Abstract
Hematopoietic stem cells (HSCs), with their dual ability for self-renewal and multilineage differentiation, constitute an essential component of hematopoietic transplantations. Human fetal liver (FL) represents a promising alternative HSC source, and we previously reported simple culture conditions allowing long-term expansion of FL hematopoietic progenitors. In the present study, we used the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse xenotransplantation assay to confirm that human FL is rich in NOD/SCID-repopulating cells (SRCs) and to show that these culture conditions repeatedly maintained short- and long-term SRCs from various FL samples for at least 28 days. Quantitative limited dilution analysis in NOD/SCID mice demonstrated for the first time that a 10- to over a 100-fold net expansion of FL SRCs could be achieved after 28 days of culture. The efficiency of this culture system may lead to an increase in the use of FL as a source of HSCs for transplantation in adult patients, as previously demonstrated with umbilical cord blood under different culture conditions. (Blood. 2004;103:1166-1170)
Introduction
There is a growing clinical need for large numbers of human hematopoietic stem cells (HSCs) for transplantation or gene therapy.1,2 In cancer patients in whom the probability of tumor cell contamination in either leukapheresis or bone marrow (BM) is high, the use of allogeneic cells is preferable. Accumulated evidence suggests that fetal liver (FL) or umbilical cord blood (CB) or both represent alternative and possibly more “universal” sources of “early” HSCs and possess a better proliferative potential and also a preimmune status that may be important in mismatched transplantation situations.3-7 However, both sources are compromised by the relatively small number of cells available. Therefore, the future clinical potential of FL and CB would be strongly enhanced if methods allowing a reliable HSC expansion, perhaps to a degree as little as 20- to 100-fold, without a loss of their engraftment ability, could be developed.8 Ex vivo expansion of CB and of adult nonobese diabetic/severe combined immunodeficiency (NOD/SCID)-repopulating cells (SRCs; Wang et al9 ) has been demonstrated (eg, Piacibello et al10 and Gammaitoni et al11 ), but successful expansion of FL SRCs has not been reported so far.
We recently published a simple and reproducible stroma-free liquid culture system allowing long-term (> 6 months) expansion of human hematopoietic cells contained within previously frozen crude FL cell suspensions. In these cultures, CD34+ cells, primitive colony-forming progenitors, and cells possessing a putative stem cell phenotype were not only present, they also underwent a continuous amplification.12 However, the maintenance and expansion of functional repopulating HSCs was not tested. This is critical given the dissociation between stem cell phenotype and in vivo repopulating function observed in hematopoietic cultures.13-15 Here, we used the NOD/SCID mouse xenotransplantation assay to quantify FL SRCs and demonstrated that our culture conditions allowed a 10- to over 100-fold net SRC expansion following 28 days of culture.
Study design
Cryopreserved human FL crude cellular suspensions were prepared from aborted fetuses (gestational weeks 12-17) as described, with the approval of the ethical committee of the Lausanne University Medical Faculty.12,16 Total nucleated FL hematopoietic cells were expanded in RPMI 1640 supplemented with 8% human AB plasma, Flt-3 ligand (50 ng/mL), interleukin 6 (10 ng/mL), megakaryocyte growth and development factor (MGDF; 10 ng/mL), and stem cell factor (SCF; 50 ng/mL). Cultures were fed and maintained as described,12 except that cells were grown in T25 flasks instead of 24-well plates. For NOD/SCID-repopulating assays, 6- to 8-week old NOD/LtSz-scid/scid (NOD/SCID) mice were sublethally irradiated (375 cGy using a 137Cs source), and cells to be tested (at the doses indicated, in about 600 μL RPMI) were injected into the lateral tail vein 4 to 24 hours later. Mice were killed after 6, 8, or 12 weeks, the BM harvested from femora, and human engraftment analyzed by flow cytometry using an antihuman CD45 antibody. In most cases, multilineage engraftment was confirmed using a combination of antibodies as described.17 For limited dilution analysis (LDA), a mouse was considered positive (engrafted) when low percentages of engraftment (< 0.5% CD45+ cells) could be unequivocally confirmed by Southern blot hybridization.9,17,18 Data from LDA experiments were analyzed by applying Poisson statistics to the single-hit model, and the SRC frequency in each cell source was calculated using the L-Calc software (StemCell Technologies, Vancouver, BC, Canada).
Results and discussion
Human HSCs possessing in vivo repopulating capability can be functionally tested in the SRC assay.9 We first determined whether FL SRCs were maintained after 4 weeks in culture, when a large number of FL hematopoietic cells, potentially sufficient for transplantation into adult patients, could be harvested from a limited supply of unexpanded FL cells. Four different FL specimens were cultured for 28 days, and cohorts of equal to or more than 5 mice per sample were given transplants with 20 × 106 expanded viable total nucleated cells (TNCs). Both short- and long-term SRCs19 were maintained in culture because all mice analyzed contained large numbers of CD45+ human hematopoietic cells in their BM, whether tested about 6 or 12 weeks after transplantation. A representative example of these analyses is shown in Table 1. Overall, engraftment levels were higher at 12 weeks than at 6 weeks, demonstrating that expanded FL samples contained SRCs capable of long-term repopulating potential. Additionally, the BM of all these mice contained cells committed to both lymphoid (positive for CD19, CD20) and myeloid lineages (CD14, CD15, CD33), as well as a significant fraction of putative primitive CD34+/CD38- hematopoietic progenitors. Interestingly, no significant differences in engraftment levels (total or lineage-specific) were observed if expansion was performed in 2 steps, that is, freezing after 7 days of culture followed by 3 more weeks of culture, or if expanded cells were stored frozen before injection (data not shown). These results demonstrate that our culture conditions repeatedly maintained short- and long-term FL SRCs capable of in vivo lymphomyeloid differentiation for at least 28 days.
To compare the maintenance and expansion of the FL repopulating stem cell compartment with that of other, more widely used HSC sources, we attempted to establish long-term hematopoietic cultures from CB or adult sources, under the same conditions we used for FL. With adult BM or mobilized peripheral blood, we never succeeded in obtaining long-term cultures that would last more than a few weeks, and after 28 days the number of cells generated was not sufficient for transplantation into NOD/SCID mice. With CB, we were only able to establish long-term cultures with about half of the samples tested, and the maintenance/expansion of SRCs in NOD/SCID mice was not tested. However, these experiments cannot be compared with published reports of successful CB or adult SRC expansion (eg, Piacibello et al10 and Gammaitoni et al11 ) because culture conditions were different; we used RPMI instead of Iscove modified Dulbecco medium (IMDM) as culture medium and expanded TNCs instead of purified/enriched CD34+ cells. We previously showed that optimal long-term expansion of FL hematopoietic cells depended not only on an adequate cytokine cocktail, but was also significantly influenced by the protein source (human plasma versus fetal calf serum), its concentration, and the culture medium.12 Thus, our culture conditions may be optimal for expanding FL TNCs but not for CB TNCs or CD34+ cells, nor for adult hematopoietic cells.
Using quantitative LDA in NOD/SCID mice,9 we then estimated the frequency of SRCs in 3 different unexpanded FL cell suspensions. Mice were given cell doses ranging from approximately 6 × 104 to 20 × 106 TNCs, and engraftment was analyzed 8 weeks later. A representative example is shown in Figure 1A. In this FL specimen, the average SRC frequency was 1 SRC in about 700 000 TNCs, or about 1 in 50 000 hematopoietic CD45+ cells because this FL sample contained about 7% CD45+ cells (Table 2). Although not directly comparable, this frequency is much higher than those estimated in CB, adult BM, or mobilized peripheral blood cells,9-11 and it correlates well with the high SRC frequency reported in human fetal blood.20 As could be expected, larger cell doses usually resulted in higher engraftment. However, the BM of mice given transplants with low cell numbers occasionally contained surprisingly high percentages of human CD45+ cells (Figure 1A). This could result from the large variability inherent to the NOD/SCID transplantation system21 or from the functional heterogeneity of the human HSC compartment, as demonstrated both in vitro and in vivo.22,23 Alternatively, this could also be stochastic, these mice having randomly received more SRCs. Data from 2 additional FL samples are summarized in Table 2, confirming that human FL is rich in SRCs.24,25
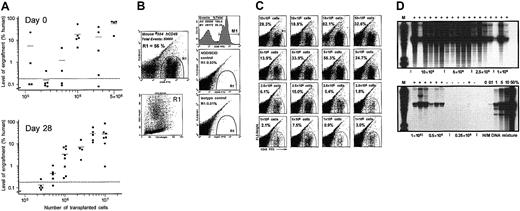
Engraftment of ex vivo expanded FL hematopoietic cells in NOD/SCID mice. (A) Summary of the levels of human cell engraftment in the BM of NOD/SCID mice given transplants with various doses of FL no. 841 cells, unexpanded (upper panel) or expanded for 28 days (lower panel). The percentage of human cells in the individual recipients was determined 8 weeks after transplantation by flow cytometry analysis of BM cells expressing human CD45. The dotted lines separate engrafted from nonengrafted animals, as confirmed by Southern blot analysis. More detailed analyses of the engraftment of expanded cells from this FL specimen are shown below. (B) Flow cytometry analysis of a highly engrafted representative mouse given transplants with 5 × 106 expanded FL no. 841 cells. About 56% of the cells in this mouse BM stained positively for the human pan-leukocyte marker CD45 (upper left panel, gate R1, and upper right panel, region M1); these human cells, once backgated to a forward/side scatter (FSC/SSC) plot, mostly fell within a typical viable (but heterogeneous) cell population (lower left panel). The middle and bottom right panels show a CD45 staining of a control (not transplanted) NOD/SCID mouse BM, and an isotypic control (mouse IgG1) of a positively engrafted NOD/SCID mouse, respectively. Both panels show only a few rare events falling in gate R1. (C) Representative examples of positive engraftment of NOD/SCID mice given transplants with 1 to 10 × 106 expanded FL no. 841 cells as indicated. Human cells appear in gate R1, and their percentages (of total BM cells) are shown in the respective panels. (D) Southern blot analysis of human cell engraftment in the BM of individual NOD/SCID mice given transplants with the indicated doses (0.25 to 10 × 106 TNCs) of FL no. 841 cells following 28 days of expansion. EcoRI-digested DNA (4 μg) was loaded in each lane, and the blot was hybridized to a human chromosome 17-specific α-satellite probe as described.9 Positive engraftment is indicated by plus signs above the respective lanes. Human/mouse (H/M) DNA controls are given as percentage of human DNA. M lanes were loaded with DNA molecular weight markers.
Engraftment of ex vivo expanded FL hematopoietic cells in NOD/SCID mice. (A) Summary of the levels of human cell engraftment in the BM of NOD/SCID mice given transplants with various doses of FL no. 841 cells, unexpanded (upper panel) or expanded for 28 days (lower panel). The percentage of human cells in the individual recipients was determined 8 weeks after transplantation by flow cytometry analysis of BM cells expressing human CD45. The dotted lines separate engrafted from nonengrafted animals, as confirmed by Southern blot analysis. More detailed analyses of the engraftment of expanded cells from this FL specimen are shown below. (B) Flow cytometry analysis of a highly engrafted representative mouse given transplants with 5 × 106 expanded FL no. 841 cells. About 56% of the cells in this mouse BM stained positively for the human pan-leukocyte marker CD45 (upper left panel, gate R1, and upper right panel, region M1); these human cells, once backgated to a forward/side scatter (FSC/SSC) plot, mostly fell within a typical viable (but heterogeneous) cell population (lower left panel). The middle and bottom right panels show a CD45 staining of a control (not transplanted) NOD/SCID mouse BM, and an isotypic control (mouse IgG1) of a positively engrafted NOD/SCID mouse, respectively. Both panels show only a few rare events falling in gate R1. (C) Representative examples of positive engraftment of NOD/SCID mice given transplants with 1 to 10 × 106 expanded FL no. 841 cells as indicated. Human cells appear in gate R1, and their percentages (of total BM cells) are shown in the respective panels. (D) Southern blot analysis of human cell engraftment in the BM of individual NOD/SCID mice given transplants with the indicated doses (0.25 to 10 × 106 TNCs) of FL no. 841 cells following 28 days of expansion. EcoRI-digested DNA (4 μg) was loaded in each lane, and the blot was hybridized to a human chromosome 17-specific α-satellite probe as described.9 Positive engraftment is indicated by plus signs above the respective lanes. Human/mouse (H/M) DNA controls are given as percentage of human DNA. M lanes were loaded with DNA molecular weight markers.
To analyze whether a net SRC expansion could be achieved, 3 FL specimens were expanded for 28 days and then analyzed in LDA experiments. For example, all mice transplanted with equal to or more than 1 × 106 expanded TNCs from FL no. 841 (Figure 1B-C), and the majority of mice injected with 0.5 × 106 cells (Figure 1D), were positively engrafted. Conversely, most mice given transplants with 0.25 × 106 expanded cells were not engrafted (Figure 1D). The calculated SRC frequency was found to be 1 in about 365 000 TNCs or CD45+ cells, because the vast majority (> 99%) of expanded cells in culture at 28 days were CD45+.12 The total cellular expansion (fold increase in viable TNCs) for this sample after 28 days of culture was about 24-fold, and the initial percentage of CD45+ cells was about 7.0% (Table 2). Assuming that only CD45+ cells in unexpanded FL suspensions contributed to the massive hematopoietic cell amplification observed in culture, this represents a CD45+ cell expansion of about 343-fold; therefore, the net SRC expansion following 28 days of culture for this FL sample could be estimated to be about 46-fold. Data from 2 additional expanded FL samples are summarized in Table 2. Although large differences in SRC frequencies were observed among FL specimens, both before and following culture, a 10-fold or more net SRC amplification was achieved in all cases.
The NOD/SCID transplantation system is extremely useful to test the repopulating potential of human hematopoietic cell populations, whether freshly isolated or after in vitro culture. However, it suffers from an important variability, both among but also within similar samples,21 making it difficult to carefully assess the potential differences in engraftment of expanded versus unexpanded cells. Despite this variability, overall no significant difference between the reconstitution capability of expanded versus unexpanded FL cells was observed in the present study. In other words, transplantation of equivalent numbers of expanded versus unexpanded FL SRCs into NOD/SCID mice usually resulted in similar engraftment levels.
Together with our previous study,12 the data presented here demonstrate for the first time that under appropriate conditions, human FL repopulating HSCs could be expanded, allowing, in a period of about 4 weeks of culture, the in vitro generation of a sufficient number of FL hematopoietic stem, progenitor, and mature cells to be used for transplantation in adults. Thus, as for CB, which is increasingly used for transplantation (for reviews, see Devine et al8 and Grewal et al26 ), FL may become a good alternative clinical source of HSCs. Our studies not only support the hypothesis that FL SRCs are uniquely different from CB and adult sources, in terms of both their in vitro expansion and in vivo repopulating capabilities,4,24,25,27,28 they also go well beyond, as the first to unambiguously demonstrate that FL HSCs can be truly expanded in simple culture conditions, while retaining their lymphomyeloid repopulating potential. Thus, FL SRCs may represent one of the best targets for HSC expansion and genetic manipulation. Although culture periods longer than 1 month may not be clinically useful, preliminary experiments suggest that FL SRCs could be maintained beyond 28 days in our culture conditions, at least until 6 weeks of culture. For example, when 20 million hematopoietic cells from FL no. 873 expanded for 42 days were tested, all NOD/SCID mice were positively engrafted 7 weeks later (n = 5), with an average engraftment of about 2.2% human CD45+ cells in the recipient BM. Whether the peak of SRC expansion is reached at 4 weeks or whether SRC expansion could still be improved at later time points awaits further LDA analyses. Because a dissociation between stem cell phenotype and repopulating function is usually observed in hematopoietic cultures,13-15 the future challenge will be to characterize expanded human FL HSCs, and to compare them to their unexpanded counterpart or to HSCs from different sources.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-06-1815.
Supported by grants from La Ligue Suisse contre le Cancer (no. 228-11-1995), La Recherche Suisse contre le Cancer (KFS 170-9-1995), and La Ligue Vaudoise contre le Cancer.
P.R. and S.K. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Prof P. Hohlfeld (Department of Gynecology and Obstetrics, University Hospital, Lausanne, Switzerland) and Prof F. Forestier (Laboratoires Marcel Mérieux, Lyon, France) for providing the FL specimens used in this study, Amgen for their generous supply of MGDF and SCF, Prof J. E. Dick for his comments on the manuscript, and Prof R. Mertelsmann for continuous support.