Abstract
2-Methoxyestradiol (2-ME), a new anticancer agent currently in clinical trials, has been demonstrated to inhibit superoxide dismutase (SOD) and to induce apoptosis in leukemia cells through a free radical–mediated mechanism. Because the accumulation of superoxide (O2−) by inhibition of SOD depends on the cellular generation of O2−, we hypothesized that the endogenous production of superoxide may be a critical factor that affects the antileukemia activity of 2-ME. In the present study, we investigated the relationship between cellular O2− contents and the cytotoxic activity of 2-ME in primary leukemia cells from 50 patients with chronic lymphocytic leukemia (CLL). Quantitation of O2− revealed that the basal cellular O2− contents are heterogeneous among patients with CLL. The O2− levels were significantly higher in CLL cells from patients with prior chemotherapy. CLL cells with higher basal O2− contents were more sensitive to 2-ME in vitro than those with lower O2− contents. There was a significant correlation between the 2-ME–induced O2−increase and the loss of cell viability. Importantly, addition of arsenic trioxide, a compound capable of causing reactive oxygen species (ROS) generation, significantly enhanced the activity of 2-ME, even in the CLL cells that were resistant to 2-ME alone. These results suggest that the cellular generation of O2− plays an important role in the cytotoxic action of 2-ME and that it is possible to use exogenous ROS-producing agents such as arsenic trioxide in combination with 2-ME to enhance the antileukemia activity and to overcome drug resistance. Such a combination strategy may have potential clinical applications.
Introduction
2-Methoxyestradiol (2-ME) is an endogenous metabolite of 17β-estradiol that is present in human urine and blood. 2-ME is produced by sequential 2-hydroxylation andO-methylation.1 This metabolic modification causes a loss of its ability to bind the estrogen receptor. 2-ME has been shown to have potent tumor-inhibiting effects in a number of cancer cell lines in vitro and in tumor xenograft models in vivo.2-9 This compound is currently in clinical trials, as a single agent or in combination with other anticancer agents, for treatment of several types of human cancer, including breast cancer, prostate cancer, and multiple myeloma. 2-ME also shows antileukemia activity in vitro with therapeutic selectivity.10
Several mechanisms of action have been proposed for the anticancer activity of 2-ME. These pharmacologic actions include the antiangiogenetic effect,3,8,11,12 inhibition of microtubule polymerization,2,13-17 induction of G2/M phase arrest,18-21 accumulation of proapoptotic protein p53,10,22-28 and inhibition of superoxide dismutase (SOD).10,29 Among these pharmacologic actions, inhibition of SOD by 2-ME and the subsequent accumulation of superoxide radical is likely a major mechanism responsible for the cytotoxic action, because forced overexpression of SOD by transfection or exogenous antioxidants are able to protect cells from the toxic effect of 2-ME.10 The p53 accumulation and G2/M cell cycle arrest likely reflect the cellular responses to free radical–mediated DNA damage, caused by 2-ME inhibition of SOD, the key enzyme responsible for metabolic elimination of superoxide radical in the cells. Our recent studies demonstrated that inhibition of SOD and accumulation of O2−led to free radical–mediated damage to mitochondrial membranes, release of cytochrome C to cytosol, and activation of the apoptotic cascade.10 Importantly, 2-ME–induced accumulation of O2− and the subsequent apoptosis were much more prominent in leukemia cells compared with healthy lymphocytes, likely because of an intrinsic oxidative stress in the leukemia cells.30 31 Because the cellular O2− content is determined by the rates of O2− generation and its elimination, we hypothesized that the cellular production of O2− is an important factor that affects the therapeutic activity of 2-ME. In the present study, we determined the O2− content in primary leukemia cells isolated from 50 patients with chronic lymphocytic leukemia (CLL) and evaluated their correlation with patient's sex, Rai stages, and clinical treatment status. We further investigated the relationship between the basal O2− levels and in vitro cytotoxic activity of 2-ME, as well as the correlation between the 2-ME–induced increase of cellular O2− and the loss of cell viability.
Mitochondrial respiration is the major biochemical pathway by which O2− is produced in the cells. Thus, the extent of 2-ME–induced cellular accumulation of O2−depends largely on the rate of O2− production in the mitochondria. We postulated that cancer cells with an active metabolic production of O2− would have to rely on SOD for elimination of the superoxide radical and thus are vulnerable to SOD inhibition by 2-ME. However, if a cancer cell has a defect in its mitochondrial oxidative phosphorylation because of certain mitochondrial alterations (mutations in the coding sequence of the mitochondria DNA leading to a defective respiration complex component), this cell would have to derive its energy mainly through glycolysis. One consequence of such metabolic alterations is a decrease of superoxide generation and resistance to 2-ME because of little superoxide accumulation even though SOD is inhibited. Because mutations in mitochondrial DNA have been observed in various cancer cells,32-35 2-ME–resistant cancer cells are likely to emerge during the course of disease progression. It has been reported that cells with deleted mitochondrial DNA (rho0 cells), which are deficient in respiration, are extremely resistant to adriamycin and photodynamic therapy involving reactive oxygen species (ROS)–mediated apoptosis.36 Thus, it is important to develop therapeutic strategies to overcome such drug resistance. We reasoned that one logical strategy is to use exogenous free radical–generating agents in combination with 2-ME to treat those leukemia cells with low O2− generation. In the present study, we tested such a possibility by treating 2-ME–resistant CLL cells with a combination of 2-ME and arsenic trioxide (As2O3, ATO), a free radical–generating agent.37-39 Because arsenic trioxide has been used in the clinical treatment of cancer, especially for the treatment of patients with refractory acute promyelocytic leukemia,40-44evaluation of its antileukemia activity in combination with 2-ME may have potential clinical implications.
Materials and methods
Chemicals and reagents
2-ME and ATO were obtained from Sigma Chemical (St Louis, MO), hydroethidine was obtained from Molecular Probes (Eugene, OR), RNase and proteinase K were from Roche Diagnostics (Indianapolis, IN), and ethidium bromide was from Bio-Rad (Hercules, CA). Annexin V–fluorescein isothiocyanate (FITC) analysis kit was obtained from PharMingen (San Diego, CA), Fico/Lite used for isolation of leukemia cells and lymphocytes was purchased from Atlanta Biologicals (Norcross, GA).
Isolation of CLL cells
Primary leukemia cells were isolated from peripheral blood samples obtained from 50 patients with CLL after appropriate informed consent. The procedures for isolation of CLL cells using a Ficoll density gradient method has been described previously.45 The isolated leukemia cells were washed twice with phosphate-buffered saline (PBS), and then resuspended in RPMI 1640 medium containing 10% fetal bovine serum (FBS; Life Technologies, Grand Island, NY) at a cell density of 2 × 106 cells/mL. Basal O2−measurement and drug treatment began after the cells were cultured in the fresh medium overnight at 37°C in a humidified 5% CO2 incubator.
Cytotoxicity assays
Cytotoxicity was determined using multiple assays. Cell survival was determined by a 3-(4,5-dimethyl thiazol-2)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously.46 The cell viability was determined after CLL cells were treated with various concentrations of 2-ME, ATO, and their combination for 72 hours. The potency of drug activity was expressed as IC50, defined as the drug concentration that induced 50% loss of cell viability. Median-effect analysis and combination index (CI) were used to evaluate the combination effect of 2-ME and ATO in CLL cells. The detail drug incubation conditions and computer-assisted analysis are described in the respective tables and figure legends.
To examine the apoptotic change in cell morphology, the control and drug-treated cells were centrifuged (550 rpm, 5 minutes) onto glass slides, using a Shandon-Elliot cytospin (London, United Kingdom). The slides were fixed with 100% methanol for 45 minutes, air-dried, and then stained with Wright-Giemsa stain solution (Biochemical Sciences, Swedesboro, NJ). Cells were examined for morphologic changes characteristic of apoptosis. Microscopic photographs were taken using a × 60 objective (Nikon, Tokyo, Japan). Drug-induced apoptosis was further confirmed by a DNA fragmentation assay. After treatment with 2-ME, approximately 5 × 106 cells were collected, and the cell pellets were digested in 1 mL buffer containing 10 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.8, 100 mM NaCl, 25 mM EDTA (ethylenediaminetetraacetic acid), 0.5% SDS (sodium dodecyl sulfate), and 10 μL proteinase K (1 mg/mL, added fresh) at 45°C overnight. The samples were analyzed on a 1.8% agarose gel in 1 × TBE buffer (100 mM Tris-borate, pH 8.3, 2 mM EDTA). After electrophoresis, the gel was incubated overnight in 400 mL of 0.1 × TBE buffer containing 20 μL RNase (500 μg/mL) and then photographed. DNA bands were quantitated using the ChemiImager 4400 imager system (Alpha Innotech Corporation, San Leandro, CA).
Apoptosis of CLL cells was quantified by a double staining with annexin V–FITC and propidium iodide (PI). Briefly, approximately 1 × 106 cells were washed with 1 × binding buffer (10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl), incubated with annexin V–FITC for 15 minutes at room temperature, and then washed once with 1 × binding buffer. The samples were then labeled with PI and analyzed by flow cytometry, using a FACSCalibur (Becton Dickinson, Mountain View, CA). The flow cytometry data were analyzed using the Becton Dickinson CellQuest Pro software package.
Measurement of cellular superoxide
Intracellular O2− contents were measured by flow cytometry analysis using hydroethidine as described previously.10 This fluorescent dye is commonly used for detection of O2−, although it may also react with other oxidants to a certain degree.47 48 CLL cells (2 × 106 per sample in 1 mL) were incubated with hydroethidine (50 ng/mL) for 1 hour, washed once with 2 mL PBS, and resuspended in 1 mL PBS. The samples were analyzed by flow cytometry using a FACSCalibur (Becton Dickinson). The flow cytometry data were analyzed using the Becton Dickinson CellQuest Pro software package.
Results
Induction of superoxide accumulation and apoptosis by 2-ME in CLL cells
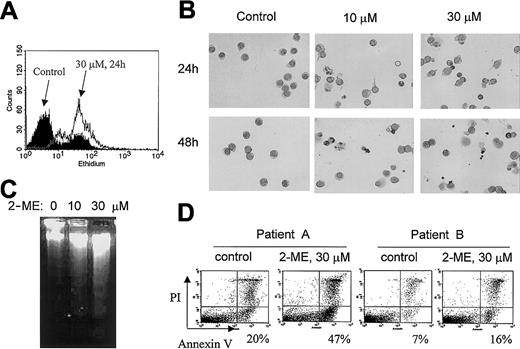
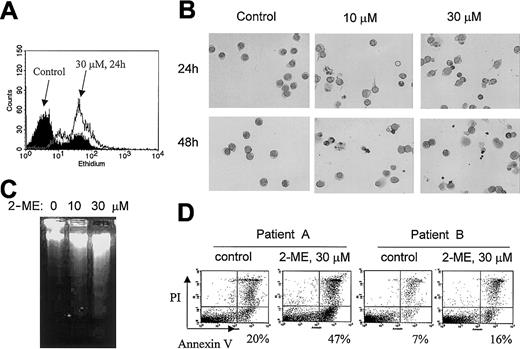
Because 2-ME is able to inhibit SOD, we first determined its effect on cellular O2− content in CLL cells. When freshly isolated CLL cells were incubated with 2-ME, a substantial accumulation of cellular O2−was observed within 24 hours, as evidenced by a right-shift of the ethidium fluorescent signal (Figure 1A). This free radical accumulation was associated with morphologic and biochemical changes typical of apoptosis. As illustrated in Figure 1B, cell shrinkage and nuclear condensation were seen 24 hours after CLL cells were incubated with 10 or 30 μM 2-ME. Fragmented nuclei and blebbing cell membranes were apparent at 48 hours. Electrophoresis of DNA isolated from 2-ME–treated cells showed a typical DNA fragmentation associated with apoptosis (Figure 1C). In the sample treated with 30 μM 2-ME for 72 hours, no intact DNA remained in the well (lane 3). When the 2-ME–treated cells were analyzed for reactivity with annexin V (an indication of phosphatidylserine exposure on the cell surface during apoptosis), a significant increase of the apoptotic signal was detected 24 hours after 2-ME incubation (Figure1D). We frequently observed various degrees of spontaneous apoptosis in CLL cells, ranging from 5% to 20% among different patients. This finding is consistent with previous observations.49 50Nevertheless, 2-ME was able to cause a significant increase of apoptosis in CLL cells that showed either high spontaneous cell death (patient A) or low spontaneous apoptosis (patient B).
Induction of O2− accumulation and apoptosis by 2-ME in CLL cells.
(A) Accumulation of O2− in primary CLL cells treated with 30 μM 2-ME for 24 hours. Primary leukemia cells freshly isolated from patients with CLL were treated with 2-ME in vitro and the O2− levels were determined by flow cytometry analysis as described in “Materials and methods.” (B) Morphologic changes characteristic of apoptosis induced by 2-ME in CLL cells. After cells were incubated with 2-ME under the conditions indicated, the samples were stained and photographed as described in “Materials and methods.” All photographs show microscopic view using × 60 objective. (C) 2-ME induced DNA fragmentation in CLL cells treated with 10 or 30 μM 2-ME for 72 hours. (D) 2-ME induced apoptosis in CLL cells as measured by annexin V reactivity using a flow cytometry analysis. The number shown below each panel indicates the percentage of annexin-positive cells.
Induction of O2− accumulation and apoptosis by 2-ME in CLL cells.
(A) Accumulation of O2− in primary CLL cells treated with 30 μM 2-ME for 24 hours. Primary leukemia cells freshly isolated from patients with CLL were treated with 2-ME in vitro and the O2− levels were determined by flow cytometry analysis as described in “Materials and methods.” (B) Morphologic changes characteristic of apoptosis induced by 2-ME in CLL cells. After cells were incubated with 2-ME under the conditions indicated, the samples were stained and photographed as described in “Materials and methods.” All photographs show microscopic view using × 60 objective. (C) 2-ME induced DNA fragmentation in CLL cells treated with 10 or 30 μM 2-ME for 72 hours. (D) 2-ME induced apoptosis in CLL cells as measured by annexin V reactivity using a flow cytometry analysis. The number shown below each panel indicates the percentage of annexin-positive cells.
CLL cells from patients with prior chemotherapy possess high levels of endogenous O2−
Because accumulation of cellular O2− is a critical event in 2-ME–induced apoptosis in leukemia cells,10 we hypothesized that the endogenous cellular production of O2− might be an important factor that affects the antileukemia activity of 2-ME. We determined the basal O2− contents in primary leukemia cells isolated from 50 patients with CLL and evaluated their correlation with patient's sex, Rai stages, and clinical treatment status. As shown in Table 1, the O2−radical levels were significantly higher in CLL cells isolated from patients previously treated with various therapeutic agents (fludarabine, cyclophosphamide, etc) than the CLL cells from previously untreated patients (P = .0056). Interestingly, CLL cells from male patients appeared to have a higher superoxide content (24.5 ± 16.1) than CLL cells from female patients (17.4 ± 8.8), although such difference is not statistically significant (P = .09). A multivariable analysis by analysis of variance (ANOVA) indicates that there was no statistically significant correlation between cellular O2−contents and the Rai stage (P = .32). The seemingly higher superoxide contents in CLL cells from stage 4 patients were not statistically different from other disease stages.
Correlation between basal O2− levels in CLL cells and their sensitivity to 2-ME
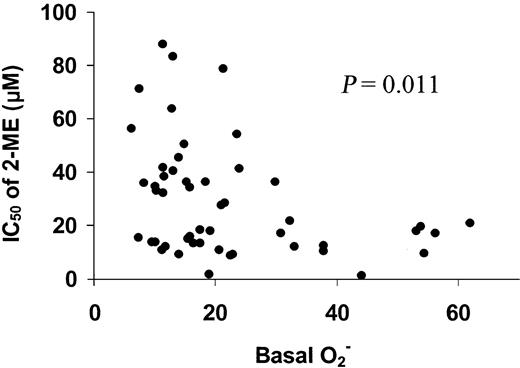
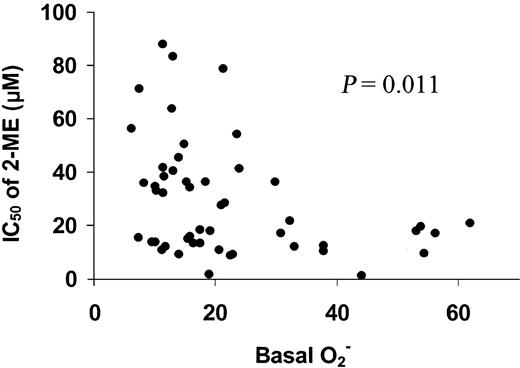
The heterogeneous levels of basal O2− in CLL cells suggest that leukemia cells from different patients with CLL have different rates of O2− generation. This heterogeneity provided a possibility to investigate the relationship between the basal cellular O2− levels and the sensitivity of CLL cells to 2-ME. Each of the freshly isolated CLL samples from the 50 patients was incubated with various concentration of 2-ME, and the sensitivity of the leukemia cells to 2-ME was determined by the MTT assay. The IC50 values were then plotted as a function of the basal O2−contents to reveal the possible correlation between these 2 parameters. As shown in Figure 2, there was an inverse correlation between the IC50 values and the cellular basal O2− contents. Statistical analysis indicates that this correlation was significant (P = .011). These data suggest that the CLL cells that were active in O2− generation (and thus had a high cellular O2− content) were more sensitive to 2-ME treatment (low IC50) than the CLL cells with lower basal O2− contents. This finding is consistent with the hypothesis that cells with an active metabolic production of O2− radicals are highly dependent on SOD for elimination of the toxic chemical species and are more vulnerable to inhibition of SOD by 2-ME.
Correlation of basal O2− levels with IC50 of 2-ME in CLL cells.
Primary leukemia cells were isolated from 50 patients with CLL. The basal O2− levels and IC50 value of 2-ME in CLL cells was determined as described in “Materials and methods.” Linear regression analysis indicates that the basal O2− levels were significantly correlated with the IC50 values of 2-ME in CLL cells (P = .011).
Correlation of basal O2− levels with IC50 of 2-ME in CLL cells.
Primary leukemia cells were isolated from 50 patients with CLL. The basal O2− levels and IC50 value of 2-ME in CLL cells was determined as described in “Materials and methods.” Linear regression analysis indicates that the basal O2− levels were significantly correlated with the IC50 values of 2-ME in CLL cells (P = .011).
Induction of O2− accumulation and loss of cell viability by 2-ME in CLL cells
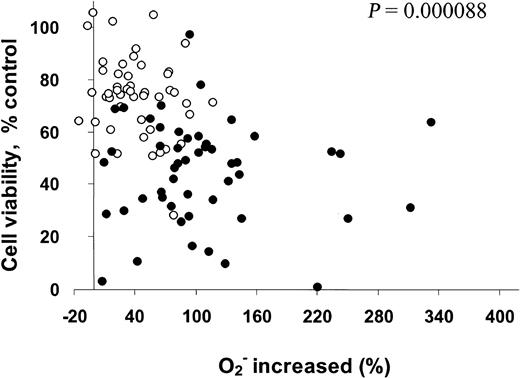
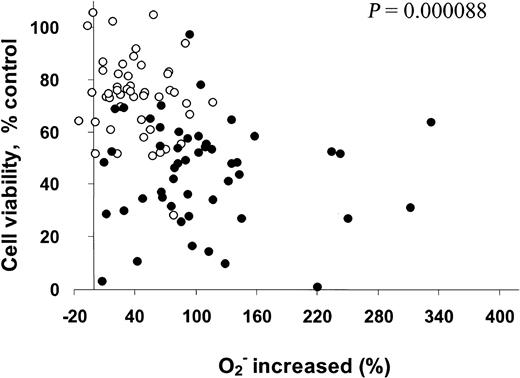
Our previous study showed that 2-ME inhibits SOD and causes O2− accumulation in leukemia cells.10 The present study further evaluated the relationship between 2-ME–induced increase of O2− and the sensitivity of CLL cells to 2-ME. Cellular O2− contents were determined before and after 2-ME incubation (10 and 30 μM, 24 hours), and the percentage of superoxide increase was calculated. 2-ME–induced loss of cell viability was determined by the MTT assay and was plotted as a function of 2-ME–induced accumulation of superoxide. As shown in Figure 3, the induction of superoxide accumulation by 2-ME and cell death were heterogeneous among the 50 patient samples (2 drug concentrations each). However, there was a highly significant correlation between the loss of cell viability and free radical increase (P = .000088). In general, a higher accumulation of superoxide was associated with a greater loss of cell viability. Thus, the cellular generation of O2− may play an important role in the cytotoxic action of 2-ME in CLL cells.
Correlation of increase in O2−with cell viability (%) in CLL cells treated with 2-ME in vitro.
Primary leukemia cells isolated from 50 patients with CLL were treated with 10 (○) or 30 (●) μM 2-ME for 24 hours. The O2− levels and cell viability were determined by flow cytometry analysis and MTT assay as described in “Materials and methods.” Linear regression analysis indicates that 2-ME–induced increase of cellular O2− is significantly correlated with the loss of cell viability (P < .001).
Correlation of increase in O2−with cell viability (%) in CLL cells treated with 2-ME in vitro.
Primary leukemia cells isolated from 50 patients with CLL were treated with 10 (○) or 30 (●) μM 2-ME for 24 hours. The O2− levels and cell viability were determined by flow cytometry analysis and MTT assay as described in “Materials and methods.” Linear regression analysis indicates that 2-ME–induced increase of cellular O2− is significantly correlated with the loss of cell viability (P < .001).
Enhancement of 2-ME activity against CLL cells by arsenic trioxide in vitro
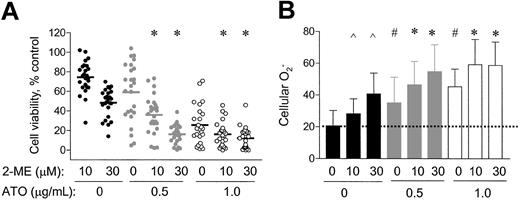
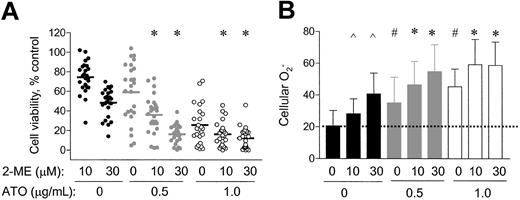
Because cellular O2− content was an important factor that affected the antileukemia activity of 2-ME, we further tested the possibility of using an exogenous ROS-generating agent to enhance the activity of 2-ME. ATO, a trivalent arsenite capable of causing ROS generation,37-39was used in combination with 2-ME to evaluate the activity against CLL cells. Primary leukemia cells freshly isolated from 25 patients with CLL were treated with 10, 30 μM 2-ME, alone or in combination with 0.5 μg/mL or 1.0 μg/mL ATO for 72 hours, and cell viability was determined by MTT assay. As shown in Figure4A, ATO at the concentrations of 0.5 and 1.0 μg/mL alone reduced cell survival to 60% and 30% of the control, respectively. Addition of these concentrations of ATO to the 2-ME–treated CLL cells significantly enhanced the cytotoxic activity (P < .05). This combination effect appears to be additive, consistent with the notion that both compounds act through a free radical–mediated mechanism. In contrast, no significant enhancement of the cytotoxic activity when 2-ME was combined with fludarabine in 3 cases of CLL cells tested (data not shown). The IC50 values of 2-ME, used alone or in combination with ATO, in 25 patient samples tested are shown in Table2.
Combined effect of 2-ME and ATO on CLL cells in vitro.
Primary leukemia cells freshly isolated from 25 patients with CLL (A) were treated with 10 or 30 μM of 2-ME, alone or in combination with 0.5 or 1.0 μg/mL ATO for 72 hours. Cell viability was determined by MTT assay. * indicates a statistically significant difference when compared with cells exposed to 2-ME alone (P < .05). Solid bars indicate average values. (B) Fresh primary leukemia cells isolated from 17 patients with CLL were treated with 10 or 30 μM 2-ME alone or in combination with 0.5 or 1.0 μg/mL ATO for 24 hours. The O2− levels were determined by flow cytometry analysis. ∧ and # indicate a statistically significant difference when compared with the control cells (P < .05); *, a statistically significant difference when compared with cells exposed to 2-ME alone (P < .05). Error bars indicate SD of the means.
Combined effect of 2-ME and ATO on CLL cells in vitro.
Primary leukemia cells freshly isolated from 25 patients with CLL (A) were treated with 10 or 30 μM of 2-ME, alone or in combination with 0.5 or 1.0 μg/mL ATO for 72 hours. Cell viability was determined by MTT assay. * indicates a statistically significant difference when compared with cells exposed to 2-ME alone (P < .05). Solid bars indicate average values. (B) Fresh primary leukemia cells isolated from 17 patients with CLL were treated with 10 or 30 μM 2-ME alone or in combination with 0.5 or 1.0 μg/mL ATO for 24 hours. The O2− levels were determined by flow cytometry analysis. ∧ and # indicate a statistically significant difference when compared with the control cells (P < .05); *, a statistically significant difference when compared with cells exposed to 2-ME alone (P < .05). Error bars indicate SD of the means.
We measured the cellular O2− levels in 17 of the 25 patient samples before and after incubation with 2-ME, ATO, and their combinations. As shown in Figure 4B, 2-ME or ATO each alone was able to cause an increase of cellular O2−, whereas the combination of both compounds appeared to have an additive effect on cellular accumulation of superoxide radicals. This finding is consistent with their additive effect on cell viability (Figure4A).
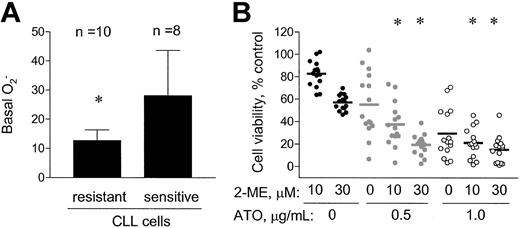
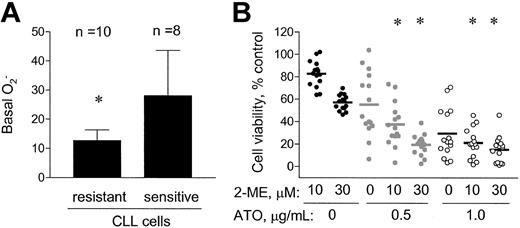
Because arsenic trioxide was able to enhance the activity of 2-ME in CLL cells, we further tested if ATO could increase the cytotoxic effect of 2-ME in CLL cells that were resistant to 2-ME alone. We identified 10 CLL patient samples that were resistant to treatment with 2-ME alone (defined as IC50 > 30 μM), and 8 CLL patient samples that were sensitive to treatment with 2-ME alone (defined as IC50 < 11 μM). As shown in Figure5A, the 2-ME–resistant cells showed significantly lower basal cellular O2−contents (12.7 ± 3.7 units) than the 2-ME–sensitive cells (28.1 ± 15.6 units, P < .01). This finding is consistent with the conclusion that cells with a higher level of intrinsic oxidative stress are more sensitive to 2-ME. When the 2-ME–resistant CLL cells were incubated with 2-ME in the presence of ATO (0.5 or 1.0 μg/mL), a significant increase in cytotoxicity was observed (Figure 5B, P < .05). At a low concentration of ATO (0.5 μg/mL), the combined effect appeared to be greater than additive, whereas a higher concentration of ATO (1 μg/mL) showed additive activity with 2-ME. For instance, 10 μM 2-ME and 0.5 μg/mL ATO alone reduced cell viability to 74% and 59%, respectively. Their combination resulted in a decrease of cell viability to 36%, compared with the expected value of 44%. Thus, the exogenous ROS-generating agent ATO appeared able to increase 2-ME cytotoxicity in 2-ME–resistant cells, which had a low level of endogenous superoxide.
ATO increased the cytotoxic effect of 2-ME in CLL cells that were resistant to 2-ME alone in vitro.
(A) A total of 10 resistant (IC50 > 30 μM 2-ME) and 8 sensitive (IC50 < 11 μM 2-ME) CLL patient samples were identified, and the basal cellular O2−contents were shown. * indicates a statistically significant difference when compared with the sensitive cells. Error bars indicate SD of the means. (B) The combined effect of 2-ME and ATO in the 2-ME–resistant CLL cells (n = 15 patients). * indicates a statistically significant difference when compared with cells exposed to 2-ME alone (P < .05).
ATO increased the cytotoxic effect of 2-ME in CLL cells that were resistant to 2-ME alone in vitro.
(A) A total of 10 resistant (IC50 > 30 μM 2-ME) and 8 sensitive (IC50 < 11 μM 2-ME) CLL patient samples were identified, and the basal cellular O2−contents were shown. * indicates a statistically significant difference when compared with the sensitive cells. Error bars indicate SD of the means. (B) The combined effect of 2-ME and ATO in the 2-ME–resistant CLL cells (n = 15 patients). * indicates a statistically significant difference when compared with cells exposed to 2-ME alone (P < .05).
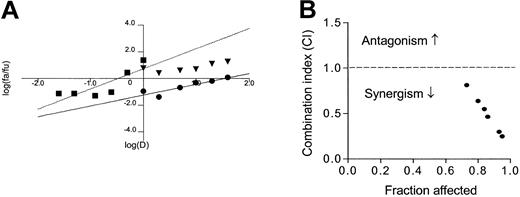
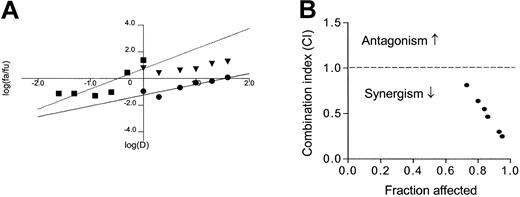
The data shown in Figures 4 and 5 suggest that the combination of 2-ME and ATO may have additive or more than additive activity. We further tested this possibility by a formal median-effect analysis and determination of CI in 6 CLL patient samples, which were each incubated with various concentrations of 2-ME and ATO, alone or in combinations. Figure 6A-B illustrates the results of such analysis in one of the patient samples. The CI values for all 6 patient samples (mean ± SD) under various drug combination conditions are summarized in Table3. A CI value of 1.0 indicates an additive effect; CI greater than 1.0 indicates antagonism, whereas CI less than 1.0 indicates synergism. It is clear that under most combination conditions, 2-ME and ATO produced more than additive effect. The 2 compounds showed additive activity at a lower 2-ME/ATO ratio (Table 3).
Analysis of median-effect and combination index (CI) in CLL cells incubated with 2-ME and arsenic trioxide.
Freshly isolated CLL cells were incubated with various concentrations of 2-ME and ATO for 72 hours. The drug effect on cell viability under various drug combination conditions was determined by MTT assay as described in “Materials and methods.” The median effect (A) and CI values (B) were calculated using a computer program by Chou and Hayball.51 In panel A ● indicates 2-ME; ▪, ATO; and ▾, 2-ME+0.5 μg/mL ATO. Dashed line at CI = 1.0 indicates an additive effect. If a CI value is below this line, it indicates synergistic activity, whereas CI values above this line suggest an antagonist effect.
Analysis of median-effect and combination index (CI) in CLL cells incubated with 2-ME and arsenic trioxide.
Freshly isolated CLL cells were incubated with various concentrations of 2-ME and ATO for 72 hours. The drug effect on cell viability under various drug combination conditions was determined by MTT assay as described in “Materials and methods.” The median effect (A) and CI values (B) were calculated using a computer program by Chou and Hayball.51 In panel A ● indicates 2-ME; ▪, ATO; and ▾, 2-ME+0.5 μg/mL ATO. Dashed line at CI = 1.0 indicates an additive effect. If a CI value is below this line, it indicates synergistic activity, whereas CI values above this line suggest an antagonist effect.
Discussion
ROS are potentially toxic “byproducts” of cellular metabolism that are generated during the production of adenosine triphosphate (ATP) by aerobic metabolism in the mitochondria, where electrons escaping from the respiratory complexes I and III may react with molecular oxygen to form oxygen radicals.52-55ROS play important roles in cell proliferation, aging, and cancer development. Because of their reactive chemical properties, ROS may cause various types of tissue injury in a wide range of human diseases, and an excessive amount of ROS can lead to cell death by apoptosis or by necrosis.56,57 The ability of ROS to damage cellular components and cause cell death suggests a possibility to exploit this chemical property for killing cancer cells through a free radical–mediated mechanism. This strategy is of therapeutic relevance, because most cancer cells are active in metabolic production of ROS and are intrinsically under increased oxidative stress, and thus more susceptible to exogenous free radical insults.31 This provides a biochemical basis to preferentially kill cancer cells.
Previous studies demonstrated that 2-ME is able to inhibit SOD, causes a substantial accumulation of O2− in leukemia cells, and preferentially induces apoptosis in malignant cells.10 These observations suggest a possibility to develop new therapeutic strategies using the free radical–mediated mechanism of 2-ME to selectively kill cancer cells. 2-ME by itself does not generate superoxide radicals, but it induces accumulation of O2− in cells through the inhibition of SOD, the key enzyme responsible for metabolic elimination of O2− radicals. Thus, the extent of 2-ME–induced accumulation of superoxide is dependent on the rates of metabolic generation of superoxide radicals in the cells. This hypothesis would predict that cells with a high level of superoxide production are more vulnerable to SOD inhibition and thus are more sensitive to 2-ME. The present study investigated the relationship between the intrinsic superoxide stress levels in CLL cells and their sensitivity to 2-ME. We demonstrated that, although the basal superoxide contents in CLL cells were heterogeneous, there was a significant correlation between the endogenous cellular O2− radical levels and 2-ME–induced cell death (Figure 2). Cells with a high O2−radical content before drug treatment (an indication of active endogenous superoxide production) were more sensitive to treatment with 2-ME than those cells with relatively low O2−radical content (Figures 2 and 5A). This finding is consistent with the earlier prediction. Furthermore, we showed that the degree of 2-ME–induced cell death is significantly correlated with the extent of drug-induced increase in O2− radicals in CLL cells (Figure 3). These observations are in agreement with the mechanism of action of 2-ME reported previously.10 29
Among the several mechanisms of actions proposed for 2-ME (antiangiogenetic effect,3,11,12 interruption microtubule assembling,2,13-17 interfering of cell cycle progression,18-21 and inhibition of SOD10,29), inhibition of SOD and the subsequent accumulation of superoxide radical is likely a major mechanism of the drug action. It has been previously observed that overexpression of SOD protects cells from the toxic effect of 2-ME, whereas decreased SOD expression by antisense oligos enhanced 2-ME toxicity.10In a separate set of experiments, we demonstrated that 2-ME caused a significant accumulation of superoxide well before apoptosis became apparent and that addition of antioxidants such asN-acetyl-L-cysteine (NAC) effectively prevented the 2-ME–induced increase of O2− and significantly suppressed apoptosis.
The reason for the high degree of heterogeneity in basal superoxide contents in primary CLL cells is not yet clear. Several possibilities may contribute to this variation. First, because superoxide is produced mainly in the mitochondria during oxidative phosphorylation for ATP generation, CLL cells at different disease stages may have different metabolic activities and thus produce various levels of superoxide radicals, depending on the energy requirement by the leukemia cells. Second, the mitochondrial oxidative phosphorylation coupling efficiency directly affects the production of superoxide. An inefficient electron transport through the respiratory chain tends to cause a “leak” of electrons from the transport chain (especially complex I and III) and generates superoxide when the escaping electrons react with molecular oxygen.58 Because the mitochondrial DNA (mtDNA) encodes 13 important protein components of the electron transport complexes, certain mutations in the mtDNA are likely to affect the electron transport and lead to an increase of superoxide formation in CLL cells. Third, the patients' individual variations such as intake of antioxidants and other medication might also contribute to the variation in free radical contents observed in CLL cells from different patients.
It is of interest to note that the CLL cells from patients with prior chemotherapy exhibited significantly higher basal superoxide contents than the CLL cells from previously untreated patients. Drug-induced mutations in mtDNA may be a possible reason for the increased O2− generation in previously treated CLL cells. It is known that fludarabine and cyclophosphamide, 2 of the most common drugs used in the clinical treatment of CLL, interact with DNA (incorporation or alkylating) and are able to cause mutations.59 60 Because mtDNA is particularly vulnerable to damage (compared with nuclear DNA) because of the lack of histone protection and a weak DNA repair capacity in the mitochondria, it is possible that prior treatments of CLL patients with such antileukemia drugs may cause mutations in the mtDNA. As discussed earlier, certain mutations of mtDNA are likely to cause an increase of superoxide generation because of inefficient respiration coupling and leakage of electrons. In future studies, sequencing of the mtDNA in CLL cells could provide direct evidence for the presence of mutations in mtDNA and reveal the relationship between such mutations and ROS generation.
The observations that CLL cells from patients with a history of prior chemotherapy contain significantly higher levels of superoxide and are more sensitive to 2-ME suggest a promising possibility to use 2-ME for the treatment of patients with CLL who failed prior therapy with other agents. This possibility warrants further investigation in both preclinical and clinical settings. However, the heterogeneity of superoxide content in CLL cells (and likely in other types of cancer cells) also presents a challenge to the use of 2-ME for treating patients with CLL, because the CLL cells with low endogenous superoxide content are less sensitive to 2-ME (Figures 2 and 5A). One strategy to overcome such drug resistance is to use exogenous free radical–generating agents in combination with 2-ME. The rationale is to use exogenous ROS-generating agents to enhance intracellular O2− radicals, and to use 2-ME to inhibit SOD, impair the cellular ability to eliminate O2−, and thus cause a severe accumulation of superoxide in the cells. However, a variety of mechanisms, including overexpression of Bcl-2, increase in multidrug-resistant (MDR) protein (MRP) expression, enhanced cellular glutathione (GSH) levels, and defects in certain apoptotic pathways, may cause a decrease in cellular sensitivity to drug treatment. Thus, the strategy of using ROS-mediated apoptosis to kill leukemia cells may not be generally applied to all cases. ROS-mediated apoptosis is likely to be most effective in those leukemia cells that contain increased levels of endogenous ROS.
Arsenic trioxide appears to be a logical choice for combination with 2-ME. ATO has been used in the clinical treatment of relapsed/refractory acute promyelocytic leukemia (APL).40-44 Oxidative damage has been suggested to be a key mechanism by which arsenic trioxide causes cell death.44 ATO-induced apoptosis has been shown to be associated with the generation of ROS in several experimental models.37-39,61 Antioxidants and free radical scavengers are able to inhibit apoptosis induced by ATO.38 On the basis of the ability of ATO to cause free radical generation in cells, one would expect that its combination with 2-ME should enhance the cytotoxic activity. In fact, the present study showed that combination of ATO with 2-ME caused an additive accumulation of superoxide in CLL cells and resulted in additive or more than additive activity against the leukemia cells. Importantly, this combination was effective in killing CLL cells that were resistant to 2-ME alone. These results suggest that it is indeed possible to combine 2-ME with ROS-generating agents to enhance therapeutic activity and overcome drug resistance. Previous studies showed that 2-ME selectively kills leukemia cells without a significant toxic effect on healthy lymphocytes.10 It would be important to further test the effect of 2-ME and its combination with other ROS-generating agents such as ATO on a variety of nontumor cells, including healthy bone marrow residents and other healthy cells such as macrophages and neutrophils that produce ROS. Studies along this line would provide important information on the relative selectivity of this therapeutic strategy. Although such a study would require the development of appropriate methods for isolation/purification of large number of the healthy cells without artificially affecting their metabolic production of ROS, its potential therapeutic implications seem to justify future research efforts in this area.
In summary, we demonstrated that the cellular production of O2− is an important factor that affects the antileukemia activity of 2-ME in vitro. Primary CLL cells from different patients exhibited heterogeneous levels of cellular O2−, with a significantly higher O2− observed in the leukemia cells from patients who had a history of prior chemotherapy. Because cells with high levels of superoxide were more sensitive to 2-ME, this compound might be potentially useful for treating patients who fail prior therapy with other drugs. For the CLL cells that contained a low level of endogenous superoxide and were resistant to 2-ME, the use of exogenous ROS-generating agents such as arsenic trioxide significantly enhanced the antileukemia activity. Thus, combination of SOD inhibitors and ROS-producing agents may provide a new strategy to enhance therapeutic activity and overcome drug resistance. Further studies are warranted to test this possibility.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-08-2512.
Supported in part by research grants CA77339, CA85563, and CA81534 from the National Cancer Institute, National Institutes of Health, and by a Cancer Center Support Grant P30 CA16672 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peng Huang, Department of Molecular Pathology, Box 089, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:phuang@mdanderson.org.