Abstract
Lymphoid deficiency after allogeneic hematopoietic cell transplantation (HCT) results in increased susceptibility to infection; however, transplantation of mature lymphocytes frequently results in a serious complication known as graft-versus-host disease (GVHD). Here we demonstrate in mice that both congenic as well as allogeneic transplantation of low numbers of highly purified common lymphoid progenitors (CLPs)—a rare population of lymphoid-lineage-committed bone marrow cells—accelerates immune reconstitution after lethal irradiation and rescue with hematopoietic stem cells (HSCs). After congenic transplantation, 3 × 103 CLPs protected against murine cytomegalovirus (MCMV) infection at a level roughly equivalent to 107 unfractionated lymph node cells. In the allogeneic model of matched unrelated donor HSC transplantation, cotransplantation of 3 × 103 CLPs protected thymus-bearing as well as thymectomized hosts from MCMV infection and attenuated disease severity. Immunohistochemistry in combination with antibody depletion of T and natural killer (NK) cells confirmed that CLP-derived as well as residual host lymphocytes contribute to antiviral protection. Importantly, transplantation of allogeneic CLPs provided a durable antiviral immunity without inducing GVHD. These data support the potential for composing grafts with committed progenitors to reduce susceptibility to viral infection following HCT.
Introduction
Myeloablative doses of chemoradiation therapy used in preparation for hematopoietic cell transplantation (HCT) lead to depletion of hematopoietic stem cells (HSCs), progenitor cells, and mature cells. The resulting immunodeficiency results in a profound susceptibility to bacterial, fungal, and viral infections. The recovery of a functional immune system after HCT is dependent upon the de novo regeneration of all hematopoietic lineages from HSCs and progenitor cells and on the function of mature cells contained in the graft. Infections after HCT typically follow a reproducible temporal pattern correlating with the kinetics of immune reconstitution. Viral infections occur later during the phase of lymphoid deficiency resulting from reactivation of herpesviruses such as cytomegalovirus (CMV), varicella zoster, and Epstein-Barr virus.1 T- and B-cell deficiency is usually protracted, resolving only after several months to years. The nature of the immunodeficiency following allogeneic HCT is further complicated by the potential to develop graft-versus-host disease (GVHD), which has been shown to contribute to thymic stroma perturbations, lymphoid hypoplasia, and B-cell dysfunction.2-6 Consequently, GVHD and the resultant administration of immunosuppressive agents are both associated with a significantly increased risk of lethal infectious complications.
One of the most common and potentially lethal manifestations of lymphoid deficiency is infection with CMV.7 Therefore, numerous clinical and animal studies were undertaken to characterize the complex immune response to CMV and to develop treatment strategies involving antiviral drugs as well as cellular therapies and vaccination.8,9 Although advances were made, new strategies to prevent infections after HCT while minimizing GVHD are still warranted. We propose that modifying grafts by inclusion of committed progenitor cells can be used to protect against infections.10 Hematopoietic cell populations at different levels of commitment have been phenotypically identified in both mice and humans and can be prospectively isolated. These populations include HSCs, common lymphoid progenitors (CLPs), common myeloid progenitors (CMPs), and, downstream of CMPs, granulocyte-monocyte progenitors (GMPs) and megakaryocyte-erythroid progenitors (MEPs).11-17 In vivo studies using competitive reconstitution assays in congenic mice showed that transplantation of CLPs, CMPs, GMPs, or MEPs resulted in accelerated reconstitution of the respective mature cell compartments compared with transplantation of HSCs alone.13,16 Our studies show that these progenitors also have functional activity in response to clinically relevant pathogens.10,18 Congenic transplantation of CMPs and GMPs protected mice against invasive aspergillosis and Pseudomonas aeruginosa infection following HSC transplantation.18
In the present study, we tested the ability of CLPs to enhance immune response to CMV infection following HCT in mice. For this purpose, we used mouse models of congenic and allogeneic HCT. We demonstrated that the kinetics of lymphoid engraftment was accelerated following cotransplantation of CLPs compared with transplantation of HSCs alone. This accelerated reconstitution resulted in more effective viral control and improved survival following lethal challenge with murine CMV (MCMV). Moreover, in allogeneic recipients, this enhanced reconstitution occurred via a mechanism that was not solely thymic dependent. In vivo depletion of residual host T and natural killer (NK) cells revealed that mixed chimerism is important for defense against this pathogen and confirmed results obtained by immunohistochemistry that CLP-derived lymphocytes as well as host-derived T and NK cells play an important role in immunity to MCMV infection. These data further support our hypothesis that inclusion of purified committed progenitor cell populations in the hematopoietic graft could be used to accelerate reconstitution of a functional immune system and to protect against infection following HCT.
Materials and methods
Animals
C57BL/Ka-Thy1.1.CD45.2 (H2b) and C57BL/Ka-Thy1.1.CD45.1 (H2b) mice were used for donor cell populations and their F1 generation was used as recipients in congenic transplantation experiments. The use of these strain combinations allowed differentiation between host and 2 independent donor cell populations. In the allogeneic transplantation experiments, the use of BALB.B (H2b, Thy1.2, CD45.2) mice as recipients, C57BL/Ka-Thy1.1.CD45.2 (H2b) as HSC donors, and C57BL/Ka-Thy1.1.CD45.1 (H2b) as CLP donors allowed the differentiation of all CLP-derived cell populations. Host- or HSC-derived T cells could be distinguished by Thy1.2 staining. All mice were bred and maintained at the animal care facility at Stanford University School of Medicine. Donor mice were used at 6 to 8 weeks and hosts at 8 to 14 weeks of age.
Cell sorting and flow cytometric analysis
To isolate HSCs, whole bone marrow cells (WBMCs) from C57BL/Ka-Thy1.1.CD45.2 mice were depleted of mature erythrocytes via ammonium chloride lysis and then stained with biotinylated rat antimouse c-Kit (3C11) followed by streptavidin immunomagnetic beads (Miltenyi Biotec, Auburn, CA) as described previously.18 C-Kit+ cells were positively selected using an autoMACS cell separator (Miltenyi Biotec). Cells enriched for HSCs were stained with phycoerythrin (PE)-conjugated anti-CD3 (145-2C11; Pharmingen, San Diego, CA), anti-CD4 (GK1.5), anti-CD5 (53-7.8), anti-CD8 (53-6.7), anti-B220 (6B2), anti-Ter119, anti-Mac-1 (M1/70), and anti-Gr-1 (RB6-8C5); and fluorescein isothiocyanate (FITC)-conjugated anti-Thy1.1 (19XE5), Texas Red (TxR)-conjugated anti-Sca-1 (E13-161), and allophycocyanin (APC)-conjugated anti-c-Kit (2B8).
For congenic transplantation, c-Kit+Thy1.1lolin–/loSca-1+ HSC were double sorted. For allogeneic transplantation, c-Kit+Thy1.1lolin–Sca-1+ long-term HSCs (LT-HSCs) were single sorted.
To isolate lin–IL-7Rα+Thy1.1–c-KitloSca-1lo CLP,13 WBMCs from C57BL/Ka-Thy1.1.CD45.1 mice were depleted of mature erythrocytes via ammonium chloride lysis and then stained with biotinylated anti-CD27 (LG.3A10; Pharmingen), followed by streptavidin immunomagnetic beads (Miltenyi Biotec). CD27+ cells were positively selected using an autoMACS cell separator (Miltenyi Biotec). After blocking with rat IgG (Sigma, St Louis, MO), cells enriched for CLPs were stained with Cy5-PE-conjugated anti-CD3 (145-2C11), anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-B220 (6B2), anti-Ter119, anti-Mac-1 (M1/70), anti-Gr-1 (RB6-8C5; eBioscience, San Diego, CA), FITC-conjugated anti-Thy1.1 (19XE5), TxR-conjugated anti-Sca-1 (E13-161), APC-conjugated anti-c-Kit (2B8), and PE-conjugated anti-interleukin-7 receptor-α (anti-IL-7Rα) (A7R34; eBioscience).
For analysis of cellular subpopulations, blood and spleen samples were depleted of mature erythrocytes via ammonium chloride lysis and then stained with antibodies in various combinations: FITC-conjugated anti-Thy1.1 (19XE5), anti-Thy1.2 (53-2.1; Pharmingen), anti-CD3 (145-2C11), PE-conjugated anti-Thy1.1 (OX7; Pharmingen), anti-CD4 (GK1.5), anti-NK1.1 (PK136; Pharmingen), TxR-conjugated anti-CD45.1 (A20.1.7), anti-CD4 (GK1.5), anti-Mac-1 (M1/70), APC-conjugated anti-B220 (6B2), anti-CD8 (53-6.7), and anti-Mac-1 (M1/70). Antibody staining and washing was performed using Hanks balanced salt solution (HBSS) with penicillin/streptomycin (Gibco BRL, Grand Island, NY) and 2% heat-inactivated fetal bovine serum (Sigma). Cells were incubated 20 to 30 minutes at 4°C and washed twice. Dead cells were excluded by propidium iodide staining. Cell sorting and analysis were performed on a dual-laser fluorescence-activated cell sorter (FACSVantage, BD Biosciences, San Jose, CA) equipped with a 488-nm argon and a 595-nm dye laser made available through the FACS shared user group at Stanford University.
Preparation of unfractionated lymph node cells
Submandibular, axillary, and inguinal lymph nodes (LN) were harvested from C57BL/Ka-Thy1.1.CD45.1 mice. Single-cell suspensions were made and filtered through a 70-μm nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ).
Cell counts
Blood samples were submitted to the diagnostic laboratory of the Department of Comparative Medicine at Stanford University where complete blood counts (CBCs) including differential counts were performed on a Cell Dyn 3500 (Abbott Laboratories, Abbott Park, IL) hematology counter. Spleen and LN cell suspensions were stained with Turks solution and counted manually using a hemocytometer.
Thymectomy, irradiation, transplantation, and MCMV infection
Thymectomies were performed at the age of 3 to 4 weeks as previously described19 at least 4 weeks before transplantation. Congenic F1 hosts were irradiated with 9.5 Gy, allogeneic BALB.B hosts with 8 Gy in 2 fractions, 3 to 4 hours apart, using a 200-kV x-ray machine (Philips RT250, Shelton, CT) and given antibiotic water (1.1 g/L neomycin sulfate and 106 U/L polymyxin B sulfate) after irradiation. Mice were anesthetized with Isoflurane (Abbott Laboratories) and cells were injected into the retro-orbital venous plexus using 0.5-cc insulin syringes with 28-gauge needles (Applied Scientific, South San Francisco, CA). On day 14 after transplantation, mice were infected intraperitoneally with 1 × 106 to 5 × 106 plaque forming units (pfu) of the lacZ-tagged MCMV RM427+.20 Mice were weighed daily and examined twice daily following infection.
In vivo host depletion
BALB.B hosts that received transplants were treated with anti-Thy1.2 (30H-12) antibody (eBioscience), which depletes host T, NK/T, a small population of activated NK cells, and plasmacytoid dendritic cells.21,22 Mice received 0.1 mg intraperitoneally on days 11, 12, and 13 after transplantation and every 7 days thereafter.
Viral load determination and histopathology
Mice were killed and liver, spleen, kidneys, lungs, and salivary glands were harvested into 5-mL polypropylene tubes (Falcon; Becton Dickinson Labware) containing Dulbecco Modified Eagle Medium and triple-sterilized dry milk (Gibco BRL). Organ weight was determined and tubes were stored at –80°C. Organs were sonicated and titered by plaque assay on 3T3 cell (American Tissue Type Collection, Manassas, VA) monolayers as previously described.23 Fragments of the liver, terminal ileum, ascending colon, and ear were frozen in optimal cutting temperature compound (Miles Diagnostics, Elkhart, IN) or fixed in 4% paraformaldehyde and embedded in paraffin. Immunofluorescent staining was performed on 5-μm tissue sections. They were incubated with rabbit anti-β-galactosidase (ICN Pharmaceuticals, Aurora, OH) overnight at 4°C, washed 3 times in phosphate-buffered saline, and then incubated with goat antirabbit Alexa 488 (Molecular Probes, Eugene, OR) for 30 minutes at room temperature. Immunohistochemistry was performed as previously described using biotinylated anti-CD45.1 and biotinylated anti-Thy1.2.24 Hematoxylin and eosin (H&E)-stained sections were coded (C.A.) and analyzed (J.P.H.) to determine the presence and severity of GVHD and/or MCMV hepatitis.
Statistical analysis
For comparison of absolute cell counts and viral titers, the rank sum test was performed. We used the log-rank test to compare groups in Kaplan-Meier survival analysis.
Results
Congenic CLP cotransplantation improves survival after MCMV infection
In order to assess whether CLP cotransplantation could improve immune function after transplantation, congenic mice received transplants of 200 highly purified c-Kit+Thy1.1lolin–/loSca-1+ HSCs alone, or they received cotransplants of 200 HSCs and varying doses of lin–IL-7Rα+Thy1.1–c-KitloSca-1lo CLPs. These mice were then challenged with MCMV on day 14 after transplantation. As shown in Figure 1, survival of mice that received cotransplants of 200 HSCs and 3000 CLPs was significantly higher than in mice that received transplants of HSCs alone (Figure 1A; 56% versus 10%, P < .0001). Uninfected control groups demonstrated full survival following transplantation (data not shown). Furthermore, cotransplantation of only 3000 CLPs proved to be as protective as adoptive transfer of 107 unfractionated LN cells (Figure 1A; P < .0001). Dose titration experiments with increasing numbers of transplanted CLPs revealed that cotransplantation of 1000 CLPs resulted in 44% survival compared with 0% after cotransplantation of 500 CLPs (Figure 1B; P = .005).
Congenic CLP cotransplantation protects against lethal MCMV infection. (A) Mice received transplants of 200 HSCs (•; n = 40, 10% survival) or 200 HSCs and 3000 CLPs (○; n = 25, 56% survival) or 200 HSCs and 107 unfractionated lymph node cells (▿; n = 37, 56% survival). The 3000 CLPs protected as well as 107 unfractionated lymph node cells against lethal MCMV infection after congenic HSC transplantation (*P < .0001 for both, ○ versus • and ▿ versus). (B) Mice that received transplants of 200 HSCs and 1000 CLPs (□; n = 19, 44% survival) were protected against lethal MCMV infection, whereas mice that received transplants of 200 HSCs and 500 CLPs (⋄; n = 9, 0% survival) were not (*P = .005).
Congenic CLP cotransplantation protects against lethal MCMV infection. (A) Mice received transplants of 200 HSCs (•; n = 40, 10% survival) or 200 HSCs and 3000 CLPs (○; n = 25, 56% survival) or 200 HSCs and 107 unfractionated lymph node cells (▿; n = 37, 56% survival). The 3000 CLPs protected as well as 107 unfractionated lymph node cells against lethal MCMV infection after congenic HSC transplantation (*P < .0001 for both, ○ versus • and ▿ versus). (B) Mice that received transplants of 200 HSCs and 1000 CLPs (□; n = 19, 44% survival) were protected against lethal MCMV infection, whereas mice that received transplants of 200 HSCs and 500 CLPs (⋄; n = 9, 0% survival) were not (*P = .005).
Derivation of splenocyte subsets after congenic CLP cotransplantation
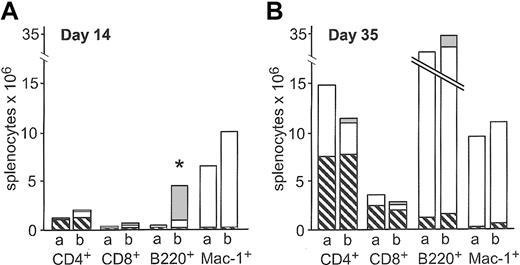
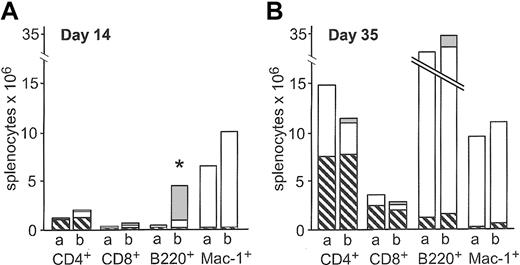
Absolute counts of splenocyte subsets were determined after congenic transplantation of 200 HSCs alone or cotransplantation of 200 HSCs and 3000 CLPs on days 14 and 35 after transplantation. CLP cotransplantation resulted in higher absolute numbers of CD4+, CD8+, B220+, and Mac-1+ splenocytes on day 14, although only the increase in B220+ splenocytes reached statistical signifi-cance (P = .007). At this time point, 80% of total B220+ cells were CLP-derived (Figure 2A). On day 35, none of the differences were statistically significant (Figure 2B). As expected, CLPs did not give rise to Mac-1+ cells, confirming the purity of the CLP sort.
Absolute counts of splenocyte subsets after congenic transplantation. Absolute counts of splenic subsets after transplantation of 200 HSCs (bar a) or 200 HSCs and 3000 CLPs (bar b) on day 14 (A, n = 5) and day 35 (B, n = 12) after transplantation. ▧ indicates residual host cells; □, HSC-derived cells; and ▦, CLP-derived cells. Data represent mean values. On day 14, CLP-derived B220+ cells accounted for the significantly higher number of total B220+ cells (*P = .007).
Absolute counts of splenocyte subsets after congenic transplantation. Absolute counts of splenic subsets after transplantation of 200 HSCs (bar a) or 200 HSCs and 3000 CLPs (bar b) on day 14 (A, n = 5) and day 35 (B, n = 12) after transplantation. ▧ indicates residual host cells; □, HSC-derived cells; and ▦, CLP-derived cells. Data represent mean values. On day 14, CLP-derived B220+ cells accounted for the significantly higher number of total B220+ cells (*P = .007).
Allogeneic cotransplantation of CLPs with HSCs leads to rapid reconstitution of the lymphoid lineages
Lymphoid deficiency is typically more pronounced and protracted following allogeneic HCT when compared with autologous HCT. Therefore, the next step was to assess the engraftment potential of CLPs in allogeneic hosts. A mouse strain combination modeling genetic disparities of a matched unrelated donor HCT in humans was chosen. In this strain combination (C57BL/Ka → BALB.B), at least 500 LT-HSCs are necessary to rescue 95% to 100% of lethally irradiated hosts (data not shown). The kinetics of immune reconstitution in mice that received transplants of 500 allogeneic c-Kit+Thy1.1lolin–Sca-1+ LT-HSCs alone versus mice that received cotransplants of 500 allogeneic LT-HSCs and 3000 allogeneic lin–IL-7Rα+Thy1.1–c-KitloSca-1lo CLPs was assessed.
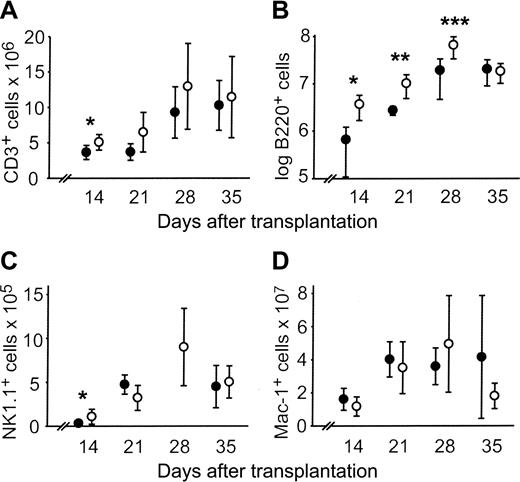
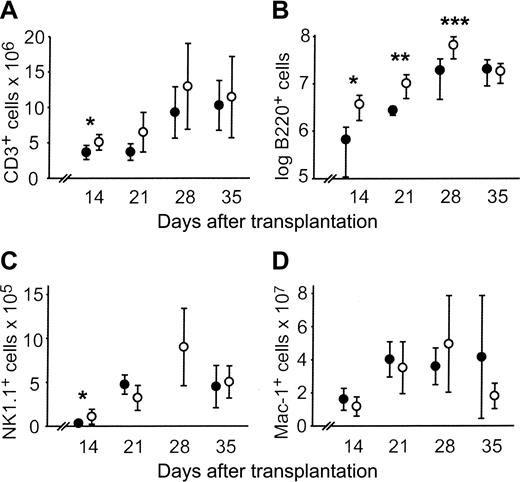
CLP cotransplantation accelerated engraftment of T cells (CD3+), NK cells (NK1.1+), and B cells (B220+) in the spleen. By day 14, the absolute numbers of all subsets were significantly higher compared with HSC controls. However, only B220+ cells were persistently increased subsequent to day 14 (Figure 3A-C). No difference in absolute numbers of Mac-1+ cells was observed (Figure 3D).
Absolute counts of splenic lymphocyte subsets are increased after allogeneic CLP cotransplantation. Absolute counts of splenic lymphocyte subsets after transplantation of 500 LT-HSCs (•) or 500 LT-HSCs and 3000 CLPs (○). Data represent mean ± SD of 5 to 9 mice in each group per time point. Statistically significant differences between the 2 groups are indicated. (A) CD3+, *P = .04. (B) B220+,*P = .02, **P = .04, ***P = .01. (C) NK1.1+,*P = .02. The NK1.1+ count for mice that received HSC transplants was not determined on day 28. (D) Mac-1+.
Absolute counts of splenic lymphocyte subsets are increased after allogeneic CLP cotransplantation. Absolute counts of splenic lymphocyte subsets after transplantation of 500 LT-HSCs (•) or 500 LT-HSCs and 3000 CLPs (○). Data represent mean ± SD of 5 to 9 mice in each group per time point. Statistically significant differences between the 2 groups are indicated. (A) CD3+, *P = .04. (B) B220+,*P = .02, **P = .04, ***P = .01. (C) NK1.1+,*P = .02. The NK1.1+ count for mice that received HSC transplants was not determined on day 28. (D) Mac-1+.
Although absolute numbers of CD3+ T cells in mice that received CLP cotransplants were significantly different from mice that received HSC transplants only on day 14, the CD8+ subset was significantly higher on days 14, 21, and 28 and the CD4+ subset only on day 21 (Figure 4). Thus, the CD4+/CD8+ ratio changed over time in the 2 transplantation groups with a skewing in favor of CD8+ cells in mice that received CLP cotransplants early after transplantation. By day 35, the CD4+/CD8+ ratio was normalized in both groups (Figure 4). The increase in splenic CD4+ and CD8+ cells demonstrated by day 21 is accounted for non-CLP-derived cells (Figure 5A-B). The increase in CD8+ cells in mice that received CLP cotransplants on days 14 and 28 is primarily due to the amount of CLP-derived CD8+ cells (Figure 5B).
Allogeneic CLP cotransplantation increases absolute numbers of splenic CD4+ and CD8+ cells. Splenic CD4+ count after allogeneic transplantation of 500 LT-HSCs (•) or 500 LT-HSCs and 3000 CLPs (○); splenic CD8+ count after transplantation of 500 LT-HSCs (•) or 500 LT-HSCs and 3000 CLPs (□). Data represent mean ± SD (n = 5-9 in each group per time point). Statistically significant differences are indicated. *P = .01 (• versus ○), *P = .008, **P = .01, ***P = .01 (• versus □).
Allogeneic CLP cotransplantation increases absolute numbers of splenic CD4+ and CD8+ cells. Splenic CD4+ count after allogeneic transplantation of 500 LT-HSCs (•) or 500 LT-HSCs and 3000 CLPs (○); splenic CD8+ count after transplantation of 500 LT-HSCs (•) or 500 LT-HSCs and 3000 CLPs (□). Data represent mean ± SD (n = 5-9 in each group per time point). Statistically significant differences are indicated. *P = .01 (• versus ○), *P = .008, **P = .01, ***P = .01 (• versus □).
Derivation of splenic CD4+ and CD8+ cells following allogeneic CLP cotransplantation. Mean splenic CD4+ (A) and CD8+ (B) count and derivation of mice that received HSC transplants (bar a) and mice that received CLP cotransplants (bar b) (n = 5-9 mice in each group per time point). ▦ indicates CLP-derived CD4+ or CD8+ cells; and •, non-CLP-derived cells. *P = .01 for non-CLP-derived cells.
Derivation of splenic CD4+ and CD8+ cells following allogeneic CLP cotransplantation. Mean splenic CD4+ (A) and CD8+ (B) count and derivation of mice that received HSC transplants (bar a) and mice that received CLP cotransplants (bar b) (n = 5-9 mice in each group per time point). ▦ indicates CLP-derived CD4+ or CD8+ cells; and •, non-CLP-derived cells. *P = .01 for non-CLP-derived cells.
In peripheral blood, reconstitution of lymphocytes was also accelerated by the inclusion of CLPs in the graft compared with mice that received HSC transplants (data not shown). Absolute lymphocyte counts were increased by day 28 (P = .06) and were significantly higher by day 35 (P = .01). Absolute numbers of CD3+ and CD4+ cells were increased on day 28 (P = .02, P = .01). Again, CD8+ cells were increased earlier, on day 21 (P = .02) and 28 (P = .04), although the CD3+ count was not.
The CD4+ or CD8+ subsets were not determined by using costaining with CD3 or TCRαβ, neither in the congenic nor in the allogeneic recipients. Therefore, the possibility remains that a proportion of CD4+ or CD8+ cells are NK, NK/T, TCRγδ, or dendritic cells. However, in separate experiments analyzing allogeneic mice that received CLP cotransplants (n = 5) on day 28, CD3 costaining showed that 99.5% (± 0.3%) or 97.3% (± 0.9%) of CD4+ cells in blood or spleen, respectively, and 96.4% (± 1.9%) or 76.9% (± 9.9%) of CD8+ cells in blood or spleen, respectively, were also CD3+ (data not shown, mean percentage ± SD).
Analysis of thymic cellularity revealed that CLP-derived thymocytes were detected as early as day 14 after transplantation, peaked at day 21 but were not detected later than day 35. In contrast, the earliest time point when HSC-derived thymocytes could be detected was day 21 (data not shown). However, there was no difference in total thymic cellularity between the 2 transplantation groups. Our findings are consistent with previous observations13 that thymic repopulation from CLPs occurs faster than from HSCs.
Absence of acute GVHD in mice after allogeneic CLP transplantation
In the mouse strain combination used, 107 T-cell enriched cells from pooled spleens and LN rapidly induced severe acute GVHD25 while transplantation of HSCs alone does not cause GVHD.26 Because transplanted CLPs give rise to T cells and 3 × 103 CLPs were similarly protective against MCMV as 107 unfractionated LN cells in our congenic experiments, mice that received transplants of 500 LT-HSCs (n = 16) or 500 LT-HSCs and 3000 CLPs (n = 17) were monitored for clinical and histologic signs of acute GVHD. Following recovery from an initial decrease in body weight temporally associated with irradiation, mice from both groups continued to gain weight and failed to demonstrate changes in posture, activity, fur texture, or skin integrity.27 Tissues from all 7 histologically analyzed mice that received transplants of LT-HSCs as well as from all 6 analyzed mice that received CLP cotransplants showed no significant histologic signs for GVHD in the terminal ileum, ascending colon, liver, or ear on day 60 (data not shown). Thus, neither transplantation of bone marrow-derived LT-HSCs alone nor cotransplantation of LT-HSCs and CLPs caused GVHD in this model.
Allogeneic CLP cotransplantation protects against lethal MCMV disease
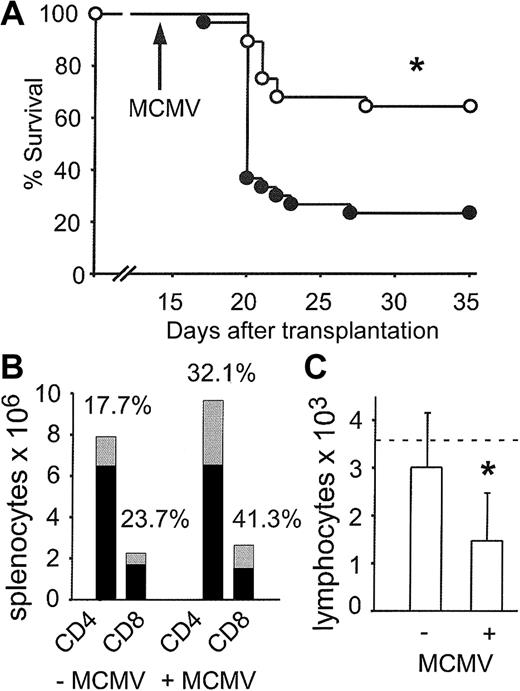
We next determined if the improved quantitative reconstitution achieved by allogeneic CLP cotransplantation also resulted in an improved functional response to MCMV infection. Groups of thymus-bearing mice were challenged with MCMV on day 14 following transplantation with 500 LT-HSCs alone or 500 LT-HSCs and 3000 CLPs. Sixty-four percent of mice that received CLP cotransplants survived compared with 23% of mice that received transplants of LT-HSCs alone (Figure 6A; P < .0001).
Allogeneic CLP cotransplantation protects thymus-bearing hosts from lethal MCMV disease. (A) Thymus-bearing mice received transplants of 500 LT-HSCs alone (•; n = 30, 23% survival) or 500 LT-HSCs and 3000 CLPs (○; n = 28, 64% survival). *P < .0001 (• versus ○). (B) Mean splenic CD4+ and CD8+ count on day 35 after CLP cotransplantation in uninfected mice (–MCMV) (n = 9) or in surviving mice after MCMV infection (+MCMV) (n = 4). ▦ indicates percentages of CLP-derived cells; and •, non-CLP-derived cells (host- and HSC-derived). (C) Absolute blood lymphocyte counts on day 35 after CLP cotransplantation in uninfected mice (–MCMV) (n = 9) or in surviving mice after MCMV infection (n = 8). The dotted line indicates the lower normal limit. Mean ± SD. *P = .01.
Allogeneic CLP cotransplantation protects thymus-bearing hosts from lethal MCMV disease. (A) Thymus-bearing mice received transplants of 500 LT-HSCs alone (•; n = 30, 23% survival) or 500 LT-HSCs and 3000 CLPs (○; n = 28, 64% survival). *P < .0001 (• versus ○). (B) Mean splenic CD4+ and CD8+ count on day 35 after CLP cotransplantation in uninfected mice (–MCMV) (n = 9) or in surviving mice after MCMV infection (+MCMV) (n = 4). ▦ indicates percentages of CLP-derived cells; and •, non-CLP-derived cells (host- and HSC-derived). (C) Absolute blood lymphocyte counts on day 35 after CLP cotransplantation in uninfected mice (–MCMV) (n = 9) or in surviving mice after MCMV infection (n = 8). The dotted line indicates the lower normal limit. Mean ± SD. *P = .01.
On day 35, cell counts in mice that received CLP cotransplants that survived MCMV infection were performed. Absolute counts of splenic CD4+ and CD8+ cells were not different compared with uninfected controls. However, the relative contribution of CLP-derived CD4+ and CD8+ cells to the total was increased without altering the CD4+/CD8+ ratio (Figure 6B). In contrast, absolute blood lymphocyte counts were significantly lower in mice that received CLP cotransplants that survived MCMV infection (Figure 6C; P = .01).
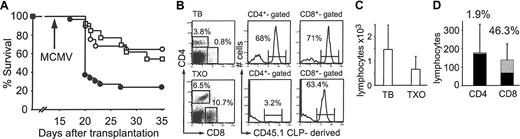
In order to assess if functional lymphoid reconstitution by CLPs is thymic dependent, groups of thymectomized mice received transplants of 500 LT-HSCs and 3000 CLPs in parallel experiments with thymus-bearing hosts that also received grafts containing both 500 LT-HSCs and 3000 CLPs. There was no significant difference in survival between thymus-bearing hosts (64%) and thymectomized hosts that received CLP cotransplants (54%) (Figure 7A; P = NS).
Thymectomized hosts are protected from lethal MCMV disease after allogeneic CLP cotransplantation. (A) Thymus-bearing (TB; ○;n = 28, 64% survival) or thymectomized (TXO; □;n = 13, 54% survival) mice received transplants of 500 LT-HSCs and 3000 CLPs and compared with thymus-bearing mice that received transplants of 500 LT-HSCs alone (•;n = 30, 23% survival). P = NS (○ versus □). (B) FACS analysis of spleen on day 35 following CLP cotransplantation. (C) Absolute blood lymphocyte counts (mean ± SD) on day 35 in thymus-bearing (n = 8) and thymectomized (n = 7) mice that received CLP cotransplants and survived MCMV infection. P = NS. (D) CD4+ and CD8+ count in blood (mean ± SD) on day 35 in thymectomized mice that received CLP cotransplants that survived MCMV infection (n = 7). ▦ indicates percentages of CLP-derived cells; and •, non-CLP-derived cells.
Thymectomized hosts are protected from lethal MCMV disease after allogeneic CLP cotransplantation. (A) Thymus-bearing (TB; ○;n = 28, 64% survival) or thymectomized (TXO; □;n = 13, 54% survival) mice received transplants of 500 LT-HSCs and 3000 CLPs and compared with thymus-bearing mice that received transplants of 500 LT-HSCs alone (•;n = 30, 23% survival). P = NS (○ versus □). (B) FACS analysis of spleen on day 35 following CLP cotransplantation. (C) Absolute blood lymphocyte counts (mean ± SD) on day 35 in thymus-bearing (n = 8) and thymectomized (n = 7) mice that received CLP cotransplants and survived MCMV infection. P = NS. (D) CD4+ and CD8+ count in blood (mean ± SD) on day 35 in thymectomized mice that received CLP cotransplants that survived MCMV infection (n = 7). ▦ indicates percentages of CLP-derived cells; and •, non-CLP-derived cells.
FACS analysis and absolute blood counts on day 35 in thymectomized mice that received CLP cotransplants that survived MCMV infection were performed. In thymectomized hosts, although the percentage of CLP-derived CD8+ cells was comparable to thymus-bearing hosts, there was a paucity of CLP-derived CD4+ cells (Figure 7B,D). Moreover, the blood lymphocyte count was lower in thymectomized mice although not statistically significant (Figure 7C) and the CD4/CD8 ratio (1.3) was skewed toward CD8+ (Figure 7D).
Histopathologic analysis of livers from thymus-bearing mice revealed differences in the nature and severity of MCMV hepatitis on day 20 by H&E and immunofluorescence staining. A representative section of a liver from a mouse that received a LT-HSC transplant (Figure 8A) shows severe acute MCMV hepatitis with typical cytomegaloviral nuclear inclusions in hepatocytes. In contrast, mice that received CLP cotransplants had fewer MCMV-infected hepatocytes and more infiltrating cells (Figure 8B). Immunofluorescence staining against β-galactosidase revealed increased presence of the lacZ-tagged virus in mice that received transplants of LT-HSCs alone compared with mice that received CLP cotransplants (Figure 8C-D). Immunofluorescent and immunohistochemical analysis of infiltrating cells revealed that residual host lymphocytes (staining against Thy1.2, data not shown) as well as CLP-derived cells (staining against CD45.1; Figure 8E) are present in the liver during acute MCMV hepatitis.
Liver histopathology after MCMV infection. (A) Severe MCMV hepatitis day 20 following LT-HSC transplantation (original magnification × 40). (B) Lymphocytic infiltration day 20 following CLP cotransplantation (original magnification × 40). Panels A and B are stained with H&E. Arrows indicate cytomegalic hepatocytes with viral inclusions. (C-D) Immunofluorescent localization of lacZ-tagged MCMV RM427+ (green; original magnification × 10) in mice that received LT-HSCs (C) and CLP cotransplants (D). (E) Immunohistochemical detection of CD45.1+ CLP-derived cells in the liver (brown, counterstained with hematoxylin).
Liver histopathology after MCMV infection. (A) Severe MCMV hepatitis day 20 following LT-HSC transplantation (original magnification × 40). (B) Lymphocytic infiltration day 20 following CLP cotransplantation (original magnification × 40). Panels A and B are stained with H&E. Arrows indicate cytomegalic hepatocytes with viral inclusions. (C-D) Immunofluorescent localization of lacZ-tagged MCMV RM427+ (green; original magnification × 10) in mice that received LT-HSCs (C) and CLP cotransplants (D). (E) Immunohistochemical detection of CD45.1+ CLP-derived cells in the liver (brown, counterstained with hematoxylin).
Viral load in liver and lungs of surviving mice was also measured by plaque assay. At day 20, the mean MCMV load in the liver of mice that received LT-HSC transplants was higher than in mice that received CLP cotransplants although the difference was not statistically significant (Figure 9A; P = .06). By day 35, viral loads of surviving mice were highly variable although it is notable that 4 of 9 mice that received CLP cotransplants had no detectable viral load. Lung titers follow a similar pattern but differences are less pronounced (Figure 9B).
MCMV load in liver and lungs. Viral load (log plaque forming units [pfu] per gram tissue) in liver (A) and lungs (B) from mice that received LT-HSCs (•) and CLP cotransplants (○). A solid line indicates the median viral load and the detection limit of the plaque assay is indicated by the dotted line. P = NS for all time points.
MCMV load in liver and lungs. Viral load (log plaque forming units [pfu] per gram tissue) in liver (A) and lungs (B) from mice that received LT-HSCs (•) and CLP cotransplants (○). A solid line indicates the median viral load and the detection limit of the plaque assay is indicated by the dotted line. P = NS for all time points.
Residual host lymphocytes contribute to the control of MCMV infection
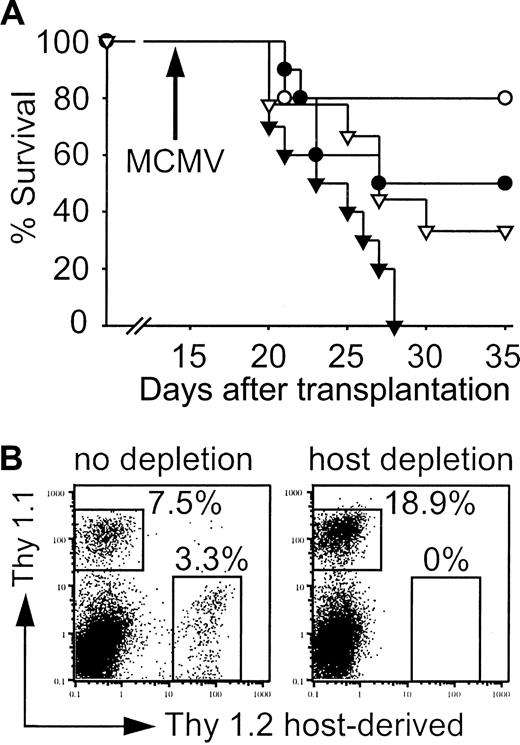
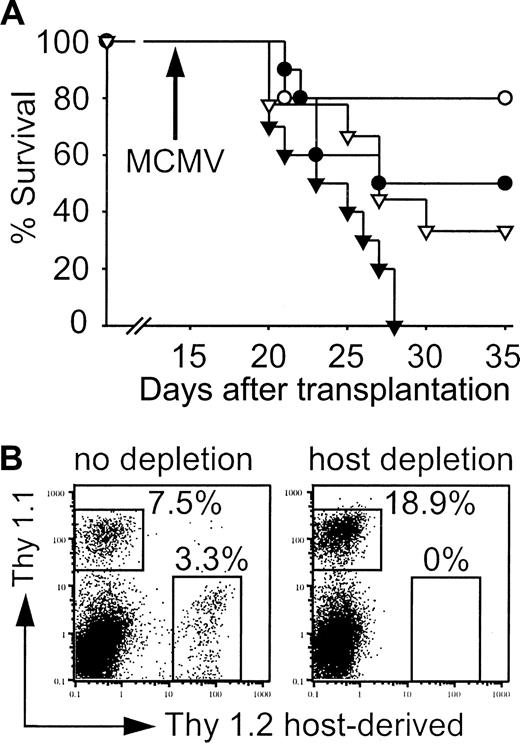
The level of T-cell chimerism early after allogeneic HCT was low and residual host T cells were detected in the livers of infected mice. We hypothesized that residual host as well as CLP-derived cells may significantly contribute to viral control in our model. Therefore, mice that received transplants were depleted of host T cells, some NK cells, and plasmacytoid dendritic cells using anti-Thy1.2 antibody prior to infection on day 14.21,22 Host depletion decreased the survival after MCMV infection in both groups (Figure 10A). However, depleted mice that received CLP cotransplants again had a survival advantage when compared with hosts that received LT-HSC transplants. FACS analysis of surviving mice on day 35 confirmed complete depletion (Figure 10B). MCMV titers in liver and lungs of moribund mice and survivors on day 35 were comparable (data not shown).
Host cells contribute to control of MCMV infection. (A) Survival of mice that received transplants with (▾, ▿) or without (•, ○) depletion of Thy1.2+ host cells. Survival of mice that received transplants of 500 LT-HSCs (•; n = 9, 55.6% survival) or 500 LT-HSCs and 3000 CLPs (○; n = 10, 80% survival) compared with mice that received transplants of 500 LT-HSCs (▾; n = 10, 0% survival) or 500 LT-HSCs and 3000 CLPs (▿; n = 9, 33.3% survival) with subsequent Thy1.2 depletion. P = .03 (• versus ▾), P = .04 (▿ versus ▾). (B) Splenic FACS analysis on day 35 of infected survivors that received CLP cotransplants confirmed complete depletion. Thy 1.1+ cells are LT-HSC and CLP derived, the majority again was CLP derived (also shown in Figure 7B).
Host cells contribute to control of MCMV infection. (A) Survival of mice that received transplants with (▾, ▿) or without (•, ○) depletion of Thy1.2+ host cells. Survival of mice that received transplants of 500 LT-HSCs (•; n = 9, 55.6% survival) or 500 LT-HSCs and 3000 CLPs (○; n = 10, 80% survival) compared with mice that received transplants of 500 LT-HSCs (▾; n = 10, 0% survival) or 500 LT-HSCs and 3000 CLPs (▿; n = 9, 33.3% survival) with subsequent Thy1.2 depletion. P = .03 (• versus ▾), P = .04 (▿ versus ▾). (B) Splenic FACS analysis on day 35 of infected survivors that received CLP cotransplants confirmed complete depletion. Thy 1.1+ cells are LT-HSC and CLP derived, the majority again was CLP derived (also shown in Figure 7B).
Discussion
We demonstrate that cotransplantation of congenic as well as allogeneic CLPs with HSCs accelerates functional immune reconstitution without inducing GVHD. CLP cotransplantation protected thymus-bearing, thymectomized, and host T- and NK-cell-depleted mice from lethal MCMV infection.
The immune control of CMV is complex, involving T cells, B cells, NK cells, and dendritic cells. Prior clinical28,29 and animal studies have characterized this complex immune response. In mice, it has been shown by antibody depletion, adoptive transfer, or vaccination, that CD4+ and CD8+ T cells,30-32 NK cells,33-35 and B cells31,36,37 all contribute to a variable degree to viral control. Dendritic cells pulsed with virus or viral antigens are capable of protecting immunocompromised mice.38,39 However, in both mouse and clinical studies, the highest susceptibility to lethal infection is seen in conditions where T-cell depletion or deficiency can be measured whether as a result of disease or medical therapy. Based on these observations, clinical studies were carried out that demonstrated that adoptive transfer of ex vivo-expanded CMV-specific CD8+ cells did not prevent CMV reactivation following HCT. However, patients were protected against CMV-disease for a limited period of time. Subsequent studies confirmed that CD4+-cell cotransfer was necessary in order to achieve sustained antiviral protection; however, the durability of this protection was not well established.40,41
Therefore, it is significant that the addition of a single population of progenitor cells not only gives rise to a full complement of hematopoietic effector cells, but it also results in protection against lethal infection. A clear dose response between numbers of transplanted CLPs and protection against MCMV was demonstrated after congenic transplantation.
Our observation that absolute lymphocyte counts in blood following MCMV infection were significantly lower compared with uninfected mice correlates with previously published observations demonstrating the inhibition of lymphopoiesis by MCMV after HCT.42 In our experiments however, splenic CD4+ and CD8+ counts were similar in infected survivors that received CLP cotransplants compared with uninfected mice on day 35 after transplantation. Moreover, the contribution of CLP-derived cells to the 2 populations was increased, suggesting a crucial role for CLP-derived cells in viral control. An important finding in our studies is that peripheral blood counts did not predict risk of death due to MCMV. Thus, splenic CD4+ and CD8+ cell numbers may be more reflective of effective antiviral immune reconstitution as has been demonstrated regarding splenic myeloid cells in control of bacterial and fungal infections.18
Lymphoid repopulation following HCT results from both thymic-dependent and thymic-independent4,43,44 pathways. The thymic-dependent pathway recapitulates lymphoid ontogeny, whereas the thymic-independent pathway consists of peripheral expansion of mature cells, contained in the graft or residual host cells, as well as de novo regeneration derived from HSCs occurring in extrathymic sites. Strategies designed to enhance each are being actively investigated. We predicted that inclusion of additional progenitor cells in the transplanted graft would augment both pathways. Our findings suggest that even in the setting of limited thymic function such as has been described following HCT,45,46 GVHD,3 or chemo-radiotherapy,5 CLP-derived cells contribute significantly to viral control. Thymectomized recipients that received CLP cotransplants were protected against lethal MCMV infection to a similar extent as thymus-bearing recipients. In thymectomized survivors, many CLP-derived CD8+ cells, whereas only very few CD4+ cells, were detected in the spleen and peripheral blood on day 35. Thus, even in the absence of a thymus, CLPs are capable of extrathymic CD8+ but not CD4+ T cell maturation. Development of CD4+ cells appears to be exclusively thymic dependent, whereas an extrathymic pathway of de novo regeneration of CD8+ cells exists as previously shown after transplantation of whole bone marrow.4,43 The phenotype of CLP-derived CD8+ cells in thymectomized mice and their specificity against MCMV are currently under investigation.
Transplantation of purified HSCs results in slower lymphocyte engraftment compared with transplantation of whole bone marrow containing the same number of HSCs.26 Therefore, following HSC transplantation, the level of T-cell chimerism is lower and the issue of residual host function is important. Thus, we assessed the role for residual host cells in response to MCMV infection by antibody depletion. Anti-Thy1.2 antibody was administered after transplantation in order to minimize duration of antibody treatment. In this setting, it is possible that inflammation via antibody-mediated lysis could contribute to an apparent increase in MCMV susceptibility. However, our observation that mice that received CLP cotransplants and were treated with anti-Thy1.2 antibody were less susceptible to MCMV than mice that received LT-HSC transplants and antibody treatment supports that CLP-derived cells contribute to antiviral protection.
Allogeneic transplantation of purified HSCs in mice did not induce GVHD.24,26 Moreover, CLPs undergo recapitulation of lymphoid cell development including selection in the host. Therefore, addition of CLPs to a graft of purified HSCs shortened the time of lymphoid deficiency after transplantation and restored functional immunity without inducing GVHD in a mouse strain combination where splenic mature T cells are capable of inducing severe lethal GVHD.25 However, when comparing splenic absolute numbers of CLP-derived T cells after congenic versus allogeneic transplantation, substantially more CLP-derived T cells are detected after allogeneic transplantation. Although clinical and histologic signs for acute GVHD are absent, this could indicate alloreactive expansion of CLP-derived T cells in allogeneic recipients.
Transplantation of high doses of purified HSCs in mice accelerated quantitative reconstitution in congenic and major mismatched allogeneic hosts.47 Because the determinants of lineage commitment are complex even in the absence of infection, transplantation of higher doses of purified HSCs may, but cannot be expected to, result in equivalent recapitulation of immune function as seen after CLP cotransplantation. Further experiments are underway to address this important question.
In conclusion, we describe here for the first time the potential of a defined highly purified committed lymphoid progenitor population to engraft rapidly in congenic and allogeneic thymus-bearing or thymectomized hosts. This population of cells accelerates reconstitution of the lymphoid compartment with regard to numbers and function as shown by protection against MCMV infection without inducing significant GVHD. Moreover, a durable immunity to infection was recapitulated. These results suggest that there is a potential role of graft engineering using purified progenitors to reduce susceptibility to infection following HCT.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2002-12-3834.
Supported by Swiss Cancer League fellowship BIL KFS 1060-9-2000 (C.A.), Amy Strelzer Manasevit Scholars Program (J.M.Y.B., J.A.S.), ASBMT/Roche New Investigator Award (J.M.Y.B.), Center for Clinical Immunology at Stanford University (J.M.Y.B.), National Institutes of Health grants 1RO1AI47458 (I.L.W.) and 2PO1CA49605 (J.M.Y.B., J.A.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Libuse Jerabek, Motonari Kondo, Ruby Wong, and Lucino Hidalgo for their invaluable assistance.







![Figure 9. MCMV load in liver and lungs. Viral load (log plaque forming units [pfu] per gram tissue) in liver (A) and lungs (B) from mice that received LT-HSCs (•) and CLP cotransplants (○). A solid line indicates the median viral load and the detection limit of the plaque assay is indicated by the dotted line. P = NS for all time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2002-12-3834/6/m_h81434639009.jpeg?Expires=1766004114&Signature=zPY9f2K4dL3Ewqp~BjKlZP9lBEh7FCZLWVZjA8kJ9aDyClVGZSOd6K2os6B0pD-nZfu51k~HQA2CIlvQ0Kb-03U0owgIkfzU7Ae7Dt8PfMFZ5hqI1G~NINGP8ZdnxUwmCQ7eqdvIBHSMO0zwnqbQdsvmYY4gy95yEZfld~pQhJ4E~qmBiHymtuAm~S7fAbP0nXyAtOVKVJ6dMFl79oSdxkeLCrt47GLVGFonphZlOf9NrE21cuROVSW2OuvECfBQt8dWCH-hfCZpa1j3lfNmkcGnKFFFelayrJvC9VfW1bcfI6IyQzoiDRQf40qhocrf7A2oSEGrWGUFJU780P4zmA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 9. MCMV load in liver and lungs. Viral load (log plaque forming units [pfu] per gram tissue) in liver (A) and lungs (B) from mice that received LT-HSCs (•) and CLP cotransplants (○). A solid line indicates the median viral load and the detection limit of the plaque assay is indicated by the dotted line. P = NS for all time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2002-12-3834/6/m_h81434639009.jpeg?Expires=1766004115&Signature=ugtAGPJ1~ZaWpLHt16t~RO~sO6LV36dnxTeEkrCUA-3YlYYLymEum58GzWw5bjX2ouywiLFXXhsGANEgwuw2Gp02SIVGFcOtNFgvM2~pi2Nlav~WMNjbSnP0xB00Kz3kE6fcBfB2JlLbA2n3Qs3G79XFoM4538ksyWLoPm93XjHnI3b1YIWXh4ZsU9pc2C3I6OSAd9bPp4Kx6hy-n2dCryvhs0SJybdx-FWTxOJFHG6I2~JsYhamrVy2miZfZi7~6zZsHHpEz7AW~ksaG2TT~ZaQS6OXOGNrtamq7fWDSRCRcuOV5LLhLCqw~vJRiJoEAwcMWji-NIn5lK5lYcnckA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)