The stem cell pool can be fractionated by using the mitochondrial dye, rhodamine-123, into Rholow hematopoietic stem cells and Rhohigh progenitors. Rholow stem cells permanently engraft all lineages, whereas Rhohighprogenitors transiently produce erythrocytes, without substantial platelet or granulocyte production. We hypothesized that the inability of the Rhohigh cells to produce platelets in vivo was due to the fact that these cells preferentially engraft in the spleen and lack marrow engraftment. Initially, we demonstrated that Rhohigh progenitors produced more megakaryocytes in vitro than Rholow stem cells did. To study the activity of the Rholow and Rhohighsubsets in vivo, we used mice allelic at the hemoglobin and glucose phosphate isomerase loci to track donor-derived erythropoiesis and thrombopoiesis. Rholow stem cells contributed to robust and long-term erythroid and platelet engraftment, whereas Rhohigh progenitors contributed only to transient erythroid engraftment and produced very low numbers of platelets in vivo. Donor-derived megakaryopoiesis occurred at higher densities in the spleen than in the bone marrow in animals receiving Rholowstem cells and peaked around day 28. Blockade of splenic engraftment using pertussis toxin did not affect the peak of splenic megakaryopoiesis, supporting the hypothesis that these megakaryocytes were derived from progenitors that originated in the bone marrow. These data emphasize that in vitro behavior of hematopoietic progenitor cell subsets does not always predict their behavior following transplantation. This study supports a major role for the spleen in thrombopoiesis following engraftment of transplanted stem cells in irradiated mice.
Introduction
Murine hematopoietic stem cells have been shown to reside within a population of cells that do not express lineage-specific markers (Linneg) and express the antigens Sca-1, c-kit, and Thy-1.1. Cells of this phenotype comprise the stem cell pool in certain strains of mice.1,2 Rhodamine-123 (Rho) is a mitochondrial dye that stains cells based on their state of activation in which the more metabolically active cells tend to fluoresce brightly and quiescent cells dimly.3 Rho has been used to subfractionate the stem cell pool into quiescent and metabolically active subsets. The Rholow subset is highly enriched for hematopoietic stem cells, to the degree that as few as 4 of these cells can reconstitute a mouse for the lifetime of the animal. Observations of the behavior of these stem cells have led to the model of the 2-tiered stem-cell pool.4 According to this model, the Rholow cells make rare contributions to the peripheral compartment, but are mobilized in response to physiologic needs, and represent most of the transplantable stem cells. In contrast, Rhohigh cells have a high rate of cell division and, thus, make frequent contributions to the peripheral compartment under normal conditions. However, Rhohigh cells perform poorly in transplantation experiments, producing mainly erythrocytes and contributing little to platelet or granulocyte recovery.5,6 All previous studies of the Rholow and Rhohigh subsets have tracked leukocyte engraftment, using mice allelic at the CD45 locus to follow donor- and host-derived hematopoiesis.5-8 Although extremely useful, this model does not allow for tracking of erythroid or platelet engraftment, because these lineages lack CD45.
Nibley and Spangrude7 defined the leukocyte engraftment kinetics of the Rholow and Rhohigh cells. Rhohigh cells, although unable to sustain long-term hematopoiesis following transplantation, rescued animals from lethal irradiation by providing a burst of robust erythropoiesis that sustained the animals until their endogenous stem cells recovered. However, Rhohigh cells failed to contribute substantially to granulocyte, lymphocyte, or platelet engraftment.7Further studies demonstrated that the Rhohigh progenitor cells preferentially engrafted the spleen with little marrow engraftment, whereas the Rholow stem cells engrafted both the spleen and the bone marrow.8 We hypothesized that the lack of megakaryocyte potential in Rhohigh compartment was due to a failure to home to the bone marrow. We further hypothesized that the microenvironment of the spleen failed to support megakaryopoiesis following stem cell transplantation. We tested this hypothesis in 3 steps. First, we examined the behavior of Rholow and Rhohigh cells in cytokines conducive to multilineage hematopoiesis to determine whether Rhohighcells could make megakaryocytes in culture. Second, we transplanted these cells into lethally irradiated mice allelic at the hemoglobin and glucose phosphate isomerase loci, allowing us to track donor and host erythrocytes and platelets. Third, we looked directly at the bone marrow and spleen following transplantation of the Rhohighand Rholow cells and measured the number of megakaryocytes per square millimeter within those 2 organs at various time points following transplantation. We found higher concentrations of megakaryocytes within the spleen during platelet recovery than we saw in the bone marrow. To determine the origin of these megakaryocytes, we blocked splenic engraftment of Rholow stem cells by using pertussis toxin.9 Pertussis toxin irreversibly blocks Gαi protein–coupled chemokine receptors and has been shown to markedly block splenic engraftment of Rholow stem cells without affecting bone marrow engraftment. Pertussis toxin did not affect the number of megakaryocytes appearing in the spleen, supporting the hypothesis that these megakaryocytes were derived from progenitors arising in the bone marrow.
Materials and methods
Mice
The murine strains C57BL/6 (B6)-Thy1.1-Ly5.1 (donor) and B6-Hbbd/Hbbd-Gpi-1a/Gpi-1a(host) were bred and maintained at the University of Utah Animal Resource Center (Salt Lake City, UT). The host animals were provided kindly by Dr D. Harrison from the Jackson Laboratory (Bar Harbor, ME). Animals were maintained on acidified drinking water, autoclaved food, and bedding.
Cell preparation
Marrow was harvested from donor mice, filtered through nylon mesh, and counted with the use of a hemocytometer. All preparative steps were performed in Hanks balanced salt solution (HBSS) containing 5% fetal calf serum (FCS) (HB/5). Red blood cells were lysed in ammonium chloride, and the remaining cells were treated for 20 minutes with a saturating solution of monoclonal antibodies specific for antigens expressed by erythroid, myeloid, and lymphoid lineages. Immunomagnetic beads (sheep antirat specific; Dynal, Great Neck, NY) were added at a bead-to-cell ratio of 1:1 and incubated on ice for 20 minutes. Lineage-positive cells were removed from the mixture by using a magnet, and the process was repeated. The lineage-depleted cells were incubated for 20 minutes with fluorescein-conjugated anti-Thy1.1 (clone 19XE5), phycoerythrin-conjugated anti–Sca-1 (Ly-6A/E), and allophycocyanin-conjugated anti–c-kit. The cells were washed, suspended in HBSS containing 10 μg/mL propidium iodide (PI), filtered, and sorted using a Becton Dickinson Vantage fluorescence activated cell sorter (FACS). The LinnegThy1.1low c-kitpos Sca-1pos subset was counted and treated with prewarmed HBSS containing 0.2 μM Rho (Molecular Probes, Eugene, OR.) After 20 minutes at 37°C, the cells were washed and resuspended in HBSS with 10% FCS at 37°C for a 20-minute efflux period. Cells were stained with PI, and the brightest and dimmest 25% of cells in the Rho-123 distribution were collected by FACS. Reanalysis confirmed high levels of purity. Dilutions were made from these cells for transplantation into irradiated animals based on the electronic count of the cell sorter.
Tissue culture
To determine the ability of Rholow stem cells and Rhohigh progenitors to differentiate into erythrocytes and megakaryocytes in tissue culture, we measured primary culture colony-forming unit (CFU-C) activity by plating 2 × 104 bone marrow cells or 100 Rholow or Rhohigh cells per 35-mm culture plate. Methylcellulose medium consisted of α MEM (minimal essential medium; Invitrogen Life Sciences, Carlsbad, CA), 1.2% methylcellulose (Shinetsu, Tokyo, Japan), 30% FCS (Life Technologies), 1% bovine serum albumin (BSA; Sigma, St Louis, MO), and 0.1 mM 2-mercaptoethanol (Mollinckrodt Chemical, Chesterfield, MO). Cultures were supplemented with the following recombinant cytokines: interleukin 3 (IL-3; 10 ng/mL) and IL-6 (20 ng/mL; Peprotech, Rocky Hill, NJ), granulocyte colony-stimulating factor (G-CSF) (10 ng/mL; Amgen, Thousand Oaks, CA), steel factor (SF) 100 μg/mL, Flt-3 ligand 10 ng/mL, and erythropoietin (5 μ/mL; Ortho Pharmaceuticals, Raritan, NJ). At the time of harvest, the colonies were stained in situ with benzidine (Fluka). Colonies containing more than 50 cells were scored based on whether they were purely benzidine positive (erythroid burst-forming units [BFUs-E]), benzidine mixes, or benzidine negative. Colonies were lifted, washed, and transferred to slides by using a cytocentrifuge at 500 rpm for 3 minutes. Slides were dried, and megakaryocytes were stained by using the acetylcholinesterase technique as described by Karnovsky and Roots10 and modified by Jackson.11 Colonies were counterstained with hematoxylin and analyzed for cell content. The proportion of colonies containing megakaryocytes and the number of megakaryocytes per colony were determined for the Rholow, Rhohigh, and bone marrow control cells at days 5, 9, and 13.
Transplantation
Recipient animals were conditioned by lethal irradiation with 1300 rad, delivered in 2 fractions (650 rad each), 3 hours apart, from a 137Cs gamma irradiator (Mark I gamma irradiator; JL Sheperd and Associates, Glendale, CA). Following anesthesia with methoxyflurane (Metofane; Mallinckrodt Veterinary, Mundelein, IL), 2000 Rholow or Rhohigh cells in 200 μL HB/5 were transplanted by retro-orbital injection. Control animals were phlebotomized under anesthesia on days 7 and 14, and they were humanely killed if they showed signs of stress.
Peripheral blood collection
Following anesthesia, a 200-μL sample was drawn from the retro-orbital sinus into a heparinized capillary tube, then dispensed into a siliconized tube containing 20 μL anticoagulant citrate dextrose solution USP formula A Fenwal (Baxter Healthcorp, Deerfield, IL). Each animal was assayed for leukocyte, erythrocyte, and platelet total blood counts at various time points following transplantation by using an automated cell counter (Serono). Normal peripheral blood count ranges were established on all experimental animal strains.
Platelet cell harvest and analysis
Platelet rich plasma (prp) was prepared by adding 400 μL of prewarmed (room temperature) phosphate-buffered saline (PBS) containing 0.33% EDTA (ethylenediaminetetraacetic acid) (PE-Buffer) to each blood sample. The tubes were then centrifuged at 130gfor 10 minutes at room temperature in a tabletop microcentrifuge. The prp was removed and washed in PE-buffer. Tubes were centrifuged at 1500g for 4 minutes, and the supernatant was removed. Platelets were stored in 10 μL distilled water at −80°C prior to processing. Chimerism was assayed by comparing electrophoretic patterns between the intracellular glucose phosphate isomerase (Gpi) isoenzymes, Gpi-1a and Gpi-1b. The Gpi isoenzymes are easily separated by agarose gel electrophoresis because of their net surface charge differences.12-14 C57BL/6 mice have the Gpi-1b allele, which encodes a protein that moves more rapidly in an electric field compared with the B6-Gpi-1acongenic strain. To observe the protein bands, an enzymatic stain specific for Gpi was mixed in hot agar and was layered over the gel surface.13 The quality of platelet preps was monitored by counting contaminating leukocytes and erythrocytes on platelet preps stained with May-Grunwald Giemsa. Levels of contamination were consistently less than 1%. Platelet preparations were electrophoresed on serum protein electrophoresis (SPE) gels (Beckman Paragon, Brea, CA) and specifically stained for Gpi. Bands were quantified by using densitometry.
Erythrocyte harvest and analysis
The erythrocyte fractions were washed twice by adding 3 mL PBS and centrifuging at 1200 rpm (200g) for 5 minutes. To separate the hemoglobin variants, erythrocyte samples were pretreated with a cystamine (cys) disulfide reagent prior to electrophoresis.15 Hemoglobin variants were separated and stained using the Paragon Hemoglobin Electrophoresis Kit (Beckman Paragon). Bands were quantified by using densitometry.
Direct visualization of megakaryocyte engraftment
Femoral bones and spleens from controls that did not receive transplants and from animals that received transplants of the Rholow and Rhohigh subsets were harvested on days 7, 14, 21, 28, 42, 56, and 182 days after transplantation. The spleens were weighed, cut in half, and either frozen in Tissue-Tek O.C.T. compound or placed in phosphate-buffered saline with 4% formaldehyde for histologic analysis. Femora were placed directly into formaldehyde prior to decalcification and sectioning. Sections were stained with hematoxylin and eosin by using standard techniques and counted by using a 5 × 5–mm reticule to determine total area counted. The number of megakaryocytes per square millimeter was determined, and at least 4 sections at different levels were analyzed per mouse. The results represent pooled data from 4 separate experiments, containing 3 to 4 mice per group, and each time point represents mice from at least 2 experiments. To better visualize megakaryocytes within the spleen, frozen spleen sections were stained using the acetylcholinesterase staining technique.10 11Slides were counterstained with hematoxylin and were scored as above. Each section was examined and content of megakaryocytes were noted, as well as the number of megakaryocytes per colony.
Pertussis toxin treatment
Pertussis toxin has been shown to block splenic engraftment of Rholow stem cells.9 Purified pertussis toxin (PT) was provided by Dr W. Cieplak (RIBI Immunochem, Hamilton, MT). PT was purified from culture filtrates of Bordetella pertussisstrain 3779 BI2S4 by affinity chromatography as previously described.16 Thylow c-kitposSca-1pos cells were incubated with PT at a concentration of 100 to 180 ng/mL along with rhodamine for 20 minutes at 37°C. Cells were washed and cultured again in PT during the 20-minute Rho efflux stage. After another wash, cells were sorted based on Rho intensity as described above. PT-treated and untreated Rholow cells were transplanted into lethally irradiated mice at a dose of 1000 cells/mouse. The animals were killed on days 14, 21, and 28, and megakaryocyte densities were determined for bones and spleens.
Statistics
Data were analyzed by using Microsoft Excel. Standard errors of the mean were determined for all time points.
Results
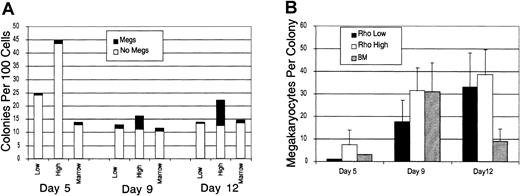
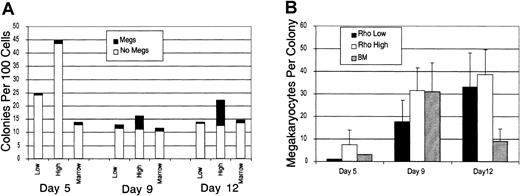
The capacity of the Rholow and Rhohighsubsets to produce megakaryocytes in tissue culture was assessed following stimulation by a cocktail of cytokines containing IL-3, IL-6, G-CSF, erythropoietin, stem cell factor (SCF), and Flt-3 ligand. Colonies were plucked, cytocentrifuged, and stained with acetylcholinesterase to determine megakaryocyte content. A total of 20 colonies were plucked per condition, in 2 separate experiments, and the results presented are representative of both experiments. Megakaryocytes appeared in small numbers in BFU-E colonies from both primitive subsets and the bone marrow control as early as 5 days in culture. By day 9, numerous megakaryocytes were present in the mixed colonies derived from both primitive cell populations. However, Rhohigh progenitors were more clonogenic and had higher megakaryocyte numbers per colony than either the Rholowstem cell or the bone marrow control colonies (Figure1). Megakaryocytes always appeared in colonies that also contained benzidine-positive cells, with the exception of a small number of colonies that were benzidine negative and contained only megakaryocytes. On day 13, most megakaryocytes had disintegrated, and colonies contained numerous macrophages containing ingested brown granules from these degenerated megakaryocytes.
Rholow, Rhohigh, and normal bone marrow cells were cultured in methylcellulose supplemented with IL-3, IL-6, G-CSF, SCF, erythropoietin, Flt-3 ligand, and IL-7.
(A) The number of colonies per 100 Rholow or Rhohigh cells plated. Controls consisted of hemolyzed bone marrow in which 2 × 104 cells were plated. (B) The number of megakaryocytes per colony. Data are mean ± SEM.
Rholow, Rhohigh, and normal bone marrow cells were cultured in methylcellulose supplemented with IL-3, IL-6, G-CSF, SCF, erythropoietin, Flt-3 ligand, and IL-7.
(A) The number of colonies per 100 Rholow or Rhohigh cells plated. Controls consisted of hemolyzed bone marrow in which 2 × 104 cells were plated. (B) The number of megakaryocytes per colony. Data are mean ± SEM.
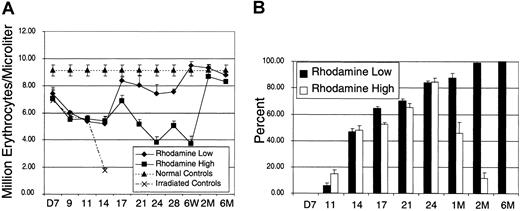
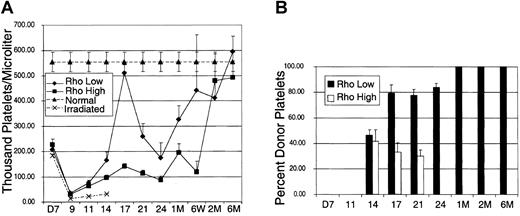
To determine the kinetics of erythrocyte and platelet engraftment following transplantation of the Rholow and Rhohigh cells, 5 separate transplantation experiments were performed to determine engraftment kinetics and chimerism data. Leukocyte, erythrocyte, and platelet cell counts were measured at various time points following transplantation. Pooled data depicting erythrocyte recovery are depicted in Figure2. Each time point represents data from at least 3 experiments and between 9 and 20 animals. The Rhohigh progenitors rescued mice from the effects of lethal irradiation by providing a burst of hematopoiesis, as evidenced by the rapid death because of anemia of irradiated control animals by day 14. Erythrocyte counts were identical in animals receiving Rholow stem cells and Rhohigh progenitor cells until day 18. The Rholow stem cells sustained erythropoiesis at levels close to those of the control animals from day 18 through the rest of the period of observation. In contrast, animals receiving Rhohigh progenitors did not achieve normal erythrocyte levels until 2 months following transplantation (Figure2A). To determine the contribution of donor- versus host-derived cells to erythroid engraftment after transplantation, we used hemoglobin electrophoresis. Figure 2B demonstrates the densitometric analysis of hemoglobin in animals that received transplants with either subset. The contribution of donor-derived hemoglobin peaked at 80% on day 25 after transplantation in animals receiving Rhohighprogenitors and then reverted to host-derived hemoglobin by 2 months after transplantation. In contrast, animals receiving the Rholow stem cells reach 100% donor-derived erythropoiesis by 2 months after transplantation.
Kinetics of erythrocyte recovery.
(A) Recipient animals were injected with 2000 Linneg c-kitpos Sca-1posThylow cells that were subfractionated based on staining with Rho-123. Normal controls are the average peripheral blood counts of 20 B6-Thy1.1-Ly5.1 animals. Irradiated controls consist of animals that received lethal irradiation and did not receive donor cells. (B) Densitometric analysis of donor-derived hemoglobin recovery. Data are mean ± SEM.
Kinetics of erythrocyte recovery.
(A) Recipient animals were injected with 2000 Linneg c-kitpos Sca-1posThylow cells that were subfractionated based on staining with Rho-123. Normal controls are the average peripheral blood counts of 20 B6-Thy1.1-Ly5.1 animals. Irradiated controls consist of animals that received lethal irradiation and did not receive donor cells. (B) Densitometric analysis of donor-derived hemoglobin recovery. Data are mean ± SEM.
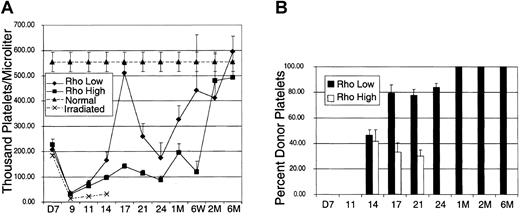
In contrast to erythrocyte engraftment, the recovery of platelets was divergent between Rholow stem cells and Rhohighprogenitors (Figure 3). Each data point represents pooled data from the same animals represented in the erythrocyte kinetics figure (Figure 2). Following a nadir at day 9, platelet counts peaked at day 17 in animals receiving either Rholow or Rhohigh subsets, albeit the contribution to platelets of Rhohigh progenitor cells was minimal. Animals receiving the Rholow stem cells had platelet counts approaching normal controls on day 17. Following the peak at day 17, the platelet counts again reached a second nadir on day 25 in animals receiving both subsets, and this was followed by a secondary increase in platelet counts. Animals receiving both subsets achieved normal platelet levels by 2 months after transplantation (Figure 3A). Densitometric analysis of Gpi electrophoresis shows that donor-derived platelets were seen by day 14 in animals that received both Rholow stem cells and Rhohigh progenitors (Figure 3B). Although the animals receiving Rholow cells showed 100% donor-derived Gpi by 1 month after transplantation, animals receiving Rhohigh progenitors never had more than 45% donor-derived Gpi and reverted completely to host Gpi by 1 month after transplantation.
Kinetics of platelet recovery.
(A) Recipient animals were injected with 2000 Linnegc-kitpos Sca-1pos Thylow cells that were subfractionated based on staining with Rho-123. Normal controls are the average peripheral platelet counts of 20 B6-Thy1.1-Ly5.1 animals. Irradiated controls consist of animals that received lethal irradiation and did not receive donor cells. (B) Densitometric analysis of donor-derived glucose phosphate isomerase recovery. Data are mean ± SEM.
Kinetics of platelet recovery.
(A) Recipient animals were injected with 2000 Linnegc-kitpos Sca-1pos Thylow cells that were subfractionated based on staining with Rho-123. Normal controls are the average peripheral platelet counts of 20 B6-Thy1.1-Ly5.1 animals. Irradiated controls consist of animals that received lethal irradiation and did not receive donor cells. (B) Densitometric analysis of donor-derived glucose phosphate isomerase recovery. Data are mean ± SEM.
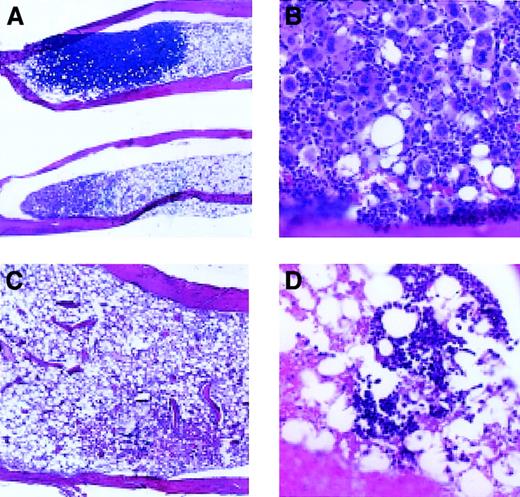
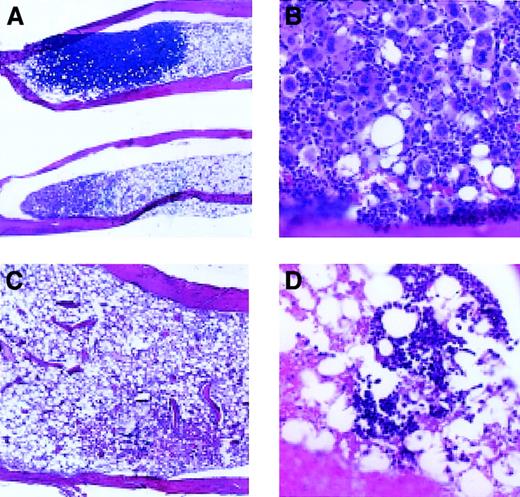
To determine whether megakaryocyte engraftment occurred preferentially in the bone marrow, we examined megakaryocytes in spleen and bone sections at various time points following transplantation (Figures4 and 5). By day 7 after transplantation, there were small colonies consisting of blast cells present in the bone marrow of animals receiving Rholow stem cells. Small numbers of megakaryocytes (host derived based on Gpi analysis of platelets) persisted in the marrow at this time. By 14 days following transplantation, most of the marrow space was empty, but there were focal areas of hematopoiesis present in the bone marrow in animals receiving Rholow stem cells (Figure 4A). These clusters varied in appearance, some containing between 15 and 130 mature megakaryocytes surrounded by erythroblasts (Figure 4B). Other areas of the marrow contained megakaryocytes scattered within dense hematopoietic areas containing cells from various lineages. In comparison, the irradiated controls on day 14 had marrow space devoid of hematopoiesis (Figure 4C). By the third week following transplantation of Rholow stem cells, the marrow space was approximately 60% the cellularity of the normal marrow, and the megakaryocytes were scattered between a variety of other cell types, similar to control animals. Marrow cellularity did not reach that of the control animals until 6 months after transplantation. In contrast, animals receiving the Rhohigh progenitors had occasional small hematopoietic colonies appearing in the bone marrow on day 14. These colonies appeared to contain erythroblasts (Figure 4D). By 4 weeks following transplantation, megakaryocytes appeared in the marrow, in colonies with large numbers of megakaryocytes, similar the to animals receiving the Rholow cells. These megakaryocytes were host derived based on concurrent Gpi analysis of platelet preps.
Hematopoiesis in the bone marrow, 14 days after transplantation.
(A) Appearance of bones following transplantation of Rholowcells (original magnification, × 20). (B) Dense megakaryocyte cluster, following transplantation of Rholow cells (original magnification, × 200). (C) Empty marrow, 14 days after transplantation of Rhohigh progenitors (original magnification, × 100). (D) Small erythroblastic colony in the marrow of animal 14 days following transplantation of Rhohighprogenitors (original magnification, × 200). Controls consisted of healthy animals and irradiated animals that did not receive a transplant.
Hematopoiesis in the bone marrow, 14 days after transplantation.
(A) Appearance of bones following transplantation of Rholowcells (original magnification, × 20). (B) Dense megakaryocyte cluster, following transplantation of Rholow cells (original magnification, × 200). (C) Empty marrow, 14 days after transplantation of Rhohigh progenitors (original magnification, × 100). (D) Small erythroblastic colony in the marrow of animal 14 days following transplantation of Rhohighprogenitors (original magnification, × 200). Controls consisted of healthy animals and irradiated animals that did not receive a transplant.
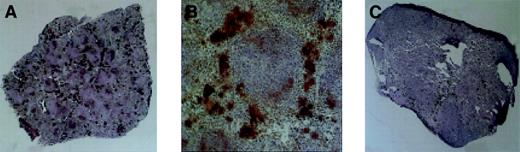
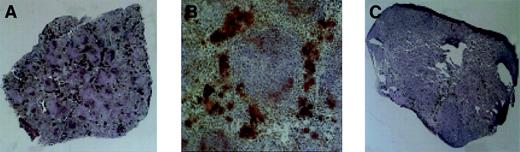
Megakaryopoiesis in the spleen, day 28 after transplantation.
Megakaryocytes stain dark as a consequence of histochemical staining for acetylcholinesterase. (A) Typical spleen of animal receiving Rholow stem cells. (B) A cluster of megakaryocytes in the perifollicular space after Rholow transplantation. (C) Animals receiving the Rhohigh progenitor have almost no splenic megakaryocyte activity. Original magnification A, × 20; B, × 100; and C, × 20.
Megakaryopoiesis in the spleen, day 28 after transplantation.
Megakaryocytes stain dark as a consequence of histochemical staining for acetylcholinesterase. (A) Typical spleen of animal receiving Rholow stem cells. (B) A cluster of megakaryocytes in the perifollicular space after Rholow transplantation. (C) Animals receiving the Rhohigh progenitor have almost no splenic megakaryocyte activity. Original magnification A, × 20; B, × 100; and C, × 20.
Acetylcholinesterase staining was used to accentuate splenic megakaryocytopoiesis (Figure 5). The splenic red pulp had prominent staining of trapped platelets within the sinusoids when the platelet count was more than 200 000, at the earliest and latest time points. There was no change in size of the day-7 posttransplantation spleens in animals 7 days after receiving a transplant of Rholow stem cells, although there were a few small colonies of hematopoietic cells evident by microscopy. Clusters of megakaryocytes consisting of between 3 and 10 cells appeared 14 days following transplantation of Rholow stem cells and increased in number through day 28 (Figure 5A). The megakaryocyte clusters tended to form in the subcapsular and perifollicular areas within the red pulp and occurred alone, or in association with erythroid progenitors (Figure 5B). The spleens of the Rhohigh progenitors increased in size and cellularity 7 days after transplantation, with confluent macroscopic nodules clearly apparent on the splenic surface. In spite of this early, robust hematopoietic activity, almost no megakaryopoiesis was observed in the spleens of animals receiving the Rhohigh progenitors until approximately day 28 (Figure 5C). A gradual increase in splenic megakaryopoiesis occurred over the next 2 weeks, with colonies similar to those seen in animals receiving the Rholow stem cells 2 weeks earlier.
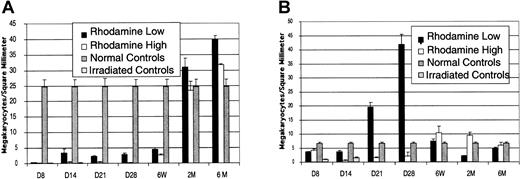
The number of megakaryocytes per square millimeter (megakaryocyte density) was measured to compare the relative contribution of megakaryopoiesis by the bone marrow and spleen (Figure6). These data are pooled from 4 separate experiments, and each time point represents between 6 and 10 mice. By day 14, bone marrows of irradiated control animals were nearly devoid of megakaryocytes. Animals receiving Rholow stem cells had levels of marrow megakaryocytes that were 17 times the density of irradiated controls and yet only 14% the density of normal subjects. Megakaryopoiesis normalized at 2 months (Figure 6A). The density of megakaryocytes in the spleen of animals receiving Rholowstem cells was more than twice that of irradiated control levels on day 8 and rapidly increased, peaking on day 28. At that time, splenic megakaryocyte density was more than 10 times bone marrow levels and 6 times the level in normal spleens. Platelets were completely donor derived at this time by Gpi analysis. Megakaryopoiesis was present above the level of the irradiated controls at day 7 in the animals receiving Rhohigh progenitors. However, megakaryocyte density dropped to irradiated control levels 14 days following transplantation. Megakaryopoiesis remained at this low level in animals receiving the Rhohigh progenitors until 6 weeks following transplantation, when splenic megakaryocyte density surpassed that of normal controls (Figure 6B). Circulating platelets at this time were completely host derived by Gpi analysis. Animals receiving both stem cells and progenitors had normal platelet levels by 2 months following transplantation.
Comparison of megakaryopoiesis in the bone marrow and spleen after transplantation of Rholow stem cells or Rhohigh progenitors.
(A) Bone marrow megakaryopoiesis. (B) Splenic megakaryopoiesis. Data are mean ± SEM.
Comparison of megakaryopoiesis in the bone marrow and spleen after transplantation of Rholow stem cells or Rhohigh progenitors.
(A) Bone marrow megakaryopoiesis. (B) Splenic megakaryopoiesis. Data are mean ± SEM.
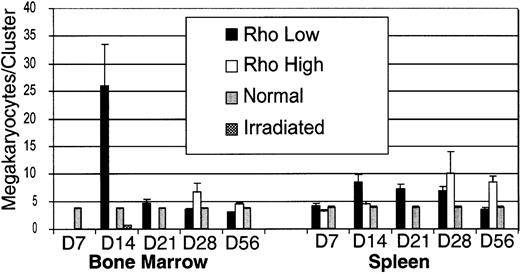
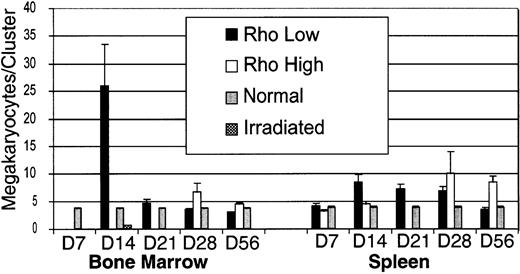
The number of megakaryocytes within each cluster was determined for each time point within the spleen and bone marrow (Figure7). Megakaryocyte clusters were identified based on cells being within 10 μm of each other, according to the criteria of Paulus et al17 for megakaryocyte colony-forming units (CFUs-meg) in vitro. Megakaryocytes are motile, and cells within colonies tend to spread. The size of clusters was greatest in the bone marrow on day 14, where a few exceeded 100 cells per section. By day 21, megakaryocytes within the marrow were more spread out, and cluster size was similar to normal controls. In the spleen, cluster size was consistently smaller than the bone marrow, and peak cluster size of the Rhohigh subset lagged behind the Rholow subset by 2 weeks (Figure 7).
Size of megakaryocyte clusters in the bone marrow and spleen after transplantation.
Data are mean ± SEM.
Size of megakaryocyte clusters in the bone marrow and spleen after transplantation.
Data are mean ± SEM.
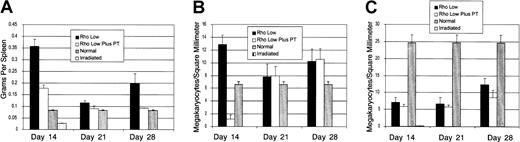
To determine the origin of the megakaryocytes that appeared in the spleen following stem cell transplantation, we used pertussis toxin (PT) to block the migration of Rholow stem cells to the spleen (Figure 8). Consistent with our prior studies,9 PT treatment did not affect the kinetics of short-term platelet engraftment. Animals receiving PT-treated stem cells had lower spleen weights and megakaryocyte densities than animals receiving untreated stem cells on day 14 after transplantation (Figure 8A), and megakaryocyte numbers were greatly decreased, reflecting decreased splenic engraftment. However, by 21 days after transplantation, splenic megakaryocytic density was equivalent among animals receiving PT-treated and untreated stem cells (Figure 8B). PT treatment had no effect on the appearance of megakaryocytes in bone marrow (Figure 8C). These data suggest that the megakaryocyte colonies that appear in the spleen are independent of initial splenic engraftment. Progenitors forming these clusters are most likely derived from the marrow and subsequently migrate to the spleen.
Effect of blockade of splenic homing of stem cells by pertussis toxin (PT).
(A) Spleen weights. (B) Splenic megakaryocyte density. (C) Bone marrow megakaryocyte density. Data are mean ± SEM.
Effect of blockade of splenic homing of stem cells by pertussis toxin (PT).
(A) Spleen weights. (B) Splenic megakaryocyte density. (C) Bone marrow megakaryocyte density. Data are mean ± SEM.
Discussion
This is the first study to carefully describe erythroid and platelet engraftment following transplantation of Rholowstem cell and Rhohigh progenitor subsets by using mice allelic at the hemoglobin and Gpi loci. The large intermediate population that falls between the dimmest 25% and brightest 25% of cells may have interesting activity and was not studied in these experiments. These studies provide, for the first time, direct evidence that Rholow cells produce long-term donor-derived erythropoiesis and thrombocytopoiesis, consistent with their previous characterization as hematopoietic stem cells. These studies also emphasize that in vitro behavior of specific hematopoietic subsets may not correlate with behavior in vivo following transplantation. Previous studies have shown that Rhohigh progenitors in limiting dilution analysis retain multilineage potential for myeloid, erythroid, and lymphoid development.5 In our study, the Rhohigh subset produced more colonies containing megakaryocytes and more megakaryocytes per colony than did Rholow stem cells in short-term tissue culture. Although the ability of this subset to produce megakaryocytes was unexpected and in contrast to its activity in vivo, the fact that it outperformed the stem cell in short-term culture belies its identity as a proliferating progenitor. In vivo, Rhohigh progenitors produced only a transient burst of erythroid activity, consistent with the engraftment pattern implied by prior studies.7,8 Although Rhohigh cells produced enough erythrocytes to rescue animals from lethal irradiation, they contributed little to platelet recovery. Presumably, differentiating progenitors proceed through the recently described common myeloid progenitor and subsequently the megakaryocyte/erythrocyte bipotent progenitor, if this is the sole pathway of erythrocyte and megakaryocyte development.18 19The in vivo behavior of the recently defined megakaryocyte-erythrocyte progenitor could be more rigorously tested by using Gpi and hemoglobin analysis. The lack of megakaryocyte production by the Rhohigh subset in vivo suggests that these cells preferentially produce erythrocytes following transplantation. Our tissue culture studies suggest that these Rhohigh cells retain megakaryocyte potential and are not erythroid committed. These cells lack the ability to home to the bone marrow. The bone marrow may provide an environment that supports the initial steps in megakaryocyte commitment. It is possible that the spleen can only support the final steps of megakaryocyte differentiation.
This study contributes to our understanding of the role of the spleen in megakaryopoiesis following transplantation. In the normal mouse, megakaryopoiesis occurs primarily in the bone marrow (our results) and is approximately 5 times more common in the marrow than in the normal spleen.20,21 However, the bone marrow contains approximately 5 × 108 cells,22 whereas the spleen contains 2 × 108 cells,23 so that in the normal mouse, the marrow accounts for 10 times the megakaryocyte mass compared with the spleen. At day 28, when spleen size and cellularity doubles, splenic megakaryocyte density is 42 cells/mm2 compared with 4 megakaryocytes/mm2 in the bone marrow. At this time, the spleen is completely effaced by hematopoietic cells, whereas the bone marrow space is about 60% cellular. In essence, the spleen is now more cellular than the bone marrow and accounts for approximately 20 times the megakaryocyte mass that the bone marrow provides at this time point. Although it is tempting to speculate that the bone marrow simply does not have room to accommodate stress megakaryopoiesis, bone marrow cellularity at the peak of splenic megakaryopoiesis was 60% of normal, and, thus, space was not the limiting factor. Some other process, such as a limitation in the ability of bone marrow stroma to support hematopoiesis, must be responsible for the mobilization of progenitors from the marrow during the recovery of platelets following stem cell transplantation.
A role for the spleen in murine hematopoiesis has been demonstrated in a number of stress states. These include exposure to cytokines such as SCF24 and G-CSF,25 antineoplastic drugs such as Actinomycin-D26 and 5-fluorouracil,27 and in response to transforming viruses such as the Friend28and Rauscher leukemia viruses.29 Finally, graft-versus-host disease has been shown to cause an increase in splenic hematopoiesis initially, followed by decreased splenic and increased hepatic hematopoiesis.30 Hepatic megakaryopoiesis was not observed in our transplantation experiments. An increased role for the spleen specifically in megakaryopoiesis has been implicated in several other studies. Increased splenic megakaryopoiesis has been observed in response to various pharmacologic agents27,31 and in leukemic32 and myeloproliferative states.33
Our study was the first to describe megakaryocyte clusters that arise in the bone marrow and spleen following transplantation of purified hematopoietic stem cells and recovery of host stem cells following irradiation. A similar phenomenon was described by Thean et al34 in the spleens of animals receiving bone marrow from animals after treatment with 5-fluorouracil, and the term spleen colony-forming unit-megakaryocyte was used to describe these clusters.34 However, colony implies derivation from single cells, and we have chosen to use the term cluster. Because we are counting cross sections of 3-dimensional clusters, size does not truly reflect the number of cells within a given cluster. However, given the difference between the behavior of Rhohigh progenitors in tissue culture and in vivo, it is important to develop ways to measure megakaryopoiesis directly within animals that received transplants, rather than relying on in vitro studies. In one of the few studies to attempt to compare the number of megakaryocyte progenitors from the bone marrow and spleen in vitro, Long and Williams21 found fewer megakaryocyte progenitors in the normal spleen than in the bone marrow. CFUs-meg derived from the bone marrow tended to be larger compared with colonies derived from the spleen, and there were more megakaryocyte progenitors in cycle in the spleen than in the bone marrow.21 The megakaryocyte clusters in our studies showed a similar pattern, with the bone marrow containing larger colonies, some containing more than 100 cells per cross section.
Our study suggests that there may be trafficking of megakaryocyte progenitors from the recovering bone marrow to the spleen in the mouse. Although trafficking of megakaryocyte progenitors has not been previously described, erythrocyte progenitors have been shown to traffic from marrow to spleen in response to G-CSF.25 A similar effect is seen in the mouse in response to phenylhydrazine, which causes a hemolytic anemia, but massive increases in splenic BFUs-E.35 Several groups have shown that irradiated mice having a small area of bone marrow shielded developed macroscopic spleen colonies, presumably from progenitors that migrated from the shielded marrow to the spleen.36-38 In humans, G-CSF has been shown to cause splenomegaly, and this cause is thought to be due to induction of extramedullary hematopoiesis, presumably from trafficking of cells derived from the bone marrow.39 40 In our study, donor-derived megakaryocyte clusters appeared in the spleens of animals that received stem cells around day 14 but peaked 2 weeks later. Similarly, in animals rescued from lethal irradiation by injection of the Rhohigh progenitors, host-derived megakaryocyte clusters appear approximately 2 weeks following the beginning of recovery of marrow hematopoiesis. This activity is opposite and distinct from the pattern of CFU-S activity. Colony-forming units spleen (CFU-S) appear following transplantation of Rhohigh progenitors on day 8 and lack megakaryocytes. One week later, CFUs-S appear in animals receiving the Rholowstem cells. It is this day 14 CFU-S activity that is greatly inhibited by exposure to pertussis toxin.
Our experiments support the hypothesis that these late megakaryocyte clusters are derived from progenitors that originate in the bone marrow. Although day 14 megakaryopoiesis is greatly inhibited by blocking splenic stem cell homing with pertussis toxin, no decrease in megakaryopoiesis is seen on day 21 or 28 following transplantation. This finding supports the hypothesis that the progenitors forming these clusters arise elsewhere, most likely in the bone marrow. It is possible that the spleen provides space for overflow hematopoiesis for specific progenitor populations at a time when the marrow space may be full. Because the bone marrow is unable to expand in size, and the spleen has an elastic capsule, it is possible that the spleen responds to hematopoietic stress by expanding the available space for hematopoiesis. The fact that the kinetics of platelet recovery is unaffected by blockade of splenic homing by stem cells suggests that the spleen does not contribute early on to platelet recovery. On days 14 to 21, the marrow space is relatively empty, and at this point there is limited megakaryocyte activity within the spleen. However, PT does not block the homing of megakaryocyte progenitors from the bone marrow to the spleen that occurs around day 28. Studies of platelet kinetics in splenectomized animals would be necessary to definitively measure the spleen's role in the later phases of platelet recovery.
In summary, these studies highlight the differences in behavior of the Rhohigh progenitor in vitro and in vivo. We have shown that the spleen plays an important role in posttransplantation platelet recovery, but that megakaryocyte colonies that appear in the spleen probably arise from the bone marrow. Finally, these observations support the hypothesis that the differences in megakaryocyte potential between the Rholow stem cells and Rhohighmultipotent progenitors are due to differences in the splenic and marrow microenvironments. Committed progenitors arising from Rholow stem cells that initially seed the bone marrow migrate to and terminally differentiate within the spleen. Because Rhohigh progenitors largely fail to colonize the marrow, they are fated to terminal differentiation within the spleen. The spleen may not support the early, proliferative phase of megakaryocyte development.
We thank Robert Goldsby MD and Linda Kelly PhD for their assistance in editing this manuscript.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-02-0490.
Supported by grants K08 HL03962 and RO1 HL56857 from the National Institutes of Health and by the Winslow Research Fellowship from the National Childhood Cancer Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William B. Slayton, Division of Pediatric Hematology Oncology, University of Florida College of Medicine, UFHSC Box 100296, Gainesville, FL 32610; e-mail: slaytwb@peds.ufl.edu.