Abstract
Telomerase activity, telomere length, stem/progenitor cell production, and function of CD34+ cells from cord blood (CB), bone marrow, and mobilized peripheral blood were evaluated in long-term cultures. CB cells were cultured either on OP-9 stromal cells transduced with an adenovector expressing thrombopoietin (TPO) or stimulated by a cytokine cocktail in the absence of stroma, with, in one method, CD34+ cells reisolated at monthly intervals for passage. Continuous expansion of stem cells as measured by in vitro cobblestone area and secondary colony-forming assays was noted for 18 to 20 weeks and by severe combined immunodeficiency (SCID)-repopulating cells (SRCs), capable of repopulating and serially passage in nonobese diabetic/SCID mice, for 16 weeks. Despite this extensive proliferation, telomere length initially increased and only at late stages of culture was evidence of telomere shortening noted. This telomere stabilization correlated with maintenance of high levels of telomerase activity in the CD34+ cell population for prolonged periods of culture. Cytokine-stimulated cultures of adult CD34+ cells showed CD34+ and SRC expansion (6-fold) for only 3 to 4 weeks with telomere shortening and low levels of telomerase. There is clearly a clinical value for a system that provides extensive stem cell expansion without concomitant telomere erosion. (Blood. 2004;103:4440-4448)
Introduction
Tissue-specific stem cells, like most somatic stem cells, possess the unique ability of self-renewal and multilineage differentiation. These combined properties are reflected in the ability of a hematopoietic stem cell (HSC) to completely and durably reconstitute hematopoiesis of a myeloablated recipient and maintain it throughout the entire life span.1,2 HSC self-renewal is not a perfect process and daughter cells have progressively reduced proliferative capacity, due in part to progressive telomere erosion with each cell division. This, in turn, leads to proliferative senescence that can be observed both in vivo and in vitro.
Telomeres are structures at the end of eukaryotic chromosomes that protect chromosomes from degradation, fusion, and recombination. In mammalian cells, they consist of hexanucleotide (TTAGGG) repeats and several associated protein components. In the absence of compensatory mechanisms, dividing cells undergo gradual telomere erosion. When telomeres reach a critical degree of shortening, cells recognize this as DNA damage and initiate proapoptotic programs or enter senescence.3 The best-characterized compensatory mechanism for maintenance of telomere length is mediated by telomerase, an enzyme that synthesizes terminal telomere repeats. CD34+ populations exhibit low telomerase activity, but activity increases significantly in both HSC and progenitor populations as cells progress from G0 into S phase.4,5 In early studies using cytokine combinations that were effective in activating stem cells into the cell cycle, but were less effective in maintaining long-term HSC proliferation, telomerase was up-regulated at early stages of culture but declined rapidly after 3 to 4 weeks.4-6 In these studies, 1 to 1.5 kb telomeric DNA was lost over 3 to 4 weeks of culture of CD34+ cells from cord blood (CB), mobilized peripheral blood (MPB), or bone marrow (BM). Thus, telomerase activity in HSCs did not prevent telomere shortening although it may have limited the extent of telomere degradation.
The use of CB as a source of HSCs for allogeneic transplantation has been limited by the HSC number present in a single harvest, which limits the utility of this source to pediatric use or to adults of low body weight.7-10 Ex vivo expansion of neonatal HSCs would be of considerable clinical utility and preliminary phase 1 results have been reported.11 The ability to maintain HSCs in vitro for prolonged periods also provides a valuable system for evaluating the role of telomerase in telomere length maintenance and cell expansion. Recent studies have demonstrated that human long-term nonobese diabetic/severe combined immunodeficiency (NOD/SCID) repopulating cells (SRCs) in CB can be expanded many-fold for up to 10 weeks of culture in the presence of ligand for c-kit (stem cell factor [SCF]), flt3/flk2 ligand (FL), c-mpl ligand thrombopoietin (TPO), and interleukin 6 (IL-6) and sustain the hematopoietic reconstitution over 3 serial passages in NOD/SCID mice.12-14 Because previous studies had indicated that progressive telomere shortening would be anticipated with in vitro expansion,4-6 comparable to up to 20 years of normal age-associated telomere loss, we chose to closely monitor telomerase activity and telomere length in the improved long-term CB cultures using the cytokine combinations optimal for SRC expansion, or in a coculture system using the mouse stromal line OP-9, which produces FL and SCF, transfected with an adenovector expressing TPO.15-18 We show that under both these culture conditions, CB CD34+ cells undergo extensive proliferation and self-renewal for 4 to 5 months with sustained elevation of telomerase activity and without concomitant significant telomere shortening. Primitive hematopoietic precursors as detected by cobblestone area-forming cell (CAFC) and by long-term culture-initiating cell (LTC-IC) assays are maintained for up to 16 to 18 weeks and greatly expand over this time. During this period, repopulating ability is maintained and the SRC number is greatly increased. After this period of telomere stabilization (0-16 weeks), telomeric DNA begins to be lost, coinciding in cytokine-stimulated cultures with a steep decline in LTC-ICs and SRCs by week 20.
Patients, materials, and methods
Human cells
Umbilical CB was obtained at the end of full-term deliveries. BM was obtained by aspiration from the posterior iliac crest of fully informed hematologically healthy donors. MPB was collected from leftovers of leukapheresis procedures from healthy volunteers donating stem cells for allogeneic transplants, who received granulocyte colony-stimulating factor (G-CSF) subcutaneously for 5 consecutive days prior to the apheresis procedure. In all cases, approved institutional procedures involving written informed consent from each patient were followed.
CD34+ cell purification
Mononuclear cells (MNCs) were isolated from CB using Ficoll Hypaque 1077 (Nyegaard, Oslo, Norway) density centrifugation. CD34+ cells were isolated using a magnetic immunoseparation device (miniMACS, Miltenyi Biotech, Gladbach, Germany). Purification efficiency was verified by flow cytometry counter staining with a CD34-phycoerythrin (PE; HPCA-2; Becton Dickinson, San Jose, CA) antibody (87%-92% CD34+).
Adenovirus
The adenovector expressing human TPO (adeno-TPO) is an E1a- and partially E1b-, E3-deficient Ad5-based vector with an expression cassette in the E1a region containing a 1.7-kb human TPO cDNA driven by the cytomegalovirus (CMV) major immediate/early promoter/enhancer. The vector was amplified, purified, and titered as described.19
Recombinant human cytokines
The following recombinant human (rh) cytokines were used: rhSCF, rh erythropoietin (rhEpo), and rhG-CSF were a gift from Amgen (Thousand Oaks, CA); rh granulocyte-macrophage colony-stimulating factor (rhGM-CSF) and rhIL-3 were from Sandoz (Basel, Switzerland); rhIL-6 were from Peprotech (Rocky Hill, NJ); rhTPO was a gift from Kirin (Kirin Brewery, Tokyo, Japan); and rhFL was provided by S. D. Lyman (Immunex, Seattle, WA).
Animals
NOD/LtSz scid/scid (NOD/SCID) mice were supplied by Jackson Laboratories (Bar Harbor, ME), maintained at the animal facilities of CIOS (Torino), and handled according to institutional regulations, under sterile conditions in cage microisolators. Mice underwent transplantation 24 hours after total body irradiation with 350 cGy, with a single intravenous injection of CB CD34+ cells obtained at the initiation of culture or harvested from expansion cultures as described. Unfractionated BM cells (10-30 × 106) from NOD/SCID mice given primary or secondary transplants were similarly injected into groups of irradiated mice. For limiting dilution analysis (LDA), mice were given transplants with decreasing numbers of CD34+ cells. Mice were killed 6 to 8 weeks after transplantation to assess the number and types of human cells detectable in murine BM.
Analysis of human cell engraftment in murine BM
BM cells were flushed from the femurs and tibias of each mouse and flow cytometric analysis performed using a FACSVantage cytometer (Becton Dickinson) after staining the cells with human-specific monoclonal antibodies.14,22 Briefly, to detect human engraftment as a first check in primary, secondary, and tertiary transplants, the presence of at least 0.1% human CD45+, CD71+, and GpA+ cells in the BM of NOD/SCID mice defined a positive engraftment. We confirmed results by Southern blot analysis as described.14,22 To detect human multilineage engraftment in secondary and tertiary transplants or as a more sensitive assay in LDA, 2 different antibody combinations were used: fluorescein isothiocyanate (FITC)-conjugated human anti-CD34 and PE-conjugated human anti-CD19 and anti-CD20 antibodies (Becton Dickinson) for the detection of human (CD34-CD19/20+) B-lineage cells, or FITC-conjugated human anti-CD15 (Becton Dickinson), anti-CD66b (Pharmacia), and PE-conjugated human anti-CD45 (Becton Dickinson) and anti-CD71 for the detection of human (CD45/71+CD15/66b+) myeloid cells. Mice with detectable human lymphoid and myeloid engraftment (5 positive events each/20 000 assessed) were counted as positive. All other mice were considered negative.20
Hematopoietic progenitor cell cultures
Freshly isolated CD34+ cells (1 × 103 cells) or expanded cells (2.5-10 × 103 cells) were plated in triplicate in methylcellulose culture as previously.5,7,20,21 After 14 days of incubation at 37°C in 5% CO2, granulocyte-macrophage colony-forming unit (CFU-GM), erythroid burst-forming unit (BFU-E), and multilineage granulocyte, erythrocyte, megakaryocyte, macrophage colony-forming unit (CFU-GEMM) progenitors were scored.
LTC-IC and CAFC assays
CAFC assays were performed as described.23 After 5 weeks of culture, CAFCs were scored using an inverted phase microscope to identify phase-dark hematopoietic areas of at least 5 cells (ie, cobblestone areas) beneath the stromal layer. The LTC-IC contents of the initial CD34 population and of the CD34+ cells isolated from LTCs at different time points (1.5 × 103 CD34+ cells) were determined by assaying for secondary colony-forming cells (CFCs) in methylcellulose culture after 5 weeks of stromal coculture, as described.12-14,22,23
Stroma-free liquid cultures
A total of 5 × 104 CB, BM, or MPB CD34+ cells/mL was resuspended in Iscove modified Dulbecco medium (IMDM; Invitrogen, Milano, Italy) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) and the following cytokines: SCF (50 ng/mL) plus FL (50 ng/mL) plus TPO (10 ng/mL) plus IL-6 (10 ng/mL), in quadruplicate tissue culture T25 or T75 flasks. Every week the culture volume was increased by adding fresh media and growth factors (to keep cell counts < 8 × 105/mL). When needed, cells were passaged in additional flasks.
“Continuous” LTCs were initiated at a cell density of 5 × 104 CD34+ cells/mL and kept for 20 weeks with weekly addition of fresh medium and growth factors. Every other week, aliquots of the total culture volume were harvested and CD34+ cells isolated for evaluation of colonies, LTC-ICs, telomere length, telomerase activity, and in vivo NOD/SCID repopulation.
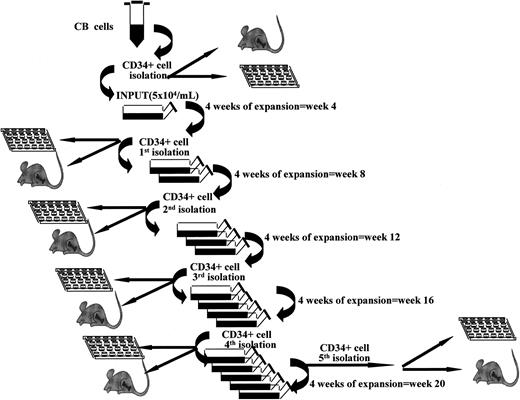
“Fractionated” LTCs were set up and kept in increasingly larger volumes. The difference was that here all cells were harvested every 4 weeks over a 20-week period. All CD34+ cells were purified and replated under the same culture conditions, as depicted in Figure 1. Briefly, at the start of culture, CB CD34+ cells were plated at 5 × 104/mL with the same media and cytokines as used in “continuous” cultures. At week 4 all cells were harvested and CD34+ cells isolated with miniMACS devices. All CD34+ cells derived from a single flask were used to initiate new expansion cultures at 5 × 104/mL with fresh media and growth factors. These cells were kept in expansion culture with addition of fresh media without cell depletion for another 4 weeks. These expansion-separation procedures were repeated 5 times (at weeks 4, 8, 12, 16, and 20) for a total of 20 weeks. At each time point, aliquots of CD34+ cells derived from parallel flasks were used to assess CFCs, LTC-ICs, telomere length, telomerase activity, and NOD/SCID mice long-term repopulating activity and for LDAs.14,22 The total number of CFCs and LTC-ICs was obtained by seeding 1000 to 1500 CD34+ cells and multiplying the observed CFC or LTC-IC frequency by the total numbers of CD34+ cells we have actually produced at that time point.
Stroma-free LTCs associated with repeated expansion-isolation procedures. Scheme of “fractionated” stroma-free LTCs with cytokines. At start of cultures, CB CD34+ cells were plated at 5 × 104/mL as explained in “Patients, materials, and methods” in triplicate or quadruplicate tissue culture flasks. Every 4 weeks all cells were harvested and CD34+ cells isolated with miniMACS devices and recultured as described in “Patients, materials, and methods.”
Stroma-free LTCs associated with repeated expansion-isolation procedures. Scheme of “fractionated” stroma-free LTCs with cytokines. At start of cultures, CB CD34+ cells were plated at 5 × 104/mL as explained in “Patients, materials, and methods” in triplicate or quadruplicate tissue culture flasks. Every 4 weeks all cells were harvested and CD34+ cells isolated with miniMACS devices and recultured as described in “Patients, materials, and methods.”
Stroma cell cultures and infection with adeno-TPO
The OP9 stroma cell line (kindly provided by Dr Kodama, Ohu University, Fukushima, Japan) was propagated in α-minimal essential medium (MEM) supplemented with 20% FBS and ascorbic acid (50 μg/mL). Based on data collected from preliminary experiments, for LTCs, stroma cells were infected with adeno-TPO at a multiplicity of infection of 15 and diluted in QBSF-60 serum-free medium (Quality Biological, Gaithersburg, MD) for 6 to 8 hours. After incubation and for the whole LTC period, serum-free medium was replaced with OP9 growth media. CB CD34+ cells were seeded 12 hours after infection at 2 × 104/flask on top of the stroma. Cultures were demi-depopulated weekly with half the media and supernatant cells harvested for progenitor assay and telomere and telomerase measurement, and fresh media added. From weeks 2 to 6 cultures were demi-depopulated twice weekly. At weeks 4, 8, 12, and 16, whole cultures containing stroma cells and expanded cells were harvested, and adherent cells were trypsinized. Supernatant and adherent cells were transferred to a new T25 flask containing a confluent OP9 layer infected with adeno-TPO.
Flow FISH
Telomere length of basal and expanded CD34+ cells was determined by telomere fluorescence in situ hybridization and flow cytometry (flow FISH),24 using a telomere-specific conjugated (C3TA2)3 PNA probe (Perseptive Biosystems, Framingham, MA). Stained cells were analyzed on a FACSCalibur cytometer. At the beginning of each analysis, fluorescence signals from FITC-labeled microbeads (Quantum-24 FITC Premix, Bangs Laboratories, Indianapolis, IN) were acquired. The resulting calibration curve was used to convert telomere fluorescence into molecule of equivalent soluble fluorochrome (MESF) units, allowing comparison of results among experiments.
Southern blot analysis of TRFs
About 0.5 to 1 × 106 cells were used for the preparation of high-molecular-weight DNA using the Nucleon BACC2 DNA extraction kit (RPN 8502, Amersham Life Science, Buckinghamshire, United Kingdom). The mean length of the terminal restriction fragment (TRF) was measured using the TeloTAGGG telomere length assay kit (Roche Molecular Biochemical, Indianapolis, IN) as previously described.25 The telomeric DNA amount was calculated by integrating the volume of each smear using MacBas V 2.5 software (Fuji, Stamford, CT).
Telomerase activity
The telomerase activity was studied by a modified version of the telomeric repeat amplification protocol (TRAP), the Intergen TRAP-eze telomerase detection kit (Intergen, Oxford, United Kingdom, or Purchase, NY).25 The TRAP assay procedure was performed according to the manufacturer's protocol. The 12.5% nondenaturing polyacrylamide gel was stained with 1× SYBR green (Molecular Probes, Eugene, OR) and analyzed by Fluor-S MultiImager (Bio-Rad, Hercules, CA). The quantitative value of telomerase activity was expressed as a total product generated (TPG) unit where: TPG = (Telomerase sample-RNase treated sample)/Internal control of sample (TSR8-negative control)/(Internal control of TSR8) × 100 (using 0.1 amole TSR8)
Alternatively, using a 32γP[ATP] end-labeled TS primer, gels were exposed to a Fuji imaging plate, scanned on a Fujifilm BAS-2500 bioimaging analyzer and then quantitated using MacBas v 2.5 (Fuji). All values were then expressed as a percentage of telomerase activity in a positive control of an extract of an immortalized neuroblastoma (NB) cell line.
Statistical analysis
The SRC frequency in a population of cells was determined by injecting cohorts of mice with several dilutions of cells. The SRC frequency was calculated from the proportions of negative mice in each cohort, using L-Calc T software program (Stem Cell Technologies, Vancouver, BC, Canada), which uses Poisson statistics and the method of maximum likelihood.
Results
Telomere length and telomerase activity of hematopoietic cells generated in long-term cocultures of CB CD34+ cells on OP9 stroma expressing human TPO
OP9 stromal cells are genetically unable to produce macrophage colony-stimulating factor (M-CSF), but express various early acting hematopoietic growth factors, among them SCF and FL, which are cross-reactive with human cells.26 The addition of TPO to OP9 cocultures, either as soluble rhTPO or following adeno-TPO infection of the stroma, supported extensive human hematopoiesis over 8 weeks using G-CSF MPB CD34+ cells. When purified CD34+ cells were cocultured on adeno-TPO-transfected OP9 stroma, hematopoiesis was sustained for 18 weeks (Figure 2).
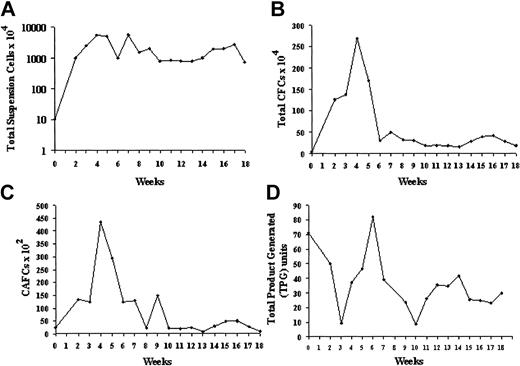
Expansion of CB CD34+ cells in stromal coculture. Cells (20 000) were plated in T25 flasks on OP9 mouse BM stroma transfected with an adenovirus-expressing TPO. (A) Nonadherent cell expansion from CB CD34+ cells. Total viable nucleated cells were counted using Trypan blue. (B) CFC expansion. Total numbers of CFCs were established by semisolid methylcellulose assays in triplicate dishes on the initial CD34+ population and on the suspension cells obtained after each weekly demi-depopulation. The results represent the mean ± SD. (C) Secondary week 5 CAFC expansion. Input CD34+ cells and weekly suspension cell populations were assayed for CAFCs by 5-week coculture on MS5 stroma. (D) Telomerase activity of input CD34+ cells and equal numbers of unseparated suspension cells obtained at weekly intervals. Activity was calculated from the TRAP signal and expressed as total product generated (TPG). The input activity corresponded to percent of the TRAP activity of an NB cell line extract used as a reference standard.
Expansion of CB CD34+ cells in stromal coculture. Cells (20 000) were plated in T25 flasks on OP9 mouse BM stroma transfected with an adenovirus-expressing TPO. (A) Nonadherent cell expansion from CB CD34+ cells. Total viable nucleated cells were counted using Trypan blue. (B) CFC expansion. Total numbers of CFCs were established by semisolid methylcellulose assays in triplicate dishes on the initial CD34+ population and on the suspension cells obtained after each weekly demi-depopulation. The results represent the mean ± SD. (C) Secondary week 5 CAFC expansion. Input CD34+ cells and weekly suspension cell populations were assayed for CAFCs by 5-week coculture on MS5 stroma. (D) Telomerase activity of input CD34+ cells and equal numbers of unseparated suspension cells obtained at weekly intervals. Activity was calculated from the TRAP signal and expressed as total product generated (TPG). The input activity corresponded to percent of the TRAP activity of an NB cell line extract used as a reference standard.
Extensive amplification of total hematopoietic cells was seen in the first 6 weeks with a maximum expansion of 500-fold, despite a twice-weekly depopulation of half the suspension cells in this period. After 8 weeks of coculture, a steady-state level of cell production was established for a further 10 weeks. (Figure 2A). Maximum CFC production was seen in the first 8 weeks with up to 150-fold expansion (Figure 2B). In addition to CFU-GMs, BFUEs, and multipotent CFU-GEMMs were generated for the entire 18 weeks of culture (4%-17% and 1%-16%, respectively, of total CFCs). Week 5 CAFCs were 4% of input CD34+ population. After 4 weeks of coculture the CAFC content of the suspension cell population was about 20-fold the input value (Figure 2C). After the initial peak, a constant level of CAFC production comparable to the input number was found. In addition, over the entire 18-week period there was a constant level of primary CAFCs by CB CD34+ cells migrating beneath the OP9 stroma. FACS analysis revealed that 5% to 10% of the suspension population was CD34+ throughout the duration of culture and at 18 weeks, 30% of the adherent CD45+ hematopoietic cells were still CD34+.
Basal telomerase activity was detected in the input CB CD34+ cells as 71 TPG units or 34% of the NB control. Telomerase activity was determined weekly from equal suspension cell amounts and, though fluctuating between 9 and 82 TPG units, it remained high, averaging 39 ± 4 TPG units or 19% of the NB control over 18 weeks (Figure 2D). Because the CD34+ population was diluted by telomerase-negative differentiating myeloid cells, the telomerase values on the suspension population underestimate the telomerase activity of stem/progenitor cells. When adjusted for CD34+ content, telomerase activity within this stem/progenitor-enriched fraction was equivalent to about 400 TPG units or 200% of the NB control. The mean telomere length (M-TRF) of the input CB CD34+ cells, determined by Southern blot analysis, was 10.46 kb (peak TRF, 11.13 kb). Analysis of mean TRF values of suspension cells harvested over 16 weeks showed an increase over baseline between 2 and 10 weeks, then shortening to a mean of 9.78 kb for an overall loss of 0.68 kb (Figure 3A-B). Measurement of peak TRF values showed no change over the duration of culture. The difference between the mean and peak TRF values indicated heterogeneity within the population, possibly reflecting subpopulations with stable telomeres admixed with populations in which telomeres were shortening.
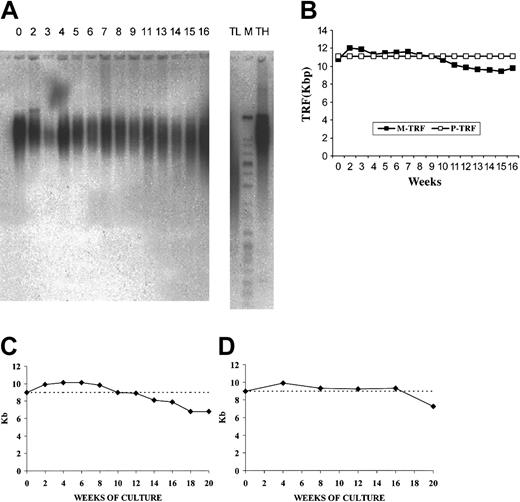
Telomere length of input CB CD34+ cells and suspension cells obtained at weekly intervals in long-term OP9/adeno-TPO stromal cell coculture and during stroma-free LTC. (A) TRF Southern blot analyses at baseline and weekly. (B) Mean or peak TRF values plotted over 18 weeks. Cultured cells showed some initial telomere elongation as measured by mean TRF values with a loss of less than 10% of initial value (1 kb). Peak TRF showed no decline, suggesting that the population was heterogeneous with a substantial subpopulation showing no telomere loss over the entire culture period. Telomere length of input CB CD34+ cells and of CD34+ cells purified from cytokine-stimulated, nonstromal cultures at intervals over 20 weeks of culture (C) and after repeated expansion-isolation procedures over 20 weeks of culture (D). Telomere length has been determined by flow FISH. Note the initial increase in telomere length over the first 8 weeks (C-D), the progressive decline beginning at 12 weeks (C) and the stabilization of the telomere size over 16 weeks and the progressive decline beginning at 20 weeks (D).
Telomere length of input CB CD34+ cells and suspension cells obtained at weekly intervals in long-term OP9/adeno-TPO stromal cell coculture and during stroma-free LTC. (A) TRF Southern blot analyses at baseline and weekly. (B) Mean or peak TRF values plotted over 18 weeks. Cultured cells showed some initial telomere elongation as measured by mean TRF values with a loss of less than 10% of initial value (1 kb). Peak TRF showed no decline, suggesting that the population was heterogeneous with a substantial subpopulation showing no telomere loss over the entire culture period. Telomere length of input CB CD34+ cells and of CD34+ cells purified from cytokine-stimulated, nonstromal cultures at intervals over 20 weeks of culture (C) and after repeated expansion-isolation procedures over 20 weeks of culture (D). Telomere length has been determined by flow FISH. Note the initial increase in telomere length over the first 8 weeks (C-D), the progressive decline beginning at 12 weeks (C) and the stabilization of the telomere size over 16 weeks and the progressive decline beginning at 20 weeks (D).
Telomere length and telomerase activity of long-term continuously cultured CB CD34+ cells in a stroma-free culture system
Similarly to small-scale expansion in “demi-depopulation” wells, large-scale long-term expansion cultures of purified CB CD34+ cells in the presence of exogenous FL, TPO, SCF, and IL-6 generated continuous production and expansion of hematopoietic progenitors and precursors for up to 20 weeks. These results prompted us to evaluate whether long-term expansion of primitive long-term repopulating cells under these conditions was associated with significant loss of telomeric DNA, as had been reported in earlier CB culture studies.4,5 From the time of initiation of large-scale cultures and at serial time points, we performed cell counts, established in vitro assays for CFCs and LTC-ICs, and inoculated NOD/SCID mice to assess the long-term repopulating ability of input and expanded cells. In addition, we measured over time the telomere length and telomerase activity of purified CD34+ cells. Cell CFC and LTC-IC outputs increased continuously for 20 and 12 weeks, respectively (Table 1); repopulating ability of NOD/SCID mice (assessed by primary and secondary serial transplants of expanded cells) was maintained for 12 weeks (Table 1; Figure 4). Telomere length of input and of purified CD34+ cells harvested at the different time points was measured by flow FISH analysis (Table 1; Figure 2) and by TRF (Table 1).
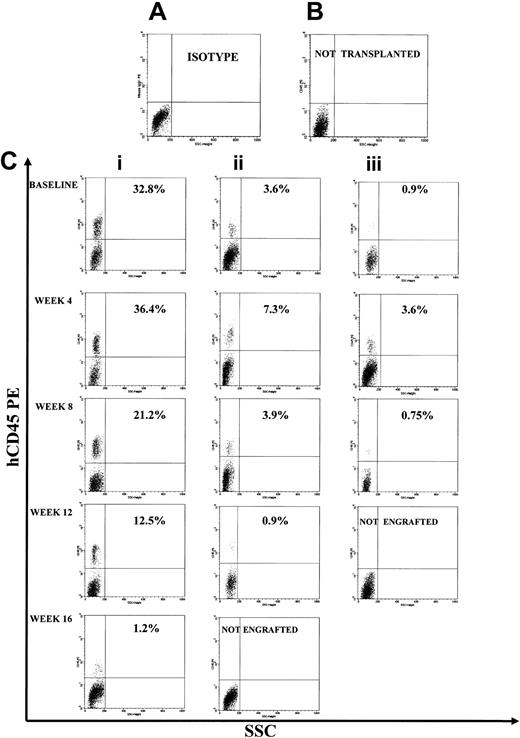
Representative FACS profile of primary, secondary, and tertiary engraftment in the BM of NOD/SCID mice after injection of human CB CD34+ cells at start of cultures and of their progeny at different times of continuous long-term expansion. (A) Isotype control. (B) Representative FACS profile of marrow cells from a NOD/SCID mouse that had not received a transplant. (C) Primary, secondary, and tertiary engraftment was evaluated in the BM of a NOD/SCID mouse injected 8 weeks previously with 3 × 105 CB CD34+ cells or 20 × 106 unseparated BM cells of a primary or secondary mouse. The level of human engraftment in the mouse BM was evaluated by FACS analysis (a positive mouse was defined by the presence of ≥ 0.1% human CD45+, CD71+, and GpA+ cells on total BM cells), confirmed by DNA analysis as described in “Patients, materials, and methods.” Total engraftment (CD45+ cells and GpA+ cells) was performed within the total, unseparated BM cells in individual NOD/SCID mice. Numbers in the upper right quadrants indicate the level of human CD45 engraftment.
Representative FACS profile of primary, secondary, and tertiary engraftment in the BM of NOD/SCID mice after injection of human CB CD34+ cells at start of cultures and of their progeny at different times of continuous long-term expansion. (A) Isotype control. (B) Representative FACS profile of marrow cells from a NOD/SCID mouse that had not received a transplant. (C) Primary, secondary, and tertiary engraftment was evaluated in the BM of a NOD/SCID mouse injected 8 weeks previously with 3 × 105 CB CD34+ cells or 20 × 106 unseparated BM cells of a primary or secondary mouse. The level of human engraftment in the mouse BM was evaluated by FACS analysis (a positive mouse was defined by the presence of ≥ 0.1% human CD45+, CD71+, and GpA+ cells on total BM cells), confirmed by DNA analysis as described in “Patients, materials, and methods.” Total engraftment (CD45+ cells and GpA+ cells) was performed within the total, unseparated BM cells in individual NOD/SCID mice. Numbers in the upper right quadrants indicate the level of human CD45 engraftment.
At the start of culture, mean telomere length by flow FISH was 9.1 ± 0.5 kb (range, 8.5-10 kb); after 12 weeks of expansion it was the same (9 ± 0.2 kb). Interestingly, in all experiments, telomere length increased significantly (P = .025) during the first 6 weeks of expansion (Figure 3C). The 4 experiments evaluated by flow FISH showed that telomere shortening began by 12 weeks and by 20 weeks, 2.2 kb telomeric DNA had been lost. The 2 experiments evaluated by TRF showed that telomeres remained in the range of 9.0 to 10.1 kb for 16 weeks, then lost 1.2 kb between 16 and 20 weeks (Table 1).
Telomerase activity of input and of purified CD34+ cells harvested at the different time points was measured by TRAP assay (Table 1). At start of culture, mean telomerase activity, expressed as TPG units, was 41.95 ± 4 (20.5% of NB control). The enzyme activity greatly increased in the first 2 weeks of culture. The enzymatic activity remained high for up to 10 weeks and was detectable through 20 weeks (Table 1).
Long-term cultured CB CD34+ cells in a stroma-free culture system associated with repeated expansion-isolation procedures: telomere length, telomerase activity, long-term in vivo repopulating ability, and SRC frequency evaluation
During long-term expansion, large numbers of differentiated mature hematopoietic cells secreting lineage-specific growth factors and cytokines have an inhibitory effect on primitive stem cell proliferation. To circumvent this problem we established a modified culture system that allowed us to selectively propagate the more primitive stem cell fractions. Large-scale suspension cultures were initiated with purified CB CD34+ cells as before, and cells were expanded for 4 weeks (Table 2). Every 4 weeks of culture we harvested and counted the cells and purified the CD34+ subpopulation by an immunomagnetic separation (Figure 1). A CD34+ cell aliquot was retained for TRAP assay and flow FISH; all remaining CD34+ cells were used to initiate new large-scale suspension cultures for an additional 4 weeks. The expansion-isolation procedure was repeated 5 times (at weeks 4, 8, 12, 16 and 20; Table 2; Figure 5).
Representative FACS profile of primary, secondary, and tertiary engraftment in the BM of NOD/SCID mice after injection of human CB CD34+ cells at start of cultures and of their progeny at different times of fractionated long-term expansion. (A) Isotype control. (B) Representative FACS profile of marrow cells from a NOD/SCID mouse that had not received a transplant. (C) Primary, secondary, and tertiary engraftment was evaluated in the BM of a NOD/SCID mouse that was injected 8 weeks previously with 3 × 105 CB CD34+ cells or 20 × 106 unseparated BM cells of primary or a secondary mouse. The level of human engraftment in the mouse BM was evaluated by FACS analysis (a positive mouse was defined by the presence of ≥ 0.1% human CD45+, CD71+, and GpA+ cells on total BM cells), confirmed by DNA analysis as described in “Patients, materials, and methods.” Total engraftment (CD45+ cells and GpA+ cells) was performed within the total, unseparated BM cells in individual NOD/SCID mice.
Representative FACS profile of primary, secondary, and tertiary engraftment in the BM of NOD/SCID mice after injection of human CB CD34+ cells at start of cultures and of their progeny at different times of fractionated long-term expansion. (A) Isotype control. (B) Representative FACS profile of marrow cells from a NOD/SCID mouse that had not received a transplant. (C) Primary, secondary, and tertiary engraftment was evaluated in the BM of a NOD/SCID mouse that was injected 8 weeks previously with 3 × 105 CB CD34+ cells or 20 × 106 unseparated BM cells of primary or a secondary mouse. The level of human engraftment in the mouse BM was evaluated by FACS analysis (a positive mouse was defined by the presence of ≥ 0.1% human CD45+, CD71+, and GpA+ cells on total BM cells), confirmed by DNA analysis as described in “Patients, materials, and methods.” Total engraftment (CD45+ cells and GpA+ cells) was performed within the total, unseparated BM cells in individual NOD/SCID mice.
We evaluated the isolated CD34+ cells for immunophenotype, CFC and LTC-IC content, telomere length, telomerase activity, and long-term NOD/SCID mouse repopulating ability (by means of primary, secondary, and tertiary serial transplants). The results of these experiments are summarized in Table 2. CD34+ cell number increased about 4-fold after the first 4 weeks of expansion; reisolated CD34+ cells, after an additional 4 weeks, expanded again 4-fold (Table 2).
Immunophenotyping of CD34+ cells demonstrated an increase of the most immature CD34+/CD38-/Lin- population for up to 16 weeks, followed by a decrease between 16 and 20 weeks. This was associated with progressive increase in the more mature CD34+/CD38+/Lin+ population and a precipitous loss of LTC-ICs by week 20 (Table 2). CFCs, including CFU-GEMMs, continued to expand through the fifth CD34+ isolation (20 weeks), whereas LTC-ICs expanded through 16 weeks, then sharply declined.
Flow FISH evaluation showed a telomere length of 9.1 ± 0.5 kb (range, 8.5-10 kb) of the input CD34+ cell population. After an initial increase in the first 4 weeks, telomere length stabilized (9.3 ± 0.28 kb) until week 16 (Table 2). Telomerase activity in the CD34+ fraction was detectable for 20 weeks but progressively declined (Figure 3D). The enzymatic activity of each monthly isolated CD34+ cells was higher than that measured at the same time points during continuous LTCs.
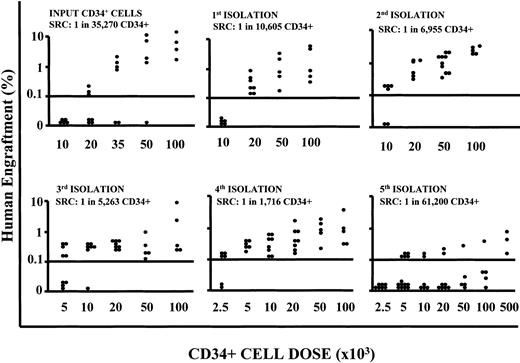
To assess whether the cells cultured under conditions where we repeatedly depleted the differentiating pool retained a long-term repopulation ability, we performed NOD/SCID mouse transplantation experiments with CD34+ cells obtained after each expansion-separation procedure. Long-term in vivo repopulation ability was retained up to 16 weeks of culture but was lost by 20 weeks (Table 2). To quantify the SRC numerical expansion, isolated CD34+ cells were transplanted in NOD/SCID mouse cohorts at decreasing doses for LDA. The frequency of SRCs in CD34+ populations increased with each expansion-separation procedure up to 16 weeks (from 1/35 270 CD34+ cells, in other words 2.8/100 000 CD34+ cells at time 0, to a maximum of 1/1716 CD34+ cells or 58.3/100 000, after 16 weeks; Figure 6) with a sharp decline in frequency at 20 weeks (Table 2). If we take into account that the cultures were initiated with 1 × 105 CD34+ cells and at 16 weeks of culture there was an absolute recovery of 30 × 106 CD34+ cells, then we calculate the actual SRC number at this time to be 17 482.
SRC frequency evaluation in LDA. Summary of the level of human engraftment (y-axis) in the BM of mice given transplants with decreasing doses of CD34+ cells (x-axis) harvested at different time points of cultures after repeated expansion-isolation procedures over 20 weeks of culture, in LDAs. Each symbol represents a mouse. Flow cytometric analyses have been performed as described in “Patients, materials, and methods.” The presence of at least 0.1% of human CD45+, CD71+, and GpA+ cells in the BM of NOD/SCID mice plus the presence of a human colony in 500 000 plated unseparated bone marrow cells defined a positive engraftment. In addition all mice were to have detectable human lymphoid (CD34-CD19/20+) and myeloid (CD45/71+CD15/66b+) engraftment (5 positive events each/20 000 assessed). The SRC frequency was calculated from the proportions of negative mice in each cohort, using L-Calc T software program (Stem Cell Technologies), which uses Poisson statistics and the method of maximum likelihood. The 95% confidence intervals were: input SRC frequency 1:35 270 (lower frequency 1:60 903; upper frequency 1:20 426), χ2 (Pearson) P = .7406; week 4 SRC frequency 1:10 605 (lower frequency 1:18 487; upper frequency 1:6,083), χ2 (Pearson) P = .3183; week 8 SRC frequency: 1:6995 (lower frequency 1:14 425; upper frequency 1:3353), χ2 (Pearson) P = .88; week 12 SRC frequency 1:5263 (lower frequency 1:9216; upper frequency 1:3006); χ2 (Pearson) P = .989; week 16 SRC frequency 1:1716 (lower frequency 1:3494; upper frequency 1:843), χ2 (Pearson) P = .9826; week 20 SRC frequency 1:83 892 (lower frequency 1:161 649; upper frequency 1:43 538), χ2 (Pearson) P = .001.
SRC frequency evaluation in LDA. Summary of the level of human engraftment (y-axis) in the BM of mice given transplants with decreasing doses of CD34+ cells (x-axis) harvested at different time points of cultures after repeated expansion-isolation procedures over 20 weeks of culture, in LDAs. Each symbol represents a mouse. Flow cytometric analyses have been performed as described in “Patients, materials, and methods.” The presence of at least 0.1% of human CD45+, CD71+, and GpA+ cells in the BM of NOD/SCID mice plus the presence of a human colony in 500 000 plated unseparated bone marrow cells defined a positive engraftment. In addition all mice were to have detectable human lymphoid (CD34-CD19/20+) and myeloid (CD45/71+CD15/66b+) engraftment (5 positive events each/20 000 assessed). The SRC frequency was calculated from the proportions of negative mice in each cohort, using L-Calc T software program (Stem Cell Technologies), which uses Poisson statistics and the method of maximum likelihood. The 95% confidence intervals were: input SRC frequency 1:35 270 (lower frequency 1:60 903; upper frequency 1:20 426), χ2 (Pearson) P = .7406; week 4 SRC frequency 1:10 605 (lower frequency 1:18 487; upper frequency 1:6,083), χ2 (Pearson) P = .3183; week 8 SRC frequency: 1:6995 (lower frequency 1:14 425; upper frequency 1:3353), χ2 (Pearson) P = .88; week 12 SRC frequency 1:5263 (lower frequency 1:9216; upper frequency 1:3006); χ2 (Pearson) P = .989; week 16 SRC frequency 1:1716 (lower frequency 1:3494; upper frequency 1:843), χ2 (Pearson) P = .9826; week 20 SRC frequency 1:83 892 (lower frequency 1:161 649; upper frequency 1:43 538), χ2 (Pearson) P = .001.
Telomere length and telomerase activity of adult unmanipulated and cultured CD34+ cells
BM and MPB HSCs can be cultured in the presence of FL, TPO, SCF, and IL-6 for up to 10 weeks, during which time they proliferate and produce large numbers of committed progenitors (up to 3000-fold).22 Primitive SRCs are expanded 6-fold after 3 weeks and retain the ability to repopulate secondary NOD/SCID mice. Telomere length from 5 BM and 9 MPB CD34+ cells was determined by flow FISH throughout the duration of culture. The telomere length of input BM CD34+ cells was 6.8 ± 1.21 kb and remained stable (6.8 ± 1.37 kb) after 1 week of culture (Table 3). The mean telomere length of input MPB CD34+ cells was 7.2 ± 1.44 kb and was slightly increased after 1 week of culture (7.4 ± 1.23 kb). After 3 weeks of expansion the telomere length was slightly reduced in both BM and MPB compared to the input population (0.3 and 0.1 kb loss, respectively). After 4 weeks of culture or more, CD34+ cell recovery was insufficient for flow FISH analysis and the vast majority of cells generated at this stage were differentiated. Telomere length was also evaluated in MPB MNCs at the start of culture and in total cells generated during the culture. A loss of 0.7 kbp was noted over 4 weeks (Table 3).
Discussion
We have demonstrated, in different culture systems, the propagation of CB CD34+ cells for months, with extensive expansion of stem cells as measured by CAFC and LTC-IC assay or NOD/SCID engraftment. Most importantly, in these systems this proliferation occurred without significant telomere loss until late stages of culture and correlated with sustained up-regulation of telomerase in the stem/progenitor compartment of CD34+ cells. These systems are critically dependent on provision of TPO, either as a recombinant factor added twice a week, or provided by transfecting the FL- and SCF-expressing OP-9 stroma cells with an adenovector-expressing TPO. In the stroma-free cultures these growth factors were exogenously added twice a week, whereas in the OP9/adeno coculture system, they were continuously provided by the stromal cells.
Accumulation of differentiated cells in the cytokine driven cultures has been shown to limit the stem cell expansion duration, hence the development of the strategy for reisolating CD34+ cells each month for repassage purposes. Similarly, long-term stem cell expansion on most murine stromal lines (eg, MS5, AFT024) is limited by accumulation of mature cells, particularly macrophages. OP9 with adeno-TPO supported human hematopoiesis without macrophage accumulation; indeed, CD14+ macrophage addition to the cultures or stimulation of macrophage production by addition of rhM-CSF leads to rapid termination of stem cell production.18
In stromal coculture the CFC kinetics and secondary CAFC production were biphasic, with considerable expansion over 4 to 5 weeks and then establishment of a steady-state level of production for a further 13 to 14 weeks. The CAFC presence in the suspension, which was subject to weekly demi-depletion, and in the adherent layer, which was not assayed, indicated that the overall stem cell expansion was considerably greater than indicated in Figure 2C. The data generated in the stroma-independent expansion-isolation procedure allowed direct evaluation of purified CD34+ cells at monthly intervals. Progenitors (CFCs and CFU-GEMMs) continued to expand throughout the 20 weeks of study, whereas stem cells as measured by in vitro LTC-IC and in vivo SRC assay expanded through week 16 but then declined sharply by week 20. LDA permitted measurements of stem cell expansion indicating a large net increase of SRCs over 16 weeks. In contrast to the CB cultures, cytokine-stimulated cultures of CD34+ cells from adult BM or G-CSF MPB did not sustain significant levels of CD34+ production beyond 4 weeks. This result points to the reduced proliferative potential of adult stem cells versus neonatal stem cells that may be due to a higher probability of differentiation than of self-renewal or to a higher frequency of stem cell apoptosis.
In this context, we have found (W.P. et al, unpublished observation, June 2003) that fetal liver CD34+ cells, which have longer telomeres than CB, show greater expansion in vitro than CB CD34+ cells with the same cytokine combination. We cannot at this stage state that telomere length directly influences ex vivo expansion and it is more likely that an ontogenetic heterogeneity or hierarchy exists at the stem cell level, with more primitive stem cells with higher telomerase activity and more limited proliferative history having long telomeres and intrinsically enhanced self-renewal potential.
In stromal coculture, telomerase activity was up-regulated in proliferating CD34+ cells and remained elevated in suspension cell populations for at least 18 weeks. It should be noted that the stromal coculture was terminated at a point when CD34+ cell production in the adherent layer was still high and both primary and secondary CAFCs remained elevated; thus, 18 weeks is an underestimate of stem cell proliferation under these culture conditions. Because telomerase activity is restricted to the CD34+ cells, the dilution by differentiating cells underestimated telomerase activity in the stem/progenitor population by a factor of 5- to 10-fold. Adjusting for this dilution by factoring in the CFC content as a measure of these early cells, the stem/progenitor population maintained telomerase activity comparable to levels found in immortal tumor cell lines throughout the culture. Levels were also comparable to those we have previously reported in hTERT-transduced human fibroblast clones that showed stable or elongated telomeres despite extensive proliferation.27,28 In stroma-free cultures of CB CD34+ cells, telomerase levels remained quite high in the first 8 weeks, comparable to levels maintained in stromal culture, but then fell to 25% of input levels between 8 and 16 weeks, and to 12% by week 20. In adult CD34+ cell cultures, the telomerase levels of the input cells and of the recovered CD34+ cells at 1 and 3 weeks were comparable but significantly lower than in CB cultures over the first 2 months.
In the majority of CB cultures, both stroma-free or OP9 adeno-TPO, telomeres remained stable for over 3 to 4 months and only at late stages did telomeres begin to shorten. Indeed, in some cultures telomere elongation was noted in the first month. At week 3, in cytokine-stimulated cultures of adult CD34+ cells, telomeres of the CD34+ subset were slightly shorter (-0.1 to -0.3 kb) than input telomere length. The great reduction in CD34+ cell recovery by week 4 precluded telomere measurement of this subset; however, telomere measurement of unseparated culture cells at this time showed a loss of 0.7 kb. This contrasted with the lengthening (+0.9-1.0 kb) seen in CB cultures over the same time period. The telomerase activity level in extracted cell protein does not take into account the role of nuclear localization of the enzyme in determining biologic activity.29 Nevertheless stable enzyme up-regulation is the most plausible explanation for telomere stabilization. Given a doubling time of 24 hours, then over 20 weeks the stem cells would have undergone 140 population doublings (PDs). With the average loss of telomeric DNA of 50 to 100 bp/PD seen in telomerase-negative cells, 7 to 17 kb of telomere would have been lost in the absence of telomerase up-regulation, and cultures would have terminated because of proliferative senescence. Even with a more conservative assumption of doubling every 48 hours, the stem cells would have lost telomere DNA equivalent to a lifetime of normal telomere erosion. In this regard, the HSCs in late passage CB cultures would more closely correspond to adult HSCs, with much reduced expansion potential in vitro, reduced telomerase levels, and loss of capacity to stabilize telomeres.4-6 Thus the 20-week culture may span a developmental stage of transition from fetal/neonatal to adult stem cell.
Does the in vitro model predict for the in vivo situation? Analysis of age-related changes in granulocyte telomere length has revealed an average loss of 39 bp/y, but in the first 6 months of life telomeres shorten at a much more rapid rate corresponding to 3 kb/y. This would correspond to 15 to 30 stem cell divisions in the first 6 months of life, followed by less than one stem cell division per year.30 This would not have been predicted from the in vitro data unless the extent of stem cell proliferation in the first 6 months was itself biphasic, with stable telomeres only for the first 3 months followed by rapid loss as HSCs transit to adult type while still actively proliferating. Numerous studies have investigated telomere shortening, particularly following autologous or allogeneic stem cell transplantation. Although significant telomere shortening is reported to occur within the first year after transplantation as yet there is no evidence that this shortening leads to impaired hematopoiesis in the majority of cases, but there are reports where late graft failure was associated with significant telomere shortening.31 Perhaps the most compelling evidence for telomere length relevance in hematopoietic cells is the recent demonstration of a highly significant association between blood leukocyte telomere length and mortality in a group of healthy elderly individuals.32 Those with the shortest telomeres had poorer survival, attributable in part to higher mortality from heart disease and infectious disease. The loss in median survival associated with possession of shorter telomeres in any of the age cohorts studied was 4.8 years for women and 4.0 years for men.
The development of an improved in vitro CB CD34+ culture system that supports a many thousand-fold amplification of HSCs has provided insight into the role of telomerase up-regulation in maintaining telomere stability in neonatal HSCs. It has also revealed an important distinction between adult and neonatal HSCs with respect to onset of proliferative senescence. The clinical potential of our observations are evident because very extensive expansion of CB HSCs can be obtained over many months without deterioration of HSC quality as measured by NOD/SCID engraftment and stable telomeres. Ex vivo expansion can extend the use of CB, not only to all adult recipients, but potentially to multiple recipients, without concern that extensive telomere shortening would compromise the long-term survival of the recipients. Particularly poor results are seen after an unrelated CB transplant when the nucleated cell dose infused is less than 1.5 × 107/kg, or 1.7 × 105 CD34+ cells/kg.9 CB harvests are generally sufficient for pediatric engraftment (median age 7 years, ∼25 kg body weight) and would be approaching the lower limits for optimal engraftment in adults up to 60 kg with engraftment problems likely in larger individuals. When patients receive lower cell doses risk of graft failure is exceedingly high and time to neutrophil recovery is prolonged. Furthermore, survival in recipients of one or 2 HLA-mismatched CB transplants is improved with higher cell doses.8,9 A recent study concluded that raising the nucleated cell dose to approximately 3 × 107/kg may offset the negative effect of one HLA-mismatch.8,9 For these reasons CB ex vivo expansion is an attractive option for adult unrelated transplantation, particularly with one or 2 HLA mismatches.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-09-3079.
Supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan, Italy); from Consiglio Nazionale delle Ricerche (CNR Progetto Strategico Oncologia); from Compagnia di San Paolo; from the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR, Rome; A.B., W.P. and M.A.); from the European Community, no. PL99-00859 (W.P.); from EUROCORD III, European Community no. QLK3-CT-2002-01918 (W.P.); from the Associazioni Donatrici Italiane Cordone Ombelicale (ADISCO); by grants NCI PO1CA 59350, U19 CA 67842, NIH HL 66952, and HL 61401 (M.A.S.M.); and from Deutsche Krebshilfe, Bonn, Germany (K.C.W.).
L.G., K.C.W., and M.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.