Abstract
The binding of frizzled (Fzd) receptors by their Wnt ligands results in the inhibition of β-catenin degradation and subsequent transcription of β-catenin/LEF–inducible genes. The β-catenin pathway is known to be involved in development, tumorigenesis, and stem cell self-renewal. In humans, the FZD9 gene lies in the region of chromosome 7q11.23 deleted in the neurodevelopmental disorder, Williams-Beuren syndrome (WBS). Fzd9-/- mice show no obvious features of WBS, but reveal a role for Fzd9 in lymphoid development and maturation. Fzd9-/- mice show pronounced splenomegaly, thymic atrophy, and lymphadenopathy with age, with accumulation of plasma cells in lymph nodes. There is a depletion of developing B cells in the bone marrow (BM), particularly in the pre-B stage where immunoglobulin heavy chains are expressed and the cells are undergoing clonal expansion prior to light chain rearrangement. The pre-B defect is partially intrinsic to the hematopoietic system; as in competitive BM reconstitution studies, Fzd9-/--derived BM exhibits defective B-cell development when implanted into a wild-type host. Mature B cells are present in normal numbers in lymph node and spleen. These findings suggest a role for Fzd9 signaling in lymphoid development, particularly at points where B cells undergo self-renewal prior to further differentiation.
Introduction
The frizzled (Fzd) family of 7-transmembrane cell surface receptors,1 paired with low-density lipoprotein–like receptors 5 and 6, mediates signaling upon binding to a family of secreted Wnt ligands. The signals are mediated by at least 3 pathways.2,3 The first, known as the canonical Wnt pathway, results in the inhibition of a constitutively active complex of axin, adenomatous polyposis coli (APC) protein, and glycogen synthase kinase-3 β (GSK-3β) that phosphorylates β-catenin and targets it for ubiquitination and degradation. As a result, cellular levels of β-catenin increase, allowing it to translocate to the nucleus where, in concert with the lymphoid enhancer-binding/T-cell factor (LEF/TCF) family of transcription factors, it induces transcription of target genes. A second signaling mechanism, the Wnt/Ca++ pathway, involves activation of phospholipase C, increasing Ca++ levels, and subsequent activation of protein kinase C, Ca++-calmodulin–dependent protein kinase II, and calcineurin. This pathway may result in nuclear accumulation of transcription factors of the nuclear factor of activated T cell (NF-AT) family and transcription of target genes. Finally, the planar cell polarity (PCP) pathway is activated by Wnt binding via small GTPases, such as RhoA and Rac, and activation of the c-Jun amino-terminal kinase (JNK), and results in effects on cell polarity and movement.
Wnt/Fzd-mediated signals, particularly those mediated via the canonical pathway involving stabilization of β-catenin, are critical in development,4 lymphoid maturation,5,6 tumorigenesis,7 and maintenance of stem cell populations in the skin,8 gut,9 and blood.10 In the stem/progenitor cell compartments, and perhaps in neoplasia, β-catenin appears to preserve the critical stem cell ability to undergo self-renewing divisions as opposed to producing 2 more terminally differentiated daughter cells following mitosis.10,11
In lymphoid cells, the role of the Wnt/Fzd pathway is just beginning to be elucidated.12 Much of the relevant information comes from studies of mice with mutations in 2 downstream transcription factor partners of β-catenin that are expressed in lymphoid cells, LEF-1 and TCF-1. Absence of TCF-1 results in premature deterioration of T-cell development (accelerated thymic atrophy) but normal immune function and peripheral T-cell numbers.13 Within the thymus, there is no block in development as seen in CD3/T-cell–receptor components14 or recombinase activating gene (RAG)15 knock-out mice, but rather a deficiency in populations that normally show high proliferation rates such as the CD4-CD8- (double negative or DN) fractions of immature thymocytes that are CD44+CD25+ (DN2) and CD44-CD25- (DN4). A complete block of thymocyte maturation at the immature single-positive stage is achieved in TCF/LEF double knock-out animals, however, suggesting that these factors have some redundancy in thymocyte (and other embryonic) development.16 Single LEF-1 knock-out mice are more profoundly abnormal than TCF-1 knock-out mice, with abnormal tooth, hair, mammary gland, and brain development; and they die within 2 weeks of birth.17 Analysis of fetal liver revealed decreased proliferation and increased apoptosis during early stages of B-cell development in these mice.18 The limitations of these “downstream” LEF/TCF knock-out animal models is that LEF/TCF interactions with other transcription factors such as ALY19 or the elimination of their transcriptional repressive activity, when paired with Groucho20,21 or C-terminal binding protein (CtBP),22 may be responsible for the observed phenotypes.
The proximal step of the signaling pathway is represented by the frizzled family of receptors, which has at least 9 members in the mouse.23 The expression patterns and precise ligand relationships with various Wnt proteins in the hematopoietic and immune system are only beginning to be elucidated. Knock-out mice have been created for frizzled 3,1 4,24 5,25 6,26 and as described in the present report, 9. Fzd3 knock-out mice die within 30 minutes of birth and have major abnormalities in central nervous system (CNS) fiber tracts.1 The hematopoietic and immune systems were not extensively analyzed, but previous data had suggested that Fzd3 expression was largely limited to the CNS.23 Absence of Fzd4, another Fzd member largely limited to CNS expression,27 results in progressive dysfunction of central and peripheral nervous systems in the cerebellum and auditory pathway, and in the esophageal musculature.24 Mice lacking Fzd5 die at day 10.75 in utero with abnormalities of the placental vasculature.25 Fzd6-/- mice have abnormal hair orientation, but detailed analyses of other tissues are not described.26
Mouse Fzd9, which is 95% identical to its human homolog at the amino acid level, is expressed in the developing CNS as well as in myotomes.28,29 In the adult mouse, expression is abundant in heart, brain, skeletal muscle, kidney, and testis as determined by Northern blot analysis.29 Our initial interest in cloning and creating a knock-out mouse model for this gene stems from the fact that the FZD9 gene lies within the genetically defined 1.4-megabase region on chromosome 7q11.23 that is deleted in Williams-Beuren syndrome (WBS). WBS is a developmental disorder characterized by elfinlike facies with small or absent teeth, cardiovascular stenoses, connective tissue abnormalities, short stature, transient hypercalcemia, mental retardation, hyperacusis, and unusual cognitive features (eg, advanced language development and auditory memory with poor visual-spatial cognition).30 Numerous other genes have been identified within this deleted region, such as ELN (elastin),31 that have only partially accounted for the complex observed phenotype.32 As Fzd9 is extensively expressed during development, particularly in the CNS, it was a logical knock-out target in an effort to develop a mouse model to dissect the complex phenotypes of WBS. As we describe here, the phenotype of the Fzd9-/- mouse appears to be most significant in the hematopoietic compartment.
Materials and methods
Mouse knock-out strategy
A 129SvJ phage genomic library (Stratagene, La Jolla, CA) was screened with a Fzd9 cDNA probe.29 There were 3 phage clones identified, and the genomic structure and physical map of the Fzd9 locus were characterized by restriction enzyme digestion and Southern blot analysis (data not shown). The complete open reading frame is included in a single exon.
The targeting vector pPNT, containing the neomycin (NEO) and thymidine kinase (TK) selectable marker cassettes,33 was used to subclone fragments flanking the Fzd9 coding exon. An 8-kb NotI 5′ upstream genomic fragment and a 1.6-kb KpnI3′ downstream fragment were inserted into the left and right arm of the NEO cassette, respectively. Homologous recombination with this targeting vector leads to deletion of the complete open reading frame of Fzd9. An external probe at the 3′ end was designed to differentiate the 6-kb wild-type allele and the 2-kb Fzd9 knock-out allele in ScaI-digested genomic DNA by Southern blot analysis. The hybridization probe was generated by polymerase chain reaction (PCR) with the following primers: 5′ GAGGT AGGGA GCTGT TGAAT 3′ and 5′ GGGAC TTCAC TCATA AATGA T 3′. PCR conditions were 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, for 30 cycles.
Recombinant embryonic stem (ES) cells from the 129/SvEv strain were transfected with the Fzd9 targeting construct to generate chimeric mice in the University of California–San Francisco Transgenic Facility as described.34 Of 200 ES cell clones screened, 6 were targeted correctly. There were 2 clones injected into C57BL/6 blastocysts; and those were implanted into pseudopregnant females. Chimeric males were obtained from both lines and gave germ-line transmission. Chimeras were bred with 129/SvEvTac females to give rise to the Fzd9-/- colony on the 129 background. A PCR protocol for genotyping of tail DNA was developed (available upon request).
Flow cytometry
Antimouse CD4 (GK1.5), CD8 (53-6.7), Ly5.1 (ALI-4AZ), Ly5.2 (A20.1.7), B220 (6B2), Gr-1, Mac-1, c-Kit (CD117, clones 3C11 and 2B8), Sca-1, and CD3 (KT31.1) were purified and conjugated in the Weissman laboratory. Antimouse CD8-α (53-6.7) APC, CD69 (H1.2F3) fluorescein isothiocyanate (FITC), 6C3 (BP1)–phycoerythrin (PE), immunoglobulin D (IgD) FITC, IgM biotin and PE, and CD138 PE were purchased from eBioscience (San Diego, CA). Antimouse CD25 FITC was purchased from Caltag Laboratories (Burlingame, CA). Antimouse CD24 (30F1) FITC and CD43-biotin were purchased from BD-Pharmingen (San Diego, CA). Streptavidin–Cascade Blue was purchased from Molecular Probes (Eugene, OR). Cells were harvested from lymphoid organs or bone marrow into single cell suspensions and stained with indicated antibodies for 30 minutes at 4°C in Hanks buffered saline solution (HBSS) + 2% fetal calf serum (FCS). Analysis was done at the Stanford Core fluorescence-activated cell sorter (FACS) facility using an LSR or FacsVantage (BD-Pharmingen), or Moflo (Cytomation, Fort Collins, CO) multilaser cytometer. The data were analyzed on FloJo (Treestar, Ashland, OR) software.
Bone marrow chimeras
Bone marrow from 6- to 12-week-old donor mice (Fzd9-/- or WT129/SvEv, Ly5.1+, or BA [C57/Bl/6-derived strain, Ly5.2+]) was obtained from femurs and tibias and enriched for CD117+ cells by magnetic-activated cell sorter (MACS) bead positive selection. CD117-enriched cells were stained for lineage (CD4, CD8, CD3, B220, Ter119, Gr1, and Mac1), CD117, and Sca-1 and were sorted by flow cytometry to isolate hematopoietic stem cells (HSCs; long term, short term, and multipotent progenitors). Equal numbers of HSCs were injected intravenously in the retro-orbital sinus into lethally irradiated (950 rads in split dose) heterozygous (HZ) recipients (C57Bl/6 derived, Ly5.1+/5.2+). Recipients were analyzed at various later time points and donor cells identified by Ly5.1 or 5.2 positivity using FACS analysis.
RT-PCR
Bone marrow B-cell subsets A to F35 were flow sorted to 98% purity using the markers B220, CD43, CD24, 6C3, IgM, and IgD and placed in Trizol (Invitrogen, Frederick, MD) with further RNA purification using Qiaquick columns (Qiagen, Valencia, CA). Cell equivalents of RNA (1000-2000) were subjected to one-step reverse transcription (RT)–PCR using an Invitrogen kit with gene-specific 3′ antisense cDNA primer for murine Fzd9 (5′-GCCATTTTTCGGTAGCACAGG-3′), with or without the addition of reverse transcriptase, followed by amplification using Fzd9 sense primer (5′-CGGAGTCTTTTCCATCCTTTACAC-3′) for 35 cycles with a DNA Engine (MJ Research, Waltham, MA) thermal cycler (58°C annealing). Beta-actin message was amplified as an internal control for RNA quality and levels using the following primers: sense, 5′-GACGGCCAAGTCATCACTATTG-3′ and antisense, 5′-AGGAAGGCTGGAAAAGAGCC-3′, for 30 cycles.
Results
Creation of Fzd9 null mice
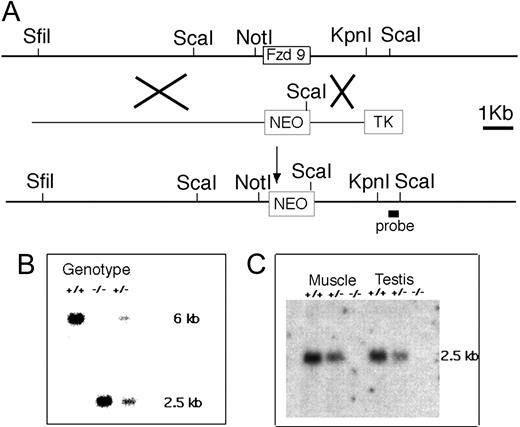
We first characterized the genomic organization of the Fzd9 locus. Like the human FZD9 homolog, the coding sequence of Fzd9 is located in a single exon. We generated a restriction map spanning 30 kb genomic DNA flanking the exon and constructed a targeting construct for homologous recombination to replace the entire exon by the Neo cassette. An external probe at the 3′ end was designed to detect the 6-kb ScaI fragment from wild type and the 2-kb fragment from the expected mutant allele (Figure 1A). Southern analysis of DNA from offspring of targeted ES cells reveals a 2-kb ScaI band in both heterozygous and homozygous mutants (Figure 1B). The absence of Fzd9 transcript was verified by Northern analysis of total RNA isolated from skeletal muscle and testis. When hybridized with a Fzd9 cDNA probe, no transcripts were detected in Fzd9-/- tissues, and a roughly 50% reduction in intensity was observed in heterozygotes (Figure 1C). These results confirm that we have created a Fzd9 null mutation.
Knock out of Fzd9 gene. (A) The restriction map of the locus surrounding the single exon of the Fzd9 gene was used to generate the targeting vector for homologous recombination. The Xs indicate sites of recombination in ES cells that are selected by neomycin (positive selection) and G418 (negative selection against TK). In the mutant chromosome, the Fzd9 gene is replaced by the neomycin-resistance (NEO) gene that introduces a new ScaI site. (B) Southern blot of ScaI-digested DNA from a wild-type (+/+), Fzd9 knock-out (-/-), and heterozygous (+/-) animal hybridized with the 3′ probe indicated in panel A. The 6-kb ScaI fragment is derived from the wild-type allele and the 2.5-kb fragment, from the mutant (KO) allele. (C) Northern blot of muscle and testis RNA from wild-type (+/+), Fzd9 knock-out (-/-), and heterozygous (+/-) animals hybridized with an Fzd9 cDNA probe and a β-actin probe as control for RNA loading. The Fzd9-specific 2.4-kb transcript is absent in homozygous mutant animals and reduced in the heterozygote.
Knock out of Fzd9 gene. (A) The restriction map of the locus surrounding the single exon of the Fzd9 gene was used to generate the targeting vector for homologous recombination. The Xs indicate sites of recombination in ES cells that are selected by neomycin (positive selection) and G418 (negative selection against TK). In the mutant chromosome, the Fzd9 gene is replaced by the neomycin-resistance (NEO) gene that introduces a new ScaI site. (B) Southern blot of ScaI-digested DNA from a wild-type (+/+), Fzd9 knock-out (-/-), and heterozygous (+/-) animal hybridized with the 3′ probe indicated in panel A. The 6-kb ScaI fragment is derived from the wild-type allele and the 2.5-kb fragment, from the mutant (KO) allele. (C) Northern blot of muscle and testis RNA from wild-type (+/+), Fzd9 knock-out (-/-), and heterozygous (+/-) animals hybridized with an Fzd9 cDNA probe and a β-actin probe as control for RNA loading. The Fzd9-specific 2.4-kb transcript is absent in homozygous mutant animals and reduced in the heterozygote.
Fzd9-/- mice have moderately reduced lifespan, splenomegaly, and accelerated thymic atrophy
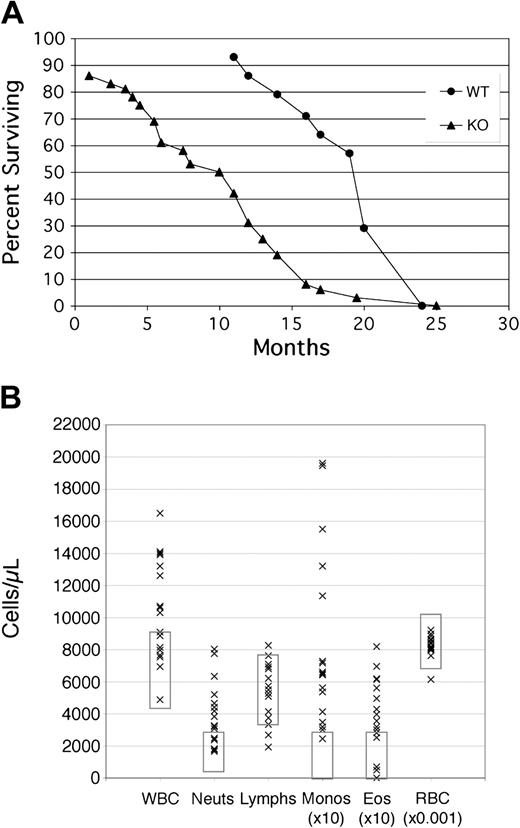
When Fzd9+/- heterozygotes were interbred to generate litters with +/+, +/-, and -/- genotypes, we observed mendelian ratios of 1:2:1, as expected from a nonlethal allele. Heterozygous and homozygous Fzd9 knock-out mice appear healthy, develop normally, and are fertile. Detailed pathologic and histologic examinations of 2- and 4-month-old animals revealed no consistent differences between the wild-type (WT) and -/- littermates. Routine blood chemistry panels were normal. On average, however, Fzd9-/- mice have reduced survival when compared with wild-type 129SvEv mice (Figure 2A). There is no obvious cause for their early demise, as autopsies of such mice do not reveal infection or neoplasia, or any histologic abnormalities of major organs, apart from those described in secondary lymphoid organs in this report. A single 7-month-old mouse was noted to have a CD8+ T-cell lymphoproliferative disorder as assessed by histologic and flow cytometric analysis.
Frizzled 9 KO mice show decreased survival and elevated WBC. (A) Survival plots of WT129 SvEv mice (• n = 14) versus Fzd9 KO (▴ n = 36). (B) Peripheral blood counts in cells/μL for 17 representative Fzd9-/- mice ranging in age from 6 to 14 months with normal value ranges denoted by rectangles for each cell type. WBC indicates total white blood cell count; Neuts, neutrophils; Monos, monocytes (× 10 for display purposes); Eos, eosinophils (× 10); and RBC, red blood cells (× 10-3).
Frizzled 9 KO mice show decreased survival and elevated WBC. (A) Survival plots of WT129 SvEv mice (• n = 14) versus Fzd9 KO (▴ n = 36). (B) Peripheral blood counts in cells/μL for 17 representative Fzd9-/- mice ranging in age from 6 to 14 months with normal value ranges denoted by rectangles for each cell type. WBC indicates total white blood cell count; Neuts, neutrophils; Monos, monocytes (× 10 for display purposes); Eos, eosinophils (× 10); and RBC, red blood cells (× 10-3).
The mice exhibit no developmental/morphologic characteristics similar to those noted in Williams-Beuren syndrome (WBS). In particular, no differences between mutant and wt littermates were observed in gross neurologic function and reflexes, behavioral screening tests,36,37 a balancing-on-the-runway test for motor and vestibular function, the elevated platform test for anxiety, and the forced swim immobility test for depression/pressure coping.38 Because hyperacusis (hypersensitivity to sound) is reported in 85% of WBS patients,39 Fzd9-/- mice and control littermates were also tested in auditory brainstem response (ABR) experiments with no significant differences observed (collaboration with Ryan Stern and Dr Anil Lalwani, University of California, San Francisco).
In the hematologic system, older Fzd-/- mice have a high frequency of peripheral blood leukocytosis (Figure 2B) due to mild neutrophilia and a more marked monocytosis and eosinophilia. Examination of Wright-stained peripheral smears reveals normal morphology of leukocytes, red blood cells, and platelets. Histologic examination of the bone marrow does not reveal abnormalities of hematopoietic or bony constituents.
Spleens in knock-out mice are enlarged to approximately twice normal size by 6 months of age (knock-out [KO] mean weight 253 ± 99 mg vs WT 147 ± 52 mg, P = .02 by Student t test, n = 8). Histologically, there is an expansion of the red pulp with increased extramedullary hematopoiesis and deposition of increased amounts of hemosiderin suggestive of accelerated red blood cell (or precursor) destruction (Figure 3A-D). The white pulp is intact, with some expansion of the marginal zones. Flow cytometric analysis shows normal percentages of CD4+ and CD8+ T cells and B220+ B cells (data not shown).
Histologic analysis of spleen and lymph node. Spleens from WT (A [× 100] and C [× 400]) and KO (B [× 100] and D [× 400]) show expanded red pulp in the KO animals with increased extramedullary hematopoiesis (note megakaryocytes) and hemosiderin deposition. Lymph nodes from WT (E) and KO (F-H) show expansion of pale staining plasma cells expanding the medullary cords (F) or in patches in the interfollicular zones (G) of the nodes at × 100. At higher power (× 1000, H), histologically normal plasma cells are identifiable by their coarse chromatin, perinuclear Hof, and cytoplasmic immunoglobulin inclusions (Russel bodies). Tissue was paraffin embedded and sections were stained with hematoxylin and eosin. Photomicrographs were taken on an Olympus BXS1 microscope (Olympus, Melville, NY) with UPlanFl 10 ×/0.30 and 40 ×/0.75 objectives using an RT Slider camera and SPOT software (Diagnostic Instruments, Sterling Heights, MI).
Histologic analysis of spleen and lymph node. Spleens from WT (A [× 100] and C [× 400]) and KO (B [× 100] and D [× 400]) show expanded red pulp in the KO animals with increased extramedullary hematopoiesis (note megakaryocytes) and hemosiderin deposition. Lymph nodes from WT (E) and KO (F-H) show expansion of pale staining plasma cells expanding the medullary cords (F) or in patches in the interfollicular zones (G) of the nodes at × 100. At higher power (× 1000, H), histologically normal plasma cells are identifiable by their coarse chromatin, perinuclear Hof, and cytoplasmic immunoglobulin inclusions (Russel bodies). Tissue was paraffin embedded and sections were stained with hematoxylin and eosin. Photomicrographs were taken on an Olympus BXS1 microscope (Olympus, Melville, NY) with UPlanFl 10 ×/0.30 and 40 ×/0.75 objectives using an RT Slider camera and SPOT software (Diagnostic Instruments, Sterling Heights, MI).
Thymi are normal in very young mice, but show accelerated atrophy with age (KO mean weight 21.4 ± 14.8 mg vs WT 54.6 ± 14.9 mg, P = .0008 by Student t test, n = 8, age 6 months). Flow cytometric analysis reveals normal percentages of CD4, CD8 double-negative (DN), double-positive, and CD4 or CD8 single-positive subsets. In addition, the DN population is normal with respect to DN I to IV subsets by CD44/CD25 staining (data not shown). Histologic analysis shows a decrease in lymphoid cells in older thymi, but normal cortical/medullary architecture (data not shown).
TUNEL (terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling) staining to detect apoptotic cells on spleen, lymph node, and thymus on mice of various ages did not indicate any significant difference in the fraction of apoptotic cells between wild-type 129/SvEv mice and age-matched Fzd9-/- mice (data not shown).
In vitro, mitogen-induced proliferation assays revealed normal T-cell responses to conconavalin-A compared with WT animals at all ages (data not shown). Although B-cell proliferation to lipopolysaccharide and anti-IgM tended to be decreased in Fzd9-/- animals, this finding was not reliably reproducible and may have been attributable to the presence of increased myeloid or other cell types in spleens from these animals.
Fzd9-/- mice have lymphadenopathy secondary to accumulation of plasma cells
Lymph nodes of Fzd9-/- mice frequently showed enlargement, even at 3 months of age, which became increasingly prevalent (> 50%) in older mice. Histologic evaluation showed variably distorted nodal architecture with an accumulation of morphologically normal plasma cells. These cells tended to fill and distend medullary cords, be present in large patches in the paracortical zones, and occasionally efface the entire node (Figure 3E-H). Their identity was confirmed by flow cytometry showing an increase in CD138+, B220-, surface Ig- cells. Staining for kappa and lambda light chain showed the normal kappa predominance in 6 of 6 mice examined (data not shown). Plasma cell numbers in the bone marrow were not increased, as determined by histology and flow cytometric analysis (data not shown). Despite the increased lymph node plasma cells, enzyme-linked immunosorbent assay (ELISA) analysis for IgM and IgG showed equivalent overall immunoglobulin levels in WT and KO mouse sera (data not shown).
Fzd9-/- mice have a paucity of early B-cell precursors
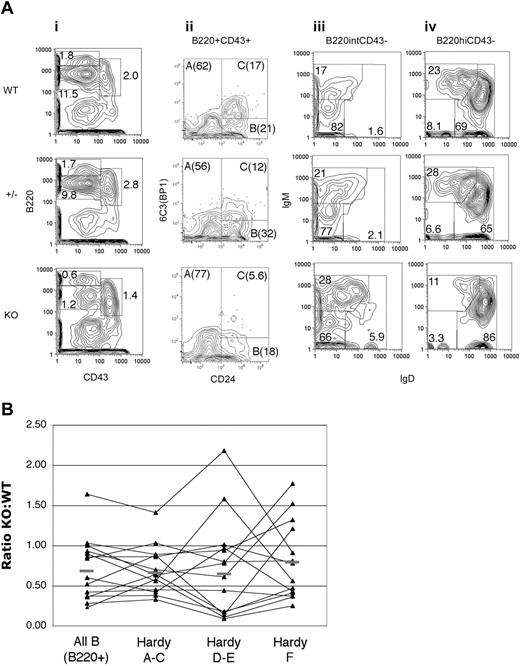
Despite the relatively normal numbers and phenotype of mature B cells in the periphery, flow cytometric analysis of the bone marrow revealed a decrease in the total B-cell number (KO B220+ total cells 66 ± 29% [mean ± SD] of that in WT mice, n = 11, P < .001 by Student t test). The total number of bone marrow (BM) cells was not significantly different between WT and KO mice. The decrease in B cells is primarily due to a reduction in CD43+B220+ (or IgM-B220+) pro/pre-B-cell populations (KO B220+CD43+ fraction 61 ± 20% of WT level, P < .001) with a particular decrease in B220intCD43intCD24+ cells in the populations of C′ and D as described by Hardy et al35 (Figure 4). In this scheme, fraction A (B220+CD43+CD24-6C3-) consists of cells committed to B-cell as well as other lineages, and fraction B (B220+CD43+CD24int6C3-) cells are beginning to rearrange immunoglobulin (Ig) heavy chain; while fraction C (B220+CD43+CD24int/hi6C3+) cells that have successfully rearranged a functional Ig heavy chain pair it with nonpolymorphic surrogate light chains, down-regulate the recombination machinery, and greatly expand prior to attempting light chain rearrangement. Fraction D (B220+CD43-IgM-IgD-CD24+) cells stop dividing, re-express RAG genes, and attempt light chain rearrangement. If light chain expression is successful, cells express surface IgM (fraction E), leave the bone marrow, and express both IgM and IgD after maturation in the periphery, recirculating to the bone marrow as fraction F cells. In the Fzd9-/- mouse, the decline in fraction C/D cells is not accompanied by a compensatory increase in absolute numbers of more immature B cells (ie, Hardy A and B) as would be expected if there were a block in maturation with accumulation of precursor cells. Rather, it appears that the expansion at the C to D transition is diminished, resulting in the presence of mature cells, but in decreased numbers. These changes are variable, but were found occasionally to be present in the youngest (3 months) and more consistently in the oldest (12 months) mice analyzed.
Decreased bone marrow B cells in Fzd9 KO mice. (A) Bone marrow from 4-month-old KO (bottom row), WT (top row), and Fzd9 heterozygous littermate (middle row) mice was stained for CD43 and B220 (i), and B220+CD43+ (ii, Hardy fractions A-C) were examined for 6C3 (BP1) and CD24 expression. B220intCD43lo/- cells (iii, predominantly Hardy fraction D-E) and B220hiCD43-/lo (iv, Hardy fraction E-F) were examined for surface IgM and IgD by flow cytometry. Percentages of total BM cells (i) or of gated cells (ii-iv) are shown. Total absolute numbers of bone marrow cells in Fzd9-/- mice varied from equal to 50% of WT levels but were not statistically significantly reduced (data not shown). In this representative experiment, bilateral tibia and femur yielded 2.15 × 107 cells in KO, 1.88 × 107 cells in heterozygotes, and 2.54 × 107 in WT mice. The CD43+B220+ gate contains essentially no sIgM+ or sIgD+ cells (data not shown). Data shown are representative of 14 pairs of KO and WT control mice studied (for CD43, B220, and sIg staining; CD24/6C3 staining was technically more variable with analyzable data in 7 pairs). (B) Individual Fzd9-/- animals are plotted as a function of the relative numbers of each B-cell subset in comparison with WT mice analyzed at the same time, with a value of 1.0 indicating equal numbers and values less than 1 indicating decreased cells in KO animals. Subsets are, from left to right, all B cells (B220+), all Hardy A to C cells (B220+CD43+), Hardy D to E (B220intCD43-), and Hardy F (B220hiCD43-); n = 14; bars denote the mean value for each subset. Individual Hardy subsets are not presented separately due to variability in the antibody combinations used in individual experiments such that data on each subset are unavailable for all data points. B220 and CD43 staining, however, was always performed, and the B220+CD43+, B220intCD43-, and B220hiCD43- subsets consistently contained a vast majority of Hardy A to C, D to E, and F cells, respectively, as determined by staining for CD25, CD24, 6C3, IgM, and IgD (panel A and data not shown).
Decreased bone marrow B cells in Fzd9 KO mice. (A) Bone marrow from 4-month-old KO (bottom row), WT (top row), and Fzd9 heterozygous littermate (middle row) mice was stained for CD43 and B220 (i), and B220+CD43+ (ii, Hardy fractions A-C) were examined for 6C3 (BP1) and CD24 expression. B220intCD43lo/- cells (iii, predominantly Hardy fraction D-E) and B220hiCD43-/lo (iv, Hardy fraction E-F) were examined for surface IgM and IgD by flow cytometry. Percentages of total BM cells (i) or of gated cells (ii-iv) are shown. Total absolute numbers of bone marrow cells in Fzd9-/- mice varied from equal to 50% of WT levels but were not statistically significantly reduced (data not shown). In this representative experiment, bilateral tibia and femur yielded 2.15 × 107 cells in KO, 1.88 × 107 cells in heterozygotes, and 2.54 × 107 in WT mice. The CD43+B220+ gate contains essentially no sIgM+ or sIgD+ cells (data not shown). Data shown are representative of 14 pairs of KO and WT control mice studied (for CD43, B220, and sIg staining; CD24/6C3 staining was technically more variable with analyzable data in 7 pairs). (B) Individual Fzd9-/- animals are plotted as a function of the relative numbers of each B-cell subset in comparison with WT mice analyzed at the same time, with a value of 1.0 indicating equal numbers and values less than 1 indicating decreased cells in KO animals. Subsets are, from left to right, all B cells (B220+), all Hardy A to C cells (B220+CD43+), Hardy D to E (B220intCD43-), and Hardy F (B220hiCD43-); n = 14; bars denote the mean value for each subset. Individual Hardy subsets are not presented separately due to variability in the antibody combinations used in individual experiments such that data on each subset are unavailable for all data points. B220 and CD43 staining, however, was always performed, and the B220+CD43+, B220intCD43-, and B220hiCD43- subsets consistently contained a vast majority of Hardy A to C, D to E, and F cells, respectively, as determined by staining for CD25, CD24, 6C3, IgM, and IgD (panel A and data not shown).
Bone marrow chimeras reveal intrinsic and nonintrinsic B-cell abnormalities
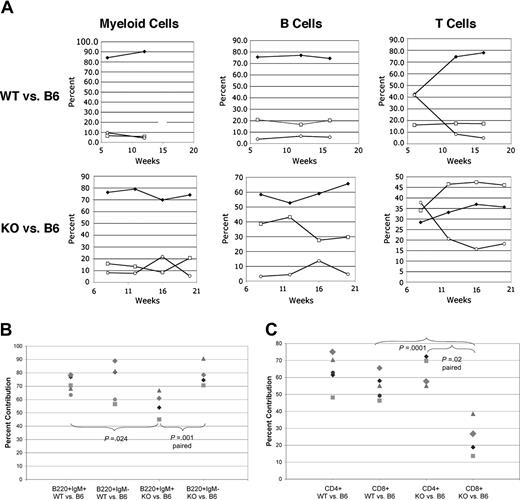
To determine whether the alterations in B-cell populations were intrinsic to hematopoietic cells, bone marrow chimeric animals were created by competitive reconstitution of lethally irradiated C57Bl/6 Ly5.1/Ly5.2 recipients with equal numbers of flow sorted hematopoietic stem cells (HSCs), as defined by lin-kit+sca+ phenotype, from Fzd9-/- or WT 129SvEv (Ly5.1+) and WT C57Bl/6 (Ly5.2+) donors. Reconstitution of B, T, and myeloid lineages was monitored in the peripheral blood over a period of weeks by staining for Ly5.1 and Ly5.2 (CD45.2 and CD45.1, respectively) and lineage markers B220 (B cells), CD3 or CD5 (T cells), and Gr-1 (neutrophils). C57Bl/6 donors and recipients were used to allow for congenic loci at the CD45 (Ly5) locus. Despite the use of equal numbers of HSCs from 129 WT or 129 Fzd9-/- and C57Bl6 WT marrow, the 129 derived (both KO and WT) cells showed a pronounced competitive advantage in the granulocytic lineage (Figure 5A) in peripheral blood. This advantage was identical for B cells and T cells derived from WT 129. Fzd9-/--derived B cells were significantly delayed in attaining this dominance, and interestingly, the KO-derived T cells failed to do so after 16 weeks (Figure 5A). In another experiment carried out for nearly 12 months, KO-derived T cells became predominant in 7 of 8 recipients, but always to a lesser extent than the B cells in the same mouse. The competitive advantage of 129SvEv-derived cells likely stems from the fact that the lin-kit+sca+ population in 129 mice contains more long-term repopulating HSCs and fewer short-term HSCs compared with C57Bl/6 mice (Amy Wagers, H.C.K.K., and E.A.R., unpublished data, December 12, 2003). Although crossing the Fzd9-/- genotype into the C57Bl/6 background is in progress, at this time comparison of 129WT and 129KO outcomes provides valid internal controls.
Bone marrow reconstitution reveals intrinsic and nonintrinsic B-cell abnormalities. Equal numbers of flow sorted Lin-Sca1+Kit+ hematopoietic stem cells (2000-5000 per recipient) from 6- to 10-week-old C57Bl/6-derived, Ly5.2+ WT mice and Fzd9-/- or +/+ mice on the 129SvEv background (Ly5.1+) were used to reconstitute lethally irradiated 6- to 10-week-old Ly5.1+5.2+ recipients (C57Bl/6 derived). Analysis of peripheral blood (A) by gating on Ly5.2+ (WT C57Bl/6, □), Ly5.1+ WT (top row,  ) or KO 129 (bottom row,
) or KO 129 (bottom row,  ), or Ly5.1+Ly5.2+ (host, ○) cells and staining for B220 (B cells), CD5 (T cells), or Gr-1+ (myeloid cells). Percent contribution from each donor source is shown for each cell population over time (weeks). (B) Analysis of bone marrow cells 20 weeks after transplantation with percent mature (B220+IgM+) and immature (B220+IgM-) B-cell progenitors derived from WT versus KO 129 donors shown. (C) Analysis of lymph node CD4+ or CD8+ T cells (CD5+B220-) as in panel B. P values by paired or unpaired Student t test are as indicated by brackets. Matching symbols across different columns represent analyses from the same animal.
), or Ly5.1+Ly5.2+ (host, ○) cells and staining for B220 (B cells), CD5 (T cells), or Gr-1+ (myeloid cells). Percent contribution from each donor source is shown for each cell population over time (weeks). (B) Analysis of bone marrow cells 20 weeks after transplantation with percent mature (B220+IgM+) and immature (B220+IgM-) B-cell progenitors derived from WT versus KO 129 donors shown. (C) Analysis of lymph node CD4+ or CD8+ T cells (CD5+B220-) as in panel B. P values by paired or unpaired Student t test are as indicated by brackets. Matching symbols across different columns represent analyses from the same animal.
Bone marrow reconstitution reveals intrinsic and nonintrinsic B-cell abnormalities. Equal numbers of flow sorted Lin-Sca1+Kit+ hematopoietic stem cells (2000-5000 per recipient) from 6- to 10-week-old C57Bl/6-derived, Ly5.2+ WT mice and Fzd9-/- or +/+ mice on the 129SvEv background (Ly5.1+) were used to reconstitute lethally irradiated 6- to 10-week-old Ly5.1+5.2+ recipients (C57Bl/6 derived). Analysis of peripheral blood (A) by gating on Ly5.2+ (WT C57Bl/6, □), Ly5.1+ WT (top row,  ) or KO 129 (bottom row,
) or KO 129 (bottom row,  ), or Ly5.1+Ly5.2+ (host, ○) cells and staining for B220 (B cells), CD5 (T cells), or Gr-1+ (myeloid cells). Percent contribution from each donor source is shown for each cell population over time (weeks). (B) Analysis of bone marrow cells 20 weeks after transplantation with percent mature (B220+IgM+) and immature (B220+IgM-) B-cell progenitors derived from WT versus KO 129 donors shown. (C) Analysis of lymph node CD4+ or CD8+ T cells (CD5+B220-) as in panel B. P values by paired or unpaired Student t test are as indicated by brackets. Matching symbols across different columns represent analyses from the same animal.
), or Ly5.1+Ly5.2+ (host, ○) cells and staining for B220 (B cells), CD5 (T cells), or Gr-1+ (myeloid cells). Percent contribution from each donor source is shown for each cell population over time (weeks). (B) Analysis of bone marrow cells 20 weeks after transplantation with percent mature (B220+IgM+) and immature (B220+IgM-) B-cell progenitors derived from WT versus KO 129 donors shown. (C) Analysis of lymph node CD4+ or CD8+ T cells (CD5+B220-) as in panel B. P values by paired or unpaired Student t test are as indicated by brackets. Matching symbols across different columns represent analyses from the same animal.
One difficulty in directly comparing C57Bl/6- and 129-derived bone marrow B-cell precursor frequencies stems from our finding that the Hardy A to D fractions stain differently, particularly with regard to B220 and CD43 levels, between the 2 strains (E.A.R., unpublished observation, July 31, 2002). If, however, we gate on pro- and pre-B cells (B220+sIgM-, Hardy fractions A-D) and more mature (B220+IgM+, Hardy fraction E-F) marrow B-cell subsets and assess the contribution of 129-derived versus C57Bl/6-derived cells, we can directly compare the performance of WT versus Fzd9-/- cells from the same strain. As shown in Figure 5B, WT129-derived cells account for 71 ± 6% of B220+sIgM+ and an identical 74 ± 14% of B220+sIgM- pro/pre-B cells in the marrow of recipients. In contrast, Fzd9-/--derived cells account for 57 ± 9% of B220+sIgM+ (P = .024 vs WT129) and 78 ± 9% of B220+sIgM- cells. Within the same recipient of Fzd9-/- HSCs, paired analysis shows a highly significant decrease in IgM+ versus IgM- marrow B-cell contribution by KO cells (P = .001). The decrease in more mature B cells mirrors that seen in peripheral blood. The normal contribution of pro/pre-B cells was surprising in light of our findings in the intact KO animals and suggests that this abnormality may not be B-cell intrinsic.
We noted an increase in lymph node plasma cells in only one of these recipients when analyzed at 15 to 16 weeks after transplantation. Interestingly, in comparison with the predominance of B cells from the Fzd9-/- donor in that mouse, the CD138+B220- plasma cells were predominantly WT derived (data not shown), hinting that the accumulation of plasma cells in the lymph nodes may not be intrinsic to the plasma cells themselves.
The bone marrow chimeras also revealed an interesting effect of absent Fzd9 on T cells. As shown in Figure 5A, T cells derived from KO HSCs do not compete as well as those from WT syngeneic HSCs after competitive reconstitution. Further analysis revealed a specific paucity of KO-derived CD8+ T cells in peripheral lymphoid organs (Figure 6C) in the animals reconstituted with KO versus B6 HSCs that was absent in 129 WT versus B6 HSCs. Although the 129-derived cells likely differed from the host at minor transplantation antigen loci more than B6-derived donor cells, the 129 WT and KO cells were both pure 129/SvEv derived and therefore should behave identically in an immunologic sense. Thus, differences in CD4 and CD8 T-cell numbers likely relate to the absence of Fzd9 in T-cell progenitors or an antigen-presenting cell population in the thymus or periphery. Further analysis will be required to understand why a paucity of CD8+ T cells is not noted in the intact KO animals.
Expression of Fzd9 mRNA in B-cell progenitor subsets. (Left) B-cell progenitors subsets were sorted by surface phenotype from WT adult C57/Bl6 and 129SvEv mice, and 1000 to 2000 cell equivalents were subjected to RT-PCR amplification of Fzd9 and beta-actin, as described in “Materials and methods.” Lanes are labeled for the Hardy B-cell subset tested, A to F, from bone marrow. The presence or absence of reverse transcriptase (RT) in the initial cDNA reactions is indicated by + or -, respectively. Mature B cells (CD19+ or B220+) from spleen and peritoneum were flow sorted for CD5 and surface IgD expression prior to RT-PCR (right panel). Splenic plasma cells (PCs) were isolated as CD138+B220lo/negIgD- cells. Data are representative of more than 4 experiments for each cell type.
Expression of Fzd9 mRNA in B-cell progenitor subsets. (Left) B-cell progenitors subsets were sorted by surface phenotype from WT adult C57/Bl6 and 129SvEv mice, and 1000 to 2000 cell equivalents were subjected to RT-PCR amplification of Fzd9 and beta-actin, as described in “Materials and methods.” Lanes are labeled for the Hardy B-cell subset tested, A to F, from bone marrow. The presence or absence of reverse transcriptase (RT) in the initial cDNA reactions is indicated by + or -, respectively. Mature B cells (CD19+ or B220+) from spleen and peritoneum were flow sorted for CD5 and surface IgD expression prior to RT-PCR (right panel). Splenic plasma cells (PCs) were isolated as CD138+B220lo/negIgD- cells. Data are representative of more than 4 experiments for each cell type.
Fzd9 expression pattern confirms a potential role in B-cell development
Cells from various mature and progenitor populations from wild-type mice were flow sorted to more than 98% purity, and one-step RT-PCR was performed on cDNA from 1000 cell equivalents. As shown in Figure 6, Fzd9 mRNA is expressed at some level throughout B-cell development, but appears to be increased in Hardy fractions B to C, consistent with our findings of a decrease in population C and downstream cells in KO mice. In addition, it is weakly expressed, at least at the RNA level, in mature CD5-IgD+ B cells and in plasma cells.
Discussion
We have described the phenotype of mice lacking expression of Fzd9 as being limited largely to the hematopoietic, and more particularly, the lymphoid compartment. Despite the presence of this gene, which is known to be expressed throughout neural development, within the 1.4-Mb deletion responsible for WBS, we do not find any evidence of WBS-associated phenotypes in mice lacking this gene in the heterozygous (analogous to WBS) or even the homozygous state. Recently, other candidate genes within the WBS deletion region on chromosome 7q11.23, including GTF2I encoding the transcription factor TFII (also known as SPIN or BAP135),40 CYLN2/CLIP-115,41 and LIMK1 (LIM kinase 1),42 have been implicated in contributing to the neurologic abnormalities in WBS. Individuals with WBS, who have heterozygous loss of the FZD9 gene, do not exhibit any overt immunologic or hematopoietic deficits.
The immunologic abnormalities detected in the Fzd9-/- mice are of interest because so little is known about the role of the Wnt/frizzled signaling system and downstream effectors such as β-catenin in lymphoid development and function. Here we show that the absence of an upstream component of this pathway results in abnormalities of B-cell and perhaps T-cell development, as well as plasma cell homeostasis. The B-cell developmental defect consists not in a block in development, but rather, we believe, in a rush through developmental checkpoints. Normally, the system takes advantage of properly created Ig heavy chains (HCs) by expanding cells that express them followed by each daughter cell expressing that HC independently attempting light chain rearrangement. This developmental system preserves antigen-binding diversity without potentially wasting one of the approximately 10% of cells that create an HC gene rearrangement that produces a protein capable of pairing with light chain. In Fzd9-/- mice, normal to modestly reduced numbers of Hardy A and B cells are present, but the expansion of the C population and the resulting numbers of D subset cells appear to be reduced. These data mirror the defects noted in T-cell development when TCF-113 or β-catenin6 are absent. They also complement the findings of Reya et al that B-cell numbers are reduced without a block in differentiation in LEF-1 knock-out mice and that LEF-1 expression was up-regulated at the Hardy B and C stages in normal B-cell development,18 similar to the developmental stage at which Fzd9 appears to be acting in our system. These findings are particularly interesting given the role of the β-catenin pathway in stem cell biology. In numerous systems, including HSCs, β-catenin signals appear to provide direction to proliferate in a “self-renewing” fashion without further differentiation.8-10 Developing B cells mirror that stem cell function after successful Ig HC rearrangement when they halt differentiation (eg, light chain rearrangement) and self-renew. Thus, we speculate that the Wnt/Fzd pathway, using Fzd9 and unidentified Wnt ligands, may activate a similar program in committed progenitors such as pre-B cells. The fact that the abnormalities in Fzd9-/- mice become more manifest with age, including increased hematopoiesis, thymic atrophy, and changes in B-cell development, suggests the possibility that the absence of Fzd9 may also result in changes in stromal niches in marrow and thymus over time that make them less able to support lymphoid development. Ongoing BM transplantation studies will directly address this question.
The plasma cell accumulations in the lymph nodes are of interest in light of recent data suggesting that malignant counterparts of plasma cells up-regulate inhibitors of the Wnt/Fzd pathway such as DKK143 and frizzled related protein44,45 and may respond to Wnt signals.46 Consistent with the finding that Wnt5a may act as a tumor suppressor in B cells,47 transformation of plasma cells appears to correlate with an attempt by the malignant clone to inhibit Wnt/Fzd signaling by soluble mediators. Similarly, loss of the Fzd9 receptor seems to result in accumulation of plasma cells in an atypical location in the Fzd9-/- mice. Although the infiltrates sometimes appear to disrupt normal architecture and occur in sheet-like patterns typical of malignant plasmacytoma, other evidence of malignancy such as Ig clonality or infiltration of other organs is thus far lacking. We are currently assessing changes in chemokine receptor expression that may affect bone marrow homing and the effects of Wnt on plasma cell proliferation and apoptosis in order to further dissect this abnormality.
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-06-2334.
Supported by the Howard Hughes Medical Institute (Y.-K.W., U.F.), National Institutes of Health (NIH) grant R01 HD39921 (H.C.K.K., U.F.), NIH postdoctoral training grant T32 GM08748 (H.C.K.K.), Howard Hughes Postdoctoral Fellowship for Physicians, and NIH grant KO8-90450-1 (E.A.R.). E.A.R. and H.C.K.K. contributed equally to the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Roger Pederson for help in generating the mutant mice; Donna Bouley, Corinne Davis, and Heinz Furthmayr for analysis of pathologic specimens; and Amy Wagers for assistance in sorting of HSCs.

![Figure 3. Histologic analysis of spleen and lymph node. Spleens from WT (A [× 100] and C [× 400]) and KO (B [× 100] and D [× 400]) show expanded red pulp in the KO animals with increased extramedullary hematopoiesis (note megakaryocytes) and hemosiderin deposition. Lymph nodes from WT (E) and KO (F-H) show expansion of pale staining plasma cells expanding the medullary cords (F) or in patches in the interfollicular zones (G) of the nodes at × 100. At higher power (× 1000, H), histologically normal plasma cells are identifiable by their coarse chromatin, perinuclear Hof, and cytoplasmic immunoglobulin inclusions (Russel bodies). Tissue was paraffin embedded and sections were stained with hematoxylin and eosin. Photomicrographs were taken on an Olympus BXS1 microscope (Olympus, Melville, NY) with UPlanFl 10 ×/0.30 and 40 ×/0.75 objectives using an RT Slider camera and SPOT software (Diagnostic Instruments, Sterling Heights, MI).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/6/10.1182_blood-2004-06-2334/6/m_zh80060575550003.jpeg?Expires=1769170851&Signature=XBCaIGwuyUSt9~CkeQ1UaQif2wLNWGxNKkIRDXuHuwKWpqsHc8qaTzRxOLjSuJsTeDIy~zisplYfoCzGAGhqXrs9wwKfJnh3AF9lNg-IxYtrVMz5LDzS2px9Uub~UikNgu~sZTwaKFIKBjmqZHasQ2j0BNSBjhM350iA8c5xc~Gy9CSoUrHD9rse1JExgiVIaqB0ifCm2KW1PfFy3AaOlSsrKSiBE30QaCdi-k0OVOcJDOdw~1uVP5UoXnB5LoM5HR~ZXMBHz21OWqqGok5tdPih2i2NsLS7IeK6FkP7IWflIZD63RsKR9jYc6UBPSB7uM3txW1pjwzX0tzQv6uOVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)