Abstract
Interleukin-3 (IL-3) and stem cell factor (SCF) are important mast cell growth and differentiation factors. Since both cytokines activate the transcription factor signal transducer and activator of transcription 5 (Stat5), a known regulator of proliferation and survival, we investigated the effects of Stat5 deficiency on mast cell development and survival. Bone marrow–derived mast cell (BMMC) populations cultured from Stat5A/B-deficient mice survived in IL-3 + SCF, but not in either cytokine alone. These cells demonstrated reduced expression of Bcl-2, Bcl-x(L), cyclin A2, and cyclin B1, with increased apoptosis and delayed cell cycle progression during IL-3 or SCF culture. Finally, the absence of Stat5 resulted in loss of in vivo mast cell development, as judged by assessments of Stat5-deficient mice and transplantation of Stat5-deficient bone marrow cells to mast cell-deficient recipient mice. These results indicate that Stat5A and Stat5B are critical regulators of in vitro and in vivo mast cell development and survival.
Introduction
In asthma and allergy, mast cell activation provides an environment in the connective and mucosal tissues conducive to chronic inflammation and resulting pathology (reviewed in Ryan and Huff1 ). A role for mast cell activation has also been shown in animal models of multiple sclerosis,2 rheumatoid arthritis,3 and coronary artery disease.4 However, recent data challenge the dogma of a pathologic role for mast cell activation, demonstrating its importance to innate immunity (reviewed in Feger et al5 ). These data make our understanding of mast cell biology more crucial than ever.
Critical aspects of mast cell biology have been brought to light through use of naturally occurring and targeted genetically deficient rodents. Data from these studies implicated stem cell factor (SCF) and interleukin-3 (IL-3) in rodent mast cell development (reviewed in Ryan and Huff1 ; Austen and Boyce6 ; and Galli and Hammel7 ). SCF and its receptor, Kit, are essential for normal mast cell development. In contrast, Lantz et al8 demonstrated that IL-3 is dispensable for mast cell development but is required for normal mast cell expansion during immune responses to intestinal pathogens.
SCF and IL-3 induce signal transduction through several pathways, including phosphoinositol 3′-kinase (PI3-K), phospholipase C, protein kinase C, the Ras-MAP kinase (MAPK) cascade, Janus (Jak) kinases, and signal transducers and activators of transcription (Stats). The relative importance of each pathway in mast cell development, survival, and proliferation is incompletely understood. Several studies have addressed these questions through use of primary bone marrow–derived mast cell (BMMC) populations or cell lines. The sum of these studies implicates PI3-K, Ras-MAPK, and Stat5 in IL-3– and SCF-induced survival and/or proliferation.9-12
While SCF has been shown to activate both Stat1 and Stat5, we find that in mast cells, only Stat5 DNA binding activity is elicited by SCF. In contrast, IL-3 activates both Stat3 and Stat5 in mast cells.13-15 Given the unique role of SCF in mast cell development, and its selective activation of Stat5, we designed experiments to assess the role of Stat5 in mast cell biology. Using in vivo and in vitro approaches, we find that Stat5 expression is an essential aspect of mast cell development, survival, and proliferation. These data emphasize the importance of Stat5 in IL-3 and SCF signal transduction, especially with respect to the mast cell lineage.
Materials and methods
Derivation of BMMC populations
BMMCs were derived from 6- to 12-week-old C57BL/6x129 mice (Taconic Farms, Germantown, NY) or from C57BL/6x129 Stat5A/B+/– or Stat5A/B–/– mice (the kind gift of James Ihle, Memphis, TN).16 BMMCs were prepared by culturing unseparated bone marrow cells at 5 × 105 cells/mL in complete RPMI supplemented with IL-3 (5 ng/mL) and SCF (50 ng/mL) for 3 weeks. Mast cell phenotype was confirmed by flow cytometry analysis with antibodies specific for Kit, immunoglobulin E (IgE), CD13, FcγRII/RIII, or T1/ST2, and by histochemical staining with toluidine blue at acid pH for 30 minutes. At the time of use, BMMC cultures were greater than 99% mast cells. BMMCs were maintained in culture for up to 6 months, but were generally used in the first 3 months of culture.
Cytokines, antibodies, and flow cytometry
Murine IL-3 and SCF were purchased from R&D Systems (Minneapolis, MN). Fluorescein isothiocyanate (FITC)–conjugated rat anti-Kit, rat anti-CD13, rat anti-FcγRII/RIII, and mouse IgE were purchased from BD Pharmingen (San Diego, CA). FITC-labeled rat anti–mouse IgE was purchased from Southern Biotechnology (Birmingham, AL). FITC-labeled rat anti–mouse T1/ST2 was purchased from Morwell Diagnostics (Switzerland). Flow cytometry was performed using a Becton Dickinson FACScan equipped with CellQuest software (BD Pharmingen, San Jose, CA).
Apoptosis analyses
For analysis of mast cell apoptosis, BMMCs were cultured at 3 × 105 cells/mL in the indicated cytokines and conditions. Cells were assessed for subdiploid DNA content by propidium iodide staining after cell fixation (PI-DNA staining) as described.12 To assess cell numbers in some cultures, cells cultured under identical conditions were analyzed in triplicate by flow cytometry with automated counting for a preset time (45 seconds per sample, with 0.1-second resolution). Live cell counts included all cells not in the subdiploid DNA region. Di(OC6)3 was purchased from Molecular Probes (Eugene, OR) and used according to the manufacturer's specifications. CaspaTag-3 and -9 kits were purchased from Intergen (Purchase, NY).
Stat5 transfection and transduction
Stat5A/B-deficient BMMCs were transfected with mouse Stat5A cDNA expressed in internal ribosomal entry site–enhanced green fluorescent protein (pIRES.EGFP) by electroporation at 400 V, 500 μF. GFP-positive cells were isolated by cell sorting after 48 hours of culture. For Stat5 transduction experiments, bone marrow cells from wild-type C57Bl/6 mice were prestimulated for 2 days in Dulbecco modified Eagle medium/15% fetal bovine serum with recombinant murine IL-3 (20 ng/mL), recombinant human IL-6 (50 ng/mL), and recombinant mouse SCF (50 ng/mL). Cells were then transduced by coculture on irradiated retroviral ecotropic producer cells for an additional 2 days in the presence of the same growth factors and polybrene (6 mg/mL). The cells were then removed and expanded in IL-3 and SCF to establish BMMC cultures. Stat5A or Stat5A Δ757 transcription from the bicistronic retroviral vectors was driven by the murine stem cell virus (MSCV) long-terminal repeat. The vectors also included an internal ribosomal entry sequence (IRES) to initiate translation of a downstream GFP gene from the same mRNA.
RNase protection assay (RPA) analysis
Total RNA harvested from 5 to 10 × 106 cells was subjected to RPA analysis using the RiboQuant System (BD Pharmingen, San Diego, CA) according to the manufacturer's specifications.
Western blot analysis
Whole cell lysates were prepared and analyzed as described previously.12 Anti–cyclin D3 and anti–Bcl-x were purchased from BD Transduction Laboratories (San Diego, CA). Anti–cyclin A, anti–cyclin B1, anti–bcl-2, antiactin, and horseradish peroxidase–conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell cycle progression analysis
For bromodeoxyuridine (BrdU) measurements of cell cycle progression, BMMCs were incubated without cytokines for 16 hours, then cultured as indicated for 48 hours. BMMCs were then incubated with 10 mM BrdU for 60 minutes, fixed in 70% ethanol, and subjected to PI-DNA staining and staining with FITC–anti-BrdU.
Transplantation of bone marrow cells to W/W-v mice
For bone marrow transplantation studies, whole bone marrow was flushed from both hind limbs (femur and tibia) into phosphate-buffered saline (PBS)/2% fetal bovine serum. The cells were then injected into W/W-v recipient mice at a donor to recipient ratio of 1:5.
Tissue preparation from Stat5A/B-deficient mice
Mouse tissues were fixed overnight in Carnoy fixative and embedded in paraffin. Sections (4 μm) were cut and processed for staining of mast cells with safranin and 1% alcian blue, pH 1.0. Some sections were similarly stained with acid toluidine blue. The number of mast cells per mm2 of tissue was determined on a Macintosh computer (Apple Computer, Sunnyvale, CA) using the public domain NIH Image program (developed at the US National Institutes of Health [Rockville, MD] and available on the Internet at http://rsb.info.nih.gov/nih-image/).
Cytokine measurements from isolated tissues
PBS supplemented with 2% bovine serum albumin was used to flush peritoneal cavities (2 mL per mouse) and to aspirate bone marrow from femurs (1 mL per mouse). SCF and IL-3 concentrations in supernatants were measured using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems and BD Pharmingen (Palo Alto, CA), respectively.
Statistical analysis
Analyses of variance were performed at α = 0.05 using Statmost software (DataMost, Salt Lake City, UT). Student t test was performed using SysStat9 software (SPSS, Chicago, IL).
Results
Stat5 is essential for in vitro mast cell development
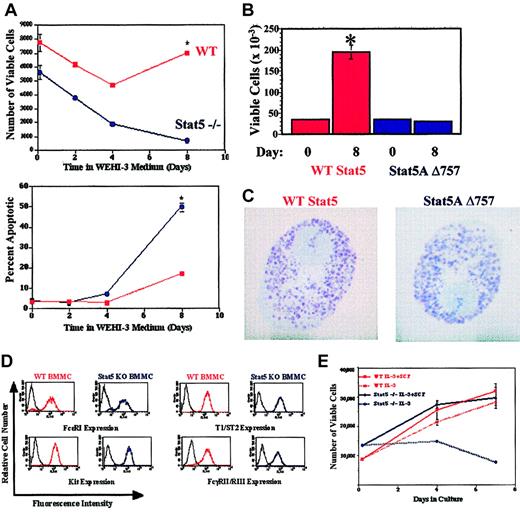
Given evidence of Stat5 involvement in IL-3 and SCF signaling, we attempted to derive BMMC populations from mice deficient in both Stat5A and Stat5B (hence referred to as Stat5-deficient). However, Stat5-deficient bone marrow cells died rapidly when cultured in WEHI-3 cell-conditioned medium, conditions that normally support BMMCs (Figure 1A). Similarly, expressing a dominant-negative Stat5A (Stat5A Δ757) in wild-type bone marrow cells greatly decreased cell proliferation in response to IL-3 + SCF, though mast cell differentiation did occur (Figure 1B-C).
Stat5 deficiency reduces survival and proliferation of developing mast cells. (A) Wild-type and Stat5-deficient bone marrow cells were cultured for the indicated times in WEHI-3CM. Live cell number and percent apoptotic cells were determined by assessing subdiploid DNA content with PI-DNA staining, using timed flow cytometry counts. (B) Wild-type bone marrow cells were infected with MSCV-based bicistronic retrovirus expressing either wild-type Stat5A or Stat5A Δ757 and an IRES-translated GFP. GFP-positive cells were isolated by cell sorting and cultured in IL-3 (5 ng/mL) + SCF (50 ng/mL). Viable cell numbers were determined by trypan blue exclusion. *P < .05 when comparing cells expressing wild-type Stat5A and Stat5 Δ757 by Student t test. (C) Toluidine blue staining of wild-type bone marrow cells expressing either wild-type Stat5A or Stat5A Δ757, after 21 days of culture in IL-3 + SCF. Final magnification shown is × 1000. (D) Expression of mast cell surface antigens on wild-type and Stat5-deficient BMMC populations after culture in IL-3 (5 ng/mL) + SCF (50 ng/mL) for 21 days. (D) Survival and proliferation of wild-type and Stat5-deficient BMMCs in IL-3 (5 ng/mL) + SCF (50 ng/mL) or in IL-3 alone (5 ng/mL). Cells were cultured in the indicated conditions and fed on day 4 of culture. PI-DNA staining with timed flow cytometry counts was used to compare numbers of viable cells. *P < .05 when comparing wild-type and Stat5-deficient cells under identical conditions by Student t test.
Stat5 deficiency reduces survival and proliferation of developing mast cells. (A) Wild-type and Stat5-deficient bone marrow cells were cultured for the indicated times in WEHI-3CM. Live cell number and percent apoptotic cells were determined by assessing subdiploid DNA content with PI-DNA staining, using timed flow cytometry counts. (B) Wild-type bone marrow cells were infected with MSCV-based bicistronic retrovirus expressing either wild-type Stat5A or Stat5A Δ757 and an IRES-translated GFP. GFP-positive cells were isolated by cell sorting and cultured in IL-3 (5 ng/mL) + SCF (50 ng/mL). Viable cell numbers were determined by trypan blue exclusion. *P < .05 when comparing cells expressing wild-type Stat5A and Stat5 Δ757 by Student t test. (C) Toluidine blue staining of wild-type bone marrow cells expressing either wild-type Stat5A or Stat5A Δ757, after 21 days of culture in IL-3 + SCF. Final magnification shown is × 1000. (D) Expression of mast cell surface antigens on wild-type and Stat5-deficient BMMC populations after culture in IL-3 (5 ng/mL) + SCF (50 ng/mL) for 21 days. (D) Survival and proliferation of wild-type and Stat5-deficient BMMCs in IL-3 (5 ng/mL) + SCF (50 ng/mL) or in IL-3 alone (5 ng/mL). Cells were cultured in the indicated conditions and fed on day 4 of culture. PI-DNA staining with timed flow cytometry counts was used to compare numbers of viable cells. *P < .05 when comparing wild-type and Stat5-deficient cells under identical conditions by Student t test.
As with the studies using Stat5A Δ757 gene transfer into wild-type bone marrow cells, Stat5-deficient BMMC populations could be derived in recombinant IL-3 + SCF. The resulting BMMC populations had a normal granulated mast cell morphology and expressed surface markers consistent with the mast cell lineage, including FcϵRI, Kit, T1/ST2, and FcγRII/RIII, at levels comparable with wild-type BMMCs (Figure 1D and data not shown). Like their wild-type counterparts, Stat5-deficient BMMCs could be stably cultured for up to 6 months in IL-3 + SCF. After deriving these BMMC cultures, we assessed their growth in IL-3 or SCF alone. Neither wild-type nor Stat5-deficient BMMCs could be maintained in SCF alone for long periods, as it appears to function largely as a comitogen for these cells (data not shown). In contrast, wild-type BMMCs could be maintained for weeks in IL-3 alone, but similar cultures of Stat5-deficient BMMCs could not be cultured for more than 4 to 7 days. Under these conditions the Stat5-deficient cells slowly lost proliferative capacity and died (Figure 1E). It appears that Stat5 is critical to the survival and expansion of developing mast cells.
Defective IL-3– and SCF-induced survival signaling in Stat5-deficient BMMCs
IL-3 and SCF are potent stimulators of mast cell survival and prevent factor withdrawal–induced apoptosis (reviewed in Mekori et al17 ). Moreover, gain-of-function c-Kit mutations are frequently observed in apoptosis-resistant, factor-independent mastocytomas.18 Stat5 function has been associated with control of survival and proliferation.19-22 Since we found a defect in Stat5-deficient BMMCs when attempting to culture them for long periods, we assessed their survival more precisely using short-term survival assays.
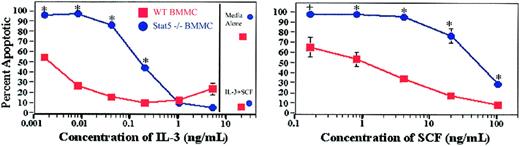
Wild-type and Stat5-deficient BMMCs were cultured in IL-3, SCF, or IL-3 + SCF for 48 hours, and apoptosis was assessed by the presence of subdiploid DNA after PI-DNA staining (Figure 2). While IL-3 + SCF allowed survival of both groups, cultures with single cytokines showed significantly different concentration-response curves. Calculation of half-maximal concentrations needed to prevent apoptosis revealed an approximate 80-fold difference in IL-3–induced signaling and a 50-fold difference in SCF-induced survival signaling.
Stat5-deficient BMMCs are defective in IL-3– and SCF-induced survival. Wild-type or Stat5-deficient BMMCs were cultured in the indicated concentrations of IL-3 or SCF for 48 hours. Apoptosis was assessed by the presence of subdiploid DNA after PI-DNA staining. Cells were also cultured in medium alone, or in IL-3 (5 ng/mL) + SCF (50 ng/mL) as death and survival controls, respectively (shown on the right of the graph). Data shown are means and standard errors from 3 to 8 samples per point, taken from 2 of 9 independent experiments. *P< .001; +P < .05 by Student t test or analysis of variance.
Stat5-deficient BMMCs are defective in IL-3– and SCF-induced survival. Wild-type or Stat5-deficient BMMCs were cultured in the indicated concentrations of IL-3 or SCF for 48 hours. Apoptosis was assessed by the presence of subdiploid DNA after PI-DNA staining. Cells were also cultured in medium alone, or in IL-3 (5 ng/mL) + SCF (50 ng/mL) as death and survival controls, respectively (shown on the right of the graph). Data shown are means and standard errors from 3 to 8 samples per point, taken from 2 of 9 independent experiments. *P< .001; +P < .05 by Student t test or analysis of variance.
Stat5-deficient BMMCs cultured in SCF or IL-3 demonstrate mitochondrial damage
The loss of survival signaling observed with Stat5-deficient BMMCs was reminiscent of factor withdrawal–induced apoptosis. This process has been shown to proceed through a mitochondrial mechanism, with loss of Bcl-x(L) and Bcl-2 expression in some cells.23-25 We therefore assessed expression of Bcl-2 and Bcl-x(L) during culture in IL-3 + SCF (positive control for survival) or in conditions where IL-3 (0.02 ng/mL) or SCF (10 ng/mL) alone was at a limiting concentration. Using IL-3 + SCF cultures as reference survival controls for each population, we found that both wild-type and Stat5-deficient BMMCs reduced Bcl-2 and Bcl-x(L) expression after culture in IL-3 or SCF alone, but that this reduction was more pronounced in Stat5-deficient populations (Figure 3A). Importantly, these mRNA data were matched by a total loss of detectable Bcl-2 and Bcl-x(L) protein in Stat5-deficient BMMCs after 36 hours of culture in IL-3 or SCF alone, as judged by Western blotting (Figure 3B).
Reduced Bcl expression in Stat5-deficient BMMCs. (A) Wild-type or Stat5-deficient BMMCs were cultured in IL-3 (0.02 ng/mL) or SCF (10 ng/mL) for 24 hours. Control cultures were maintained in IL-3 (5 ng/mL) + SCF (50 ng/mL). Total RNA was subjected to RPA analysis. For each culture, the ratio of Bcl-x(L) or Bcl-2 expression to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and L32 loading controls was determined. These ratios were then used to determine relative changes in Bcl-x(L) or Bcl-2 expression in cultures containing IL-3 or SCF alone to the same cells cultured in IL-3 + SCF. This percentage of Bcl-x(L) and Bcl-2 expression relative to IL-3 + SCF is plotted in the bar graph next to the autoradiogram. These data are from 1 of 3 experiments that gave similar results. (B) Wild-type and Stat5-deficient BMMCs were cultured as in panel A for 36 hours, and total cell lysates were subjected to Western blot analysis for the indicated Bcl proteins. The same filter was stripped and reprobed for actin to show protein loading.
Reduced Bcl expression in Stat5-deficient BMMCs. (A) Wild-type or Stat5-deficient BMMCs were cultured in IL-3 (0.02 ng/mL) or SCF (10 ng/mL) for 24 hours. Control cultures were maintained in IL-3 (5 ng/mL) + SCF (50 ng/mL). Total RNA was subjected to RPA analysis. For each culture, the ratio of Bcl-x(L) or Bcl-2 expression to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and L32 loading controls was determined. These ratios were then used to determine relative changes in Bcl-x(L) or Bcl-2 expression in cultures containing IL-3 or SCF alone to the same cells cultured in IL-3 + SCF. This percentage of Bcl-x(L) and Bcl-2 expression relative to IL-3 + SCF is plotted in the bar graph next to the autoradiogram. These data are from 1 of 3 experiments that gave similar results. (B) Wild-type and Stat5-deficient BMMCs were cultured as in panel A for 36 hours, and total cell lysates were subjected to Western blot analysis for the indicated Bcl proteins. The same filter was stripped and reprobed for actin to show protein loading.
In keeping with reduced Bcl-2 and Bcl-x(L) expression, we observed a greater loss of mitochondrial membrane potential and a greater activation of caspases -9 and -3 over a 24- to 48-hour period after culturing wild-type and Stat5-deficient BMMCs in limiting IL-3 or SCF (Figure 4). Stat5-deficient BMMC cultures generally had twice the percentage of cells expressing active caspases -9 or -3 when cultured in IL-3 or SCF alone as did wild-type cells. Collectively these data indicate that Stat5 expression is necessary for properly maintaining Bcl family gene expression and mitochondrial membrane stability.
Loss of Stat5 accentuates mitochondrial damage and caspase activation. (A) Wild-type or Stat5-deficient BMMCs were cultured in IL-3 (0.02 ng/mL), SCF (10 ng/mL), or IL-3 (5 ng/mL) + SCF (50 ng/mL) for 48 hours, and assessed for changes in mitochondrial membrane potential by Di(OC6)3 staining. Data shown are representative of at least 3 wild-type and Stat5-deficient populations assessed in 3 independent experiments. (B) Cells were cultured as in panel A for 24 hours and assessed for caspase-9 or caspase-3 activation. Numbers on the right of each histogram indicate means of 4 samples from one experiment. Data shown are representative of 2 independent experiments.
Loss of Stat5 accentuates mitochondrial damage and caspase activation. (A) Wild-type or Stat5-deficient BMMCs were cultured in IL-3 (0.02 ng/mL), SCF (10 ng/mL), or IL-3 (5 ng/mL) + SCF (50 ng/mL) for 48 hours, and assessed for changes in mitochondrial membrane potential by Di(OC6)3 staining. Data shown are representative of at least 3 wild-type and Stat5-deficient populations assessed in 3 independent experiments. (B) Cells were cultured as in panel A for 24 hours and assessed for caspase-9 or caspase-3 activation. Numbers on the right of each histogram indicate means of 4 samples from one experiment. Data shown are representative of 2 independent experiments.
Stat5-deficient BMMCs exhibit delayed cell cycle progression and cyclin expression in response to IL-3 and SCF stimulation
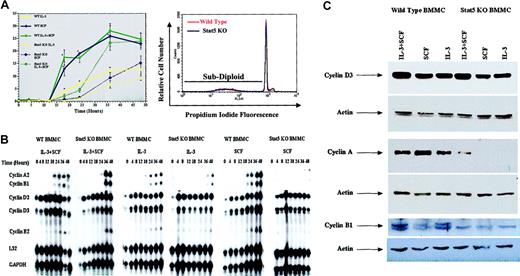
To investigate the role of Stat5 in IL-3– and SCF-induced cell cycle progression, we used dual PI-DNA and BrdU staining. BMMCs were synchronized in G0/G1 by incubation in medium without cytokines for 16 hours, then stimulated with high concentrations of IL-3 (5 ng/mL) and/or SCF (50 ng/mL), and the percentage of cells in S phase was determined over 48 hours (Figure 5A). Under these short-term incubation conditions, culture in IL-3 or SCF alone did not lead to an increase in apoptosis, as judged by subdiploid DNA content (for example, Figure 5A, bottom).
Stat5-deficient (Stat5 KO) BMMCs exhibit delayed cell cycle progression and cyclin up-regulation. (A) Wild-type or Stat5-deficient BMMCs were cultured in IL-3 (5ng/mL), SCF (50ng/mL), or IL-3 + SCF for the indicated times, and assessed for S-phase progression by PI/BrdU staining. Data shown are means and standard error values from 6 to 9 measurements taken from 5 independent experiments. *P < .001 when comparing wild-type and Stat5-deficient cells under identical conditions. The lower panel shows a representative PI-DNA staining profile for BMMCs cultured in IL-3 alone for 48 hours. Under these conditions, subdiploid content (apoptotic cells) represented approximately 5% of wild-type or Stat5-deficient populations. (B-C) Cells were cultured as in panel A for the indicated times (B) or for 36 hours (C). Total cellular RNA or protein was assessed for cyclin expression by RPA analysis (B) or Western blot analysis (C). Western blots were stripped and reprobed for actin expression to show equal protein loading.
Stat5-deficient (Stat5 KO) BMMCs exhibit delayed cell cycle progression and cyclin up-regulation. (A) Wild-type or Stat5-deficient BMMCs were cultured in IL-3 (5ng/mL), SCF (50ng/mL), or IL-3 + SCF for the indicated times, and assessed for S-phase progression by PI/BrdU staining. Data shown are means and standard error values from 6 to 9 measurements taken from 5 independent experiments. *P < .001 when comparing wild-type and Stat5-deficient cells under identical conditions. The lower panel shows a representative PI-DNA staining profile for BMMCs cultured in IL-3 alone for 48 hours. Under these conditions, subdiploid content (apoptotic cells) represented approximately 5% of wild-type or Stat5-deficient populations. (B-C) Cells were cultured as in panel A for the indicated times (B) or for 36 hours (C). Total cellular RNA or protein was assessed for cyclin expression by RPA analysis (B) or Western blot analysis (C). Western blots were stripped and reprobed for actin expression to show equal protein loading.
Wild-type BMMCs exhibited a consistently sharp increase in S-phase progression after 12 to 18 hours, reaching approximately 25% of the population by 36 hours in cultures containing IL-3 + SCF or SCF alone. In experiments using primary BMMCs from a variety of sources, we have found this to be the maximum percentage of cells able to enter S phase at any point in culture, even when the cells are synchronized prior to cytokine stimulation. Cultures containing IL-3 alone exhibited a similar profile, but peaked at 12% of the population. Surprisingly, Stat5-deficient BMMCs entered S phase in all culture conditions. This S-phase progression was substantially delayed, requiring 24 to 36 hours to occur. The delay could be noted in the time required for half-maximal entry into S phase. For example, SCF-stimulated wild-type cells reached this point in approximately 18 hours, while Stat5-deficient BMMCs required 33 hours.
To investigate the mechanism for this delay in cell cycle progression, we examined cyclin mRNA and protein expression. We found that cyclin D2 and D3 mRNAs were up-regulated with similar time courses in wild-type and Stat5-deficient BMMCs (Figure 5B). Western blot studies corroborated these results, demonstrating similar cyclin D3 expression in wild-type and Stat5-deficient BMMCs after 36 hours of stimulation (Figure 5C).
In contrast to the cyclin D family, we observed reduced expression of downstream cyclins in Stat5-deficient BMMCs cultured in IL-3 or in SCF alone, compared with wild-type control cells. RPA and Western blot analyses were comparable, exhibiting a striking difference in both mRNA and protein (Figure 5B-C). While wild-type BMMCs demonstrated cyclin A2 mRNA induction within 18 hours of IL-3 and/or SCF stimulation, this required 36 hours in Stat5-deficient BMMCs. Moreover, Western blotting of cells stimulated for 36 hours revealed expression of cyclin A protein in wild-type but not Stat5-deficient BMMCs stimulated with IL-3 or SCF alone. Expression of the mitotic cyclin B1 was similarly delayed at the RNA level. Western blotting of cyclin B1 protein proved less sensitive than cyclin A. Despite this reduced signal, cyclin B1 was detected in wild-type (WT) BMMCs under all conditions tested, but not in Stat5-deficient BMMCs even after costimulation with IL-3 + SCF (Figure 5C). These data are consistent with the delay in G1-to-S transition we observed in Stat5-deficient BMMCs, since loss of cyclin A could retain cells in the G1 phase.
Stat5 deficiency prevents in vivo mast cell development
Given the defects in BMMC survival and proliferation, it seemed possible that in vivo mast cell development and survival could be compromised in the absence of Stat5. We assessed mast cell development by 2 methods. First, mast cell-deficient W/W-v mice received transplants of wild-type or Stat5-deficient bone marrow cells. Mast cell numbers in the peritoneal cavity were determined 12 weeks after transplantation. While wild-type bone marrow cells gave rise to 0.68% ±16% (SEM) mast cells, no histologically identifiable mast cells were present in mice receiving Stat5-deficient bone marrow cells (Figure 6A). The inability to detect mast cells did not appear to be due to defective granulation, since flow cytometry staining for Kit and FcϵRI expression also demonstrated very few cells expressing these mast cell markers (Figure 6B). It appears that Stat5-deficient bone marrow cells are unable to repopulate the mast cell compartment of W/W-v mice.
Stat5-deficient bone marrow fails to repopulate mast cells in W/W-v mice. (A) Wild-type or Stat5-deficient bone marrow cells were transplanted to W/W-v mice. At 12 weeks after transplantation, peritoneal cells were harvested and stained with toluidine blue. Arrow indicates mast cell morphology, present in wild-type, but not Stat5-deficient, recipients. Final magnification is × 200. (B) Representative flow cytometry profiles of peritoneal cells from W/W-v mice (control) or W/W-v mice that received transplants of wild-type or Stat5-deficient bone marrow cells. Samples were stained for IgE receptor and Kit expression. Background staining with control antibodies averaged 0.05%.
Stat5-deficient bone marrow fails to repopulate mast cells in W/W-v mice. (A) Wild-type or Stat5-deficient bone marrow cells were transplanted to W/W-v mice. At 12 weeks after transplantation, peritoneal cells were harvested and stained with toluidine blue. Arrow indicates mast cell morphology, present in wild-type, but not Stat5-deficient, recipients. Final magnification is × 200. (B) Representative flow cytometry profiles of peritoneal cells from W/W-v mice (control) or W/W-v mice that received transplants of wild-type or Stat5-deficient bone marrow cells. Samples were stained for IgE receptor and Kit expression. Background staining with control antibodies averaged 0.05%.
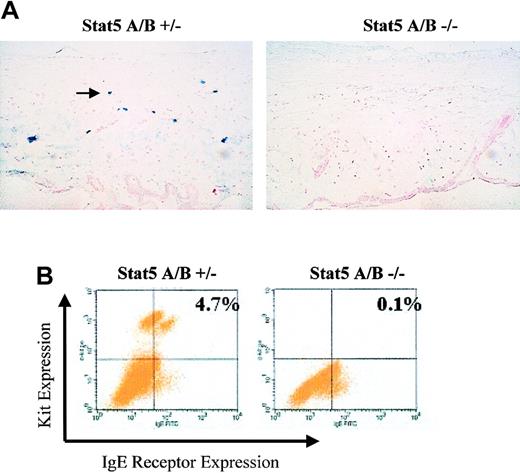
We next assessed mast cell numbers in tissue sections from 12-week-old Stat5A/B+/– (lacking one chromosomal copy of both Stat5A and Stat5B) or Stat5A/B–/– littermate mice. Since mast cells are numerous in mucosal and connective tissues, we analyzed peritoneal fluid, skin, stomach, and spleen tissue by alcian blue or toluidine blue staining. Stat5-deficient mice had no detectable mast cells after staining with alcian blue or toluidine blue in any tissue (Figure 7A; Table 1). As with the W/W-v transplantation studies, flow cytometry analysis demonstrated that the lack of detectable mast cells was not due to changes in morphology, such as loss of granule composition. In these studies, heterozygous mice averaged 1.7% IgE receptor–positive/Kit-positive mast cells, but Stat5-deficient mice were essentially devoid of such a population (0.04% ± 0.03%, Table 1). Hence by 2 in vivo analyses, the absence of Stat5 appears to result in mast cell deficiency.
Stat5-deficient mice lack detectable mast cells. (A) An example of alcian blue staining from ear skin of Stat5A/B+/– and Stat5A/B–/– littermate animals. Original magnification, × 200. (B) Flow cytometry profile of Kit and FcϵRI expression on peritoneal cells extracted from Stat5A/B+/– or Stat5A/B–/– littermate animals at 12 weeks of age. Background staining with control antibodies was approximately 0.05%.
Stat5-deficient mice lack detectable mast cells. (A) An example of alcian blue staining from ear skin of Stat5A/B+/– and Stat5A/B–/– littermate animals. Original magnification, × 200. (B) Flow cytometry profile of Kit and FcϵRI expression on peritoneal cells extracted from Stat5A/B+/– or Stat5A/B–/– littermate animals at 12 weeks of age. Background staining with control antibodies was approximately 0.05%.
Although the maturation and survival defects we observed in Stat5-deficient BMMCs offer the most likely explanation for the in vivo mast cell deficiency, an alternative explanation is reduced growth factor production due to loss of Stat5. We assessed this possibility by measuring IL-3 and SCF levels in bone marrow and peritoneal fluid, the tissues where all mast cells begin their development or where many reside, respectively. As shown in Table 2, IL-3 and SCF concentrations in these tissues were consistently low, well below the nanogram/milliliter concentrations used in vitro. Importantly, only one measure, reduced IL-3 levels in the peritoneal fluid of Stat5-deficient mice, achieved statistical significance. Since IL-3–deficient mice have normal basal mast cell numbers,8 this is an unlikely explanation for the loss of mast cell development in Stat5-deficient mice.
Discussion
These results indicate that Stat5 is an essential regulator of mast cell development and survival, whose absence results in lack of tissue mast cells in vivo and altered maturation, survival, and cell cycle progression in vitro. The defect in maturation and proliferation can be overcome in vitro by costimulation with IL-3 + SCF. The mechanism by which costimulation supports survival remains elusive. We suspect that synergistic induction of a survival/proliferation pathway occurs under these conditions but have not yet identified the pathway involved. For example, preliminary studies found wild-type and Stat5-deficient BMMCs to be equally sensitive to the apoptotic effects of phosphatidylinositol 3-kinase inhibitors (data not shown). Despite the lack of knowledge about this pathway, the morphology and surface marker expression of Stat5-deficient BMMCs indicate that combined signaling by IL-3 + SCF is supporting normal mast cell differentiation without Stat5.
It is interesting to note that studies of mice lacking SCF or IL-3 production have shown distinct roles for these cytokines as mast cell growth factors. For example, Sl/Sld mice, lacking membrane-bound SCF, are nearly devoid of mast cells, but IL-3–deficient mice have normal mast cell development and peripheral mast cell numbers.1,8 Infection of IL-3–deficient mice with intestinal pathogens revealed that IL-3 is important in mast cell hyperplasia during the immune response.8 Consequently, it appears that SCF is essential for mast cell development and survival, while the nonredundant functions of IL-3 relate to peripheral mast cell expansion. While many other growth factors such as nerve growth factor and IL-9 have been implicated in mast cell survival (reviewed in Ryan and Huff1 ), our understanding of how these cytokines collectively regulate mast cell development in vivo is far from complete. The lack of tissue mast cells in Stat5-deficient mice would appear to indicate that the costimulation allowing mast cell development in vitro does not exist in vivo.
Complex in vivo environments can resist interpretations inferred from reductionist in vitro assays, and the use of Stat5 by many cytokines and growth factors makes indirect effects quite possible. For example, Stat5-deficient mice have defects in peripheral T-cell proliferation.21 This could cause cytokine alterations, possibly reducing mast cell numbers. However, our ELISA studies (Table 2) revealed little change in IL-3 or SCF levels when assessing Stat5-deficient mice. Additionally, athymic mice or IL-3–deficient mice have normal numbers of connective tissue mast cells.8,26 Therefore, a peripheral T-cell defect or lack of IL-3 alone is unlikely to explain the lack of mast cells we observe in Stat5-deficient mice. Further, a general loss of SCF should lead to other observable phenotypes, such as coat color defects,1 which are not found in Stat5-deficient mice. Given these findings, we believe that the lack of tissue mast cells in Stat5-deficient mice is not due to reduced growth factor production. Further supporting this theory, Stat5-deficient bone marrow cells failed to repopulate the mast cell lineage in W/W-v mice, whereas wild-type bone marrow cells were able to do so. This experiment points to a cell-intrinsic defect in the etiology of Stat5-dependent mast cell deficiency, rather than microenvironmental conditions. Lastly, the use of Stat5A Δ757 demonstrated that inhibiting Stat5 function greatly decreases proliferation without preventing mast cell maturation. Collectively, these data argue that Stat5 activation by IL-3 and SCF is required for normal proliferative/survival signals, the absence of which dampen mast cell development sufficiently to preclude their detection in vivo.
Supporting the theory that Stat5 is important to mast cell survival signaling, we found Stat5-deficient BMMCs very sensitive to growth factor withdrawal. The reduction in Bcl-2 and Bcl-x(L) expression, concomitant with mitochondrial membrane potential dysfunction, agrees with data derived from studies of IL-3 or SCF withdrawal-induced apoptosis. For example, Bojes et al24 found that IL-3 withdrawal elicited a loss of mitchondrial potential in 32D cells, with resulting caspase-3 activation. Li et al27 showed that Akt activation, which could lead to inactivation of proapoptotic Bad protein, prevented IL-3 withdrawal–induced apoptosis in 32D cells. Mekori et al25 demonstrated that SCF deprivation caused a reduction of both Bcl-2 and Bcl-x(L) in human mast cells that preceded apoptosis, and that expression of these proteins was maintained in transformed, apoptotic-resistant mast cells. Lastly, Zhou-Li et al28 found a correlation between increased Stat1 and Stat5 expression and survival of IL-3–deprived 32D cells. Collectively, these data illustrate a connection between IL-3 or SCF signaling, Stat activation, and maintenance of mitochondrial membrane potential through Bcl proteins.
Importantly, our data do not show that Stat5 directly controls transcription of Bcl-2 or Bcl-x(L) in mast cells, a topic that has sparked some debate.29-32 Unfortunately, we find Stat5-deficient BMMCs to be very poor transfection subjects, making reporter assay systems difficult to use. What we can say with certainty is that Stat5-deficient mast cells are much more sensitive to IL-3 or SCF deprivation, and die coincidental with loss of mitochondrial membrane potential and caspases -9 and -3 activation. These data, coupled with reduced expression of Bcl-2/Bcl-x(L), fit the profile of the mitochondrial pathway for apoptosis that would be expected from withdrawal of IL-3 or SCF signaling. This points to Stat5 as a critical component in maintaining IL-3– and SCF-mediated survival.
Several studies have implicated Stat5 in control of cyclin D expression,21,33-35 which could explain the delayed S-phase progression of Stat5-deficient BMMCs. However, our assessment of cyclin gene expression in these cultures yielded distinctly different results. These cultures, prepared in the same manner as our PI/BrdU (Figure 4) studies, made it apparent that loss of cyclin D induction does not explain the observed delay in S-phase progression. The discrepancy in cyclin D induction between previous studies and our own could lie in the experimental systems used. BMMCs are primary cells at a nearly complete stage of differentiation. Previous studies of Stat5-mediated cyclin D regulation have generally used transformed cell lines, such as CTLL2, BaF3, and F-36P-mpl.33-35 However, Moriggl et al21 found that primary T cells lacking Stat5 also failed to induce cyclin D, as well as cyclin A, proteins. It is possible that control of cyclin D expression is lineage-dependent, maturation-dependent, or is differentially regulated in transformed cell lines.
In place of a cyclin D defect, our RPA and Western blot analyses found reduced expression of the downstream cyclins A and B. The lack of cyclin A induction was consistent with the delayed S-phase transition we observed. These results indicate that Stat5 expression is required for normal cyclin A induction, but that a delayed induction is possible through a Stat5-independent pathway. This entry into S phase appeared to be at best a faltering one, since Stat5-deficient BMMCs could not be maintained in IL-3 alone (Figure 1). It is interesting to note that Stat5-deficient bone marrow has been found to have a poor competitive repopulating ability in transplantation assays,36,37 and that bone marrow population–enriched stem cells are 10-fold less responsive to IL-3 + SCF stimulation than wild-type cells.38 These results also fit with a defect in cell cycle progression and/or survival. At present we do not know whether the in vivo loss of mast cells found in Stat5-deficient mice or in the W/W-v transplantation model is due to defects in proliferation, survival, or both.
Stat5 is the most ubiquitously activated member of the Stat family, used by the receptors for IL-2,39,40 IL-3,41 IL-4,42 IL-5,41 IL-7,43 IL-9,44 IL-15,45 granulocyte-macrophage colony-stimulating factor (GM-CSF),41,46 erythropoietin (EPO),47,48 thrombopoietin (TPO),49 prolactin,50 growth hormone,51,52 and SCF.13,14 Collectively these receptors control almost all aspects of the immune and hematopoietic systems. Our studies show that Stat5 is a central regulator of IL-3 and SCF signaling with overt effects on mast cell biology. Its absence precludes proper control of mast cell development, survival, and proliferation such that mast cell development fails in vivo. It is interesting to note that the mast cell deficiency, combined with the sterility16 and hematopoietic defects31,36,38 observed in Stat5-deficient mice, gives a phenotype reminiscent of mutations in the Kit (W) and SCF (Sl) loci (reviewed in Ryan and Huff1 ). This makes it apparent that Stat5 is a critical component of Kit signaling. The use of Stat5 by a range of cytokines and growth factors makes understanding its biologic functions important to many areas of clinical relevance, including hematopoiesis and cancer.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-11-3490.
Supported in part by generous grants to the Ryan lab from The American Cancer Society (IN-105), the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust (J-457), the Horsley Cancer Research Fund, the National Institute of Allergy and Infectious Diseases (1R01-AI43433), and the National Cancer Institute (1R01-CA91839).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Lawrence B. Schwartz for thoughtful review of this manuscript.