A total of 109 patients (aged 6-66 years; median, 46 years) with myelodysplastic syndrome (MDS) were treated with busulfan (BU) targeted to plasma concentrations of 800 to 900 ng/mL plus cyclophosphamide (CY), 2 × 60 mg/kg, and hemopoietic stem cell (HSC) transplantation from related (n = 45) or unrelated donors (n = 64). At the time of transplantation, 69 patients had less than 5% myeloblasts in the marrow, and 40 patients had more advanced disease. All but 2 evaluable patients had engraftment. The Kaplan-Meier estimates of 3-year relapse-free survival (RFS) were 56% for related and 59% for unrelated recipients. The cumulative incidences of relapse were 16% for related and 11% for unrelated recipients. Nonrelapse mortality (NRM) at 100 days (3 years) was 12% (28%) for related and 13% (30%) for unrelated recipients. The only factor significant for RFS was the etiology of MDS (de novo better than treatment related;P = .03). Factors significantly correlated with relapse were advanced French-American-British classification (P = .002) and International Prognostic Scoring System score (P = .009), poor-risk cytogenetics (P = .03), and treatment-related etiology (P = .03). None of the factors examined was statistically significant for NRM. Patient age and donor type had no significant impact on outcome. RFS tended to be superior in patients receiving transplants with peripheral blood rather than marrow stem cells. Thus, a targeted BUCY regimen provided effective transplant conditioning for patients with MDS receiving transplants from HLA-identical siblings or alternative donors. Although there was still considerable nonrelapse morbidity and mortality, the present regimen was used successfully even in patients older than 60 years of age.
Introduction
The myelodysplastic syndromes (MDSs) are clonal hemopoietic disorders, characterized by ineffective hemopoiesis resulting in single or multilineage peripheral blood cytopenias, dysplastic morphology in single or multiple lineages, and, in many patients, clonal cytogenetic abnormalities. The French-American-British (FAB) classification has subdivided the disease into refractory anemia (RA; < 5% marrow blasts), RA with ringed sideroblasts (RARS), RA with excess blasts (RAEB; 5%-20% blasts), and RAEB in transformation (RAEB-T; 21%-30% blasts).1 A World Health Organization proposal suggests that patients with more than 20% blasts should now be considered as having acute myeloid leukemia (AML).2Chronic myelomonocytic leukemia (CMML) was previously considered an MDS, but has recently been reclassified as a myeloproliferative disorder. In an attempt to improve prognostic accuracy for patients with MDS, a team of investigators has proposed the International Prognostic Scoring System (IPSS), which considers, in addition to the proportion of blasts, clonal chromosomal abnormalities and the number of peripheral blood cytopenias.3 Depending on the IPSS score, a patient's median life expectancy may be as long as a decade or as short as a few months.
Currently, the only established therapy with curative potential for MDS is a hemopoietic stem cell transplant (HSCT).4Investigators generally agree that for patients with advanced disease, particularly those of younger age, HSC transplantation is the treatment of choice. Transplantation for less advanced disease, especially in older patients and with the use of unrelated volunteer donors, has remained controversial. The major reason for this controversy is the incidence of posttransplantation nonrelapse mortality (NRM), which in some trials has been as high as 50% to 60%.5-11
We have developed a transplant-conditioning regimen that combines oral busulfan (BU) targeted to predetermined plasma levels in combination with cyclophosphamide (CY). Observations in patients with chronic myelocytic leukemia indicated that this approach was associated with reduced toxicity and with a low probability of leukemia relapse.12 Encouraged by those observations, we conducted the present trial in patients with MDS. Preliminary data from this trial suggested that NRM was reduced, and as a result, relapse-free survival (RFS) improved compared to RFS achieved with total body irradiation (TBI)–conditioning regimens. Here, we present a comprehensive analysis of this trial enrolling patients with less advanced, as well as more advanced, MDS who received HSCTs from related or unrelated donors.
Patients and methods
Patients
From January 1993 to December 2000, 109 patients (57 male, 52 female), 6 to 66 years of age (median, 46 years), were enrolled. Patient and disease characteristics are summarized in Table1. The interval from diagnosis to transplantation was 1 to 128 months (median, 10 months). The study was initially designed for patients with less than 5% myeloblasts in the marrow (RA and RARS) who had volunteer unrelated donors. However, as concurrent trials in patients with HLA-identical related donors were completed,10,13,14 the present trial was also opened for transplants from related donors. Furthermore, because preliminary results with the BUCY regimen in older patients with more advanced disease were encouraging,13 the present study was also opened to patients with MDS with more than 5% myeloblasts in the marrow. Overall, 69 patients fulfilled the criteria for RA/RARS, 24 had RAEB, 10 had RAEB-T/transformation into AML (tAML), and 6 had CMML or unclassifiable MDS. In 78 patients, MDS arose de novo; 14 had previously received chemotherapy, irradiation, or both for treatment of Hodgkin disease (n = 6), non-Hodgkin lymphoma (n = 3), acute lymphocytic leukemia (n = 1), rheumatoid arthritis (n = 1), or solid tumors (n = 3). Seventeen patients had pre-existing hematologic disorders, including aplastic anemia (n = 13), paroxysmal nocturnal hemoglobinuria (n = 2), or essential thrombocythemia (n = 2). By IPSS criteria, 63 patients had good-risk, 16 intermediate-risk, and 29 poor-risk cytogenetics (material was insufficient for determination in 1 patient). By overall IPSS scores, 16 patients were considered low risk; 54, intermediate-1; 24, intermediate-2; and 8, high risk at the time of transplantation. In 7 patients, the score was undetermined.
All patients had received transfusions of red blood cells, platelets, or both. Additional therapy before referral in some patients included administration of granulocyte colony-stimulating factor (G-CSF), erythropoietin, or additional growth factors for variable periods of time. Others had received androgens or glucocorticoids in the recent or distant past. Six patients (5 with tAML and 1 with RAEB-T) received induction-type chemotherapy (generally cytosine arabinoside combined with daunorubicin or topotecan) before transplantation. Five achieved a remission as defined by marrow morphology, although in 2 patients peripheral blood cell counts had not reached normal values. One of the 6 had 10% blasts in the marrow at the time of transplantation.
Conditioning regimen
Patients were prepared with a regimen of oral BU at starting doses of 1 mg/kg to be given every 6 hours for 16 doses, followed by CY intravenously, 60 mg/kg per day on 2 consecutive days. Details of the chemical (gas chromatography/mass spectrometry) and pharmacokinetic analyses of BU to provide accurate assessments of BU exposure during conditioning have been described.12,15,16 Blood samples were collected at 0, 1, 2, 4, and 6 hours after the morning doses on days 1, 2, and 3 of BU administration, and dose adjustments were made for subsequent doses to maintain steady-state plasma levels in the range of 800 to 900 ng/mL. The BU_Css values given were the means of the values observed in each patient on those 3 days.12 Phenytoin was administered to all patients at loading doses of 15 mg/kg before BU administration, and maintenance dosing of 300 mg orally per day was continued until 24 hours after the last dose of BU.
Donors
Donor and transplant characteristics are summarized in Table2. Forty-five patients received transplants from related donors (HLA-identical siblings [n = 41]; HLA-phenotypically matched relative [n = 1]; DQB1-mismatched relative [n = 1]; DRB1-mismatched relative [n = 1]; and 1 from a syngeneic donor), and 64 from volunteer unrelated donors (HLA-matched by intermediate- or high-resolution typing in 53 cases; mismatched for one HLA-A antigen in 4, one HLA-B antigen in 1, one DR antigen [allele] in 1, one HLA-A plus one HLA-B antigen in 3, and one HLA-B plus one HLA-C antigen in 2 cases).17 18
Source of stem cells
The source of stem cells was marrow in 81 patients and peripheral blood after G-CSF mobilization in 28 patients (19 HLA-identical siblings, 6 HLA-identical unrelated donors, and 3 HLA-nonidentical donors).
Graft-versus-host disease prophylaxis
Prophylaxis for acute graft-versus-host disease (GVHD) consisted of methotrexate (MTX) and cyclosporine (CSP) in 97 patients, CSP plus mycophenolate mofetil (MMF) in 6, and MTX and FK506 in 5 patients.19,20 One patient had a syngeneic donor and received no GVHD prophylaxis. Adjustments for MTX, CSP, MMF, and FK506 were made if deemed clinically necessary. Acute and chronic GVHD were diagnosed and graded using established criteria.21,22Standard therapy for acute GVHD consisted of methylprednisolone. Steroid-refractory GVHD was treated according to various protocols involving the use of antithymocyte globulin, monoclonal antibodies, rapamycin (sirolimus), or MMF.23-25
Engraftment and rejection
The day of engraftment was defined as the first of 3 consecutive days on which the neutrophil count exceeded 0.5 × 109/L.17 Evidence of graft rejection was sought when the absolute neutrophil count (ANC) failed to reach 0.5 × 109/L, when the ANC declined after initial recovery, and when relapse was diagnosed. Patients who died before day 28 were considered nonevaluable for engraftment. When the donor and patient were of different gender, fluorescence in situ hybridization with Y or X chromosome–specific probes was performed on bone marrow and peripheral blood mononuclear cells (PBMCs).26,27 When patient and donor were of the same gender, DNA from marrow and PBMCs was amplified for several variable number tandem repeat loci for identification of informative host and donor markers.28
Relapse
All patients were scheduled to have marrow samples examined morphologically and by cytogenetic and flow cytometric analyses on days 28 and 84 after transplantation, and then annually or as clinically indicated. Relapse was defined morphologically as the detection of metaphases in the marrow that showed the same clonal marker(s) identified before transplantation, or as the reemergence of blasts or aberrant precursors identified by flow cytometry.29
Infection
Blood samples were examined weekly for evidence of cytomegalovirus (CMV) antigenemia. Interstitial pneumonia was diagnosed on the basis of radiographic findings, by use of culture, histologic, or histochemical analysis of bronchoalveolar lavage fluid, open lung biopsy, or at autopsy. Strategies to prevent infectious diseases included the prophylactic use of systemic antibiotics, fluconazole, acyclovir, and ganciclovir. All CMV-seronegative patients received CMV− blood products. Acyclovir prophylaxis was given to all patients who were seropositive for herpes simplex or herpes zoster virus. Ganciclovir was given to all CMV-seropositive recipients at engraftment or at the first documentation of antigenemia.30
Causes of death
Deaths that occurred after posttransplantation relapse were categorized as due to relapse regardless of the proximate causes; deaths in the absence of relapse were categorized as NRM. Infection was considered the cause of death when a bacterial, viral, or fungal infection other than interstitial pneumonia was the proximate cause of death in patients who had not had relapse. Infections were further categorized according to whether or not they were associated with GVHD and with organ failure. Multiorgan failure was considered the cause of death if decompensation occurred in at least 2 organ systems (eg, liver and kidneys or liver and lungs) and could not be attributed to GVHD or infection alone.
Statistical analysis
The probabilities of RFS were estimated using the Kaplan-Meier method.31 The incidences of relapse and NRM were expressed in terms of cumulative incidence (CI).32 Cox regression was used to analyze risk factors related to the hazard rates for these outcomes. In these analyses, relapse and NRM were considered competing events. The time to these outcomes was censored at the time of the competing event. Multivariate models were constructed by a forward selection procedure. At each step, the most significant factor at a level of at least 0.05 was added. Multivariate P values refer to the significance of the factor after adjusting for other factors in the final multivariate model. All P values are based on likelihood ratio statistics from Cox regression models and are 2-sided. Results were analyzed as of June 30, 2001.
Results
Conditioning
The target range steady-state plasma level for BU was 800 to 900 ng/mL. The actual BU_Css levels reached were 635 to 1140 ng/mL (median, 883 ng/mL) with 65 patients (60%) in the prescribed target range. Although 13% of patients achieved this level with the prescribed doses, 78% required BU dose reductions, and 9% required increments. The actual total dose of BU administered ranged from 9.6 to 20.2 mg/kg (mean, 14.3 mg/kg). The doses in 7 patients younger than 10 years were not significantly different from the remainder of the study cohort. All patients received the prescribed dose of CY, 2 × 60 mg/kg.
Engraftment
Five patients died before day 28 and were not considered evaluable for engraftment. A total of 104 patients achieved engraftment, as determined by an ANC of 0.5 × 109/L at 10 to 30 days (median, 19 days), and 90 reached platelet counts of more than 20 × 109/L at 7 to 137 days (median, 21 days) after transplantation. Two patients showed recovery of host cells, and both died, one after 2 additional attempts at transplantation. Engraftment was sustained in the remaining 102 patients.
GVHD
Acute GVHD of grades II to IV developed in 78 patients for CIs 64% among patients with HLA-identical sibling donors, 68% among patients with HLA-matched unrelated donors, and 100% among patients with HLA-nonidentical related or unrelated donors. The incidence of GVHD of grades III to IV was 7% after HLA-identical sibling transplants, 19% after matched unrelated transplants, and 29% after HLA-nonidentical transplants. In all but one patient, GVHD developed before day 30.
Chronic GVHD occurred in 47% of patients, and incidence rates were similar with related and unrelated donors, and with HLA-identical and -nonidentical recipients.
RFS
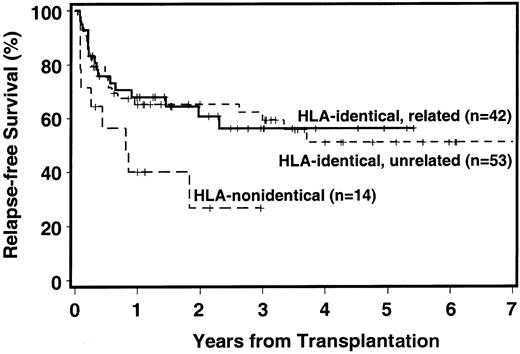
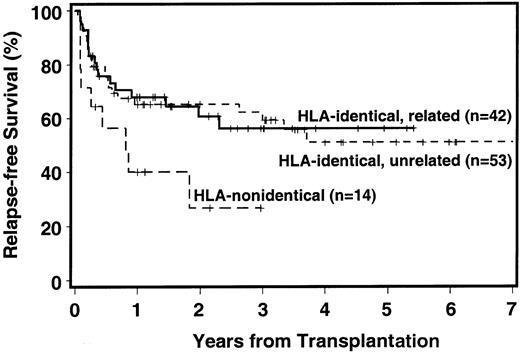
Currently, 64 patients are surviving, 62 in continued complete remission. Overall relapse incidence, RFS, and NRM for HLA-matched donor transplants are summarized in Table3. The probability of 3-year RFS was 56% with HLA-identical sibling donors, 59% with HLA-matched unrelated donors, and 27% with HLA-mismatched donors (Figure1). Among recipients of HLA-identical sibling transplants, RFS at 3 years was 68% for patients with RA/RARS, 45% for patients with RAEB, and 33% for patients with more advanced disease. Corresponding figures for patients with matched unrelated donors were 70%, 40%, and 17%, respectively.
Disease-free survival among recipients of HLA-identical related, HLA-identical unrelated, and HLA-nonidentical (related or unrelated) transplants
. The + indicates censored patient.
Disease-free survival among recipients of HLA-identical related, HLA-identical unrelated, and HLA-nonidentical (related or unrelated) transplants
. The + indicates censored patient.
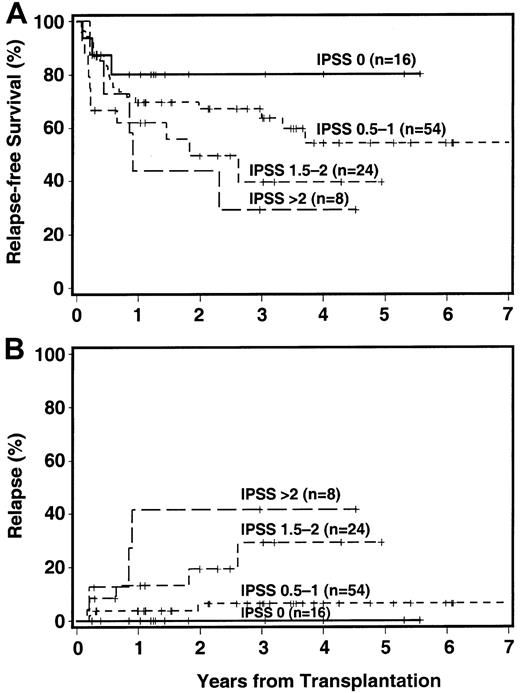
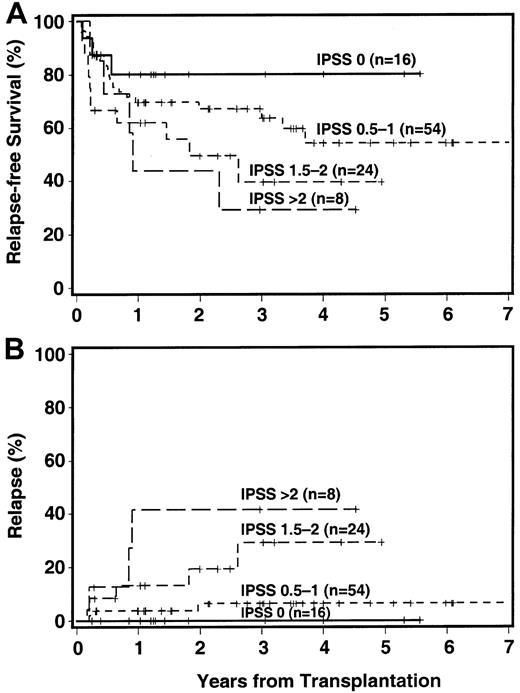
Relapse-free survival by IPSS category is shown in Figure2A. The 3-year RFS was 80% among patients in the low-risk group (IPSS score 0), and progressively decreased with increasing scores to 29% among patients with an IPSS score higher than 2.
Impact of IPSS score on outcome.
The + indicates censored patient; in 7 patients, an IPSS score could not be assigned. (A) RFS. (B) CI of relapse.
Impact of IPSS score on outcome.
The + indicates censored patient; in 7 patients, an IPSS score could not be assigned. (A) RFS. (B) CI of relapse.
Relapse
Thirteen patients had recurrent or progressive disease, reaching a cumulative incidence of 14% at 3 years, and 11 of these have died (Table 4). The 3-year incidence of relapse was strongly correlated with cytogenetic findings (9%, 13%, and 26%, for good, intermediate, and poor risk, respectively), with FAB category (5%, 34%, and 32% for RA/RARS, RAEB, and RAEB-T/tAML, respectively), and IPSS score (0%, 6%, 29%, and 42% for low, intermediate-1, intermediate-2, and high risk, respectively, Figure 2B).
NRM
The NRM rate among all patients was 16% by day 100, and 31% by 3 years, 12% and 28%, respectively, for HLA-identical sibling transplants, 13% and 30%, respectively, for matched unrelated donor transplants, and 36% and 52%, respectively, for recipients of HLA-nonidentical transplants.
Forty-five patients have died. Causes of death are summarized in Table4. The most frequent causes of death, in addition to relapse, were organ failure, in particular veno-occlusive disease of the liver, GVHD with bacterial infections or disseminated aspergillosis, and pneumonia/idiopathic pneumonitis syndrome.
Effect of source of hemopoietic stem cells
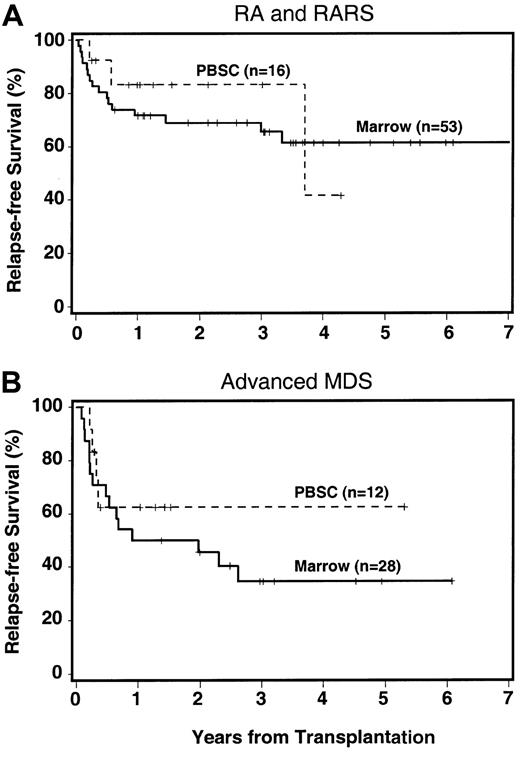
Among 28 patients receiving PBSC transplants, the 3-year RFS was 68%, compared to 48% for patients receiving marrow transplants. Differences were observed in all MDS categories (Figure3; RA/RARS, 83% versus 61%; advanced MDS, 63% versus 32%; IPSS score ≤ 1, 82% versus 61%; IPSS score > 1, 80% versus 38%). These differences were not affected by year of transplantation.
RFS by source of cells.
RFS by source of hemopoietic stem cells (marrow versus PBSCs) among patients with RA/RARS (A) and among patients with more advanced MDS (B). The + indicates censored patient.
RFS by source of cells.
RFS by source of hemopoietic stem cells (marrow versus PBSCs) among patients with RA/RARS (A) and among patients with more advanced MDS (B). The + indicates censored patient.
Statistical analyses
Results of univariate analysis are shown in Table5. The most significant risk factors for relapse were the etiology of MDS (hazard ratio for prior chemotherapy or radiotherapy 5.4, and for prior hematologic diagnosis, 0.8; as compared to de novo MDS; P = .03), cytogenetic risk group (hazard ratio 2.1 for intermediate risk, and 5.2 for high risk as compared to good risk; P = .03), the FAB category (hazard ratio 8.5 for RAEB, and 8.6 for RAEB-T/tAML, as compared to RA;P = .002), and the IPSS score (P = .002; if the IPSS score was considered a continuous variable; Figure 2B). For NRM, none of the pretransplantation risk factors had a significant impact. However, NRM was higher in patients who developed acute GVHD grades II to IV (P = .006). For RFS, the etiology of MDS was significant (hazard ratio for prior chemotherapy or radiotherapy 2.8, and for prior hematologic disorder 1.3, relative to de novo MDS;P = .03); and IPSS score, if analyzed as a continuous variable (P = .04; Figure 2A). FAB category (P = .08) showed a trend for significance.
In multivariate analysis, after adjusting for etiology of MDS, no other factors, including the source of hemopoietic stem cells and patient age, were significantly associated with RFS. Similarly, no other factors were significantly associated with relapse after adjusting for either IPSS score or FAB classification.
Discussion
This study shows that targeted BU plus CY provided effective conditioning for patients with MDS before HSC transplantation from related and unrelated donors. Outcomes with HLA-identical related and unrelated donors were similar. Engraftment was achieved in all but 2 patients, and early treatment-related mortality was low. The strongest predictors for relapse were FAB and IPSS classifications, and this effect on relapse was reflected in RFS. Patients with de novo MDS or MDS developing from pre-existing hematologic disorders fared better than those with therapy-related MDS.
Although the BUCY regimen was originally designed for patients with RA/RARS, this trial showed that patients with more advanced/high-risk disease could undergo successful transplantation with this regimen, in agreement with earlier reports by O'Donnell et al5 and by Ratanatharathorn et al.6 In fact, RFS among patients with RAEB and RAEB-T in the present study was comparable or superior to that reported with a more intensive regimen of BU plus TBI.10Although the relapse incidence in patients with more advanced MDS in the present study was slightly higher than reported after BU plus TBI conditioning,10 NRM was substantially lower (12%-15% at 100 days). As a result, 3-year RFS among patients with RA/RARS was 68% for HLA-matched related and 70% for unrelated transplant recipients, whereas for patients with more advanced disease (RAEB and RAEB-T/tAML), the figures were 45% and 33% for related, and 40% and 17% for unrelated transplants, respectively. Results in the small group of patients receiving transplants from HLA-nonidentical related or unrelated donors (overall RFS 27%) were similar to those reported by other investigators.33
The present data with HLA-identical transplants compare favorably with results reported recently by the European Group for Blood and Marrow Transplantation showing a 3-year RFS of 36% for patients with HLA-identical sibling donors.8,34 That study showed a high incidence of treatment-related mortality, similar to our past experience with high-dose TBI regimens.10,11 35
In agreement with previous reports, the cumulative incidence of posttransplantation relapse in the present study was twice as high in patients with poor-risk cytogenetics than in the remaining cohort.7 36 Our results indicate that the impact of cytogenetic abnormalities was of the same order of magnitude as an increase in marrow blasts. Thus, future trials, similar to studies in AML, should consider the marrow karyotype as well as the blast count in the study design.
The role of pretransplantation “debulking” in patients with MDS is not well defined. Although some reports show a benefit as determined by improved posttransplantation RFS,37 other studies fail to show such an advantage, apparently due to increases in transplant-related toxicity.38 In the present study, the cumulative overall incidence of relapse was 16% for related and 11% for unrelated transplant recipients, respectively. Considering this low relapse incidence, it appears unlikely that pretransplantation debulking or induction chemotherapy would benefit a large proportion among all patients with MDS. A recent cooperative European study of MDS showed a 4-year RFS rate of 31% among patients with HLA-identical sibling donors, when the transplantation was done during complete remission after induction chemotherapy.34 This compares to 3-year RFS rates in the present study of 45% and 33% for patients with RAEB and RAEB-T/tAML, respectively, who had HLA-identical sibling donors, when the transplantation was done without preceding induction therapy. Whether there are subgroups of patients at high risk for relapse who might derive an advantage from pretransplantation chemotherapy or whether debulking therapy followed by reduced-intensity conditioning regimens might be advantageous, remains to be determined.
In the current trial, over the course of 3 years, approximately one third of patients died from nonrelapse causes, such as organ failure, GVHD, and infections, especially with fungal organisms. The high frequencies of infections and organ failure are consistent with previous reports,8,10,11,34 although the causes are not clear. In part, complications may be related to the disease course before transplantation, including colonization with infectious organisms, and side effects of prolonged transfusion support. In contrast to several previous reports, the present study did not identify disease duration as a risk factor for outcome.14,35 Conceivably, the adjustments of BU doses to reach a predetermined plasma target range, which was the unique feature of the present trial, improved the tolerability of the regimen and thereby reduced NRM as suggested in previous reports.12 13
Because regimen-related toxicity has generally been high in older patients, reduction in conditioning-related toxicity may also be a major reason why patient age did not have a significant impact on outcome in this study. Although the incidence of relapse tended to increase with age, NRM did not, and RFS among 23 patients older than 55 years of age (8 of these were 60 years or older) was similar to that in younger patients. These data confirm an earlier preliminary report from our center13 and are in agreement with a recent study of more than 500 patients with MDS who had unrelated donors.39
Although the overall incidence of acute GVHD was high, it was not different from that in patients with acute leukemia undergoing transplantation in Seattle, and acute GVHD of grades III to IV was infrequent (7% and 19% for matched related and unrelated transplants, respectively). Further, there was no significant difference in the incidence of GVHD between related and unrelated recipients of HLA-matched transplants, supporting the findings from other studies that show that with donor selection based on intermediate- or high-resolution HLA typing, results with unrelated donors are comparable to those with HLA-identical sibling donors.18However, in agreement with previous studies, patients who developed acute GVHD experienced significantly higher transplant-related mortality and had a lower RFS.
Determination of the best source of hemopoietic stem cells for allogeneic transplants has remained a major focus of transplantation research.40-43 Our protocol originally prescribed the use of marrow. With increasing experience with the use of PBSCs, however, and because of frequent patient requests, the protocol was modified to allow the use of PBSCs. Differences in RFS in favor of PBSCs were observed in all subgroups of patients. Although these differences were not significant, the data suggest an advantage for PBSCs in transplantation for patients with MDS as observed for other disease groups.41-43
Thus, conditioning with targeted BUCY was effective in preparing patients with MDS for transplantation from related and unrelated donors. Relapse rates were similar to those observed with high-dose TBI regimens, whereas the incidence of treatment-related mortality was lower, and as a result, RFS was improved. FAB and IPSS classifications showed strong correlations with posttransplantation relapse. GVHD remained a problem, and novel preventive regimens deserve to be tested in patients with MDS. Although the present study did not address the feasibility of autologous transplantation in patients with MDS, the observations that recipients of unrelated donor transplants did as well as patients receiving transplants from HLA-identical siblings suggest that allogeneic transplantation should be offered more widely to patients with MDS. The fact that patient age, spanning 6 decades, was not a significant risk factor for RFS further suggests such a conclusion. Whether results as obtained with the present regimen of targeted BUCY can be improved with the use of modified preparative regimens aimed at further reducing NRM remains to be determined.
We thank Amy Mellon, Mary Beauchamp, Elizabeth Soll and Christine Kane for maintaining the patient database, Kate Rupert for help with cytogenetics, Bonnie Larson and Helen Crawford for manuscript preparation.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-02-0527.
Supported in part by grants HL36444, CA18029, CA18221, CA15704, and CA87948 from the National Institutes of Health, Bethesda, MD. E.H.W. is also supported by a Lilly Clinical Investigator Award from the Damon Runyon Cancer Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. Joachim Deeg, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109; e-mail: jdeeg@fhcrc.org.