Abstract
Primitive hematopoietic cells from several species are known to efflux both Hoechst 33342 and Rhodamine-123. We now show that murine hematopoietic stem cells (HSCs) defined by long-term multilineage repopulation assays efflux both dyes variably according to their developmental or activation status. In day 14.5 murine fetal liver, very few HSCs efflux Hoechst 33342 efficiently, and they are thus not detected as “side population” (SP) cells. HSCs in mouse fetal liver also fail to efflux Rhodamine-123. Both of these features are retained by most of the HSCs present until 4 weeks after birth but are reversed by 8 weeks of age or after a new HSC population is regenerated in adult mice that receive transplants with murine fetal liver cells. Activation of adult HSCs in vivo following 5-fluorouracil treatment, or in vitro with cytokines, induces variable losses in Rhodamine-123 and Hoechst 33342 efflux activities, and HSCs from mdr-1a/1b-/- mice show a dramatic decrease in Rhodamine-123 efflux ability. Thus, the Rhodamine-123 and Hoechst 33342 efflux properties of murine HSCs fluctuate in the same fashion as a number of other HSC markers, suggesting these are regulated by a common control mechanism that operates independently of that regulating the regenerative function of HSCs. (Blood. 2004;103:4487-4495)
Introduction
Throughout adult life the hematopoietic system maintains a population of hematopoietic stem cells (HSCs), each of which is capable of producing large numbers of all blood cell types for many years.1,2 The durability of the multilineage output potential of HSCs is believed to reflect their ability to execute self-renewal divisions; that is, an ability to proliferate without activation of a latent readiness to differentiate along any one of the blood cell lineages. The molecular mechanisms that regulate this defining property of HSCs are still poorly understood, although various studies have indicated that events downstream of the c-kit,3 gp130,4,5 and Wnt signaling pathways6 are involved, at least some of which may target members of the Hox gene family.7-9
Such studies are highly dependent on methodologies to generate, manipulate, and track the progeny of purified populations of cells with functionally defined HSC activity. Characterization of the cell surface profile of HSCs from healthy adult mouse bone marrow has led to strategies that now allow these cells to be obtained at purities of 20% to 50%.10-14 However, the expression of many of the markers targeted in these purification procedures, including AA4.1, Mac-1, CD34, and CD38, are now known to alter during ontogeny or when adult mouse HSCs are stimulated to proliferate.15-22 This has made it more difficult to obtain comparably purified populations of HSCs from fetal sources or after various in vitro and in vivo manipulations of adult HSCs. Other properties of HSCs present in steady-state adult mouse bone marrow include a verapamil-sensitive ability to efflux Rhodamine-12323 and Hoechst 33342.24 In the latter case, this causes the HSCs to be found in the rare side population (SP) of dimly fluorescent cells seen when Hoechst 33342-stained adult mouse bone marrow cells are exposed to UV light and examined at 2 emission wavelengths simultaneously.25 The low fluorescence exhibited by Rhodamine-123- and Hoechst 33342-stained HSCs has been attributed to their selective expression of 2 different ABC transporters, P-glycoprotein26,27 and Abcg2,28 respectively. In an earlier study, it was noted that the HSCs in murine fetal liver differ from their adult counterparts in their inability to efflux Rhodamine-123 efficiently.21 Similarly, it was found that the activation of HSCs in adult mice that occurs by 4 days after the administration of 150 mg/kg 5-fluorouracil (5-FU) also leads to a loss of HSC Rhodamine-123 efflux ability.16 These findings suggested to us that similar changes might affect other sources of activated HSCs and that the Hoechst 33342-determined SP phenotype might also be unstable. To address these possibilities, we examined the HSC content of Rhodamine-123- and Hoechst 33342-stained fetal liver and bone marrow cells from mice at different stages of development, or after being stimulated in vivo or in vitro. Use of a rigorous long-term in vivo repopulation assay to quantitate the number and distribution of HSCs in populations showing no or low versus some fluorescence demonstrated that both the Rhodamine-123 and the Hoechst 33342 efflux abilities of adult HSCs are acquired around or after 4 weeks of age, and later, when adult HSCs are activated, both properties may be lost.
Materials and methods
Mice
C57Bl/6J-Ly5.2 (B6) mice, congenic C57Bl/6J:Pep3b-Ly5.1 (Pep3b), and C57Bl/6J W41/W41-Ly5.2 (W41) mice, and NOD/LtSz-scid/scid (NOD/SCID [nonobese diabetic/severe combined immunodeficiency]) mice were propagated from breeders originally obtained from Dr L. Schultz and Dr J. Barker at the Jackson Laboratory (Bar Harbor, ME). FVB and FVB-mdr-1a/b-/- mice were generated from breeders obtained from Taconic (German-town, NY). All mice were housed in microisolator units and provided with sterilized food and water. Following irradiation, all mice were given HCl-acidified water (pH 3), and for NOD/SCID mice 100 mg/L ciprofloxacin (Bayer, Leverkusen, Germany) was also added to the drinking water. Adult donor mice were used between 8 and 10 weeks of age, juvenile mice at 3 weeks of age. Recipients were at least 6 weeks old when irradiated.
Cell preparation
Murine fetal liver cells were obtained at 14.5 days after coitus and minced, and the cells were forced through a 70-μm sieve to obtain a single-cell suspension. Some bone marrow cells were obtained from mice injected intravenously 2 or 4 days previously with 150 mg/g body weight 5-FU (Faulding, Vaudreuil, Quebec, Canada) dissolved in phosphate-buffered saline (PBS). Bone marrow cells were obtained by flushing femurs and tibiae with Hanks balanced salt solution containing 2% fetal bovine serum (HF; StemCell Technologies, Vancouver, BC, Canada). Where indicated, Sca-1+ cells were isolated immunomagnetically from adult bone marrow cells (using EasySep; StemCell Technologies) and then cultured at 104 cells/mL for 7 days in a serum-free medium (SFM) consisting of Iscoves modified Dulbecco medium (IMDM), a bovine serum albumin-insulintransferrin-serum substitute (BIT; StemCell Technologies), 10-4 M 2-mer-captoethanol (Sigma Chemicals, St Louis, MO) and 40 μg/mL low-density lipoproteins (Sigma), to which 300 ng/mL murine Steel factor (SF; expressed in COS cells and purified in the Terry Fox Laboratory), 20 ng/mL human interleukin-11 (IL-11; Genetics Institute, Cambridge, MA), and 1 ng/mL human flt3-ligand (Immunex, Seattle, WA) were added.
Flow cytometry
For characterization and assays of HSCs in fetal liver, juvenile bone marrow, and healthy or manipulated adult bone marrow preparations, the red blood cells (RBCs) were first lysed by incubating cells with NH4Cl. For analysis of Rhodamine-123 and Hoechst 33342 efflux activity, cells were then incubated at 106 cells/mL in SFM containing 0.1 μg/mL Rhodamine-123 (Molecular Probes, Eugene, OR) for 30 minutes at 37°C, washed once with HF, and resuspended at the same cell concentration in prewarmed SFM plus 5 μg/mL Hoechst 33342 (Molecular Probes). After incubation for 90 minutes at 37°C, cells were washed, resuspended in ice-cold HF plus 5% rat serum (Sigma) and 3 μg/mL Fc receptor blocking antibody (2.4G2)29 at 107 cells/mL, followed by staining for 30 minutes on ice with the following biotinylated antilineage (lin) antibodies: anti-Gr1 (RB6-8C5, granulocytes), anti-B220 (RA3-6B2, B lymphocytes), and anti-Ly1 (53-7.3, T lymphocytes), all of which were prepared in the Terry Fox Laboratory, and TER-119 (Becton Dickinson [BD], San Jose, CA). Except for the staining of steady-state adult bone marrow cells, anti-Mac1 (M1/70, monocytes and macrophages) was purposefully omitted from this anti-lin antibody cocktail because Mac1 expression has been shown to be up-regulated on activated HSCs.19-21 A parallel aliquot of cells was stained with Hoechst 33342 in the presence of 50 μM verapamil (Sigma) to set the gates for SP cells.25 As indicated, cells were also stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD45.1 antibody (anti-Ly5.1, clone A20, prepared in the Terry Fox Laboratory). Cells stained with biotinylated antibodies were washed and incubated for a further 20 minutes on ice with streptavidinphycoerythrin (PE) or streptavidin-allophycocyanin (APC) (both from BD), as indicated, and then washed again first in HF alone and then in HF plus 2 μg/mL propidium iodide (PI; Sigma). Cells were then analyzed and sorted on a FACStar+ or FACS Vantage SE (BD). Gates to define cells retaining Rhodamine-123 (Rho+ cells) or stained positively with a given antibody were set at a level of fluorescence that excluded at least 99.9% of the viable cells in an unstained sample of the same cell suspension being analyzed.
Assays for hematopoietic activity
For detection and quantitation of long-term repopulating HSCs, the competitive repopulating unit assay was used.30 Briefly, varying dilutions of cells from B6, Pep3b, or FVB donors were transplanted into Pep3b, W41, or NOD/SCID mice irradiated with 900, 400, or 350 cGy, respectively. Pep3b recipients were coinjected with 106 healthy adult Pep3b bone marrow cells to protect them from the lethal effects of the 900 cGy. Peripheral blood (PB) cells collected from all mice 16 weeks after they received transplants (and in many cases again 24 weeks after transplantation) were analyzed for test cell-derived white blood cells (WBCs) after NH4Cl lysis of the RBCs. To detect B6 cells in Pep3b mice, the WBCs were first incubated with biotinylated anti-Gr1 and anti-Mac-1, or biotinylated anti-B220, or biotinylated anti-Ly1, and then incubated with streptavidin-PE plus FITC-conjugated anti-CD45.2 (anti-Ly5.2, clone HIS41, prepared in the Terry Fox Laboratory). To detect Pep3b cells in W41 mice, the same protocol was followed but using streptavidin-PE plus FITC-conjugated anti-CD45.1. Reconstitution of NOD/SCID mice with adult bone marrow cells from FVB donors was detected by costaining the WBCs with the same biotinylated lin+ antibodies and streptavidin-PE plus a FITC-labeled anti-H2Kq antibody (BD). For all analyses, at least 5000 gated events were analyzed on a FACSCalibur (BD). Recipients were considered to be repopulated with at least one HSC only if all of the following conditions were met: at least 1% of PI- WBCs were donor-derived with donor-derived B220+cells, Ly1+ cells and Gr1+ and/or Mac1+ cells each at 0.1% levels or more. Gates for positive cells were set as those that excluded more than 99.98% of unstained cells. HSC frequencies in the populations transplanted were calculated from the proportions of mice considered negative by using the L-calc software program (StemCell Technologies) that uses Poisson statistics and the method of maximum likelihood. It has been previously shown that the frequency of Pep3b repopulating cells detected in congenic sublethally irradiated W41 mice is the same as when these cells are assayed in radioprotected B6 given 900 cGy or when B6 cells are assayed in radioprotected Pep3b mice given 900 cGy. In addition the engraftment levels and lineage ratios obtained are similar in all 3 of these donor-host combinations.4,31,32 Therefore, we pooled the results from the assays performed in W41 and B6 recipients.
For secondary transplantations, bone marrow cells were harvested from both femurs and tibiae of primary mice 8 to 11 months after transplantation, and the cells were then stained with Hoechst 33342 prior to isolation of the SP and non-SP fractions as described earlier. The cells in these preparations were then injected at varying dilutions into irradiated mice of the same strain as the primary recipients. The presence of regenerated WBCs of the same genotype originally injected into the primary mice was assessed 4 to 6 months later, and HSC frequencies were determined as described earlier.
Results
Hoechst 33342 and Rhodamine-123 efflux activities of murine HSCs are developmentally regulated
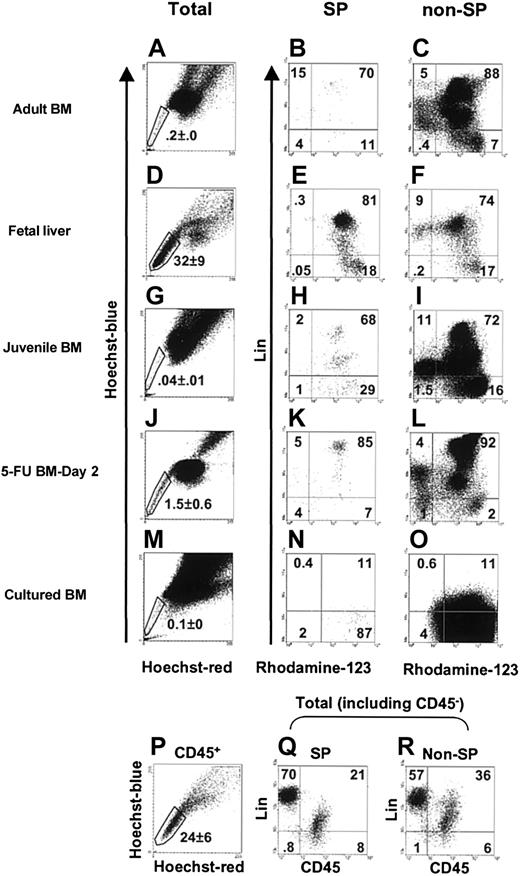
Figure 1 shows a comparison of the fluorescence profiles of cells from different sources after staining with Hoechst 33342, Rhodamine-123, and antibodies to B220, Ly1, Gr-1, and Ter119 (and Mac1 for adult bone marrow cells only), as described in “Materials and methods.” Aliquots of the day-14.5 fetal liver cells were also stained with anti-CD45 antibody to determine the proportion of nonhematopoietic cells present as indicated by a lin- CD45- phenotype. These analyses showed the proportionof such cells in suspensions of day-14.5 fetal liver to be less than 2% (Figure 1H,I). SP cells defined by their verapamil-sensitive ability to efflux Hoechst 33342 under the staining conditions used were readily detected in all 3 primary tissues examined: day-14.5 fetal liver, juvenile bone marrow (from 3-week-old mice), and adult bone marrow (from 8- to 10-week-old mice). However, there were also significant (P < .05) changes in the relative size of this population in these different hematopoietic tissues during ontogeny. SP cells were the most prevalent in day-14.5 fetal liver (mean ± SEM = 32% ± 9% of the total nucleated cells, n = 4, Figure 1D and 24% ± 6% of the total CD45+ cells, Figure 1P), least prevalent in juvenile bone marrow (0.04% ± 0.01% of the total nucleated cells, n = 4, Figure 1G), and intermediate in adult bone marrow (0.2% ± 0.0% of the total nucleated cells, n = 5, Figure 1A). The SP values obtained for adult bone marrow are similar to those we have previously reported for FVB27 and B614 mice using the same methodology but are somewhat higher and include a higher proportion of lin+ cells than reported by others.25,26 The majority of the lin- cells in the SP (and non-SP) fractions of all 3 tissues were Rho+ (Figure 1, second column of profiles). However, in each case, a small lin- Rho- SP fraction could be identified, with these being rarest in day 14.5 fetal liver.
The variable prevalence of SP cells in different hematopoietic cell populations. Cells were costained with lin+ antibodies, Hoechst 33342, and Rhodamine-123 as described in “Materials and methods.” Representative FACS profiles are shown for adult bone marrow (A-C), day-14.5 fetal liver (D-F and P-R), juvenile bone marrow (G-I), day-2 5-FU adult bone marrow (J-L), and adult Sca-1+ bone marrow cells cultured for 7 days with SF, IL-11, and flt3-ligand (M-O). The first column of FACS profiles shows the dual-wave Hoechst 33342 emission fluorescence of the total population of PI- cells for each population examined. The second column of profiles (B-N) shows the subpopulations of SP cells according to their expression of lin+ markers and Rhodamine-123 fluorescence. For the analyses of adult bone marrow cells (B-C), Mac1 was included in the lin+ antibody cocktail, but in all other analyses Mac1 was omitted. The third column of profiles (C-O) shows the corresponding subsets in the non-SP cells from each of the suspensions tested. The gates shown in the panels in the second and third columns were used to isolate cells for HSC assays. For fetal liver, some cells were also stained with CD45 (which does not stain either hepatic cells or mature erythroid cells), and representative profiles are shown in panels Q-R. Because maturing erythroblasts make up most of the cells in the day 14.5 fetal liver, a large lin+CD45- population is seen (Q-R). The values shown in all of the profiles show the percentages of cells in each subset of the population examined (total cells in the first column, SP cells in the second column, non-SP cells in the third column, and as indicated in panels P-R). Note that in the experiments with cultured adult bone marrow cells, the streptavidin-APC used to visualize the lin+ cells gave a very weak staining of these cells and hence a likely underestimation of their numbers (N-O).
The variable prevalence of SP cells in different hematopoietic cell populations. Cells were costained with lin+ antibodies, Hoechst 33342, and Rhodamine-123 as described in “Materials and methods.” Representative FACS profiles are shown for adult bone marrow (A-C), day-14.5 fetal liver (D-F and P-R), juvenile bone marrow (G-I), day-2 5-FU adult bone marrow (J-L), and adult Sca-1+ bone marrow cells cultured for 7 days with SF, IL-11, and flt3-ligand (M-O). The first column of FACS profiles shows the dual-wave Hoechst 33342 emission fluorescence of the total population of PI- cells for each population examined. The second column of profiles (B-N) shows the subpopulations of SP cells according to their expression of lin+ markers and Rhodamine-123 fluorescence. For the analyses of adult bone marrow cells (B-C), Mac1 was included in the lin+ antibody cocktail, but in all other analyses Mac1 was omitted. The third column of profiles (C-O) shows the corresponding subsets in the non-SP cells from each of the suspensions tested. The gates shown in the panels in the second and third columns were used to isolate cells for HSC assays. For fetal liver, some cells were also stained with CD45 (which does not stain either hepatic cells or mature erythroid cells), and representative profiles are shown in panels Q-R. Because maturing erythroblasts make up most of the cells in the day 14.5 fetal liver, a large lin+CD45- population is seen (Q-R). The values shown in all of the profiles show the percentages of cells in each subset of the population examined (total cells in the first column, SP cells in the second column, non-SP cells in the third column, and as indicated in panels P-R). Note that in the experiments with cultured adult bone marrow cells, the streptavidin-APC used to visualize the lin+ cells gave a very weak staining of these cells and hence a likely underestimation of their numbers (N-O).
To determine whether the HSCs in fetal liver and juvenile bone marrow share the SP and/or Rho- properties of most of the HSCs in adult bone marrow, proportional dilutions of FACS-sorted cells from the different subsets of Hoechst 33342- and Rhodamine-123-stained cells were transplanted into irradiated Ly-5-congenic recipients. The blood of these mice was then assessed 16 and, in most mice, again 24 weeks later for the presence of fetal liver or juvenile bone marrow-derived T, B and granulocyte-macrophage (GM) cells. The proportions of mice not showing such multilineage test cell-derived repopulation were then used to calculate the frequency and hence the total number and distribution of HSCs in the initial subsets of cells analyzed. The results for fetal liver HSCs are given in Table 1 and for juvenile bone marrow HSCs in Table 2. In the fetal liver, only a few (∼1%) of the HSCs were detected in the SP fraction of CD45+ cells and most (∼99%) were found in the non-SP fraction of CD45+ cells. HSCs were also more highly enriched in the non-SP fraction of CD45+ fetal liver cells (∼40 ×, P < .05). Similarly, only Rho+ HSCs were detected in the day 14.5 fetal liver, and, at the resolution of the experiments performed, these accounted for more than 99% of all the HSCs present in this tissue.
In juvenile bone marrow, many HSCs were still found in the non-SP fraction, although this varied considerably between experiments (Table 2). Nevertheless, because of the reduced numbers of other types of SP cells in juvenile bone marrow, the frequency of HSCs in the lin- SP fraction of cells from this latter source was consistently much higher than in the non-SP fraction of lin- cells. Combined analysis of the Rhodamine-123 and Hoechst 33342 efflux properties of the HSCs in juvenile bone marrow showed that many were also still unable to efflux Rhodamine-123 regardless of their SP phenotype.
These findings demonstrate that the ability of adult bone marrow HSCs to efflux Hoechst 33342 efficiently is a phenotype acquired relatively late in murine development. To further test this, the phenotype of HSCs regenerated in mice that received transplants with fetal liver HSCs was examined. Accordingly, 2 irradiated adult W41 mice received transplants with day 14.5 fetal liver cells from Pep3b (Ly5.1) mice, one with 2.5 × 104 lin- non-SP cells and the other with 4 × 103 lin- non-SP CD45+ cells. These inocula contained an estimated 52 and 8 HSCs, respectively, based on the HSC frequency values shown in Table 1. Then 8 or 11 months after transplantation, the number of HSCs regenerated from the injected (Ly5.1) fetal liver cells in the bone marrow of these primary hosts was determined by performing limiting dilution repopulation assays of the harvested cells in irradiated secondary W41 mice. As detailed in Table 3, 85% and 88% of the PB cells of the 2 primary mice were derived from HSCs regenerated from the original fetal liver cells transplanted. Moreover, in both experiments, more than 50% of all of the fetal liver-derived HSCs regenerated in the primary mice had a SP phenotype at the time of analysis. However, fetal liver-derived HSCs could also be readily identified in the non-SP fraction.
Reversibility of the Hoechst 33342 and Rhodamine-123 efflux activities of HSCs in healthy adult mouse bone marrow
In light of previous studies showing adult HSCs stimulated to divide in vivo or in vitro reactivate expression of markers exclusive to fetal HSCs (eg, CD34, CD38, and Mac1), it was of interest to determine whether the Rhodamine-123 and Hoechst 33342 efflux properties of HSCs in adult bone marrow might also change when these cells are activated. We examined the Hoechst 33342 and Rhodamine-123 efflux activities of HSCs in the bone marrow of 8- to 10-week-old mice 2 or 4 days after they had been injected with 150 mg/kg 5-FU. As expected, injection of 5-FU decreased the total number of cells present in the bone marrow (2 tibiae and 2 femurs) approximately 2-fold after 2 days and another 2- to 3-fold after 4 days. After 2 days, 1.5% ± 0.6% of all the bone marrow cells could be visualized in the SP fraction, and the lin- subset of these included approximately equal numbers of Rho- and Rho+ cells (Figure 1J-L). Transplantation assays of the Rho+ and Rho- subsets of the SP and non-SP cells in the 2-day post-5FU bone marrow revealed that almost all of the HSCs (≥ 91%) were still contained within the SP population and many (at least 56%) were also still Rho- (Table 4). However, the proportion that was Rho+ (at least 35%) was already markedly increased in comparison to healthy adult bone marrow (11% Rho+14). Four days after the 5-FU treatment, the proportion of all cells exhibiting a SP phenotype was, on average, slightly lower (0.8% ± 0.1%) than after 2 days and by 4 days 71% of these cells had become Rho+. Analysis of the proportions of HSCs in the SP and Rho- fractions showed that most (at least 94%) were still contained within the SP fraction, but again many (at least 71%) were Rho+ (Table 5). These experiments demonstrate the rapidity with which adult HSCs lose their ability to efflux Rhodamine-123 in 5-FU-treated mice and show that this can happen prior to any apparent change in Hoechst 33342 efflux ability.
In a further series of experiments (n = 3), we investigated the Rhodamine-123 and Hoechst 33342 efflux properties of the HSCs generated in 7-day-old cultures of adult Sca-1+ mouse bone marrow cells containing a potent growth factor cocktail that stimulates adult bone marrow HSC expansion.32,33 As shown in Figure 1M-O, some SP cells were still detectable in these 7-day cultures (0.1% ± 0%), but very few of these were Rho- (< 3%). In vivo assays of the cells present in different subsets isolated from these cultures showed that most of the HSCs (> 63%) were in the non-SP fraction, and, regardless of their SP phenotype, only Rho+ HSCs were detected (Table 6).
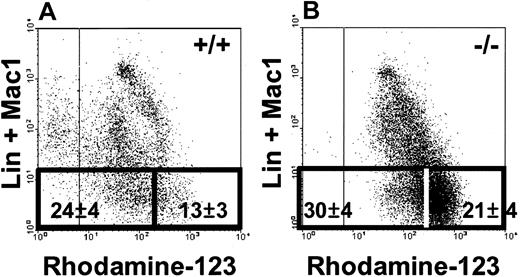
The Rho phenotype of HSCs is determined by mdr-la/lb
Previous studies have shown that the bone marrow (and adult liver) of FVB-mdr-la/lb-/- mice contain normal numbers of HSCs that are predominantly SP, but the Rhodamine-123 efflux capacity of the lin- SP cells is defective.26,27 This suggested that P-glycoprotein, the product of the mdr-la/lb genes, is essential for the Rho- phenotype of HSCs in the bone marrow of healthy adult mice. To test this directly, bone marrow cells from 8- to 10-week-old adult FVB-mdr-la/lb-/- or FVB-+/+ mice were stained with lin antibodies, Hoechst 33342, and Rhodamine-123, and then isolated subsets were analyzed for HSC activity by using sublethally irradiated NOD/SCID mice as recipients.27 As expected, the SP fraction of bone marrow cells from the FVB-mdr-la/lb-/- (but not the FVB-+/+) mice contained reduced numbers of Rho- and Rho-/low cells (in both the lin- and lin+ subsets, Figure 2A-B). The results of the HSC in vivo assays are given in Table 7. The distribution of HSCs between the Rho-/low and Rhohigh subsets of SP cells from FVB-+/+ bone marrow was the same as previously found for B6 bone marrow; that is, none of the HSCs were found in the Rhohigh fraction.14 For unknown reasons the frequency of the HSCs detected in the bone marrow of the FVB-mdr-la/l-/-mice in these experiments was much lower than in the FVB-+/+ mice and also lower than those documented previously using the same assay procedure.27 However, 33% of the HSCs detected in the bone marrow of the FVB-mdr-1a/1b-/- mice exhibited a Rhohigh phenotype. These results provide direct evidence that the Rho efflux ability of HSCs from FVB-+/+ mice is largely determined by the activity of P-glycoprotein.
SP cells from FVB-mdr-1a/b-/-bone marrow contain reduced numbers of Rho-/low cells. SP cells from both FVB-+/+ (A) and FVB-mdr-1a/b-/- (-/-) mice (B) were analyzed for their expression of lin+ markers (including Mac1) and Rhodamine-123 efflux capability. The gates used to distinguish the Rho-/low and Rhohigh fractions of lin- SP cells and the percentages of lin- SP cells (mean ± SEM from 3 experiments) found within these gates are indicated.
SP cells from FVB-mdr-1a/b-/-bone marrow contain reduced numbers of Rho-/low cells. SP cells from both FVB-+/+ (A) and FVB-mdr-1a/b-/- (-/-) mice (B) were analyzed for their expression of lin+ markers (including Mac1) and Rhodamine-123 efflux capability. The gates used to distinguish the Rho-/low and Rhohigh fractions of lin- SP cells and the percentages of lin- SP cells (mean ± SEM from 3 experiments) found within these gates are indicated.
Discussion
Properties of HSCs that are altered when they begin to differentiate are of great interest because the molecules involved may serve as indicators of mechanisms that determine their stem cell status. In many instances, the identification of such differentiation-associated changes has been exploited to develop improved strategies for isolating highly enriched (20%-50% pure) populations of HSCs from healthy adult mouse bone marrow.10-14 These purified HSC populations, in turn, are now being widely used to examine the full spectrum of HSC differentiation potentialities, their self-renewal control by exogenous stimuli, their different gene expression profiles, and their proliferation kinetics in response to different cytokines. In addition, the properties used to obtain these highly purified HSC suspensions offer the possibility of describing a HSC-specific phenotype, which if stable could allow HSCs to be enumerated directly. However, this latter goal has proven surprisingly elusive. This appears to be due in part to changes in the expression on HSCs of a number of antigens depending on the source of the HSCs being examined. In particular the expression of a number of antigens whose presence (or absence) characterizes HSCs in steady-state adult mouse bone marrow has proven to be dependent on the quiescent status of these HSCs. Thus, when they are activated, expression of these antigens may be either up-regulated (eg, CD34 and Mac1) or down-regulated (eg, CD38) without affecting the regenerative potential of the HSCs.18,19,22
The abilities of HSCs from adult mice to efflux Rhodamine-123 and Hoechst 33342 efficiently are properties that are lost rapidly as these cells begin to differentiate.23,24 The ability of HSCs to efflux Hoechst 33342 has received particular attention because this property is shared with other primitive cell types, including muscle and cardiac stem cells,34,35 neural/retinal stem cells,36,37 and subsets of ES cells,26 as well as populations of primitive hematopoietic cells from other species, including humans.38-40 Rhodamine-123 efflux properties have not been as widely studied, although this has been known for many years to also be a feature of primitive healthy human hematopoietic cells.41-43 Interestingly, earlier studies had suggested that the ability of murine HSCs from different sources to efflux Rhodamine-123 was inconsistent,16,21 raising the possibility that this phenotype might also vary according to the activation status of HSCs. Here, we have confirmed and extended these observations to show that loss of Rhodamine-123 efflux activity is a very sensitive indicator of HSC “activation.” In addition, we show that an ability to efflux Hoechst 33342 is another unreliable indicator of the regenerative potential of HSCs. Thus, in contrast to adult mouse bone marrow in which most of the HSCs are Rho- SP cells, essentially all of the HSCs in the liver of day 14.5 mouse embryos have an opposite Rho+ non-SP phenotype, despite the fact that the fetal liver contains proportionately one of the largest CD45+ SP populations. Moreover, a Rho+ non-SP phenotype is retained by most of the HSCs during the expansion of the hematopoietic system that occurs until young adulthood is reached (by 8 weeks of age). A similar transition from a Rho+ non-SP phenotype to a Rho- SP phenotype occurs following the regeneration of a new HSC population in myeloablated mice that receive transplants of mouse fetal liver cells. Thus, the Rho- SP phenotype of steady-state adult HSCs is acquired late in development and may then be altered to varying degrees after stimulation by different treatments in vivo or in vitro.
Initially it was thought that both the Rhodamine-123 and Hoechst 33342 efflux activities of primitive hematopoietic cells were due to their high expression of P-glycoprotein because both of these efflux activities were inhibited by verapamil.25 Subsequent studies,26 in addition to those reported here, have shown that the Rhodamine-123 efflux activity of adult HSCs is, indeed, mediated by P-glycoprotein. However, their Hoechst 33342 efflux activity is mediated by a different ABC transporter, Abcg2.26,28 In healthy adult bone marrow, both dye efflux activities are mirrored by the presence of transcripts for these 2 transporters in HSC-enriched populations and their absence in fractions containing later cell types.26 Future studies with highly purified populations of preadult and activated adult HSCs will be required to determine whether the changes in dye efflux activity these cells exhibit when they are activated are regulated at the mRNA level. Because these changes appear to parallel those described for AA4.1, CD34, CD38, and Mac1 expression on murine HSCs,18,19,22 it is inviting to speculate that all of these are mediated by a common pathway that is regulated in HSCs by a cell cycle checkpoint at the Go/G1 interface.
At first glance, these findings may appear at odds with those of Nadin et al44 who demonstrated the presence of a SP population containing many, and in some cases, all of the clonogenic hematopoietic progenitors in mouse embryos from day 9.5 to day 11.5 after coitus. However, their study did not assess the phenotype of HSCs with long-term in vivo repopulating activity. Cells with long-term in vivo repopulating activity do not appear until 10 to 11 days after coitus and are detectable first in the embryo and shortly thereafter in the yolk sac and fetal liver45 and hence should have been present in the suspensions evaluated. It is also known that a subset of clonogenic hematopoietic progenitor cells in the adult display an SP phenotype.27,46 It is thus possible that during early development, the regulation of Hoechst 33342 efflux ability is not the same in HSCs and progenitor cells able to proliferate in vitro.
The present findings also serve to emphasize important differences between murine and human HSCs in the regulation of properties thought to be characteristic of these cells. For example, CD34 is expressed on murine HSCs until the mice reach adulthood.18 In contrast, in humans, expression of CD34 on at least some HSCs with long-term in vivo repopulating ability persists into adulthood47,48 but is also reversible.49 Here, we have shown that most of the murine fetal liver HSCs do not have an SP phenotype, which is directly opposite to the situation in human fetal liver, in which all of the HSCs assessed by their ability to repopulate NOD/SCID mice were found to have an SP phenotype.40 Whether HSCs from adult human tissues also have an SP phenotype has been difficult to establish because of their low frequency.50,51 The Rhodamine-123 efflux activity of fetal human HSCs has not yet been defined. However, Smeets et al52 did report that growth factor activation of human CD34+ bone marrow cells increased the proportion of Rho+ cells consistent with a decrease in Rhodamine-123 efflux ability following human HSC activation. Interestingly, Smeets et al52 also reported a similar finding when Rho- blasts from patients with acute myeloid leukemia were activated. Taken together, their findings and the present results predict that the activities of both P-glycoprotein and Abcg2 in primitive human hematopoietic cells will be subject to variation according to their cell cycle as well as their differentiated status, with important implications for future therapies that involve the use of agents that can be effluxed by either of these transporters.
Supported by grants from the Stem Cell Network, the National Cancer Institute of Canada (NCIC) with funds obtained from the Terry Fox Run, and from the National Institutes of Health (NHLBI P01-55435). N.U. received fellowship support from Kirin Brewery, Inc, and the Canadian Institutes of Health Research. B.D. was supported by studentships from the National Science and Engineering Research Council of Canada, the University of British Columbia, the Stem Cell Network, and the NCIC. K.L. was a recipient of a summer studentship from the British Columbia Cancer Foundation. F.L. was a recipient of a summer studentship from the Canadian Liver Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, February 26, 2004; DOI 10.1182/blood-2003-11-3989.
We thank the staff of the flow cytometry facility for assistance in cell sorting, the staff of the animal facility for breeding and care of mice, Rozmina Premji for help in preparing the manuscript, and P. Lansdorp, Genetics Institute, Immunex, and StemCell for valuable gifts of reagents.