Secreted growth factors are integral components of the bone marrow (BM) niche and can regulate survival, proliferation, and differentiation of committed hematopoietic stem cells (HSCs). However, downstream genes activated in HSCs by early-acting cytokines are not well characterized. To better define intracellular cytokine signaling in HSC function, we have analyzed mice lacking expression of both signal transducer and activator of transcription 5a (STAT5a) and STAT5b (STAT5ab−/−). These studies specifically avoided possible autoimmune and/or splenomegaly disease-mediated indirect effects on HSC function by using 2 independent approaches: (1) by crossing onto the C57Bl/6 RAG2−/− background, and (2) by generation of wild-type chimeric mice reconstituted with transplanted STAT5ab−/− BM cells. These experiments demonstrated that STAT5-deficient HSCs have cell autonomous defects in competitive long-term repopulating activity. Furthermore, in the chimeric mice, injected wild-type BM cells showed a progressive multilineage competitive repopulating advantage in vivo, demonstrating that steady-state hematopoiesis was also highly STAT5-dependent. Consistent with the in vivo repopulating deficiency, when Sca-1+c-kit+lin− (KLS) cells were isolated and stimulated with growth factors in vitro, up to a 13-fold reduced expansion of total nucleated cells was observed in response to cocktails containing interleukin 3 (IL-3), IL-6, stem cell factor (SCF), Flt3 ligand, and thrombopoietin. Notably, a 10-fold reduction in expansion was observed with IL-3 and SCF. However, STAT5 activation was not required for regeneration of the KLS pool in vivo following transplant or for secondary repopulating ability. These studies support a major role for STAT5 activation as a cellular determinant of cytokine-mediated HSC repopulating potential but not self-renewal capacity.
Introduction
Hematopoietic stem cells (HSCs) have tremendous proliferative potential and are required to generate differentiated progeny cells of all hematopoietic lineages in response to short-term myelosuppression. In addition, HSCs are defined clonally by their ability both to differentiate and to self-renew. Regulation of HSC commitment is not well-defined but is believed to be stochastic and not regulated by the microenvironment.1,2 However, HSC survival and self-renewal are likely regulated by secreted factors within the bone marrow (BM) niche.3 Following commitment, early-acting hematopoietic growth factors can drive cell cycle activation and promote differentiation of HSCs. Growth factor signals can promote HSC survival and proliferation during in vitro stimulation, leading to enhanced oncoretroviral-mediated gene transfer.4 However, little is known about the intracellular signal transduction pathways that are necessary for proliferation and differentiation or for self-renewal division of HSCs.
Our studies have focused on activation of Janus kinase (JAK) and the latent signal transducer and activator of transcription (STAT) pathway. The JAK/STAT signaling axis is a conserved pathway involved in diverse aspects of development. Recent studies inDrosophila have highlighted the role of the lone STAT protein in self-renewal of germ cells during spermatogenesis.5,6 Furthermore, murine STAT3 knockout leads to early embryonic lethality,7 and its activation is essential for embryonic stem cell self-renewal in vitro.8,9 In contrast, STAT5 expression is first evident during differentiation of embryonic stem (ES) cells.10HSCs from mice lacking expression of both STAT5a and STAT5b (STAT5ab−/−) can contribute to hematopoiesis at both short-term and long-term time points following transplantation,11 but STAT5-deficient HSCs are noncompetitive with wild-type HSCs.11,12 Despite a multilineage competitive repopulating defect, STAT5-deficient BM does not show a reduction in the number of cells with the HSC phenotype11,12 or a reduced homing ability following transplantation.12
Difficulties in the characterization of HSC defects in adult STAT5ab−/− mice have been the expansion of erythroid (Ter119+) precursor cells in the spleen13,14and accumulation of activated phenotype T lymphocytes.13 The splenomegaly and possible autoimmune phenotypes reduce the life span of homozygous double-mutant C57Bl/6 background mice. The etiology of this disease is complex, and it was not the goal of this study to explore the disease phenotype. However, demonstration that adult STAT5-deficient mice show greater cell cycle activation in BM Sca-1+lin−hematopoietic progenitors12 suggested that microenvironmental factors might increase cell cycle status, resulting in decreased long-term engraftment following transplantation. Such a phenotype could be due to an observed age-dependent increase in T lymphocytes accumulating in the adult BM (K.D.B., unpublished observations, March 2001). It is important to note that STAT5-deficient mice share some common phenotypes with the IL-2–receptor β chain knockout mouse, which has an activated T-cell phenotype that alters repopulating activity.15 To specifically avoid these potentially confounding features of the STAT5-deficient mouse, we have used 2 independent approaches. First, the STAT5 mutations were crossed onto the RAG2−/−background. This provided a model for testing the cell intrinsic repopulating ability of the graft in the absence of adult T lymphocytes. Second, the ability of STAT5-deficient HSCs to fully reconstitute lethally irradiated recipient mice avoided possible cell nonautonomous effects by reconstituting STAT5-deficient HSCs into a wild-type microenvironment.
Methods
Mice genotyping and drug injections
The STAT5 mouse colony (C57Bl/6; Ly-5.2/Hbs) was maintained by crossing heterozygote STAT5ab+/− mice to yield viable STAT5ab−/− pups that were genotyped by polymerase chain reaction (PCR) as previously described11 16 The congenic mice B6.C-H1b/By (HW80) and B6.SJL-PtprcaPep3b/BoyJ (Ly-5.1) were obtained from The Jackson Laboratory (Bar Harbor, ME). These congenic mouse strains have polymorphic changes at the hemoglobin locus (Hb) and the CD45 (Ly5) locus that facilitate tracking of transplanted donor cells. C57Bl/6 RAG2−/− mice (B6.SJL-Ptprca/BoCrTac-[KO]Rag2N10) were purchased from Taconic Labs (Germantown, NY). Clinical grade 5-fluorouracil (5-FU; ICN Pharmaceuticals, Costa Mesa, CA) was diluted 1:5 from the 50 mg/mL stock solution in sterile phosphate-buffered saline (PBS) to 10 mg/mL for intraperitoneal injection at a dose of 150 mg/kg.
Bone marrow transplantation and peripheral blood analyses
BM was harvested from both hind limbs (tibias and femurs) of either STAT5ab−/− or littermate wild-type mice. For competitive repopulation assays, the cell mixtures were injected via the lateral tail vein into lethally irradiated (1100 rad) recipient mice. The minimum cell dose injected was 2 × 106 cells for each BM graft. For generation of chimeric mice, equal donor equivalents of BM cells were injected for each primary recipient mouse. For some experiments, BM was harvested from primary recipients at times 12 to 16 weeks following transplantation and injected via the lateral tail vein into lethally irradiated secondary recipients (1100 rad). In other experiments, the secondary transplanted BM cells were a mixture of Ly-5.1/wild-type versus Ly-5.2/STAT5ab−/− BM cells from primary recipients. Beginning at 8 weeks after transplantation, peripheral blood was obtained following puncture of the retro-orbital venous sinus using a microcapillary tube. Microcapillary tubes were spun in a microcentrifuge (Stat-Spin, Norwood, MA), and hematocrits were read manually. Hemoglobin patterns were analyzed from packed peripheral red blood cells by electrophoresis on cellulose acetate gels. To calculate the relative proportions of single and diffuse donor hemoglobin in peripheral blood from reconstituted mice, the hemoglobin gels were digitized using a ScanJet IIcx/T scanner (Hewlett Packard, Palo Alto, CA). Data files were quantitated by densitometry using ImageQuant software (Amersham Biosciences, Piscataway, NJ). For experiments where donor grafts were competed using the Ly-5.1/Ly-5.2 system, mice were analyzed by flow cytometry as described below.
Antibody staining and flow cytometry
BM cells were lineage depleted using a magnetically labeled antibody kit (StemSep; Stem Cell Technologies, BC, Canada), which was followed by staining with a cocktail of phycoerythrin (PE)–conjugated antibodies to lineage markers that included Ly-6G (Gr-1), CD11b (Mac-1), CD45R/B220, CD4 (L3T4), CD8 (Ly-2), Ter119/Ly-76, CD90.2 (Thy1.2), and NK1.1 (NKR-P1B and NKR-P1C). The cells also were stained with antibodies to fluorescein isothiocyanate (FITC)–conjugated Ly-6A/E (Sca-1) and biotin-conjugated CD117 (c-kit). The biotinylated c-kit antibody was detected using a secondary streptavidin-phycoerythrin-Cy5 conjugate (SA-PE-Cy5). All antibodies for these studies were obtained from BD Pharmingen (San Diego, CA). Cells were then analyzed on a fluorescence activated cell sorter (FACS)Vantage SE flow cytometer equipped with an INNOVA 70C laser providing 488 nm of excitation at 70 mW output power (BD Biosciences, San Jose, CA) . For peripheral blood analyses, the percentage of Ly-5.2 donor engraftment in Ly-5.1 recipient mice was quantitated using an FITC-conjugated CD45.2 (anti–Ly-5.2) antibody in combination with either direct PE-conjugated or biotinylated PE-Cy5–conjugated lineage antibodies. Some analyses also were performed on a BD LSR flow cytometer (BD Biosciences). For 4-color analyses of KLS cells and Ly-5.1 on the BD LSR, allophycocyanin (APC)–conjugated antibody to c-kit and PE-Cy5–conjugated antibody to CD45.1 (Ly-5.1) were used.
Southern blot analyses
Genomic DNA was prepared from BM and spleen tissues from mice that received transplants as previously described.11 DNA was then extracted with an equal volume of phenol/chloroform/isoamyl alcohol 25:24:1 and precipitated with 2.5 volumes of ice-cold ethanol and 1/10 volume sodium acetate. Overnight, 5 μg DNA was digested withNcoI enzyme and separated on a 0.8% agarose gel by electrophoresis. Gels were blotted overnight onto Hybond N+nylon membrane (Amersham, Arlington Heights, IL), UV cross-linked, and hybridized with a [32P]-labeled mouse Y-chromosome probe. Blots were washed at a final stringency of 0.5X sodium citrate (SSC)/0.5% sodium dodecyl sulfate (SDS) at 65°C, exposed overnight, and autoradiographic images were obtained using a Storm phosphorimager (Amersham Biosciences) and x-ray film (Eastman Kodak, Rochester, NY). [32P]-dCTP was obtained from Amersham.
Results
Long-term repopulating defects are intrinsically dependent on STAT5 activation
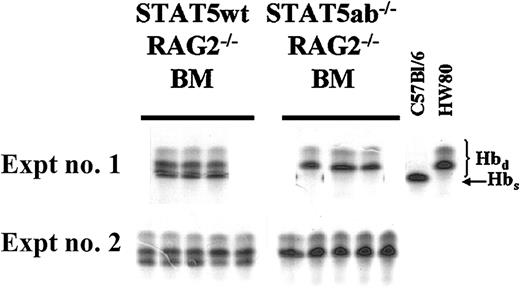
To eliminate lymphocytes, the C57Bl/6 STAT5ab+/−genotype was crossed onto the C57Bl/6 RAG2−/− background where splenomegaly disease and autoimmune phenotypes were not observed. BM cells were isolated from both hind limbs of donor C57Bl/6 (hemoglobin single, Hbs) RAG2−/−STAT5ab+/+ or RAG2−/−STAT5ab−/− littermate mice and mixed 1:1 with congenic wild-type HW80 (hemoglobin diffuse; Hbd) mouse BM cells using the approach previously described.11 In all RAG2−/− mice, absolute lymphocyte counts were virtually zero as determined on peripheral blood smear (data not shown). Lethally irradiated BM transplant (BMT) recipients were analyzed 19 and 18 weeks later for experiment no. 1 and experiment no. 2, respectively (Figure1).
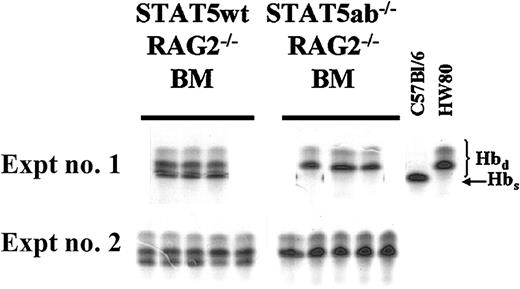
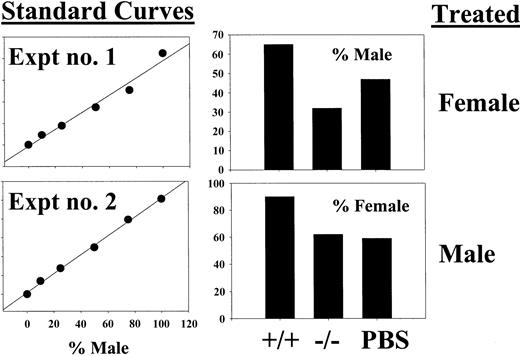
Competitive repopulation of STAT5-deficient HSCs after crossing onto a RAG2−/− background.
C57Bl/6 RAG2−/− mice were crossed onto the C57Bl/6 STAT5ab+/− background to allow for generation of triple knockout mice for RAG2, STAT5a, and STAT5b. BM was collected from young RAG2−/− STAT5ab−/− or littermate RAG2−/− STAT5ab+/+ mice and mixed at a 1:1 ratio with wild-type HW80 BM cells (Hbd). The cells were transplanted into lethally irradiated recipient mice, and reconstitution was measured by hemoglobin electrophoresis. The results of two experiments are shown with the STAT5 wild-type (wt) competition on the left and the STAT5ab−/− competition on the right. At the far right are positive controls for both the Hbs and Hbd patterns.
Competitive repopulation of STAT5-deficient HSCs after crossing onto a RAG2−/− background.
C57Bl/6 RAG2−/− mice were crossed onto the C57Bl/6 STAT5ab+/− background to allow for generation of triple knockout mice for RAG2, STAT5a, and STAT5b. BM was collected from young RAG2−/− STAT5ab−/− or littermate RAG2−/− STAT5ab+/+ mice and mixed at a 1:1 ratio with wild-type HW80 BM cells (Hbd). The cells were transplanted into lethally irradiated recipient mice, and reconstitution was measured by hemoglobin electrophoresis. The results of two experiments are shown with the STAT5 wild-type (wt) competition on the left and the STAT5ab−/− competition on the right. At the far right are positive controls for both the Hbs and Hbd patterns.
At all time points after transplantation, no Hbs was detectable from the RAG2−/− STAT5ab−/−graft, indicating a very severe engraftment disadvantage. As a control, RAG2 deficiency alone (RAG2−/− STAT5ab+/+) did not affect repopulating potential, resulting in levels of engraftment of 48% ± 2% in experiment no. 1 and 38% ± 6% in experiment no. 2. However, RAG2−/−STAT5ab−/− mice still had an abnormally reduced life span and thus were not useful as an alternative to increase the yield of surviving adult C57Bl/6 STAT5ab−/− mice.
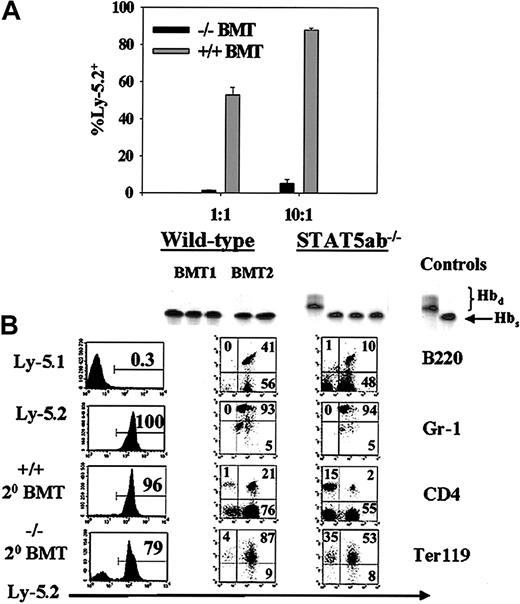
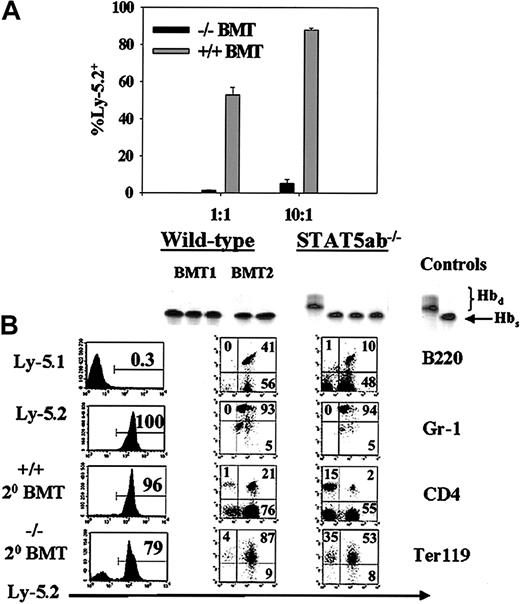
Since we previously showed that STAT5-deficient HSCs could engraft transplant recipients in the absence of a competitor marrow,11 a second approach was to generate chimeric mice by transplanting Ly-5.2/Hbs STAT5ab−/− or littermate Ly-5.2/Hbs wild-type BM cells into congenic HW80 (Ly-5.2/Hbd) recipients. Control mice were simultaneously generated by transplanting wild-type Ly-5.1/Hbs BM cells into normal HW80 recipients. All sets of primary mice that received transplants were generated by injection with the same donor equivalent of cells. The hemoglobin markers were used for analysis of chimerism. At 16 weeks after transplantation, BM was collected from all 3 sets of chimeric primary mice that received transplants. Secondary competitive repopulation experiments were then set up using BM cells from primary recipients: STAT5ab−/− (Ly-5.2) versus wild-type (Ly-5.1), referred to as −/−BMT in Figure2A, or littermate wild-type BM cells (Ly-5.2) versus wild-type (Ly-5.1), referred to as +/+ BMT in Figure2A. This approach provided a normal nonhematopoietic microenvironment for all 3 sets of stem cells to reside in the primary recipients and allowed for comparison of secondary repopulating potential. The relative engraftment with the donor Ly-5.2 STAT5ab−/−cells was markedly reduced at 1:1 (0.4% ± 0.05%; n = 4) and even at a 10:1 (4% ± 2%; n = 4) mixing ratio (P < .001), which was highly biased toward the Ly-5.2 graft. The engraftment defects were of similar magnitude in multiple lineages, as determined by antibody staining (data not shown). As expected, the control wild-type BM competition showed roughly the input ratio of cells with 49% ± 4% at 1:1 mixing and 84% ± 4% at 10:1 mixing. In separate transplants in the absence of a competitor, STAT5ab−/− HSCs were always able to reconstitute secondary recipients at levels roughly equivalent to primary recipients (Figure 2B). Shown are representative separate transplants into HW80 or Ly-5.1 recipients. Although increased chimerism was observed in mature red blood cells, Ter119+ erythroid progenitors, and CD4+ T lymphocytes, lymphomyeloid engraftment of B220+ B lymphocytes and Gr-1+myeloid cells remained complete. Additionally, the hematocrits of secondary BMT mice of 41% ± 2% for STAT5ab−/− and 49% ± 4% for wild-type mice that received transplants were essentially the same as following primary transplant.11
Secondary transplantation of STAT5-deficient BM cells with and without competition with wild-type BM cells.
Chimeric mice were first generated by primary transplant of either wild-type or STAT5-deficient BM cells into irradiated HW80 recipients. (A) For the competitive secondary transplant experiment, BM was collected 16 weeks following transplantation and mixed at a 1:1 ratio with Ly-5.1 BM from identically derived primary recipients. Sixteen weeks following secondary transplant, mice were analyzed for peripheral blood percentage of Ly-5.2–positive cells. (B) For the noncompetitive secondary transplant experiments, BM cells were injected into irradiated recipients, and secondary engraftment was documented following transplantation into either HW80 recipients using hemoglobin electrophoresis (top panels) or following transplantation into Ly-5.1 recipients using FACS for total Ly-5.2 (panels labeled Ly-5.1, Ly-5.2, +/+ 20 BMT, −/− 20 BMT) or multilineage reconstitution (panels labeled B220, Gr-1, CD4, Ter119 under the columns for wild-type and STAT5ab−/−).
Secondary transplantation of STAT5-deficient BM cells with and without competition with wild-type BM cells.
Chimeric mice were first generated by primary transplant of either wild-type or STAT5-deficient BM cells into irradiated HW80 recipients. (A) For the competitive secondary transplant experiment, BM was collected 16 weeks following transplantation and mixed at a 1:1 ratio with Ly-5.1 BM from identically derived primary recipients. Sixteen weeks following secondary transplant, mice were analyzed for peripheral blood percentage of Ly-5.2–positive cells. (B) For the noncompetitive secondary transplant experiments, BM cells were injected into irradiated recipients, and secondary engraftment was documented following transplantation into either HW80 recipients using hemoglobin electrophoresis (top panels) or following transplantation into Ly-5.1 recipients using FACS for total Ly-5.2 (panels labeled Ly-5.1, Ly-5.2, +/+ 20 BMT, −/− 20 BMT) or multilineage reconstitution (panels labeled B220, Gr-1, CD4, Ter119 under the columns for wild-type and STAT5ab−/−).
STAT5-deficient HSCs are not 5-FU sensitive and are noncompetitive during steady-state hematopoiesis
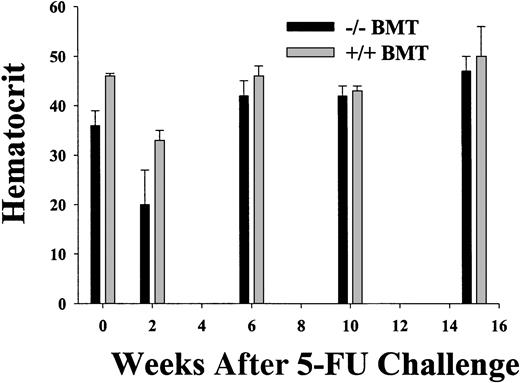
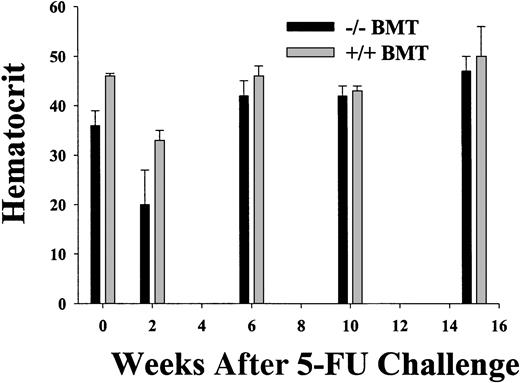
Using the BM chimera approach, engraftment was complete at all times from 8 weeks after transplantation, except for the previously described STAT5ab−/− T-lymphocyte chimerism.11 To determine the sensitivity of STAT5-deficient HSCs to a cell cycle–active drug, between 12 and 16 weeks after transplantation the chimeras were treated with a single intraperitoneal injection of 5-FU and analyzed serially for the hematocrit and the engraftment measured by hemoglobin electrophoresis (Figure 3). Although the myelosuppression was severe in the STAT5ab−/− chimeras, the recovery from the nadir was not delayed. At 2 weeks after transplantation for experiment no. 1, the hematocrit of 20% ± 7% for STAT5ab−/− was lower than the 33% ± 2% for wild-type chimeras. However, in both experiment no. 1 and experiment no. 2, the hematocrit was able to rebound to levels that unexpectedly were not different from the wild-type chimeras. In experiment no. 1 at 6 weeks, the hematocrit was normal (42% ± 3% for STAT5ab−/−versus 46% ± 2% for wild-type), and at 15 weeks the STAT5ab−/− chimeras had a hematocrit of 47% ± 3% (n = 3), and the wild-type chimeras had a hematocrit of 50% ± 6% (n = 4). In experiment no. 2 at 13 weeks, the STAT5ab−/− chimeras had a hematocrit of 42% ± 2% (n = 3), and the wild-type chimeras had a hematocrit of 43% (n = 2). Engraftment was always 100% at all time points as determined by hemoglobin electrophoresis (data not shown).
Recovery from myelosuppression in wild-type and STAT5-deficient chimeric mice following a single injection of 5-fluorouracil.
Chimeric mice were generated by transplantation of wild-type or STAT5ab−/− BM cells into lethally irradiated recipient HW80 mice. Reconstitution of irradiated recipients was determined by hemoglobin electrophoresis. After reconstitution, mice were injected once with 150 mg/kg 5 fluorouracil (5-FU). One week prior to the 5-FU injection and at the indicated times after injection, the mice were bled and the hematocrit was determined. A second experiment was also performed with fewer of the intermediate time points (data not shown). At all time points hemoglobin electrophoresis was also performed to check for donor engraftment (data not shown).
Recovery from myelosuppression in wild-type and STAT5-deficient chimeric mice following a single injection of 5-fluorouracil.
Chimeric mice were generated by transplantation of wild-type or STAT5ab−/− BM cells into lethally irradiated recipient HW80 mice. Reconstitution of irradiated recipients was determined by hemoglobin electrophoresis. After reconstitution, mice were injected once with 150 mg/kg 5 fluorouracil (5-FU). One week prior to the 5-FU injection and at the indicated times after injection, the mice were bled and the hematocrit was determined. A second experiment was also performed with fewer of the intermediate time points (data not shown). At all time points hemoglobin electrophoresis was also performed to check for donor engraftment (data not shown).
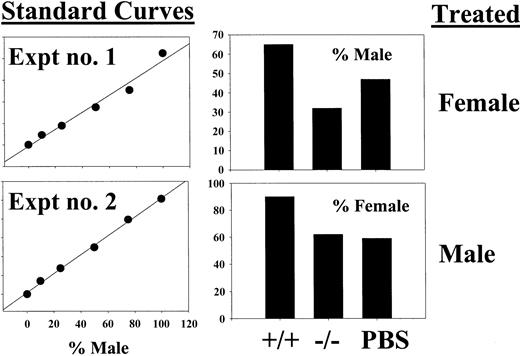
Although the HSC pool was not ablated by 5-FU treatment in the context of the chimeric mouse, we also wanted to determine whether STAT5-deficient repopulating cells might be more 5-FU sensitive than wild-type HSCs. For these experiments, we treated both mutant and wild-type young (3-5 weeks old) mice that had not received transplants with 150 mg/kg 5-FU, and 2 days later harvested the BM for competitive repopulation against PBS control–treated BM cells. Two experiments were performed, one with male treated mice and the other with female treated mice. In both cases, the male versus female competitive repopulation also was performed with both PBS-treated mice as a control. The mice were reconstituted for 16 weeks and then killed for BM collection. Pooled BM cells were then analyzed by Southern blot for the percentage of male DNA as determined using a standard curve of male DNA mixed into mouse Y-negative female DNA (Figure 4, left panels). Toxicity from the 5-FU treatment was reflected by an increase in the reconstitution with PBS-treated competitor as shown in the right panels. Modest 5-FU toxicity was observed on wild-type BM cells, however, relative to the wild-type samples, the STAT5-deficient 5-FU–treated samples competed on average 1.7-fold better against its own PBS-treated competitor. Thus, STAT5 deficiency does not increase 5-FU sensitivity but rather may decrease sensitivity even in the 3- to 5-week old mutant microenvironment. The average overall engraftment of the donor cell pool was used as the measure of repopulating potential to control for possible variation between individual recipients of STAT5ab−/− BM cells, which were likely limiting in total HSC activity.
Competitive repopulation of BM from wild-type and STAT5-deficient mice 48 hours following a single 5-FU injection.
Two separate experiments were performed using a male versus female competitive repopulation assay. In experiment no. 1, female donor mice were 5-FU treated, and in experiment no. 2, male donor mice were treated. In each case, the BM cells were mixed with the opposite sex cells harvested from PBS control–treated mice. BM cells were mixed at a 1:1 ratio, and the relative levels of male engraftment were determined by Southern blot analysis of pooled BM DNA obtained from 2 to 5 recipient mice. Two blots were probed with a male-specific probe, and a standard curve of male DNA mixed with female DNA was run on each gel (left panels). The y-axis for the standard curves is arbitrary units defined from the band volumes using ImageQuant software (Amersham Biosciences). On the right are the results of densitometry showing the percent contribution of the PBS-treated competitor.
Competitive repopulation of BM from wild-type and STAT5-deficient mice 48 hours following a single 5-FU injection.
Two separate experiments were performed using a male versus female competitive repopulation assay. In experiment no. 1, female donor mice were 5-FU treated, and in experiment no. 2, male donor mice were treated. In each case, the BM cells were mixed with the opposite sex cells harvested from PBS control–treated mice. BM cells were mixed at a 1:1 ratio, and the relative levels of male engraftment were determined by Southern blot analysis of pooled BM DNA obtained from 2 to 5 recipient mice. Two blots were probed with a male-specific probe, and a standard curve of male DNA mixed with female DNA was run on each gel (left panels). The y-axis for the standard curves is arbitrary units defined from the band volumes using ImageQuant software (Amersham Biosciences). On the right are the results of densitometry showing the percent contribution of the PBS-treated competitor.
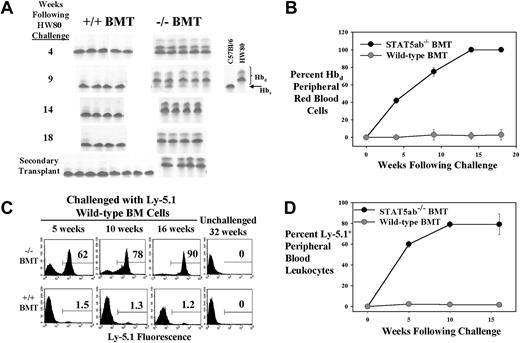
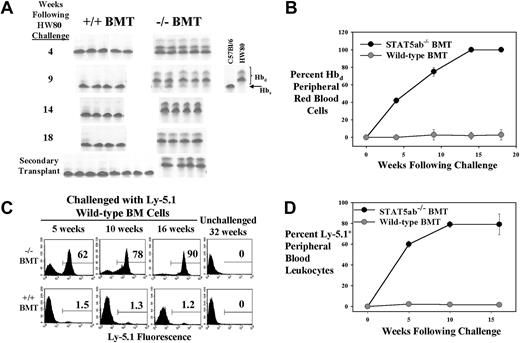
To test whether STAT5-deficient chimeras would be favorable recipients for a wild-type BM challenge, chimeric mice were again generated. STAT5ab−/− or wild-type littermate BM cells (Ly-5.2/Hbs) were transplanted into either Ly-5.1/Hbs recipients (experiment no. 1) or HW80 (Ly-5.2/Hbd) primary recipients (experiment no. 2). Engraftment with donor cells was documented in the lethally irradiated hosts by either Ly-5.2 FACS (experiment no. 1) or by hemoglobin electrophoresis (experiment no. 2). Mice in experiment no. 1 were challenged with a dose of 5 × 106 wild-type HW80 BM cells (Hbd). Analysis of engraftment with the HW80 challenge cells was then tracked over time by hemoglobin electrophoresis. Figure 5A-B shows results of serial analysis of all mice challenged with HW80 BM cells. Mice in experiment no. 2 were challenged with a dose of 5 × 106 wild-type Ly-5.1 cells, and analysis of engraftment was by FACS. A representative example of the serial FACS analysis is shown in Figure 5C. The average of all mice analyzed is shown in Figure 5D (n = 4). In both experiments, the donor cells had a competitive advantage in the STAT5ab−/− chimeric mice but not in the wild-type chimeric mice. Engraftment levels were observed in multiple hematopoietic lineages (Gr-1+, 86% ± 6%; B220+, 90% ± 4%; CD4+, 62% ± 48%; Ter119+, 83% ± 8%). The CD4+ T-lymphocyte variability was likely due to competition with the endogenous host T lymphocytes that were not replaced after the primary transplant. In contrast, minimal levels of engraftment were detected in challenged wild-type chimeras lineages (Gr-1+, 0.4% ± 0.2%; B220+, 1.8% ± 0.6%; CD4+, 1.4% ± 0.6%; Ter119+, 1.3% ± 0.3%) with unchallenged mice showing a background of 0.2% ± 0.1% as an average of all lineages. After 16 weeks, the mice were killed, and the BM was collected. Ly-5.1 FACS analyses on the BM from experiment no. 2 of the mice showed 81% ± 6% Ly-5.1+ cells in the STAT5ab−/−chimera but only 0.23% ± 0.03% in the wild-type chimera. Secondary transplant recipients from experiment no. 1 (Figure 5A) and experiment no. 2 (data not shown) showed multilineage reconstitution with the wild-type donor cells, confirming the stem cell level engraftment advantage.
Challenge of fully reconstituted BM chimeric mice with an injection of wild-type BM cells.
(A-B) Chimeric mice were generated in the Ly-5.1 background and were then challenged with a dose of wild-type HW80 BM cells. Following injection, the mice were bled serially and analyzed for the presence of Hbd red blood cells by hemoglobin electrophoresis with analyses continued for up to 18 weeks. At the time of killing, BM cells from some mice were transplanted into lethally irradiated Hbs secondary recipients (panel A, bottom). (C-D) Chimeric mice were generated in the HW80 background and then challenged with a dose of wild-type Ly-5.1 BM cells. Following injection, the mice were bled serially and analyzed for the presence of Ly-5.1–positive cells by flow cytometry for up to 16 weeks following challenge.
Challenge of fully reconstituted BM chimeric mice with an injection of wild-type BM cells.
(A-B) Chimeric mice were generated in the Ly-5.1 background and were then challenged with a dose of wild-type HW80 BM cells. Following injection, the mice were bled serially and analyzed for the presence of Hbd red blood cells by hemoglobin electrophoresis with analyses continued for up to 18 weeks. At the time of killing, BM cells from some mice were transplanted into lethally irradiated Hbs secondary recipients (panel A, bottom). (C-D) Chimeric mice were generated in the HW80 background and then challenged with a dose of wild-type Ly-5.1 BM cells. Following injection, the mice were bled serially and analyzed for the presence of Ly-5.1–positive cells by flow cytometry for up to 16 weeks following challenge.
STAT5-deficient KLS cells are defective in long-term repopulating activity in vivo and have reduced cytokine responsiveness in vitro
We next wanted to determine whether the effects observed in vivo would be evident in an enriched HSC population isolated from the BM of the STAT5-deficient mice. For some experiments, Sca-1+c-kit+lin− (KLS) cells were transplanted at limiting dilution into lethally irradiated recipient mice along with 2 × 105 competitor cells (Table1). In all 3 transplants with wild-type KLS cells, long-term repopulating activity was seen with cell doses as low as 250 cells. However, in 2 separate KLS transplants using mutant cells, no detectable engraftment was observed with 10- to 20-fold higher KLS cell doses. Even following injection of lineage-negative non-KLS cells from experiment no. 3, no engraftment was detected in 3 mice receiving 14 000 cells, while 1 of 4 mice receiving 43 000 wild-type lineage-negative non-KLS cells did show some engraftment (6%). This result demonstrates the complete loss of competitive long-term repopulating activity despite a normal KLS phenotype.
In other experiments, 200 to 800 KLS cells/well were put into liquid suspension culture in the presence of cytokine cocktails to stimulate proliferation in vitro over a 6-day incubation period. Consistent with the defective repopulating activity in vivo, the KLS cells showed a reduced expansion potential in vitro (Table2).
As expected, IL-3 supported the greatest proliferation and was required for maximal levels of cell expansion. Stimulation with cocktails containing other early-acting growth factors such as IL36S, IL3S, and IL3SFT revealed the greatest deficiency for STAT5-deficient KLS cells. These growth factor combinations are known to synergize with IL-3 to promote cell proliferation and survival during short-term ex vivo culture. As described previously, in all experiments the absolute number of hind limb KLS cells was not different between wild-type and STAT5-deficient mice. Interestingly, analysis of KLS numbers from 3 separate experiments 16 weeks following transplantation also showed equivalent numbers of KLS cells between both hind limbs of the mice receiving transplants of wild-type BM cells (7945 ± 4738), STAT5ab−/− BM cells (4367 ± 1168), and unmanipulated wild-type mice (4703 ± 1836). All KLS cells from mice receiving transplants of BM cells were 98% ± 1% Ly-5.2+ and thus donor derived. Therefore, complete regeneration of the KLS pool following transplant did not require STAT5 activation.
Discussion
This study sought to determine whether the competitive disadvantage of STAT5-deficient HSCs was due primarily to a cell intrinsic requirement for STAT5 activation or to the other life-threatening phenotypes observed in these mice.13,15Since previous reports have shown antiapoptosis15 and cell cycle–related12 defects in the adult STAT5-deficient mouse, we have clarified a critical issue regarding the role of STAT5 in long-term repopulating activity. The STAT5-deficient mouse is severely affected by many phenotypes that reduce the life span and that may likely contribute to an autoimmune-mediated effect on HSC survival within the BM cavity. The results of this study indicate that STAT5 activation is required downstream of early-acting cytokines to promote cytokine-mediated repopulating activity of HSCs. The inability to rescue this defect by elimination of lymphocytes or by transplant into a “normal” wild-type microenvironment demonstrates that the primary mechanism for the defect is cell intrinsic.
We isolated KLS fractions from young STAT5ab−/− mice and tested these cells for repopulating activity in vivo and cytokine responsiveness in vitro. Consistent with the whole BM competitive repopulation experiments, the sorted KLS cells were unable to contribute to hematopoiesis even at very high numbers in the competitive repopulation assay. This was also consistent with the report by Snow et al using sorted Sca-1+lin−BM cells.12 In contrast, good engraftment was observed in all 3 experiments where positive control wild-type KLS cells were injected. HSC surface phenotype can change associated with activation, resulting in c-kit down-regulation,17 Mac-1 up-regulation,17 or CD34 up-regulation.18Although we showed the inability of lineage-negative non-KLS cells from STAT5-deficient mice to engraft, we cannot rule out that the STAT5-deficient HSCs had acquired Mac-1, since an antibody to Mac-1 was used in our lineage cocktail. However, the 5-FU studies in Figures 3and 4 strongly suggest that STAT5-deficient repopulating cells are not “activated” but instead may be more quiescent. Consistent with the transplant data, the in vitro cytokine stimulation was highly reduced in the STAT5-deficient KLS cells. The 10-fold deficiency in response to IL-3 and SCF provides evidence that STAT5 may be a key intermediate in SCF signaling in primitive hematopoietic cells. Further studies will be required to determine the degree to which SCF signaling may be specifically impaired in these mice. In the BM challenge experiments, the ability of wild-type BM cells to take over the hematopoietic system also had similar kinetics with that described by others following injection into c-kit signaling deficient W/Wv mice, which have dominant defects in the white spotting locus (W).19Repopulating deficiencies were therefore not due to a homing defect since injected competitor cells could take over even after prior engraftment with the primary transplant and in the absence of further host conditioning. However, we cannot rule out that migration and competition for the BM niche during steady-state hematopoiesis20 might be defective. c-kit is known to interact with both JAK2 and SH2-containing protein tyrosine phosphatase-1 (SHP-1), which act as positive and negative regulators, respectively, for modulating downstream signaling21,22 and possibly modulating STAT5 activation in stem cells. Some evidence exists that SCF can activate STAT5 in mast cells23 and some cell lines in vitro,24 although evidence supporting STAT5 activation downstream of SCF/c-kit in HSCs has not been previously reported.
Bromodeoxyuridine-labeling studies have shown that within 30 days, all murine HSCs will have gone through one cell division.25,26Therefore, rather than a clonal succession model with both proliferating and deeply quiescent HSC populations, HSCs are currently believed to be slowly cycling, but at any one point in time only 4% to 5% of the HSC population will be in S/G2/M. Here we show by competitive repopulation that repopulating STAT5-deficient HSCs are less sensitive to the mild 5-FU effects than wild-type HSCs. This result suggests that less cell cycle activation reduced 5-FU toxicity27 or 5-FU–mediated HSC activation,17which has been associated with decreased repopulating activity.28 Further studies using 5-FU in combination with SCF will be necessary to test more HSC toxic regimens.27Possible downstream targets of STAT5 in HSCs could be the cell cycle–associated genes such as the cyclins.29,30 Cyclin D2 is expressed at high levels in long-term repopulating HSCs.26 While we were unable to obtain sufficient numbers of STAT5-deficient mice and KLS cells for cell cycle analysis by FACS, future studies on differential gene expression might identify important downstream genes that are dysregulated. STAT5 is a mediator of p210Bcr-abl signaling in transduced cells,31 and chronic myelogenous leukemia is known to be a clonal stem cell proliferative disease. Constitutively activated mutants of STAT332 and STAT533 are also oncogenic. Therefore, the decreased cytokine responsiveness reported here for STAT5-deficient HSCs provides further support for targeting STAT5 as a treatment for at least some leukemias. However, STAT5 is not essential for myeloproliferative disease in chronic myeloid leukemia models, since STAT5-deficient mice could still develop disease.34 In an acute myeloid leukemia (AML) model for the Tel-JAK2 translocation, STAT5 activation was essential for leukemogenesis,33 and STAT5 activation is associated with AML.35 36
In these studies we have not determined whether antiapoptosis gene expression is dysregulated in the absence of STAT5. This might also comprise a significant component of the deficiency, although increased apoptosis in the HSC compartment would be predicted to reduce the pool size. Survival signaling pathways mediated through c-mpl37and/or Flt338 might also require STAT5 activation in HSCs. Other signaling components downstream of c-kit could also compensate for the STAT5-mediated defects, possibly resulting in a similar but less severe phenotype than reported for the W/Wv mouse. Our data demonstrate that STAT5 activation is not essential for HSC self-renewal, since the KLS pool was restored to levels equivalent with wild-type mice following transplantation and hematopoietic reconstitution following 5-FU treatment or secondary transplant was not severely reduced. This finding was consistent with the idea that SCF may not be essential for murine HSC self-renewal39-41 but capable of promoting cell cycle entry.42 However, differences might exist between fetal and adult HSCs in this regard.43 Direct demonstration of STAT5 activity in the specific proliferative or antiapoptotic responses to cytokine stimulation will require further study. In summary, this study demonstrates that overall stem cell cytokine responsiveness and repopulating potential are highly STAT5 dependent and that in the STAT5-deficient background, wild-type BM cells have a dominant selective advantage in multiple lineages. Inversely, it remains to be determined whether conditional STAT5 activation might be used to confer a competitive engraftment advantage on wild-type BM cells. Recent demonstration of STAT5-dependent expansion of multipotential hematopoietic cells by signals from JAK2 and c-kit or Flt3 supports this concept.44
The authors would like to thank James N. Ihle for helpful discussions and Robert G. Hawley for critical review of the manuscript. We also thank Albert Forero for technical assistance with the mouse genotyping PCR analyses.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-05-1602.
Supported by National Institutes of Health grant R01DK059380, The Lauri Strauss Leukemia Foundation (K.D.B.), and the American Red Cross.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kevin D. Bunting, Hematopoiesis Department, American Red Cross Holland Laboratory, 15601 Crabbs Branch Way, Rockville, MD 20855; e-mail:buntingk@usa.redcross.org.