Abstract
Hematopoietic stem cell transplantation (SCT) is the only proven cure for chronic myeloid leukemia (CML), a rare disease in childhood. We report outcomes of 314 children with Philadelphia-chromosome–positive (Ph+) CML undergoing SCT from HLA-matched siblings (n = 182) or volunteer-unrelated donors (VUD; n = 132). Three-year overall survival (OS) and leukemia-free survival (LFS) rates were 66% and 55% (n = 314). For 156 children in first chronic phase (CP1) who underwent transplantation from HLA-identical siblings, OS and LFS rates were 75% and 63%. For 97 children who underwent SCT in CP1 from VUD, 3-year OS and LFS rates were 65% and 56%, reflecting higher transplantation-related mortality (TRM) after VUD SCT (35% vs 20%; multivariate hazard ratio [HR], 1.9; 95% confidence interval [CI], 1.0-3.5; P = .05). In a multivariate model for OS and LFS, outcomes were superior in CP1 than in advanced phase (AP/CP1) (OS HR, 2.0; 95% CI, 1.3-3; P = .001; LFS HR, 1.8; 95% CI, 1.2-2.6; P = .003). For relapse, donor source (VUD/sibling) (HR, 0.38; 95% CI, 0.19-0.76; P = .006) and disease stage (AP/CP1) (HR, 2.4; 95% CI, 1.36-4.3; P = .003) were significant. This is the first large series to show that SCT confers long-term LFS in most children with CML and helps assess alternative therapy, including tyrosine kinase inhibitors.
Introduction
Chronic myeloid leukemia (CML) constitutes approximately 3% to 5% of all childhood leukemias and is characterized by the same molecular, cytogenetic, and morphologic features observed in adults with classical Philadelphia-positive (Ph+) CML.1 The incidence in childhood is less than 1 in 100 000.2 As in adults, allogeneic stem cell transplantation (SCT) is the only proven curative treatment for children with CML.3 For adults leukemia-free survival (LFS) ranges from 45% to 80%.4-9 However, few studies have specifically analyzed outcomes in children, and the number of cases in these studies is small.10-12 In some studies children have been included in adult series, but they form a small proportion of the patient group, and their outcomes are not separately considered.4,5,8,13
A number of prognostic factors have been identified for outcome after allogeneic SCT. These include disease stage,14-16 degree of HLA disparity,17-19 recipient/donor sex mismatch,20 and duration of disease before transplantation.4-6,14 Increased recipient age may also be an unfavorable risk factor.8,21,22 However, there are conflicting data regarding whether prior treatment with interferon-α (IFN-α)21,23-25 or cytomegalovirus (CMV) serostatus26,27 affects the outcome of subsequent SCT. In addition, the effects of these factors have not been specifically assessed in the pediatric population. The worsening outcome of transplantation with increasing numbers of risk factors is attributed to an increase in transplantation-related mortality (TRM).28
Since the introduction of new and potentially highly effective treatment, such as tyrosine kinase inhibitors,29 the risk-benefit assessment for allogeneic SCT is increasingly difficult to estimate. The aims of this study were to more accurately determine overall survival (OS), LFS, and TRM in a large group of children with CML undergoing allogeneic SCT and to identify factors influencing outcome. We have, therefore, analyzed data from the 314 children with Ph+ CML for whom a complete data set was reported to the Chronic Leukemia Registry of the European Group for Blood and Marrow Transplantation (EBMT) between 1985 and 2001.
Patients, materials, and methods
We conducted a retrospective analysis on behalf of the EBMT Chronic Leukemia and Pediatric Working Parties. Children (younger than 18 years) reported to the EBMT Registry who had undergone allogeneic SCT for Ph+ CML (CML) between January 17, 1985 and December 5, 2001 were included. The analysis was completed from information available as of August 23, 2002. Additional information regarding the use of IFN-α before SCT, characteristics of the donor, conditioning regimens, and clinical outcomes was sought by questionnaire from all 95 participating centers.
The EBMT Registry contains data on 530 children who have undergone allogeneic SCT, but this analysis relates to the 314 children with Ph+ CML for whom we have detailed information. The source of stem cells was bone marrow for all children; donors were HLA-identical siblings for 182 children and matched volunteer-unrelated donors (VUDs) for 132 children. Bone marrow transplantation (BMT) was carried out in first chronic phase (CP1) for 253 children (156 sibling donors, 97 VUDs) and in advanced-phase (AP) disease (CP2, accelerated phase, and blast crisis [BC]) for 61 children. Approximately half (52%) the SCTs were performed before 1995. The median follow-up time of children who underwent sibling or unrelated donor SCT was 49 months (range, 1-202 months) or 21 months (range, 1-193 months), respectively. Characteristics of the patients are provided in Table 1.
HLA typing and donor matching
Analysis of HLA matching was based on typing for HLA-A and -B antigens by serologic methods and for HLA-DR by serologic typing or DNA techniques (restriction fragment length polymorphisms or sequence-specific oligonucleotide probes) as reported by institutions performing the transplantations. All recipients of VUD SCT were “fully matched” according to the HLA typing strategy performed at the time of donor identification in each center, and definitions of HLA-matching were considered with respect to reagents available when typing was performed.
Disease characteristics before SCT
Recipient/donor age. The median age of recipients at transplantation was 14 years (range, younger than 12 months to 17 years) and was not significantly different for children undergoing SCT from sibling or unrelated donors. Information about donor age was available for 156 (86%) siblings and 81 (61%) VUDs, and the median age of the former was significantly lower than for VUD recipients (13 vs 33 years; P < .001) (Table 1).
Sex. Of the 314 children included in this study, 168 (54%) were boys and 146 (46%) were girls with a male-female ratio of 1.15:1.0, which is similar to that observed in adults. A similar proportion of sibling (27%) and VUD (23%) transplantations was performed for the combination male recipient/female donor.
Disease phase. The phase of disease at the time of transplantation in the 314 study patients was defined according to the International Bone Marrow Transplant Registry (IBMTR) criteria.30 Before transplantation, most (253; 81%) patients were in CP1. This represented 86% of all HLA-identical sibling transplantations and 73% of the transplantations from matched VUD (P = .007) (Table 1). Of the 61 children who underwent transplantation in more advanced phases of CML, 25 were in CP2, 26 were in accelerated phase, 10 were in BC.
Treatment before SCT
Information about the use of IFN-α before SCT was available for 270 patients, of whom 98 received the drug. Regarding those for whom we have data, 39 (24%) of 162 recipients of sibling and 59 (55%) of 108 recipients of VUD SCT were given IFN-α before treatment (P < .001).
Interval from diagnosis to SCT
The interval from diagnosis to transplantation was 0 to 6 months in 100 (32%) children, 6 to 12 months in 89 (28%) children, and longer than 12 months in 125 (40%) children. SCT was performed earlier in a significantly higher proportion of children with sibling donors—within 6 months and 1 year of diagnosis in 44.5% and 71%, respectively, compared with only 19% and 45% of those with VUDs (P < .001).
CMV status donor/recipient
Data regarding the CMV serostatus of recipients and their donors were available for 277 children (156 siblings, 121 VUDs). A similar proportion of children in the 2 groups were CMV seronegative and had seronegative donors (37% siblings, 37% VUDs), whereas more seronegative recipients of VUD SCT had seropositive donors (22% vs 9%; P = .008).
Conditioning regimen
Choice of conditioning therapy before transplantation was undertaken independently at each institution. Of the 314 patients in the study, 214 (68%) underwent preparative regimens containing total body irradiation (TBI) and 100 did not receive TBI. A higher proportion (87%) of VUD recipients underwent TBI compared with 54% of siblings (P < .001) (Table 1).
GVHD prophylaxis/supportive therapy
T-cell depletion strategies were adopted in a higher proportion of children undergoing VUD transplantation (61%) than for those undergoing sibling allograft (14%). In contrast, cyclosporin A (CsA) alone was used for 54 (30%) children undergoing SCT from HLA-identical donors compared with only 1 VUD recipient (0.8%), and methotrexate was administered to a higher proportion of VUD recipients (90.8%) than to children who underwent SCT from HLA-identical siblings (64%).
Outcome
Outcomes analyzed were hematopoietic recovery, acute and chronic graft-versus-host disease (GVHD), TRM, relapse risk (Rel), OS, and LFS. The date of neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) above 0.5 × 109/L. The date of platelet recovery was defined as the first of 7 consecutive days with a platelet count higher than 50 × 109/L without transfusion. Acute and chronic GVHD were classified according to published criteria.31 Only those patients surviving more than 100 days after transplantation were considered evaluable for chronic GVHD. Rel was defined as molecular, cytogenetic, or hematologic (according to the definition accepted at each center). Patients in continuous complete remission were censored at death or, for survivors, at last contact. TRM was defined as death in continuous complete remission; patients were censored at time of relapse or at last follow-up evaluation. LFS was defined as survival in continuous complete remission.
Statistical methods
The EBMT Registry contains data on 530 children who underwent allogeneic SCT. All analyses for this study were conducted on the data set of all 314 children with complete information for age at SCT, donor relation, stage of disease at transplantation, donor-recipient sex mismatch, time interval between diagnosis and transplantation, calendar year of SCT, GVHD prophylaxis, and conditioning regimen (TBI vs non-TBI–containing regimens). To ensure that analyses of these 314 children did not introduce any clinically relevant selection bias with respect to our main conclusions, we compared (data not shown) the unselected cases (n = 216) and the selected cases. Given that the 2 main risk factors (stage and donor relation) are complete for all 530 children, we were able to check that there was no difference in the effect of both risk factors among the selected and the unselected cases on OS and LFS (by verifying that all interaction terms representing such a differential effect have P values greater than .50).
Associations between discrete variables were evaluated using χ2 analysis on the appropriate cross-tabulations. Subgroups were compared with respect to continuous variables using Student t test or the Mann-Whitney U test in case of nonnormal distributions or the presence of outliers.
For the analysis of OS and LFS, Kaplan-Meier curves were used to describe the entire population and to compare subgroups without adjustment for other covariates. Cumulative incidence curves were created for relapse incidence and TRM because they are competing risks. Tables 2 and 3 report the estimated 3-year OS and LFS probabilities (Kaplan-Meier) and the 3-year Rel and TRM cumulative incidence estimates.
Multivariate Cox models were used to evaluate the adjusted effects of a small number of risk factors, expressed as (adjusted) hazard ratios (HRs) for comparison with the existing literature. The final Cox models are reported in Table 4. Only the significant factors for each outcome are reported, but all the models include donor relation, stage of disease at transplantation, donor-recipient sex mismatch, time interval between diagnosis and transplantation, calendar year of SCT, and GVHD prophylaxis. Recipient age and TBI were initially included in the models but were later removed because they did not influence any outcome in the multivariate analysis. (Donor age was not included because it was missing for 77 donors. However, in the setting of HLA-identical sibling donors, donor age was highly correlated with recipient age, and its omission from the models is unlikely to have led to appreciable bias in the estimation of the other coefficients.)
The influence of CMV serostatus and the use of IFN-α before SCT on outcome were analyzed separately given that the information was incomplete (data were missing for 37 and 44 children, respectively). Univariate analysis was first reported, and then the 2 variables were separately incorporated into the multivariate models. SPSS version 10 (SPSS, Chicago, IL) was used for all statistical calculations, except for the estimation of the cumulative incidence, which was performed in NCSS 2001 (Number Cruncher Statistical Systems, Kaysville, UT).
Results
Hematopoietic recovery
One hundred seventy-six (97%) siblings and 124 (95%) VUD recipients successfully engrafted. Neutrophil engraftment occurred at a median of 23 days after HLA-identical sibling transplantation and 22 days after VUD allograft (P = NS). Platelet engraftment was achieved at a median of 32 and 28 days after HLA-identical sibling and VUD transplantation, respectively (P = NS).
Graft-versus-host disease
Moderate to severe acute (grades 2-4) GVHD occurred in 37% of children undergoing transplantation from HLA-identical siblings and was significantly more frequent in children undergoing VUD transplantation (52%) (P = .009) despite more frequent usage of T-cell depletion (TCD). Data relating to the incidence and severity of chronic GVHD showed that of the 247 evaluable patients, 50 (20%) had extensive GVHD, 60 (24%) had limited GVHD, and 137 (56%) had none. There was no significant difference in the incidence (P > .1) or severity (P = .076) of chronic GVHD between the 2 groups.
Transplantation-related mortality
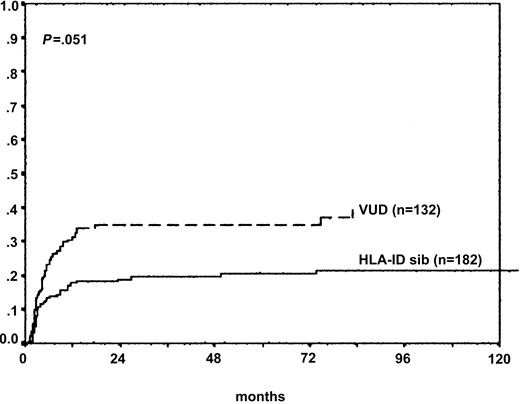
Unadjusted cumulative incidence estimates of TRM at 3 years for children who underwent SCT in CP1 from siblings and unrelated donors were 20% and 31%, respectively. For patients who underwent transplantation in the advanced phase, the results were 16% and 46%, respectively. TRM for the whole cohort was 26%. Multivariate analysis demonstrated that recipients of VUD SCT had significantly higher TRM than those from sibling donors (HR, 1.9; 95% CI, 1.0-3.5; P = .05) (Figure 1). The use of methotrexate was associated with significantly reduced TRM for sibling and unrelated donor transplantation on multivariate analysis (HR, 0.56; 95% CI, 0.3-0.97; P = .04). The effect of calendar year on TRM was also evaluated by multivariate analysis and was estimated to be on the same order of magnitude as in adults (HR, 0.98; 95% CI, 0.92-1.05), indicating that there was a reduction over time, but this failed to reach statistical significance (Figure 1).
Transplantation-related mortality (donor source). TRM for VUD recipients (n = 132) was significantly higher than for children with HLA-identical sibling donors (n = 182) (adjusted HR, 1.9; 95% CI, 1.0-3.5; P = .051).
Transplantation-related mortality (donor source). TRM for VUD recipients (n = 132) was significantly higher than for children with HLA-identical sibling donors (n = 182) (adjusted HR, 1.9; 95% CI, 1.0-3.5; P = .051).
Leukemic relapse
Crude cumulative incidence estimates of relapse at 3 years for sibling and VUD recipients who underwent transplantation in CP1 were 17% and 13%, respectively. For children who underwent transplantation in the advanced phase, cumulative incidence estimates of relapse at 3 years were 49% for sibling recipients and 20% for children with unrelated donors. When the whole cohort was analyzed, cumulative incidence estimates of relapse at 3 years was 19%. On multivariate analysis, the use of TCD strategies was associated with an increased incidence of leukemic relapse (adjusted HR, 1.8; 95% CI, 1.0-3.3; P = .057). In the multivariate Cox model for relapse, donor source (Figure 2) and stage of disease at SCT significantly affected outcome (VUD vs siblings HR, 0.38; 95% CI, 0.19-0.76; P = .006; AP vs CP1 HR, 2.4; 95% CI, 1.36-4.3; P = .003) (Figure 2).
Leukemic relapse (donor source). Children with VUD donors were less likely to relapse than recipients who underwent HLA-identical sibling transplantation (adjusted HR, 0.38; 95% CI, 0.19-0.76; P = .006).
Leukemic relapse (donor source). Children with VUD donors were less likely to relapse than recipients who underwent HLA-identical sibling transplantation (adjusted HR, 0.38; 95% CI, 0.19-0.76; P = .006).
Overall survival
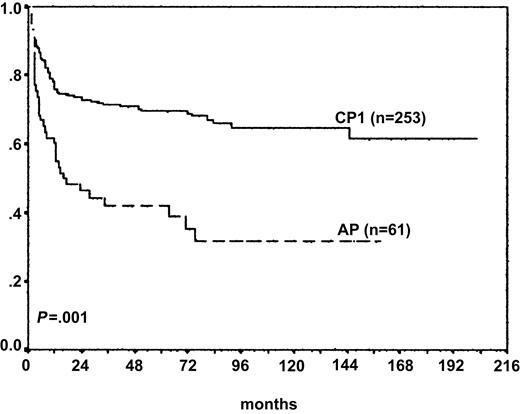
The 3-year OS rate was 66% for the whole cohort. The OS rate was significantly higher in children undergoing SCT in CP1 from sibling compared with unrelated donors on univariate analysis. Crude Kaplan-Meier estimates of OS at 3 years for sibling and VUD recipients who underwent transplantation in CP1 were 75% and 65%, respectively. Corresponding figures for children who underwent transplantation in advanced phase from sibling and unrelated donors were 46% and 39%, respectively. The outcome of SCT was inferior for the 61 children in the advanced phase in the multivariate model (Figure 3) (adjusted HR, 2.0; 95% CI, 1.3-3.0; P = .001). Use of methotrexate was associated with superior outcome in the multivariate analysis for sibling and unrelated donor transplantation (adjusted HR, 0.6; 95% CI, 0.38-0.97; P = .038). Donor source did not significantly affect outcome in the multivariate model (VUD/sibling adjusted HR, 1.4; 95% CI, 0.8-2.4; P = .18) (Figure 3).
Overall survival (stage of disease at SCT). OS was significantly higher for children who underwent transplantation in CP1 (n = 253) than for children who underwent transplantation in AP (n = 61) (adjusted HR, AP:CP1, 2.0; 95% CI, 1.3-3.0; P = .001).
Overall survival (stage of disease at SCT). OS was significantly higher for children who underwent transplantation in CP1 (n = 253) than for children who underwent transplantation in AP (n = 61) (adjusted HR, AP:CP1, 2.0; 95% CI, 1.3-3.0; P = .001).
Leukemia-free survival
The 3-year LFS was 55%, respectively, for the whole cohort. LFS was significantly higher in children undergoing SCT in CP1 than in those in the advanced phase. Crude Kaplan-Meier estimates of LFS at 3 years for children who underwent SCT in CP1 from siblings and VUDs were 63% and 56%, respectively, compared with 35% and 34%, respectively, for children in the advanced phase. Inferior outcomes for children who underwent transplantation in AP was also observed in the multivariate model (adjusted HR, 1.8; 95% CI, 1.2-2.6; P = .003).
Children who received a TCD graft had reduced LFS, although this was of borderline statistical significance (HR, 1.46; 95% CI, 0.98-2.2; P = .064). Inferior outcome was observed for children who underwent transplantation more than 6 months after diagnosis (6-12 months: HR, 1.6; 95% CI, 1.0-2.5; P = .037; more than 12 months: HR, 1.5; 95% CI, 0.98-2.4; P = .064). Outcomes for children who underwent HLA-identical sibling and VUD transplantation are shown in Tables 2 and 3, respectively.
CMV serostatus and the use of IFN-α before SCT
The influence of CMV serostatus and the use of IFN-α before SCT on outcomes were initially excluded from the multivariate models reported above because the information was incomplete. Findings from univariate analysis of the influence of the 2 variables on the outcomes for the whole cohort were reported first. Both variables were then incorporated into the multivariate models and are reported in the rest of this section.
CMV serostatus
The CMV serostatus of recipients and their donors (R/D CMV serostatus) affected outcome (TRM, OS, and LFS) after SCT on univariate analysis. TRM was significantly higher in CMV-negative recipients with CMV-positive donors (CMV–/+) compared with those with CMV-negative donors (CMV–/–) (HR, 2.1; 95% CI, 1.1-3.9; P = .02). Poorer OS and LFS were observed in CMV–/+ patients than in the CMV–/– R/D combination (OS HR, 2.2; 95% CI, 1.3-3.8; P = .003; LFS HR, 1.6; 95% CI, 0.99-2.6; P = .057). No significant influence of CMV serostatus on the incidence of leukemic relapse was observed.
No significant difference in any outcome measurement was observed between CMV+/+, CMV+/–, and CMV–/– combinations on univariate analysis. When incorporated into the multivariate models, CMV serostatus significantly affected OS only. CMV–/+ patients had significantly reduced OS compared with CMV–/– patients (adjusted HR, 1.8; 95% CI, 1.0-3.1; P = .04). No difference in OS between CMV+/+, CMV+/–, and CMV–/– patients was observed.
Pre-SCT IFN-α
The use of IFN-α was associated with higher TRM (HR, 1.6; 95% CI, 1.0-2.5; P = .05) but also with a reduced likelihood of leukemic relapse (HR, 0.53; 95% CI, 0.28-1.0; P = .05) on univariate analysis. No significant effect on OS or LFS was observed.
When use of IFN-α was incorporated into the multivariate models, it was shown to influence the likelihood of leukemic relapse only. Children who received IFN-α before SCT were significantly less likely to have relapses (adjusted HR, 0.4; 95% CI, 0.2-0.8; P = .012).
Discussion
This is the largest reported series to specifically evaluate the outcome of allogeneic SCT for CML in children. The current study shows that most children who undergo transplantation in chronic phase (CP1) are free of leukemia at 3 years. The results of this study confirm the importance of disease stage,14,16 donor type,8,32 and GVHD prophylaxis33 as prognostic factors for outcome after allogeneic SCT. A number of interesting issues are raised.
Improved outcomes in CP1 compared with outcomes in more advanced phases of disease confirm the findings of earlier studies that demonstrate a less favorable outcome for patients with evidence of disease progression.8,14,16 SCT is still the best treatment option for children with advanced-phase disease because the LFS was more than 30% at 3 years in our study, which is considerably better than any results achievable by chemotherapy alone.34 We also found inferior LFS in children who underwent transplantation more than 6 months after diagnosis. These observations suggest that for children with HLA-identical donors, SCT must be planned early in the course of the disease.
The optimal treatment for children who have no HLA-identical donor remains contentious. The superior LFS observed in this study in children who underwent transplantation less than 6 months after diagnosis may reflect a selection bias in some transplantation centers. Because information about factors influencing outcome after SCT for CML in children has been unavailable to date, it is possible that knowledge of the known risk factors for adults had an impact on the decision making about the timing of transplantation in children.4,6,14 In that way, candidates considered “less than ideal” might have received recommendations to proceed to transplantation within the first year. The information collected in the registry precludes any further identification of the reasons for the timing of transplantation in any single patient or the identification of factors that might have categorized a patient as less than ideal. One possible explanation is that the timing of transplantation might have been perceived to be a more important risk factor than the degree of match of the donor (with respect to high-resolution typing or cytotoxic T-lymphocyte precursor [CTL-p] frequency) or CMV serostatus of the donor. In addition, candidates with increasing instability of disease, though failing to meet the criteria for disease acceleration, might have been encouraged to proceed to allograft within a year of diagnosis. However, superior outcomes were observed in the children who underwent transplantation in CP1 (OS and LFS) and within 6 months of diagnosis (LFS); thus, the treatment of choice for a child lacking an HLA-identical sibling donor is allogeneic SCT early after diagnosis if a molecularly matched unrelated donor is identified. Prospective, randomized studies are needed to address this complex issue.
The main reasons for treatment failure after SCT for CML were relapsed disease and TRM, and the outcome curves (Figures 2 and 1, respectively) might not yet have reached plateau, especially for the VUD cohort in which the median follow-up time is relatively short (21 months). Factors that influenced the incidence of relapse were donor relation, stage of disease, use of TCD, and pre-SCT treatment with IFN-α. Although LFS in the study (55% at 3 years, including all stages of disease) reflects the high relapse rate over the 16-year study period, it is likely that this will improve with time given that molecular monitoring of minimal residual disease and the subsequent use of donor lymphocyte infusion (DLI)35,36 and imatinib37 are anticipated to have a similar impact in children as they do in adults. Unfortunately, we are unable to describe the current LFS rate of children in this study because these data are not routinely reported to the EBMT. When incorporated into the multivariate model, treatment with IFN-α pre-SCT was associated with a significantly reduced likelihood of relapse. However, a higher proportion of children undergoing VUD transplantation (55% VUD vs 24% siblings) were given IFN-α before SCT, and VUD recipients were significantly less likely to relapse.
The TRM was significantly higher in children who underwent transplantation from a VUD who had a 35% chance of fatal transplantation-related complications compared with 20% for recipients of sibling allografts. The reasons for this are unclear, although there are a number of possibilities. First, severe acute GVHD (grades 2-4) affected significantly more VUD recipients (52%) than children receiving grafts from sibling donors (37%; P = .009). Most pediatric studies report lower rates of GVHD20,38 than we have observed, and the results in our study may reflect the relatively high median age of the patients (14 years) and a reluctance to use TCD because of the well-recognized risk for relapse in CML.39-41 In addition, many of the VUD cohort underwent SCT before the introduction of molecular typing for HLA class I and II alleles.18,19 TRM rates were lower (20%) for children who underwent transplantation during CP1 from HLA-identical siblings and only 8% for the subgroup with no or mild acute GVHD (grades 0-1), suggesting that the prevention of GVHD should be prioritized when devising conditioning regimens in view of the potential for rescue of relapse by DLI,35,36 imatinib, or both.37 The use of IFN-α in children before SCT was shown to be associated with higher rates of TRM on univariate but not on multivariate analysis. This may reflect the fact that a higher proportion of VUD recipients received the drug (55% vs 24% siblings).
The 3-year OS rates for children who underwent transplantation in CP1, whether with sibling donors (75%) or with VUD (65%), are comparable to figures reported for adults.4,6,42 This is perhaps surprising given the well-recognized differences between children and adults in the incidence and severity of GVHD and TRM after SCT for acute leukemia.43,44 Again this may, at least in part, reflect the older median age (14 years) of patients undergoing transplantation for CML. CMV serostatus of patient and donor also impacted OS in multivariate analysis: CMV-negative patients who underwent transplantation from CMV-positive donors had a significantly reduced OS compared with the other CMV R/D combinations (adjusted HR, 1.8; 95% CI, 1.0-3.1; P = .04). Other groups have also demonstrated that CMV serology significantly affects overall survival.26,27 Although we reported greater TRM in CMV–/+ patients using univariate analysis, this did not reach significance in the multivariate model. The association between CMV positivity and higher TRM has been reported more frequently in the setting of T-cell depletion.26,27 In our study, a higher proportion of VUD recipients were of the combination CMV–/+ (22% vs 9% siblings), and a higher proportion of VUD SCTs were T-cell depleted (61% vs 14% siblings). Our data support the suggestion of others that a seronegative donor should be selected for seronegative patients.27
Some unanswered questions remain, and further studies are indicated for this age group. First, the role of tyrosine kinase inhibitors, such as imatinib, must be defined in children as in adults,29 particularly for those children without HLA-identical hematopoietic stem cell donors. The decision to proceed to SCT is difficult for the patient without an HLA-identical sibling donor. Although other treatment options, such as imatinib, IFN-α, or combination therapy, may be considered first because they lack the immediate risks associated with transplantation from an unrelated donor, there is no evidence that IFN-α or imatinib is curative in children; therefore, long-term survival would be predicted to be superior with SCT from either a sibling donor or a VUD. Second, it is important to analyze the impact on outcome of SCT for children with CML using donors fully matched at a molecular level versus donors with 1 or more subtype mismatches.
Finally, because allogeneic SCT offers a high chance of cure, the long-term sequelae must also be considered. Few studies have addressed the issue of optimal conditioning regimens for children undergoing allogeneic SCT. Two prospective, randomized studies in adults, which have included a small number of children, have demonstrated comparable survival and cure rates after TBI-containing (cyclophosphamide [CY]/TBI) and busulfan-containing (BU/CY) preparative regimens in patients with CML-CP undergoing HLA-identical sibling SCT.45,46 Our data support this observation because the type of conditioning regimen was shown not to influence any outcome in multivariate analysis. Furthermore, evidence shows that prepubertal children receiving BU experience less growth impairment and that long-term toxicity may be less than what is incurred in TBI regimens.47
We conclude that the outcome for children who undergo transplantation for CML is comparable to that observed for adults. SCT is curative for most children, but TRM remains a problem, particularly after VUD SCT. Long-term survival is also affected by the stage of the disease at SCT, with significantly better outcomes seen in CP1. This, together with the finding that LFS is significantly better for children who undergo transplantation within 6 months of diagnosis, suggest that it is important to proceed to SCT as soon as an HLA-identical donor has been identified. Further studies to better identify prognostic factors that predict SCT outcome should prove invaluable in elucidating the clinical role of SCT, particularly the timing of the procedure and the choice of alternative donors, when balanced against the results achieved with novel medical therapy such as tyrosine kinase inhibitors. Because CML is a relatively rare disease in childhood, it is imperative that cooperative studies be initiated so that sufficient numbers of patients can be recruited to allow these issues to be addressed.
Appendix
Participating centers and physicians in the EBMT-CML study were: I. Roberts, Hammersmith Hospital, London, England; E. Gluckman, Hopital St. Louis, Paris, France; M. Al Jurf, King Faisal Specialist Hospital, Riyadh, Saudi Arabia; J. M. Vossen, Leiden University Hospital, The Netherlands; F. Locatelli, Policlinico San Matteo IRCCS/Pediatrica, Pavia, Italy; W. Arcese, University La Sapienza, Rome, Italy; A. Vitek, Institute of Hematology and Blood Transfusion, Prague, Czech Republic; A. Bacigalupo, Ospedale San Martino, Genova, Italy; E. Montserrat, Hospital Clinic, Barcelona, Spain; C. Uderzo, Ospedale San Gerardo, Monza, Italy; G. Dini, Institute G. Gaslini, Genova, Italy; R. Powles, Royal Marsden Hospital, Sutton, England; J. J. Ortega, Hospital M. Infantil Vall d'Hebron, Barcelona, Spain; C. De Souza, Cidade Universitaria Zeferino Vaz, Campinas, Brazil; J. P. Jouet, Hopital Claude Huriez, Lille, France; C. Heilmann, Rigshospitalet, Copenhagen, Denmark; F. Zintl, University of Jena, Germany; L. A. Noens, University Hospital Gent, Belgium; J. Holowiecki, Silesian Medical Academy, Katowice, Poland; D. Sommelet, CHU de Nancy Hopital de Brabois, Vandoeuvre, France; M. Michallet, Hopital E. Herriot, Lyons, France; A. Gratwohl, Kantonsspital, Basel, Switzerland; J. Wachowiak, K. Marcinkowski University of Medical Sciences, Poznan, Poland; W. Siegert, Charite-Virchow Klinikum d.Humboldt-University, Berlin, Germany; F. Guilhot, Hopital La Miletrie, Poitiers, France; A. Iriondo, Hospital Universitario Marqués de Valdecilla, Santander, Spain; A. Torres Gomez, Cordoba Hospital—Reina Sofia, Córdoba, Spain; D. Beelen, University of Saarland—University Hospital, Hamburg, Germany; O. Aleinikova, Belorussian Center for Pediatric Oncology and Hematology, Minsk, Belarus (Rep); D. Niethammer, University Hospital, Tübingen, Germany; T. Ruutu, Helsinki University Central Hospital, Finland; C. Coze, Hopital d'Enfants de la Timone, Marseilles, France; B. Hertenstein, Medical School of Hannover, Germany; P. Di Bartolomeo, Ospedale Civile, Pescara, Italy; U. Píhkala, Children's Hospital University of Helsinki, Finland; N. Patton, Canterbury Health Laboratory, Christchurch, New Zealand; A. O'Meara, Our Lady's Hospital for Sick Children, Dublin, Ireland; B. Rotoli, University of Napoli, Italy; A. Grañena, Institut Catala d'Oncologia, Barcelona, Spain; J. Hansz, K. Marcinkowski University of Medical Science, Poznan, Poland; J. H. Bourhis, Institut Gustave Roussy, Villejuif, France; E. Vilmer, Hôpital Robert Debré, Paris, France; N. Harhalakis, Evangelismos Hospital, Athens, Greece; H. Koc, Ankara University, Ibni Sina Hospital, Turkey; G. Visani, Pesaro Hospital, Italy; H. Gadner, St Anna Kinderspital, Vienna, Austria; R. E. Clark, Royal Liverpool University Hospital, England; F. Fagioli, Ospedale Regina Margherita, Torino, Italy; A. Fasth, Queen Silvia Children's Hospital, Goeteborg, Sweden; J.-O. Bay, Centre Jean Perrin-CHU, Clermont-Ferrand, France; J. J. Sotto, Hopital A. Michallon, Grenoble, France; J. Reiffers, Hopital Haut-Leveque, Pessac, France; B. Chapuis, Hopital Cantonal Universitaire, Geneva, Switzerland; S. McCann, St James Hospital, Trinity College, Dublin, Ireland; S. Tura, Hospital San Orsola, Bologna, Italy; J.-Y. Cahn, Hopital Jean Minjoz, Besancon, France; H. G. Prentice, Royal Free Hospital and School of Medicine, London, England; P. Ljungman, Huddinge University Hospital, Sweden; A. Uyttebroeck, University Hospital of Leuven, Belgium; A. Fischer, Hopital Necker, Paris, France; H. Rubie, CHU de Purpan, Toulouse, France; J. P.Vannier, Hôpital Charles Nicolle, Rouen, France; C. Niemeyer, University Children's Hospital, Freiburg, Germany; M. Caswell, Alder Hey Children's Hospital, Liverpool, England; T. Masszi, St László Hospital, Budapest, Hungary; M. Bayik, Marmara Universitesi Hastanesi, Istanbul, Turkey; A. Fassas, G. P. General Hospital of Thessaloniki, Exokhi, Greece; A. Bosi, Ospedale di Careggi, Firenze, Italy; C. Cordonnier, Hopital Henri Mondor, Creteil, France; F. Rodeghiero, S. Bortolo Hospital, Vicenza, Italy; P. Macchia, University of Pisa, Italy; M. N. Fernández, Clinica Puerta de Hierro, Madrid, Spain; Y. Beguin, University of Liege, Belgium; N. H. Russell, Nottingham City Hospital, England; P. Dufour, Hopital de Hautepierre, Strasbourg, France; H. Guy, Hopital de Bocage, Dijon, France; W. Schroyens, Universiteit Antwerpen, Antwerp Edegem, Belgium; A. Ghavamzadeh, Shariati Hospital, Teheran, Iran; C. Urban, University Children's Hospital, Graz, Austria; A. Lange, K.Dluski Hospital and Institute of Immunology and Experimental Therapy, Wroclaw, Poland; M. Andolina, Istituto Per. L'Infanzia Burlo Garofolo, Trieste, Italy; J. Cornish, Bristol Hospital for Sick Children—St Michael's Hill, Bristol, England; R. Seger, University Children's Hospital, Zürich, Switzerland; M. Abecasis, Institute Portugues Oncologia, Lisbon, Portugal; T. Izzi, Spedali Civili, Brescia, Italy; C. Messina, Centro Leucemie Infantili, Padova, Italy; B. Simonsson, University Hospital, Uppsala, Sweden; P. J. Gravett, The London Clinic, England; D. L. Barnard, St James' University Hospital, Leeds, England; J. L. Harousseau, Hotel Dieu, Nantes, France; D. Guyotat, CHRU de Saint-Etienne Hopital Nord, Saint-Etienne, France; I. Franklin, Glasgow Royal Infirmary, Scotland; A. Ferrant, Cliniques Universitaires St. Luc, Brussels, Belgium; J. M. Davies, Western General Hospital, Edinburgh, Scotland; L. Douay, Hopital d'Enfants Armand Trousseau, Paris, France.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-12-3637.
A complete list of the members of the Paediatric and Chronic Leukaemia Working Parties of the European Group for Blood and Marrow Transplantation appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.