compared with 124 ng/mL for IV ATO at day 8), and the AUC0-24 at day 15 was 2140 ng.h/mL (compared with 1302 ng.h/mL for IV ATO at day 8). ORH-2014 was subsequently renamed SY-2101, and the dose recommended for further study was 15 mg/d. However, clinical development of SY-2101 in APL was suspended in 2023 and there are currently no clinical trials of SY-2101 registered at www.ClinicalTrials.gov.
An oral arsenic formulation that has satisfactory bioavailability and the potential for multinational regulatory agency approval constitutes an unmet need for patients with APL. This gap in the therapeutic armamentarium provided the rationale for the development of such a product in Australia through a collaboration between Phebra/Eupharma and the ALLG. This study capitalized on the success of protocols that use IV ATO for both SR and HR disease by incorporating a bioavailability assessment of oral ATO within a standard-of-care consolidation regimen for patients with APL in first CR.
In a small pilot study (part 1), the bioavailability of oral ATO 0.15 mg/kg per day was comparable with IV ATO 0.15 mg/kg per day when PK profiles for both formulations were assessed sequentially; no adjustments in oral dosing for individual patients were required in week 1 of cycle 4. In part 2 of the study, which randomized patients to alternative scheduling of the oral and IV PK assessments, bioequivalence of oral ATO at a dose of 0.15 mg/kg per day was confirmed for total arsenic in WB and PL as assessed by AUC0-24 and Cmax. Intracycle accumulation of both oral and IV ATO was documented, consistent with previous serial PK data for ORH-2014 and IV ATO,23 but no accumulation was observed between sequential cycles. In an exploratory analysis of arsenic metabolites, bioequivalence of oral ATO was also shown in PL for the clinically active metabolite As(III) as assessed by the primary bioavailability end point, AUC0-24. However, bioequivalence requirements for the secondary end point, Cmax, were not satisfied because the lower boundary of the 90% CI for the estimate of the geometric mean of the oral-to-IV ratio fell just below 0.80. The significance of this observation is uncertain, but data from other studies of oral arsenic suggest it will not be clinically relevant. In the PK data for the Hong Kong oral ATO product, AUC0-24 was comparable with IV ATO, but Cmax was lower,38 prompting the investigators to speculate that this would provide greater safety because of a lower risk of cardiac toxicity (QTc prolongation ± torsades de pointe).39 Whether this speculation is justified is arguable, because the frequency of grade 1/2 QTc prolongation in a multicenter randomized study of oral Realgar-Indigo Naturalis Formula and IV ATO was identical with the different formulations (19% in each arm).20
Although bioequivalence of our oral formulation for total arsenic AUC0-24 and Cmax (and for As(III) AUC0-24) has been established, several limitations of the study have been recognized. These include the short duration of oral ATO exposure and the inability to clearly distinguish AEs associated with oral vs IV ATO due to the study design. Nevertheless, the first limitation is offset to some extent by each patient being assessed for bioavailability of both the oral and IV formulations on multiple occasions (days 1 and 4 of the first week of each cycle of consolidation). With regards to the question of differential toxicity, although there was an excess of grade 1 nausea and vomiting in cycles that commenced with oral ATO, that difference did not reach statistical significance. Attribution of those AEs specifically to oral ATO is not possible, and there was no overall excess of serious AEs with the oral formulation.
In conclusion, we have shown that a novel oral formulation of ATO satisfies bioequivalence criteria specified by the EMA with regards to total arsenic in WB and PL, and also satisfies criteria for As(III) in PL for AUC0-24. This novel encapsulated therapeutic can be easily dosed on a per-kilogram basis and has the potential to positively affect the delivery of ATO for patients with APL by simplifying the treatment experience and sparing valuable health service resources.
Acknowledgments
The authors thank the patients who participated, and the nurses, data coordinators, and medical officers involved. The molecular monitoring was predominantly performed by Shane Supple at the Royal Prince Alfred Hospital Molecular Haematology Laboratory. Phebra provided oral arsenic trioxide and research support to the Australasian Leukaemia and Lymphoma Group. Helpful discussions with Jessica Altman and Martin Tallman before the commencement of the study were greatly appreciated.
Authorship
Contribution: H.J.I., J.R., and A.H.W. designed the study; H.J.I., L.K., A.H.W., S.F., S.W.L., N.W., R.G., T.A., R.H., A.-M.W., P.T., R.F., F.K., W.S., S.Y., and A.P.N. facilitated implementation of the study and recruited patients; A.V.B. and H.B.S. performed the pharmacokinetic analysis; J.R. performed the statistical analysis; R.H., J.R., A.V.B., P.M., and H.J.I. served on the trial management committee that provided oversight of the trial operations; L.R. coordinated the trial data collection; H.J.I., J.R., A.V.B., and H.B.S. wrote the first version of the manuscript; and all authors read, reviewed, and approved the manuscript.
Conflict-of-interest disclosure: H.J.I. has served on an advisory committee for Syros. A.H.W. has served on advisory boards of, and/or consulted for, and/or served on speakers bureaus for, Novartis, AstraZeneca, Astellas, GlaxoSmithKline, Janssen, Jazz, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead, Bristol Myers Squibb, BeiGene, Shoreline, and Aculeus; and is an employee of the Walter and Eliza Hall Institute (WEHI) and receives a financial benefit from milestone and royalty payments via WEHI related to the development of venetoclax. The remaining authors declare no competing financial interests.
A complete list of the members of the Australasian Leukaemia and Lymphoma Group appears in supplemental Appendix.
Correspondence: Harry J. Iland, Institute of Haematology, Royal Prince Alfred Hospital, Missenden Rd, Camperdown, NSW 2050, Australia; email: harry.iland@health.nsw.gov.au.
References
Author notes
The study metadata are listed at https://www.allg.org.au/datasets/. Requests for deidentified individual participant data that support the findings of this study can be directed to info@allg.org.au.
The full-text version of this article contains a data supplement.