Abstract
Most bone marrow (BM) malignancies develop in association with an angiogenic phenotype and increased numbers of endothelial cells. The molecular mechanisms involved in the modulation and recruitment of BM endothelium are largely unknown and may provide novel therapeutic targets for neoplastic diseases. We observed that angiogenic stimulation of BM endothelial cells activates mTOR and engages its downstream pathways 4E-BP1 and S6K1, which are inhibited by the mTOR-specific blockers rapamycin and CCI-779. Both mTOR blockers significantly inhibit growth factor- and leukemia-induced proliferation of BM endothelium by inducing G0/G1 cell-cycle arrest. This effect is associated with down-regulation of cyclin D1 and cdk2 phosphorylation, and up-regulation of the cdk inhibitors p27kip1 and p21cip1. Under conditions that reproduce the biomechanical fluidic environment of the BM, CCI-779 is equally effective in inhibiting BM endothelial-cell proliferation. Finally, simultaneous blockade of mTOR and NF-κB pathways synergize to significantly inhibit or abrogate the proliferative responses of BM endothelial cells to mitogenic stimuli. This study identifies mTOR as an important pathway for the proangiogenic stimulation of BM endothelium. Modulation of this pathway may serve as a valid therapeutic intervention in BM malignancies evolving in association with an angiogenic phenotype.
Introduction
The formation of new vessels in normal and pathologic conditions requires the activation of quiescent endothelial cells (ECs), a process triggered by proangiogenic factors that are generally elevated in cancer patients.1,2 Bone marrow endothelial cells (BM-ECs) and their precursors play important roles in the neovascularization associated with malignancies developing in the bone marrow (BM)3-5 and seem to be implicated in cancers evolving in other tissues.6,7 Of interest, ECs purified from tumor-infiltrated BM exhibit an activated, angiogenic phenotype.8 Studies in a leukemia model showed that specific targeting of the EC markedly inhibited tumor development, suggesting a critical role for the BM endothelium in leukemia biology.9 More recently, it has been shown that endothelial microdomains in the BM play important roles in leukemia-cell homing and maintenance.10 Taken together, these studies suggest that BM endothelium plays an important role in the development and maintenance of tumors evolving in the BM, and that strategies targeting BM-ECs may provide a therapeutic advantage.
A significant number of reports have evaluated the molecular events and pathways involved in EC responses to extrinsic stimuli. PI3K/Akt, MAPK/ERK, Jak/STAT, and small GTPases, as well as NF-κB pathways,11-13 seem to play significant roles in the endothelial-cell responses to mitogenic stimuli and in the switch to an angiogenic phenotype. How these multiple, distinct signals are integrated within ECs needs further evaluation. Moreover, most signaling studies were performed in umbilical vein endothelial cells (HUVECs), and little is known on the signaling machinery activated in other ECs, particularly in BM-ECs. This is relevant, as ECs from different tissues/organs, and even within the same tissue, possess variable phenotypic, metabolic, and functional properties, including their responsiveness to extrinsic stimuli.14,15 For example, BM-ECs differ from HUVECs in their ability to support adhesion of hematopoietic progenitors16 and cancer cells.17 Also, ECs from different tissue beds respond differentially to biomechanical stimuli,18 which translates into activation of distinctive transcriptional profiles and results in different functional phenotypes.19
The mammalian target of rapamycin (mTOR) pathway coordinates cell growth and cell-cycle progression by integrating growth factor signals and nutrient availability,20,21 modulating the protein translation machinery through inhibition of 4E-BP1 and activation of S6K1 and its substrate S6 ribosomal protein (S6RibP). The mechanism(s) involved in growth factor stimulation of mTOR pathway are still a matter of controversy. However, recent studies indicate that mTOR nutrient sensing ability crosstalks with PI3K-regulated growth factor signaling. In this model, PI3K lays both upstream and in parallel to mTOR and shares common downstream targets.20,21 The mTOR-specific blocker rapamycin exerts antitumor activity by disrupting tumor angiogenesis.22,23 Also, mTOR blockade by rapamycin induces PKB/Akt degradation,24 whereas VEGF-induced activation of PI3K/Akt/mTOR stabilizes PKB/Akt, promoting EC survival.
Here, we show that activation of BM endothelium by proangiogenic factors triggers mTOR, activating its downstream pathways 4E-BP1 and S6K1. Specific blockade of mTOR by rapamycin or CCI-779 abrogates the cytokine- or leukemia-promoted stimulation of mTOR pathway in BM-ECs and inhibits their proliferation by modulating critical mediators of cell-cycle progression. The inhibitory effects of CCI-779 on BM-ECs are also observed under flow conditions that recapitulate the biochemical environment of the BM. Finally, simultaneous blockade of mTOR and NF-κB pathways results in the synergistic inhibition of BM endothelium.
Materials and methods
Endothelial cells and leukemia specimens
BM-ECs were isolated from the BM of healthy donors by purification with CD105 microbeads (Miltenyi, Auburn, CA), followed by culture in EGM2-MV medium (Cambrex, Walkersville, MD; containing FGF-2, VEGF, EGF, IGF-1, and 5% fetal bovine serum [FBS]) at 37°C, 5% CO2. Phenotypic and functional studies confirmed the endothelial lineage of these cells. Cells were used at passages 2 to 5. Diagnostic plasmas were collected from children with acute lymphoblastic leukemia (ALL). Appropriate informed consent was obtained in accordance with the Declaration of Helsinki and the guidelines of the Dana-Farber Cancer Institute's Institutional Review Board. Leukemia plasmas used were “angiogenic,” as demonstrated by their ability to stimulate BM-EC proliferation and morphogenesis and to promote angiogenesis in a Matrigel-plug assay (J. Pedro Veiga, L.F.C., and A.A.S., manuscript submitted, “Leukemia-stimulated bone marrow endothelium promotes leukemia cell survival,” October 2005). HUVECs and bovine aortic ECs (BAECs) were obtained from Cambrex and Cell Systems (Kirkland, WA), respectively.
BM-EC proliferation
Proliferation was measured using an MTS-based assay (CellTiter Proliferation Assay; Promega, Madison, WI). BM-ECs (1.5-2 × 103 cells/well) were cultured in 96-well plates in EGM2-MV medium. For measuring BM-EC responses to tumor stimuli, cells were seeded in EBM2 basal medium (Cambrex) prior to the addition of leukemia plasma (10% vol/vol). In experiments with inhibitors, rapamycin (Calbiochem, La Jolla, CA), CCI-779 (kindly provided by Wyeth, Madison, NJ), SC514 (Calbiochem), or the appropriate controls were used at the concentrations indicated. Cultures were carried out for 48 to 72 hours, after which MTS was added and plates were incubated at 37°C for 3 hours. Absorbance was measured at 490 nm in a SpectraMax spectrophotometer (Molecular Devices, Sunnyvale, CA). All conditions were tested in triplicate.
Cell-cycle analysis and apoptosis
Cell-cycle analysis was performed using bivariate distributions of bromodeoxyuridine (BrdU; BD Pharmingen, San Diego, CA) incorporation versus DNA content measured by propidium iodide (PI; Molecular Probes, Eugene, OR). BM-ECs stimulated with EGM2-MV or leukemia plasma were treated with rapamycin (10 and 100 ng/mL) or CCI-779 (10 nM) for 12 hours prior to addition of BrdU (10 μM). Cells were harvested at 6, 12, or 24 hours; fixed in 70% ethanol; and preserved at 4°C. BrdU-labeled cells were treated with 2 N HCl and stained with anti-BrdU Ab (BD Pharmingen) followed by goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated antibody (Southern Biotechnology, Birmingham, AL) and 50 μg/mL PI. Samples were acquired in a Cytomics FC500 flow cytometer (Beckman-Coulter, Fullerton, CA). Apoptosis was determined using standard Annexin V/PI staining (BD Pharmingen), according to the manufacturer's specifications.
Immunoblotting
BM-EC lysates were prepared from cells cultured under the experimental conditions and time periods indicated. Cells were washed with cold PBS and lysed on the plate with 500 μL lysis buffer (50 mM Tris [pH 7.6-8.0], 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1% Protease inhibitor cocktail [Sigma, St Louis, MO], 1% Phosphatase inhibitor cocktail-1 (Sigma), 1% Phosphatase inhibitor cocktail-2 σ, and 1% PMSF). Following centrifugation at 16 000g, lysates were concentrated using Microcon10 columns (Millipore, Billerica, MA). For analysis of cell-cycle regulators, cells were trypsinized and washed twice in cold phosphate-buffered saline (PBS), and cell pellets were lysed using the same lysis buffer. Equal amounts of protein were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 4%-15% gradient gels; BioRad, Melville, NY) and blotted onto nitrocellulose membranes (BioRad). Membranes were probed with Abs for the following: Phospho-4E-BP1(Ser65), Phospho-eIF-4G(Ser1108), Phospho-p70S6kinase(Thr389), Phospho-S6RibP(Ser235/236), S6RibP, Phospho-Akt/PKB(Ser473), Phospho-IκBα(Ser32), cyclin D1 (DCS6), Phospho-cdk2(Thr160) (Cell Signaling, Beverly, MA); p21/Cip1/WAF-1 (Upstate Biotechnology, Lake Placid, NY); p27/Kip1 (BD Biosciences, San Jose CA); and Actin (Sigma-Aldrich, St Louis, MO). Immunodetection was performed using horseradish peroxidase-conjugated Abs specific for mouse IgG, rabbit IgG, or goat IgG (Promega). Blots were developed by chemoluminescence using either Western Lightning reagent (Perkin Elmer, Boston, MA), followed by X-ray film exposition, or ChemiGlow reagent (Alpha Innotech, San Leandro, CA) with blot images acquired in a FluorChem 8900 Imaging System (Alpha Innotech).
BM-EC proliferation in perfusion bioreactor
The perfusion bioreactor18 is composed of 12 independent channels of Silastic laboratory tubing (Dow Corning, Midland, MI) forming closed loops, each divided into 3 segments: (1) a 40-cm inlet-length silicone rubber tubing; (2) a 9-cm length test segment seeded with BM-ECs; and (3) a 40-cm outlet-length silicone rubber tubing. A programmable peristaltic Ismatec pump (Cole-Parmer, Vernon Hills, IL) built in across the side wall of an incubator (37°C, 5% CO2) propels the media from 1.5 to 177 mL/min at a maximum pressure of 22 psi. A custom-developed analog circuit designed to generate a 0 to 5 VDC square waveform, with a 60% duty cycle, allows the regulation of the flow profile. An ultrasound flowmeter (Transonic Animal Research, Ithaca, NY) is used to monitor instantaneous and average volumetric flow. Three distinct flow patterns were generated for the experiments: venouslike (steady flow of 2 dyn/cm2 SS), arterial-like (1 Hz pulsatile flow of 18 dyn/cm2 SS), and BM-like flow (steady flow of 0.5dyn/cm2 SS).
Prior to cell seeding, test segments were rinsed with successive 20-minute incubations in 0.2% SDS solution (Fluka, Munich, Germany) and distilled water in an ultrasonic bath. After autoclave sterilization, the Silastic tubes were coated with 100 μg/mL fibronectin (Sigma) in PBS for 2 hours while rotating at 2.33 × 10-6g at 37°C. For EC seeding (BM-ECs, HUVECs, or BAECs), cells were added to the fibronectin precoated tubing at 3500 cell/cm2 and cultured for 24 hours in EGM2 media, while rotating axially at 10 rph. The initial attached cell number was determined 24 hours after seeding using a Beckman-Coulter cell and particle counter (day 0). EC-seeded tubes were either placed within the perfusion system and exposed to a defined flow (venouslike, arterial-like, or BM-like flow) or mounted in the system without flow as a sham control for the duration of the experiment (up to 4 days). Final cell number was measured after trypsinization using a particle counter (Beckman-Coulter, Miami, FL). The results are presented as the ratio between cell number at day 0 and the final cell number at the conclusion of the experiment. The effect of CCI-779 on cell proliferation was studied by adding 100 nM CCI-779 to the perfusate in the bioreactor for the whole duration of the experiment. Independent channels without CCI-779 were carried out as negative controls.
Dose effect and statistical analysis
To determine potential synergism between inhibition of mTOR and NF-κB signaling, dose response curves were defined using the MTS assay and the data analyzed using CalcuSyn software (Biosoft, Ferguson, MO), a widely used tool to quantify the effects of drug combinations and to determine potential synergisms. This algorithm performs multiple drug dose-effect calculations using the median effect methods described by Chou and Talalay25 and calculates combination index values that are indicative of synergy, additivity, or antagonism between 2 agents. Combination indexes of 0.1 to 0.3 units are defined as “strongly synergistic”; 0.3 to 0.7, as “synergistic”; and 0.7 to 0.85, as “moderately synergistic.”
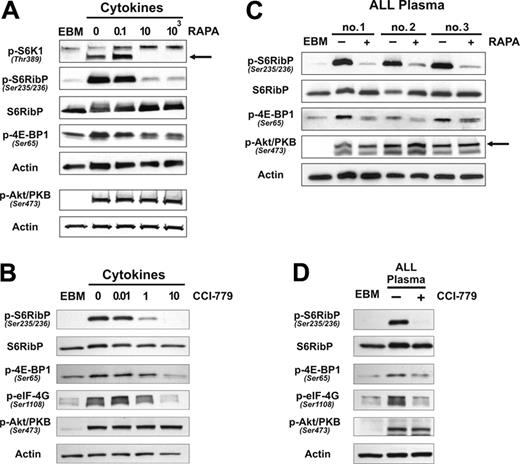
Cytokine and leukemia stimulation of BM-ECs triggers the mTOR pathway. Quiescent BM-ECs were stimulated and analyzed for activation of mTOR downstream substrates (S6RibP, 4E-BP1) and activation of Akt/PKB by Western blot. (A) BM-ECs cultured overnight in EBM + 1% FBS were stimulated for 15 and 30 minutes with a cocktail of proangiogenic cytokines (FGF-2, VEGF, IGF-1, and EGF). Data are representative of 2 BM-EC donors tested and 2 independent experiments. (B) Cells were cultured overnight in EBM2 and stimulated for 15 minutes with 10% vol/vol of plasma from leukemia patients. The arrow indicates the correct band. Three representative cases of 7 patients tested.
Cytokine and leukemia stimulation of BM-ECs triggers the mTOR pathway. Quiescent BM-ECs were stimulated and analyzed for activation of mTOR downstream substrates (S6RibP, 4E-BP1) and activation of Akt/PKB by Western blot. (A) BM-ECs cultured overnight in EBM + 1% FBS were stimulated for 15 and 30 minutes with a cocktail of proangiogenic cytokines (FGF-2, VEGF, IGF-1, and EGF). Data are representative of 2 BM-EC donors tested and 2 independent experiments. (B) Cells were cultured overnight in EBM2 and stimulated for 15 minutes with 10% vol/vol of plasma from leukemia patients. The arrow indicates the correct band. Three representative cases of 7 patients tested.
Statistical significance was determined by the paired t test (2-tailed), using GraphPad Prism software (version 4.0a; GraphPad Software, San Diego, CA). Differences between study groups were considered statistically significant when P values were less than or equal to .05.
Results
Stimulation of BM endothelium activates mTOR pathway and is abrogated by rapamycin or CCI-779
To define putative molecular targets in human BM endothelial cells, we evaluated the role of the critical signaling integrator mTOR20 in BM-EC responses to potent angiogenic stimuli. Quiescent BM-ECs were stimulated with a cocktail of proangiogenic cytokines, which have been shown to be elevated in cancers evolving in the BM and that play significant roles in the biology of such cancers.1,3,26,27 This stimulation triggered activation of mTOR pathway by increasing the phosphorylation of its downstream substrates S6RibP and 4E-BP1 (Figure 1A). In basal conditions, phosphorylation levels of these molecules were either low or absent (Figure 1A-B). We also evaluated whether plasma from leukemia patients, which contains proangiogenic factors, activates mTOR signaling in BM-ECs. We have shown previously that leukemia plasma, but not plasma from healthy donors, stimulates BM-EC proliferation and promotes de novo angiogenesis in vivo (J. Pedro Veiga, L.F.C., and A.A.S., manuscript submitted). We observed that stimulation of BM-ECs with leukemia plasma also resulted in marked phosphorylation of both S6RibP and 4E-BP1 (Figure 1B), an effect seen in all cases tested (n = 7). Since Akt/PKB has been identified as a critical mediator of mTOR activation,20,21 we evaluated whether these stimuli also engaged the PI3K/Akt pathway in BM-ECs. As shown in Figure 1A-B, proangiogenic stimuli triggered activation of Akt/PKB, demonstrated by the markedly increased phosphorylation of residue Ser473, a crucial event for the transduction of Akt/PKB signals.28
Rapamycin/FKBP12 complexes specifically interact with mTOR, blocking the activation of its downstream substrates.29 We observed that rapamycin (10 ng/mL or higher) effectively inhibited the cytokine-mediated phosphorylation of S6K1, S6RibP, and, to a lesser degree, 4E-BP1 in BM-ECs (Figure 2A). No effect was observed in the activation pattern of Akt/PKB (Figure 2A). Similar findings were observed when BM-ECs were treated with the rapamycin analog, CCI-779. Significant inhibition or abrogation of mTOR signaling was attained at 10 nM (Figure 2B). Equivalent experiments were performed on BM-ECs stimulated with leukemia plasma, and both rapamycin (Figure 2C) and CCI-779 (Figure 2D) reduced or abrogated tumor-promoted phosphorylation of mTOR targets. These results indicate that rapamycin or CCI-779 can effectively inhibit or prevent the transduction of mTOR signals stimulated by recombinant cytokines or, more importantly, by tumor stimuli.
mTOR blockade by rapamycin or CCI-779 inhibits BM-EC proliferation through cell-cycle arrest
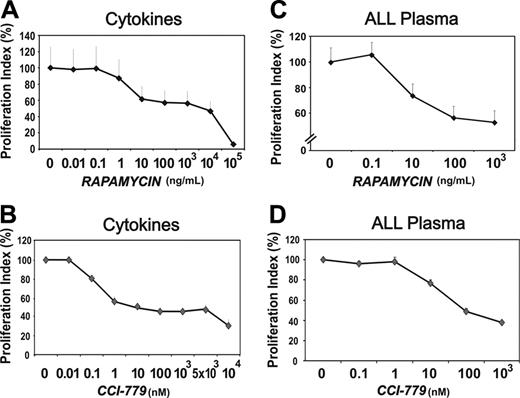
Since rapamycin and CCI-779 inhibited mTOR molecular events triggered by stimulation of BM-ECs, we assessed the impact of mTOR blockade on the proliferation and survival of these cells. At doses that effectively block activation of mTOR substrates, rapamycin (10 ng/mL, P < .02) and CCI-779 (10 nM, P < .005) significantly inhibited BM-EC proliferation induced by recombinant cytokines (Figure 3A-B). This inhibition was dose dependent, with a plateau at 10 to 103 ng/mL for rapamycin and at 10 × 103 to 5 × 103 nM for CCI-779. These effects were not due to solvent toxicity (DMSO; data not shown), which was evident only at doses corresponding to the highest amount of rapamycin tested (105 ng/mL). Of importance, in all cases tested (10 and 8, respectively), rapamycin (100 ng/mL, P < .001) and CCI-779 (100 nM, P < .001) were equally effective in inhibiting the mitogenic activity of leukemia plasmas on BM-ECs (Figure 3C and D, respectively). Of note, the inhibition of plasma-mediated BM-EC proliferation required a 10-fold higher dose of rapamycin (100 ng/mL) or CCI-779 (100 nM) than that induced by recombinant cytokines, indicating that the leukemia milieu may protect BM-ECs from inhibitory agents. A similar effect was observed in tumor B cells, with autologous leukemia plasma promoting their resistance to DNA-damaging agents.30 To evaluate whether mTOR blockade affected the assembly of BM-ECs into capillary-like structures, which recapitulates a morphogenetic event critical in angiogenesis, experiments were performed using rapamycin at 10 to 104 ng/mL. No significant inhibition of cytokine- or leukemia-promoted BM-EC morphogenesis was observed (data not shown), suggesting that mTOR signaling is not essential for polarization and functional differentiation of these cells in Matrigel.
Rapamycin and CCI-779 effectively inhibit cytokine- or leukemia-triggered mTOR signaling. Quiescent BM-ECs were stimulated for 15 minutes with cytokine media/EGM2 (A-B) or 10% vol/vol leukemia plasma (C-D) and analyzed by Western blot for activation of mTOR downstream substrates (S6K1, S6RibP, 4E-BP1, eIF-4G), and activation of Akt/PKB in the presence of mTOR blockers—rapamycin (A,C) or CCI-779 (B,D). (A) Three BM-EC donors tested. (B) Representative of 4 BM-EC donors tested. (C) Three cases of 5 patients tested. (D) One representative case of 7 patients tested.
Rapamycin and CCI-779 effectively inhibit cytokine- or leukemia-triggered mTOR signaling. Quiescent BM-ECs were stimulated for 15 minutes with cytokine media/EGM2 (A-B) or 10% vol/vol leukemia plasma (C-D) and analyzed by Western blot for activation of mTOR downstream substrates (S6K1, S6RibP, 4E-BP1, eIF-4G), and activation of Akt/PKB in the presence of mTOR blockers—rapamycin (A,C) or CCI-779 (B,D). (A) Three BM-EC donors tested. (B) Representative of 4 BM-EC donors tested. (C) Three cases of 5 patients tested. (D) One representative case of 7 patients tested.
Rapamycin and CCI-779 inhibit cytokine- and leukemia-induced BM-EC proliferation. BM-ECs were cultured in cytokine media/EGM2 (A-B) or 10% vol/vol leukemia plasma after a period of starvation (C-D); proliferation was measured using an MTS assay. Rapamycin (RAPA; A,C) and CCI-779 (B,D) were tested at the indicated doses. As control for putative solvent toxicity, DMSO was tested at concentrations equivalent to those present in the respective doses of RAPA, with no significant toxicity observed up to the DMSO amount present in 104 ng/mL of the drug (data not shown). Results presented as proliferation index in percentage of control condition ± SEM of experiments using BM-ECs from 4 to 6 different donors. Plasmas from 8 to 10 different leukemia patients were used.
Rapamycin and CCI-779 inhibit cytokine- and leukemia-induced BM-EC proliferation. BM-ECs were cultured in cytokine media/EGM2 (A-B) or 10% vol/vol leukemia plasma after a period of starvation (C-D); proliferation was measured using an MTS assay. Rapamycin (RAPA; A,C) and CCI-779 (B,D) were tested at the indicated doses. As control for putative solvent toxicity, DMSO was tested at concentrations equivalent to those present in the respective doses of RAPA, with no significant toxicity observed up to the DMSO amount present in 104 ng/mL of the drug (data not shown). Results presented as proliferation index in percentage of control condition ± SEM of experiments using BM-ECs from 4 to 6 different donors. Plasmas from 8 to 10 different leukemia patients were used.
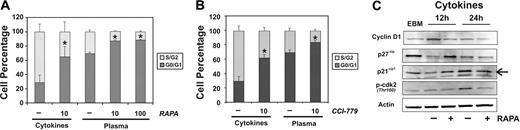
To define the mechanisms mediating the inhibitory effects of mTOR blockers in BM-ECs, apoptosis and cell-cycle analyses were performed. No significant induction of cell apoptosis by rapamycin was observed, even at higher doses (1000 ng/mL; 84.4 ± 4.3% viable cells in rapamycin vs 85.8 ± 2.9 in control DMSO; P > .5). Cell-cycle analysis using BrdU incorporation showed that the antiproliferative effects of rapamycin (Figure 4A) and CCI-779 (Figure 4B) were mediated through modulation of BM-EC cell-cycle progression. Significant increases in cells arrested in G0/G1 were observed in BM-ECs cultured with rapamycin and CCI-779 treatment in both cytokine-stimulated (rapamycin, P < .03; CCI, P < .02) and leukemia-stimulated (rapamycin, P < .001; CCI, P < .001) cells. These studies indicate that blockade of mTOR signaling in BM-ECs prevents cell-cycle progression, thus effectively inhibiting cell proliferation.
Rapamycin and CCI-779 induce G0/G1 arrest of BM-ECs. Cell-cycle analyses were performed at 24 hours, using flow cytometry bivariate distributions of BrdU incorporation versus DNA content (PI). BM-ECs were stimulated with cytokine media/EGM2 or 10% vol/vol leukemia plasma and treated with rapamycin (10, 100 ng/mL) (A) or with CCI-779 (10 nM) (B). Data represent mean ± SEM of experiments using BM-ECs from 3 different donors and 10 different ALL patients. *Significance level of P < .05 using a 2-tailed paired t test. (C) BM-ECs were cytokine/serum deprived for 12 hours (EBM) and then stimulated with cytokine media/EGM2 with or without rapamycin (10 ng/mL) for 12 or 24 hours. Protein levels of cyclin D1, p27kip1, and p21cip1 and the phosphorylation status of cdk2 were determined by Western blot. Arrow indicates correct band. Results are representative of 3 (12-hour time point) or 5 (24-hour time point) different BM-ECs tested.
Rapamycin and CCI-779 induce G0/G1 arrest of BM-ECs. Cell-cycle analyses were performed at 24 hours, using flow cytometry bivariate distributions of BrdU incorporation versus DNA content (PI). BM-ECs were stimulated with cytokine media/EGM2 or 10% vol/vol leukemia plasma and treated with rapamycin (10, 100 ng/mL) (A) or with CCI-779 (10 nM) (B). Data represent mean ± SEM of experiments using BM-ECs from 3 different donors and 10 different ALL patients. *Significance level of P < .05 using a 2-tailed paired t test. (C) BM-ECs were cytokine/serum deprived for 12 hours (EBM) and then stimulated with cytokine media/EGM2 with or without rapamycin (10 ng/mL) for 12 or 24 hours. Protein levels of cyclin D1, p27kip1, and p21cip1 and the phosphorylation status of cdk2 were determined by Western blot. Arrow indicates correct band. Results are representative of 3 (12-hour time point) or 5 (24-hour time point) different BM-ECs tested.
To determine the molecular mechanisms by which mTOR blockade mediates cell-cycle arrest in BM-ECs, we analyzed for changes in the expression levels or phosphorylation status of key players in the cell cycle: cyclin D1, cdk2, and the cdk inhibitors p27kip1 and p21cip1. Quiescent BM-ECs were stimulated with proangiogenic cytokines for 12 or 24 hours in the presence or absence of rapamycin. Cell-cycle progression in BM-ECs was accompanied by increased expression of cyclin D1 and phosphorylation of cdk2, which were inhibited by rapamycin (Figure 4C). Blockade of mTOR activation by rapamycin also prevented the cytokine-mediated down-regulation of p27kip1 and p21cip1, which was evident at 12 hours (Figure 4C). In contrast, at 24 hours, cytokine-stimulation of BM-ECs resulted in increased levels of both p27kip1 and p21cip1, which was abrogated by rapamycin. These observations indicate that inhibition of mTOR prevents molecular events essential for the G1-S transition in BM-ECs, highlighting the critical role of mTOR signaling in these cells.
mTOR blockade inhibits BM-EC proliferation in conditions recapitulating the BM fluid mechanic environment
The biomechanical environment modulates a wide spectrum of cellular functions in tissues, and ECs subjected to blood flow must react to rapid changes in fluid shear stress, cyclic stretch, and pressure.19 Since exposure of ECs to distinct biomechanical conditions translates into activation of different transcriptional profiles,31 we investigated the effect of mTOR blockade on the BM-EC proliferation under conditions that most closely recapitulate the biomechanical environment of the BM. We started by evaluating the proliferation of BM-ECs under different hemodynamic conditions in a perfusion bioreactor, in comparison with venous ECs (HUVECs) and arterial ECs (BAECs). Of interest, BM-ECs showed optimal proliferation at steady flow, 0.5 dyn/cm2 (Figure 5A; P < .001 vs venouslike flow; P < .005 vs arterial-like flow; P < .001 vs no flow), a flow regime that more closely reproduces the fluid mechanical environment of the BM.32,33 In contrast, no significant effect on BM-EC proliferation was observed under flow regimes that support optimal expansion of venous ECs (2 dyn/cm2; steady) or arterial ECs (18 dyn/cm2; 1 Hz pulsatile) (Figure 5A). We then used the bioreactor to test the efficacy of the mTOR blocker CCI-779 on the BM-EC response to cytokines. As shown in Figure 5B, CCI-779 (10 or 100 nM; 40%-48% inhibition; P < .005) markedly inhibited BM-EC proliferation, with an efficacy comparable with that observed under no flow conditions (Figure 2A). These results demonstrate that blockade of mTOR pathway is an effective strategy to target BM-ECs even at flow conditions that recapitulate their biomechanical microenvironment.
Blockade of mTOR and NF-κB pathways synergize to effectively inhibit BM endothelial cells
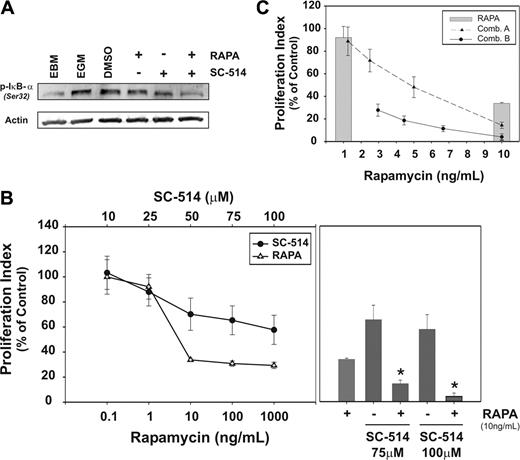
NF-κB pathway plays an important biologic role and may constitute a molecular target for tumor-associated angiogenesis.11,12 Cytokine stimulation of BM-ECs engages this pathway as shown by the phosphorylation of the inhibitory protein IκB-α at Ser32 (Figure 6A), an essential step in the activation of NF-κB.34 Therefore, we evaluated whether blockade of NF-κB signaling could strengthen the inhibitory effects of mTOR blockade in BM-ECs. The IKK-2 inhibitor SC-514 inhibits IκB-α phosphorylation (Figure 6A), an effect that is more pronounced when combined with mTOR blockade. Of importance, inhibition of NF-κB pathway by SC-514 decreases the mitogenic effect of cytokines on BM-ECs, in a dose-dependent manner (Figure 6B). At optimal inhibitory doses for both agents, combination of SC-514 (75 μMor 100 μM) and rapamycin (10 ng/mL) significantly inhibits BM-EC proliferation (Figure 6B, right panel; P < .02 and P < .01), abrogating the mitogenic effect of this cytokine cocktail (85% to 95% reduction in cell proliferation). To determine potential synergism/antagonism in the activity of these drugs on BM-ECs, 2 constant ratio combination curves were generated, using their respective effective doses (combination A: 10 ng/mL rapamycin and 75 μM SC-514, 2-fold dilutions; or combination B: 10 ng/mL rapamycin and 100 μM SC-514, 1.5-fold dilutions; Figure 6C and Table 1). Data were analyzed using the CalcuSyn algorithm to retrieve combination index (CI) values and to determine whether the effects of drug combination were synergistic, additive, or antagonistic. At higher doses, the inhibitory effect of the combined blockade of mTOR and NF-κB is moderately synergistic (Table 1; .7 < CI < .85 for both combinations). Of interest, at lower doses (Table 1; combination B: 4.44 ng/mL/44.44 μM and 66.67 ng/mL/66.67 μM of rapamycin/SC-514, respectively), these agents synergize (.3 < CI <.7) to significantly inhibit BM-EC proliferation (P < .01), with more than 80% reduction of BM-EC proliferation. These results demonstrate that simultaneous blockade of mTOR and NF-κB pathway effectively inhibits or abrogates the proliferative responses of BM-ECs to mitogenic stimuli.
Antiproliferative effects of mTOR blockade are equally effective under BM-like biomechanical conditions. (A) HUVECs, BAECs, and BM-ECs were cultured for 24 hours in a 12-channel perfusion bioreactor in cytokine media/EGM2 and subjected to venouslike (2 dyn/cm2, steady flow), arterial-like (18 dyn/cm2, 1 Hz pulsatile flow), or BM-like flow (0.5 dyn/cm2, steady flow). As control, cells subjected to no-flow conditions were also tested. The results are presented as the ratio between the number of seeded cells and the final number of collected cells (cell proliferation expressed as fold increase). Error bars indicate SD. *Significance level of P < .005 compared with arterial-like flow. ▴Significance level of P < .001 compared with venouslike flow or no flow. (B) BM-ECs were cultured for 3 days in the perfusion bioreactor under BM-like flow conditions in cytokine media/EGM2 alone (Control) or with CCI-779 (10 or 100 nM). Data represent the percentage of proliferation inhibition of drug-treated versus untreated BM-ECs, and error bars indicate SD. The results were obtained with BM-ECs from 3 different donors. *Significance level of P < .005 using a 2-tailed paired t test.
Antiproliferative effects of mTOR blockade are equally effective under BM-like biomechanical conditions. (A) HUVECs, BAECs, and BM-ECs were cultured for 24 hours in a 12-channel perfusion bioreactor in cytokine media/EGM2 and subjected to venouslike (2 dyn/cm2, steady flow), arterial-like (18 dyn/cm2, 1 Hz pulsatile flow), or BM-like flow (0.5 dyn/cm2, steady flow). As control, cells subjected to no-flow conditions were also tested. The results are presented as the ratio between the number of seeded cells and the final number of collected cells (cell proliferation expressed as fold increase). Error bars indicate SD. *Significance level of P < .005 compared with arterial-like flow. ▴Significance level of P < .001 compared with venouslike flow or no flow. (B) BM-ECs were cultured for 3 days in the perfusion bioreactor under BM-like flow conditions in cytokine media/EGM2 alone (Control) or with CCI-779 (10 or 100 nM). Data represent the percentage of proliferation inhibition of drug-treated versus untreated BM-ECs, and error bars indicate SD. The results were obtained with BM-ECs from 3 different donors. *Significance level of P < .005 using a 2-tailed paired t test.
Discussion
We identified mTOR signaling as a molecular event triggered during functional responses of BM endothelium to proangiogenic stimuli and showed that simultaneous blockade of mTOR and NF-κB signaling synergizes to significantly inhibit BM-EC proliferation. We also showed that the mTOR blocker CCI-779 is equally effective in inhibiting BM-ECs under flow conditions that recapitulate the biomechanical environment of the BM.
An obstacle to the generalization of signal transduction and functional studies to different endothelial cells is the diversity of molecular signatures, phenotypes, and responsiveness to biomechanical stimuli of ECs from distinct types, vascular beds, or tissues.14,15,35,36 For example, the differential activation of STAT proteins by VEGF in distinct ECs suggests the existence of different regulatory mechanisms in these cells.37,38 Also, gene-profiling analyses of purified ECs from 2 distinct organs showed differential expression of genes involved in signal transduction, including growth factor receptors, receptor tyrosine kinases, transcription factors, and transcription repressors.39 It is possible that this diversity is even more pronounced between BM endothelial precursors and “mature” ECs, as the former have the ability to undergo distinct programs of cell differentiation and specification. It has been shown that endothelial precursors respond differentially to angiogenic stimuli (VEGF) during differentiation.40 Also, molecular events critical for EC differentiation and cell-type specification, such as Notch signaling, Sonic hedgehog, and the Ephrin-Eph axis, have different effects on distinct endothelium, modulating their responses to proangiogenic stimuli.36,41 We have recently observed that engagement of Notch signaling through Dll4 differentially affects EC proliferation and cell-cycle progression, in a manner that is EC-type specific and intensity dependent (M. João Tavares and A.A.C.; “Delta-4-induced Notch signaling as negative regulator of endothelium”; manuscript submitted October 2005). Therefore, studies are necessary to dissect the molecular circuitry that critically mediates or regulates BM endothelium, namely, on decisions affecting release from quiescence, proliferation, differentiation, morphogenesis, and, in defined conditions, exit from the BM.
mTOR is an integrator of signaling cascades that regulates protein synthesis and RNA biogenesis affecting critical cell functions, such as cell-cycle progression, cell growth, and proliferation.20 Therefore, it is an obvious candidate target for interventions aiming at disrupting tumor cells and their tumor-supportive microenvironment. The direct antitumor effect of mTOR blockade in tumor models of distinct cancers, including B-ALL and other BM malignancies, substantiates this concept.42-44 A few studies also reported that blockade of mTOR, in different tumor models, abrogates tumor-induced angiogenesis, inhibiting metastatic and primary tumor size.22,45 In our studies in bone marrow ECs, we observed that mTOR blockade by rapamycin or CCI-779 significantly inhibited cell proliferation but did not affect BM-EC morphogenesis in a Matrigel assay, in response to either recombinant growth factors or leukemia plasma (data not shown). This is in contrast with studies showing that rapamycin inhibits VEGF-promoted tubelike formation by HUVECs in Matrigel.22 Whether these differences are due to EC type or distinct experimental conditions is unclear. However, our observations are in concordance with reports showing that (1) the formation of capillary-like structures in Matrigel does not involve cell division and is associated with down-regulation of events critical for cell proliferation,46 and (2) actin reorganization processes, which are required for EC morphogenesis,47 are controlled by the rapamycin-insensitive mTOR complex 2 and do not involve the rapamycin-sensitive mTOR complex 1.48
NF-κB inhibitor SC-514 potentiates rapamycin effect on BM-EC proliferation. (A) Semiconfluent BM-ECs were cultured for 12 hours in EBM2 and pretreated for 1 hour with rapamycin (10 ng/mL), SC514 (100 μM), or DMSO (vehicle control). Cells were then stimulated for 15 minutes with cytokine media/EGM2, lysed, and analyzed for activation of NF-κB pathway inhibitory protein IκB-α by Western blot. Data are representative of 4 BM-EC donors tested. (B) BM-ECs were cultured in cytokine media/EGM2 in the presence of rapamycin or SC514 (tested at indicated doses; left panel). Proliferation was measured using an MTS assay, and data are presented as proliferation index in percentage of control condition. At optimal inhibitory doses for both agents, combination of SC-514 (75 μM or 100 μM) and rapamycin (10 ng/mL) was tested (right panel; *significance level of P < .02 and P < .01, respectively). (C) Two constant ratio combinations of rapamycin and SC-514 were tested, with 2-fold (combination A) or 1.5-fold (combination B) dilutions from the highest dose combination (see rows marked with an asterisk in Table 1). Dose-effect analysis was performed using CalcuSyn software, with the combination index (CI) determined by the Chou-Talalay method. Data in panels B and C show a representative case from 4 independent experiments using BM-ECs from 3 to 6 donors. Error bars represent SEM.
NF-κB inhibitor SC-514 potentiates rapamycin effect on BM-EC proliferation. (A) Semiconfluent BM-ECs were cultured for 12 hours in EBM2 and pretreated for 1 hour with rapamycin (10 ng/mL), SC514 (100 μM), or DMSO (vehicle control). Cells were then stimulated for 15 minutes with cytokine media/EGM2, lysed, and analyzed for activation of NF-κB pathway inhibitory protein IκB-α by Western blot. Data are representative of 4 BM-EC donors tested. (B) BM-ECs were cultured in cytokine media/EGM2 in the presence of rapamycin or SC514 (tested at indicated doses; left panel). Proliferation was measured using an MTS assay, and data are presented as proliferation index in percentage of control condition. At optimal inhibitory doses for both agents, combination of SC-514 (75 μM or 100 μM) and rapamycin (10 ng/mL) was tested (right panel; *significance level of P < .02 and P < .01, respectively). (C) Two constant ratio combinations of rapamycin and SC-514 were tested, with 2-fold (combination A) or 1.5-fold (combination B) dilutions from the highest dose combination (see rows marked with an asterisk in Table 1). Dose-effect analysis was performed using CalcuSyn software, with the combination index (CI) determined by the Chou-Talalay method. Data in panels B and C show a representative case from 4 independent experiments using BM-ECs from 3 to 6 donors. Error bars represent SEM.
Mechanistically, we show that growth factor stimulation of quiescent BM-ECs induces cell-cycle progression by up-regulating cyclin D1 expression and cdk2 phosphorylation, an effect reversed by mTOR blockade. These results are consistent with previous reports in murine ECs and smooth muscle cells.49,50 However, in their system, mTOR blockade decreases p21cip1 expression, suggesting that this cdk inhibitor (cdki) is not implicated in the rapamycin-triggered cell-cycle arrest. In BM-ECs, cell-cycle progression was accompanied by down-regulation of the cdki p21cip1 and p27kip1, an effect that was prevented by mTOR blockade at a 12-hour time point, but not at 24 hours. Our data point to the involvement of p21cip1 and p27kip1 in BM-EC early responses to proangiogenic cytokines, and suggest that the inhibitory effects of rapamycin on cell-cycle progression are, at least partially, mediated through the modulation of these cdki's.
Since blockade of mTOR in BM-ECs induces cell-cycle arrest but does not seem to promote significant EC apoptosis, a possible interventional strategy is the combination of mTOR blockers with known cytotoxic agents or other signaling inhibitors. This strategy offers the potential advantages of strengthening the blockade of crucial survival signals and of preventing drug resistance associated with signaling redundancy or cross talk between distinct pathways.51 NF-κB has been implicated in the tumorigenic process, including in tumor angiogenesis. It promotes angiogenic/metastatic gene expression in colorectal cancer,52 where NF-κB/RelA and VEGF are significantly overexpressed,12 and mediates the upregulation of the proangiogenic factors IL-8/CXCL8 and VEGF in prostate cancer cells.53 In BM endothelium, stimulation by proangiogenic stimuli triggers activation of NF-κB, which is reversed by the combination of IKK2-specific inhibitor SC-514 and rapamycin (Figure 6A; L.F.C. and A.A.C., unpublished observations, January 2004); mTOR blockade alone shows no effect on NF-κB activation. A study in HUVECs showed that mTOR blockade accelerates thrombin-induced NF-κB activity in these cells.54 Whether this reflects differences in the overall signaling machinery engaged by these distinct stimuli (proangiogenic cytokines vs thrombin) or variability between the ECs tested (BM-ECs vs HUVECs) is unknown. As mTOR and NF-κB pathways represent distinct signaling cascades transducing activating stimuli into BM-ECs, their simultaneous blockade was likely to strengthen their individual impact on EC proliferation. The demonstration that blockade of NF-κB synergizes with rapamycin to effectively inhibit BM-EC proliferation suggests new therapeutic possibilities for targeting ECs and tumor angiogenesis. The synergistic effects of the simultaneous blockade of these pathways may also allow the use of lower doses of inhibitors of mTOR and NF-κB, with lower risk of undesirable side effects. It has been reported previously that mTOR blockers synergize with other agents to more effectively inhibit malignant cells,55,56 but no comparable studies have been reported on endothelial cells. This report is the first demonstration that mTOR and NF-κB blockade targets a critical component of the tumor microenvironment in cancers evolving in the BM.
Although genetic factors play essential roles in vascular development and EC differentiation, these cell fate decisions are modulated by extrinsic factors.41,57 The mechanical environment dictates a wide spectrum of cellular functions in tissues, with mechanical signals translated into functional responses through the engagement of signaling events regulating cell-cycle progression, migration, and cell-fate decisions.18,58,59 The mitogenic responses of ECs to angiogenic stimuli are significantly influenced by hemodynamic and rehologic conditions. While aortic ECs exhibit signaling peaks under pulsatile, high shear stress flow conditions,18 venous ECs are most active at steady, lower shear stress conditions. To our best knowledge, this is the first demonstration that BM-ECs optimally proliferate under laminar flow conditions that more closely reflect the BM fluid mechanical environment (0.5 dyn/cm2 shear stress steady flow) and show suboptimal expansion under venoustype or arterial-type conditions. The demonstration that blockade of mTOR by CCI-779 is effective in inhibiting BM-EC proliferation to angiogenic stimuli validates the use of the perfusion bioreactor as a valuable tool for evaluating the efficacy of antiangiogenic agents, under experimental conditions that more closely reproduce the biomechanical environment of distinct ECs. This is particularly relevant as it has been proposed that angiogenic intervention may be more effective if tailored for the specific EC type.60
The present study demonstrates that BM endothelial cells' response to proangiogenic stimuli engages mTOR signaling and that its specific targeting significantly inhibits BM-EC proliferation. Blockade of the mTOR pathway may represent an effective strategy to target not only the malignant cell43,44,61 but also its tumor-permissive BM microenvironment. Further studies are necessary to explore the synergistic effects of mTOR and NF-κB inhibition in the angiogenesis evolving in association with BM tumors, and to assess their therapeutic potential in these malignancies.
Prepublished online as Blood First Edition Paper, September 1, 2005; DOI 10.1182/blood-2005-06-2208.
Supported by grants from the National Institutes of Health (P01-CA68484 to L.M.N.; HL49309; and HL62456 to E.R.E.) and the Fundação para Ciência e Tecnologia (FCT-Portugal; SAU/13240 to A.A.C.). L.F.C. is supported by a scholarship from FCT-Portugal.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs W. Nicholas Haining and Ana Limón for the critical reading of the paper. We thank Dr J. Andres Yunes for suggestions and technical advice.